A Wireless Implant for Gastrointestinal Motility Disorders
Abstract
:1. Introduction
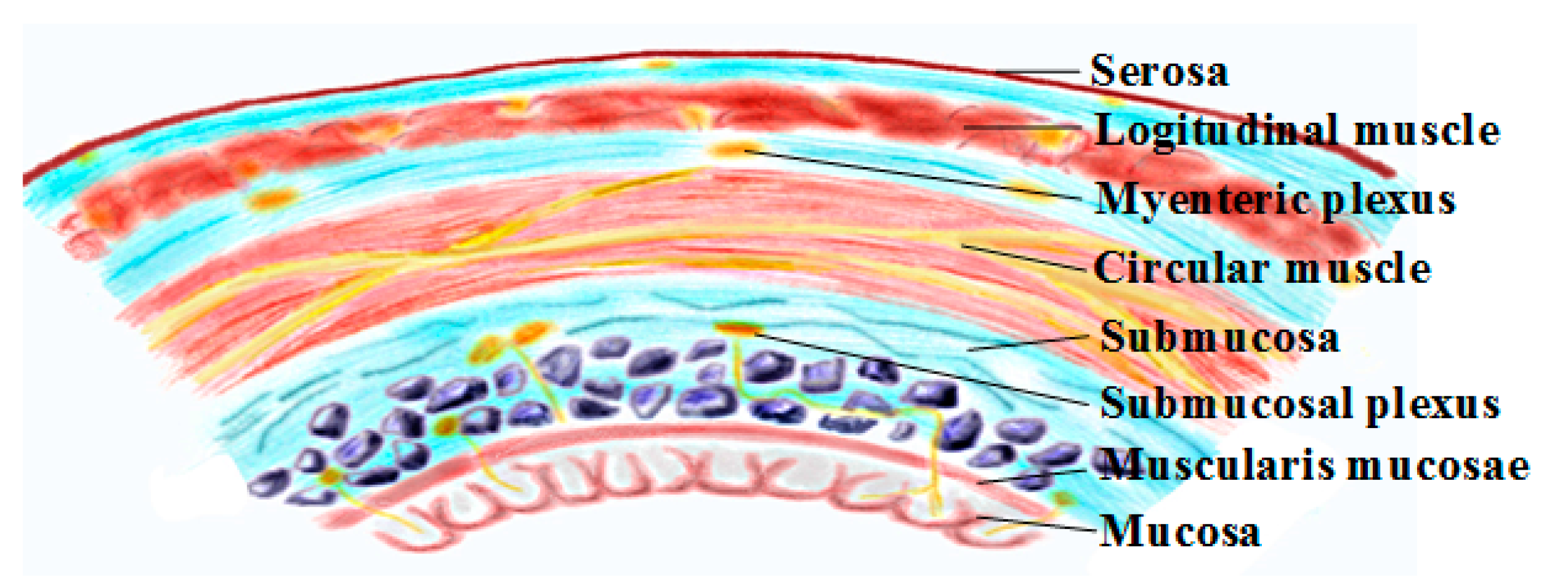
2. Gastrointestinal Physiology and Smooth Muscle Electrophysiology
3. System Design Consideration
3.1. Versatile Stimulation Pattern
3.2. Wireless GI Motility Recording
3.3. Flexible, Electrochemically Safe Electrode and Miniaturized Package
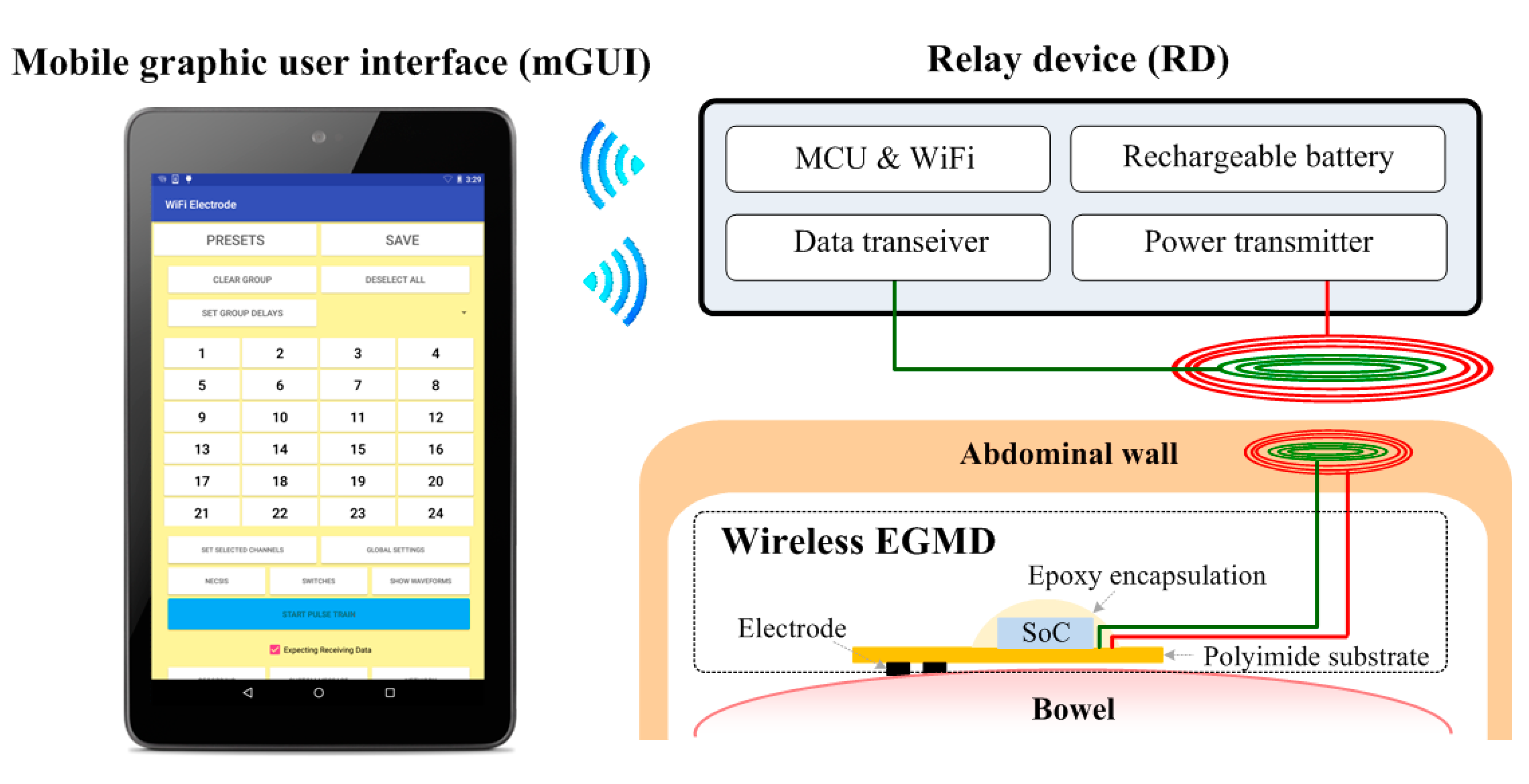
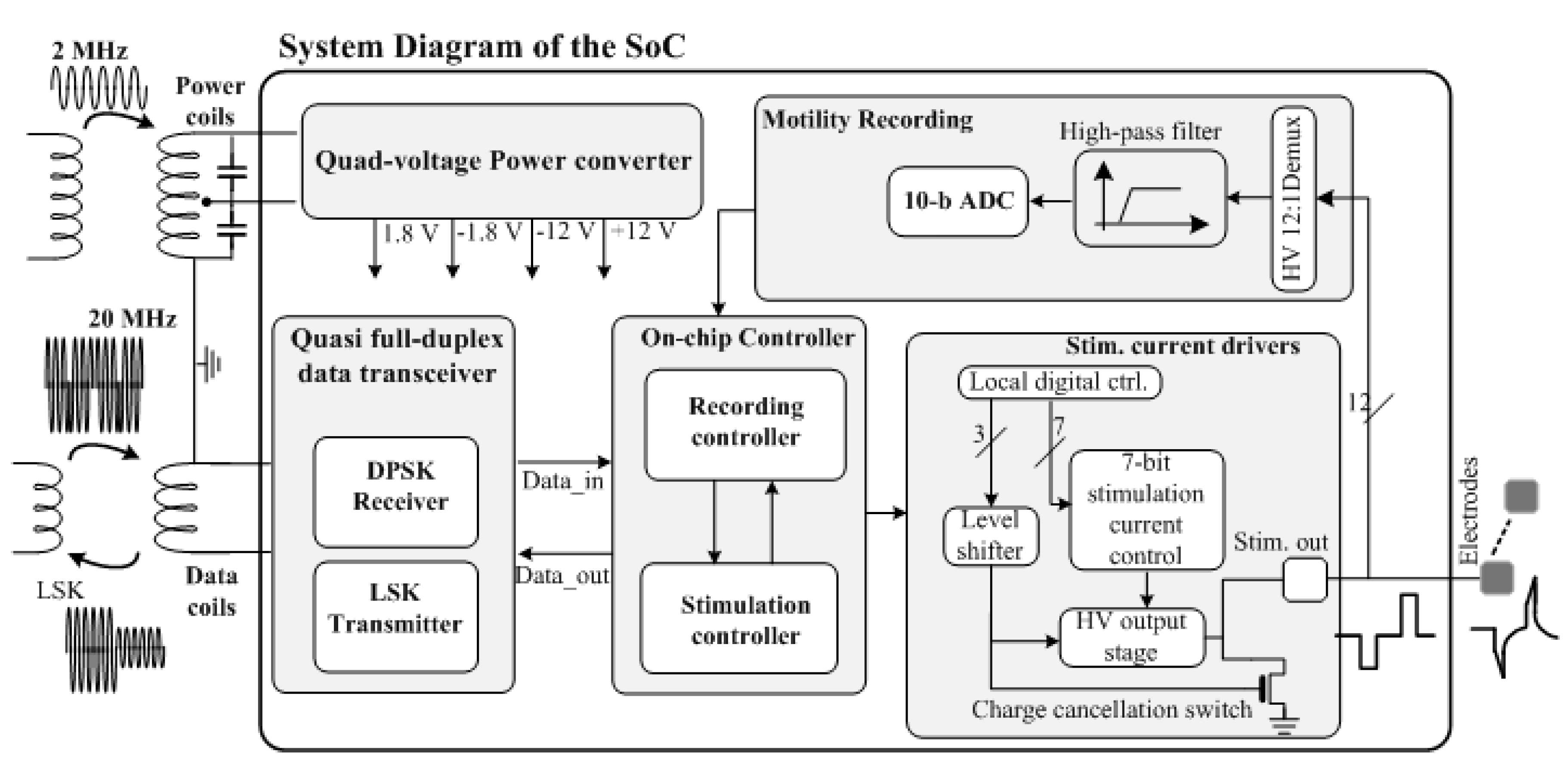
4. Wireless Extraluminal Gastrointestinal Modulation Device (EGMD)
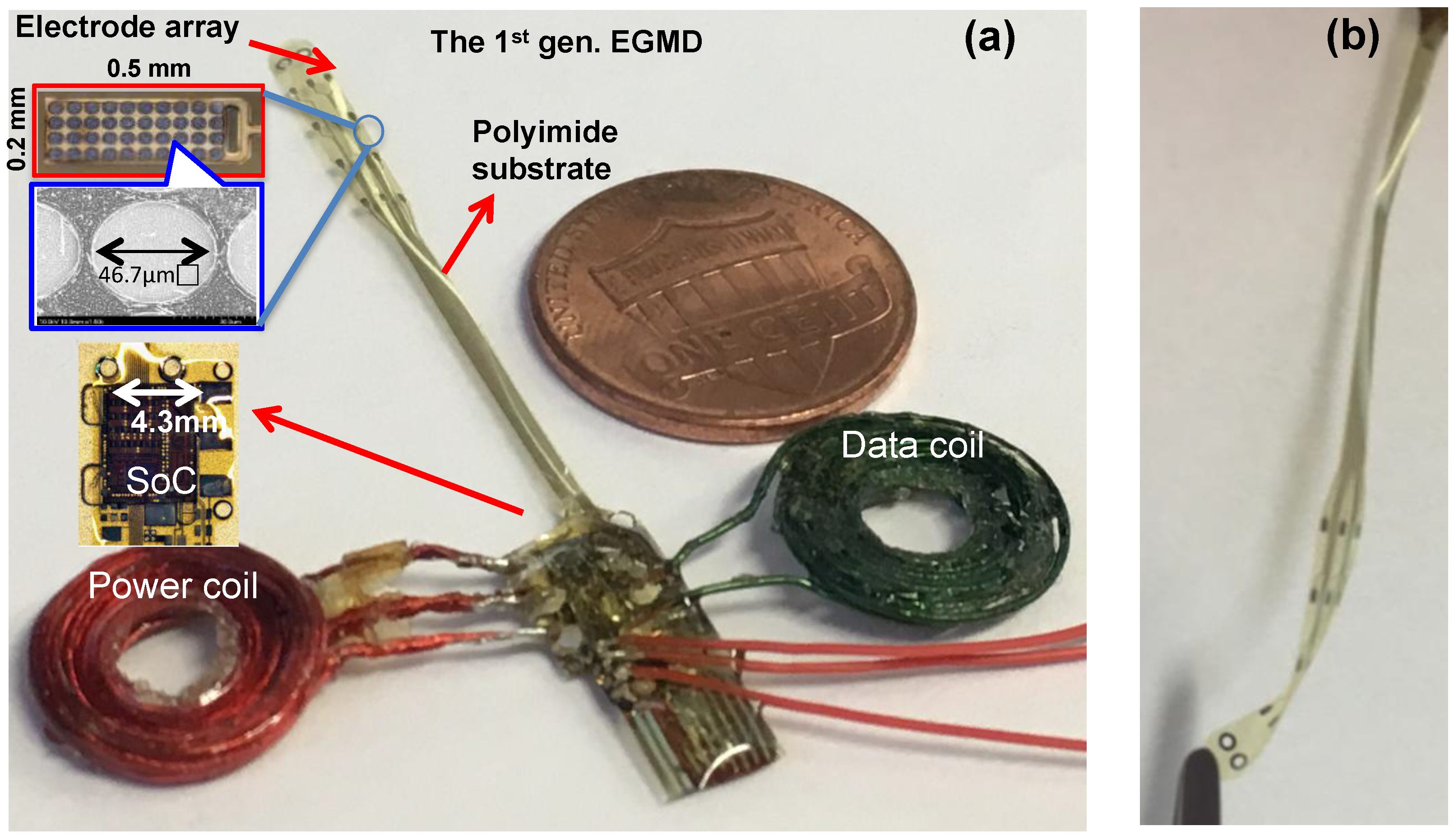
5. Experimental Results
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Halder, S.L.; Locke, G.R.; Schleck, C.D.; Zinsmeister, A.R.; Melton, L.J.; Talley, N.J. Natural history of functional gastrointestinal disorders: A 12-year longitudinal population-based study. Gastroenterology 2007, 133, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Woodward, M.N.; Foley, P.; Cusick, E.L. Colostomy for treatment of functional constipation in children: A preliminary report. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Bilgutay, A.M.; Wingrove, R.; Griffen, W.O.; Bonnabeau, R.C., Jr.; Lillehei, C.W. Gastro-intestinal Pacing A New Concept in the Treatment of Ileus. Ann. Surg. 1963, 158, 338. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Felsher, J.; Brody, F.; Soffer, E. Gastric electrical stimulation significantly increases canine lower esophageal sphincter pressure. Dig. Dis. Sci. 2005, 50, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Cigaina, V. Gastric pacing as therapy for morbid obesity: Preliminary results. Obes. Surg. 2002, 12, 12S–16S. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Chen, J.D. Excitatory effects of synchronized intestinal electrical stimulation on small intestinal motility in dogs. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G1190–G1195. [Google Scholar] [CrossRef] [PubMed]
