Bio-Inspired Microdevices that Mimic the Human Vasculature
Abstract
1. Introduction
2. 2D Microdevices
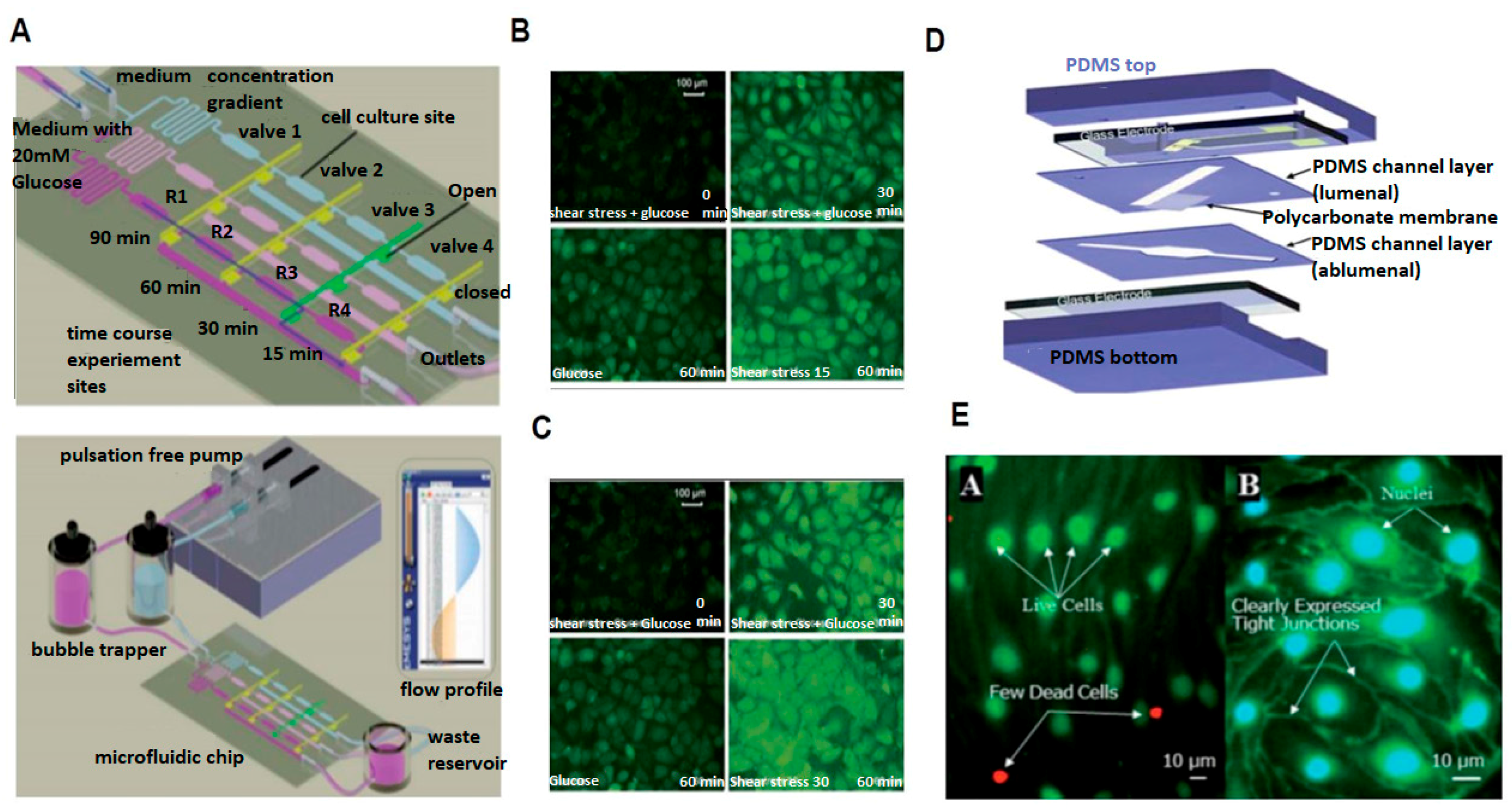
2.1. Probing Cellular Biochemical Response