- Amaris, M.; Rashev, P.; Mintchev, M.; Bowes, K. Microprocessor controlled movement of solid colonic content using sequential neural electrical stimulation. Gut 2002, 50, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Sevcencu, C.; Rijkhoff, N.J.; Sinkjaer, T. Colon emptying induced by sequential electrical stimulation in rats. IEEE Trans. Neural Syst. Rehabilit. Eng. 2005, 13, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Chen, J.D. Mechanisms and potential applications of intestinal electrical stimulation. Digest. Dis. Sci. 2010, 55, 1208–1220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, J. Systematic review: Applications and future of gastric electrical stimulation. Aliment. Pharmacol. Ther. 2006, 24, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, L.; Rodriguez, P.; Gómez, B.; Ayala, J.C.; Oxenberg, D.; Perez-Castilla, A.; Netto, M.G.; Soffer, E.; Boscardin, W.J.; Crowell, M.D. Two-year results of intermittent electrical stimulation of the lower esophageal sphincter treatment of gastroesophageal reflux disease. Surgery 2015, 157, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Wang, Y.; Yan, G.; Jiang, P.; Liu, Z. A gastrointestinal electrical stimulation system based on transcutaneous power transmission technology. Gastroenterol. Res. Pract. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- De Csepel, J.; Goldfarb, B.; Shapsis, A.; Goff, S.; Gabriel, N.; Eng, H. Electrical stimulation for gastroparesis. Surg. Endosc. Other Interv. Tech. 2006, 20, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E. Guyton and Hall Textbook of Medical Physiology; Elsevier Health Sciences: Philadelphia, PA, USA, 2015. [Google Scholar]
- Vantrappen, G.; Janssens, J.; Coremans, G.; Jian, R. Gastrointestinal motility disorders. Digest. Dis. Sci. 1986, 31, 5–25. [Google Scholar] [CrossRef]
- Lo, Y.-K.; Chen, K.; Gad, P.; Liu, W. A fully-integrated high-compliance voltage SoC for epi-retinal and neural prostheses. IEEE Trans. Biomed. Circ. Syst. 2013, 7, 761–772. [Google Scholar]
- Lin, Y.-P.; Yeh, C.-Y.; Huang, P.-Y.; Wang, Z.-Y.; Cheng, H.-H.; Li, Y.-T.; Chuang, C.-F.; Huang, P.-C.; Tang, K.-T.; Ma, H.-P.; et al. A battery-less, implantable neuro-electronic interface for studying the mechanisms of deep brain stimulation in rat models. IEEE Trans. Biomed. Circ. Syst. 2016, 10, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Ortmanns, M.; Rocke, A.; Gehrke, M.; Tiedtke, H.-J. A 232-channel epiretinal stimulator ASIC. IEEE J. Solid-State Circ. 2007, 42, 2946–2959. [Google Scholar] [CrossRef]
- Rhew, H.-G.; Jeong, J.; Fredenburg, J.A.; Dodani, S.; Patil, P.G.; Flynn, M.P. A fully self-contained logarithmic closed-loop deep brain stimulation SoC with wireless telemetry and wireless power management. IEEE J. Solid-State Circ. 2014, 49, 2213–2227. [Google Scholar] [CrossRef]
- Sevcencu, C.; Rijkhoff, N.; Gregersen, H.; Sinkjær, T. Propulsive activity induced by sequential electrical stimulation in the descending colon of the pig. Neurogastroenterol. Motil. 2005, 17, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Familoni, B.O.; Abell, T.L.; Voeller, G.; Salem, A.; Gaber, O. Case report: Electrical stimulation at a frequency higher than basal rate in human stomach. Digest. Dis. Sci. 1997, 42, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Abell, T.L.; van Cutsem, E.; Abrahamsson, H.; Huizinga, J.D.; Konturek, J.; Galmiche, J.P.; VoelIer, G.; Filez, L.; Everts, B.; Waterfall, W.E.; et al. Gastric electrical stimulation in intractable symptomatic gastroparesis. Digestion 2002, 66, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Soffer, E.; Abell, T.; Lin, Z.; Lorincz, A.; McCallum, R.; Parkman, H.; Policker, S.; Ordog, T. Gastric electrical stimulation for gastroparesis-physiological foundations, technical aspects and clinical implications. Aliment. Pharmacol. Ther. 2009, 30, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Abell, T.; McCallum, R.; Hocking, M.; Koch, K.; Abrahamsson, H.; LeBlanc, I.; Lindberg, G.; Konturek, J.; Nowak, T.; Quigley, E.M.M.; et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology 2003, 125, 421–428. [Google Scholar] [CrossRef]
- Dagdeviren, C.; Javid, F.; Joe, P.; von Erlach, T.; Bensel, T.; Wei, Z.; Saxton, S.; Cleveland, S.; Booth, L.; McDonnell, S.; et al. Flexible piezoelectric devices for gastrointestinal motility sensing. Nat. Biomed. Eng. 2017, 1, 807. [Google Scholar] [CrossRef]
- Lo, Y.-K.; Wagner, J.; Chang, C.-W.; Rouch, J.D.; Dunn, J.; Liu, W. Single-electrode colon stimulation and impedance monitoring in an intestinal aganglionosis model. Gastroenterology 2015, 148, S-121–S-122. [Google Scholar] [CrossRef]
- Arriagada, A.; Jurkov, A.; Neshev, E.; Muench, G.; Andrews, C.; Mintchev, M. Design, implementation and testing of an implantable impedance-based feedback-controlled neural gastric stimulator. Physiol. Meas. 2011, 32, 1103. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.-K.; Chang, C.-W.; Liu, W. Bio-impedance characterization technique with implantable neural stimulator using biphasic current stimulus. In Proceedings of the 2014 36th Annual International Conference of the Engineering in Medicine and Biology Society (EMBC), Chicago, IL, USA, 26–30 August 2014; pp. 474–477. [Google Scholar]
- Lo, Y.-K.; Chang, C.-W.; Kuan, Y.-C.; Culaclii, S.; Kim, B.; Chen, K.; Gad, P.; Edgerton, V.R.; Liu, W. 22.2 A 176-channel 0.5 cm3 0.7 g wireless implant for motor function recovery after spinal cord injury. In Proceedings of the 2016 IEEE International Solid-State Circuits Conference (ISSCC), San Francisco, CA, USA, 31 January–4 February 2016; pp. 382–383. [Google Scholar]
- Wang, W.F.; Yin, J.Y.; Chen, J.D.D. Acceleration of small bowel transit in a canine hypermotility model with intestinal electrical stimulation. J. Digest. Dis. 2015, 16, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Enterra ii Neurostimulator. Available online: http://www.medtronic.com/us-en/patients/treatments-therapies/neurostimulator-gastroparesis/enterra-2-neurostimulator.html (accessed on 2 January 2018).
- Endostim Lower Esophageal Sphincter Stimulation System Clinician Manual (With EndoStim II LES Stimulator Model 1006). Available online: https://www.dropbox.com/sh/qo8gdeuq0xj92ok/AAB0KGI_K1drewRI5lKWzlYta/UK%2C%20HK%2C%20NZ?dl=0&preview=CM-09-ENG+Rev+B+EndoStim+II+Clinician+Manual.pdf (accessed on 2 January 2018).
- Lo, Y.-K.; Kuan, Y.-C.; Culaclii, S.; Kim, B.; Wang, P.-M.; Chang, C.-W.; Massachi, J.-A.; Zhu, M.; Chen, K.; Gad, P.; et al. A fully integrated wireless SoC for motor function recovery after spinal cord injury. IEEE Trans. Biomed. Circ. Syst. 2017, 11, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-W.; Lo, Y.-K.; Gad, P.; Edgerton, R.; Liu, W. Design and fabrication of a multi-electrode array for spinal cord epidural stimulation. In Proceedings of the 2014 36th Annual International Conference of the Engineering in Medicine and Biology Society (EMBC), Chicago, IL, USA, 26–30 August 2014; pp. 6834–6837. [Google Scholar]
- Wagner, J.P.; Sullins, V.F.; Khalil, H.A.; Dunn, J.C. A durable model of Hirschsprung’s colon. J. Pediatr. Surg. 2014, 49, 1804–1808. [Google Scholar] [CrossRef] [PubMed]
- Hajer, J.; Novák, M. Development of an autonomous endoscopically implantable submucosal microdevice capable of neurostimulation in the gastrointestinal tract. Gastroenterol. Res. Pract. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Deb, S.; Tang, S.-J.; Abell, T.L.; McLawhorn, T.; Huang, W.-D.; Lahr, C.; Filip, S.D.; Easter, J.; Chiao, J.-C. Development of innovative techniques for the endoscopic implantation and securing of a novel, wireless, miniature gastrostimulator (with videos). Gastrointest. Endosc. 2012, 76, 179–184. [Google Scholar] [CrossRef] [PubMed]


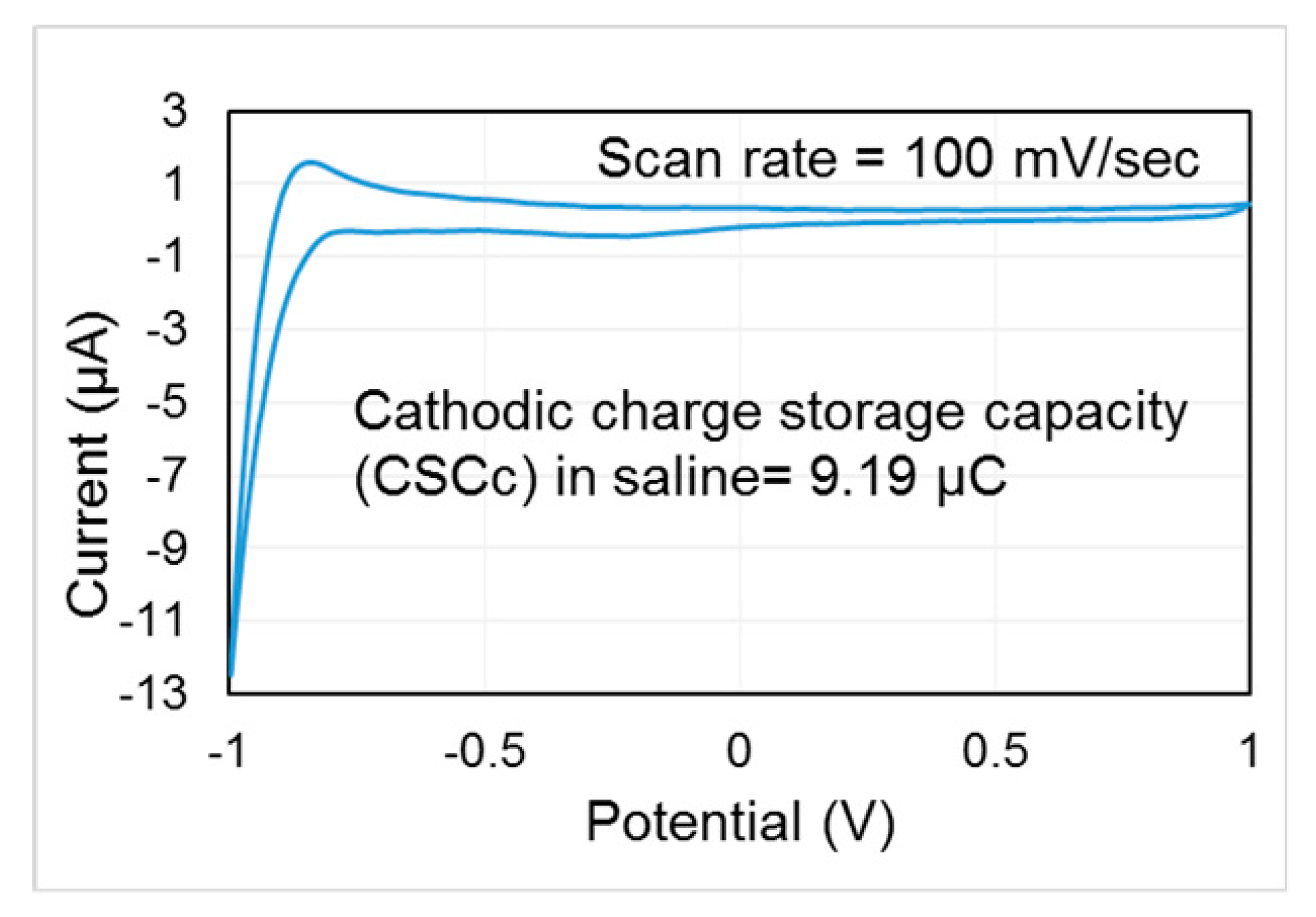
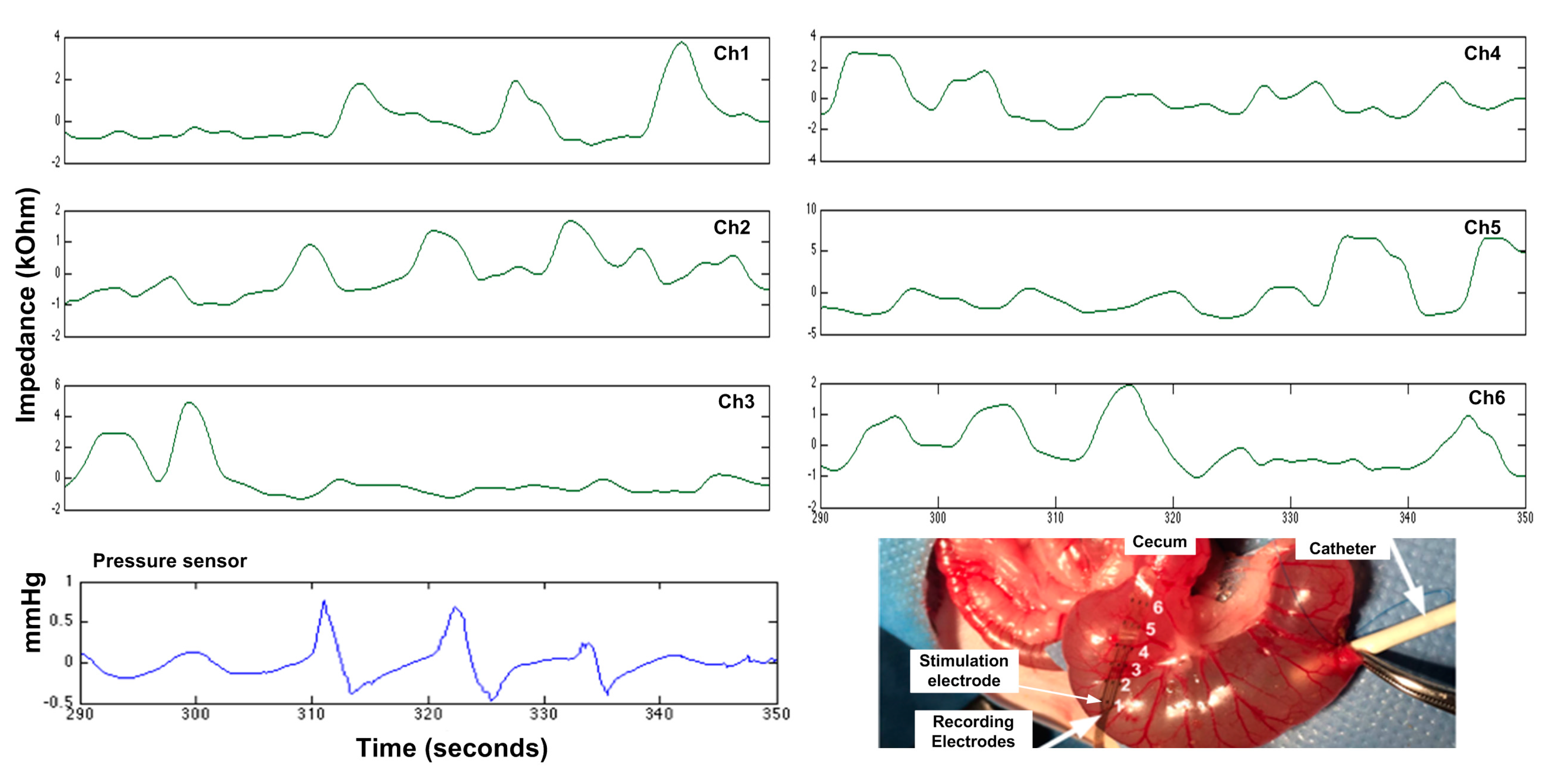

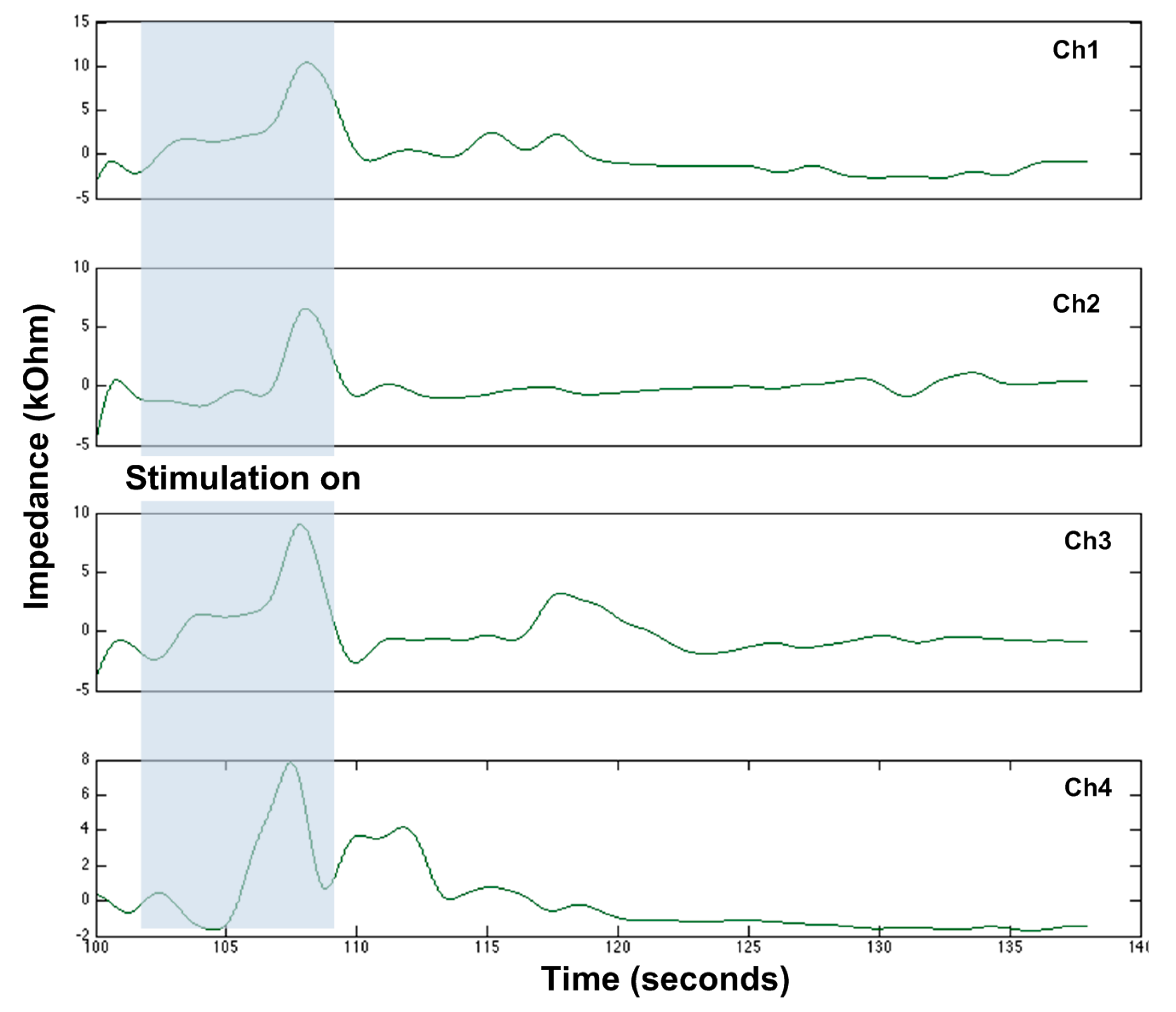
| Reference | [29] | [30] | [25] | [36] | [37] | This Work |
| Power source | Battery 1 | Battery 1 | Battery 1 | Battery 1 | Wireless power | Wireless power |
| Stimulation mode | Current | Current | Voltage | Current | Current | Current |
| Amplitude (mA) | 5 | 2–10 | 4–10 | 5 | 5 | 0.003–20 2 |
| Stimulation frequency (Hz) | 2.1–130 | 2–80 | 10–10 | 2.1–130 | 14 | User defined |
| Stimulation pulse width (ms) | 0.06–0.45 | 0.03–0.975 | 1–100 | 0.06–0.45 | 0.33 | 0.01–User defined |
| Compliance voltage (V) | 10.5 | N/A | 10 | 3 | 3.3 | ±12 |
| Continuous stimulation | Yes | No | No | N/A | No | Yes |
| Pulse train stimulation | Yes | Yes | Yes | Yes | Yes | Yes |
| Residual charge removal | N/A | N/A | N/A | N/A | N/A | Yes |
| Motility recording | No | No | Impedance | No | EGG | Impedance |
| Implant size (cm3)/weight (g) | 37/42 | 20.3/28.5 | 53.4/48.9 | 3.3/3.1 | 2.8/5.2 | ~0.5/~0.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo, Y.-K.; Wang, P.-M.; Dubrovsky, G.; Wu, M.-D.; Chan, M.; Dunn, J.C.Y.; Liu, W. A Wireless Implant for Gastrointestinal Motility Disorders. Micromachines 2018, 9, 17. https://doi.org/10.3390/mi9010017
Lo Y-K, Wang P-M, Dubrovsky G, Wu M-D, Chan M, Dunn JCY, Liu W. A Wireless Implant for Gastrointestinal Motility Disorders. Micromachines. 2018; 9(1):17. https://doi.org/10.3390/mi9010017
Chicago/Turabian StyleLo, Yi-Kai, Po-Min Wang, Genia Dubrovsky, Ming-Dao Wu, Michael Chan, James C. Y. Dunn, and Wentai Liu. 2018. "A Wireless Implant for Gastrointestinal Motility Disorders" Micromachines 9, no. 1: 17. https://doi.org/10.3390/mi9010017
APA StyleLo, Y.-K., Wang, P.-M., Dubrovsky, G., Wu, M.-D., Chan, M., Dunn, J. C. Y., & Liu, W. (2018). A Wireless Implant for Gastrointestinal Motility Disorders. Micromachines, 9(1), 17. https://doi.org/10.3390/mi9010017




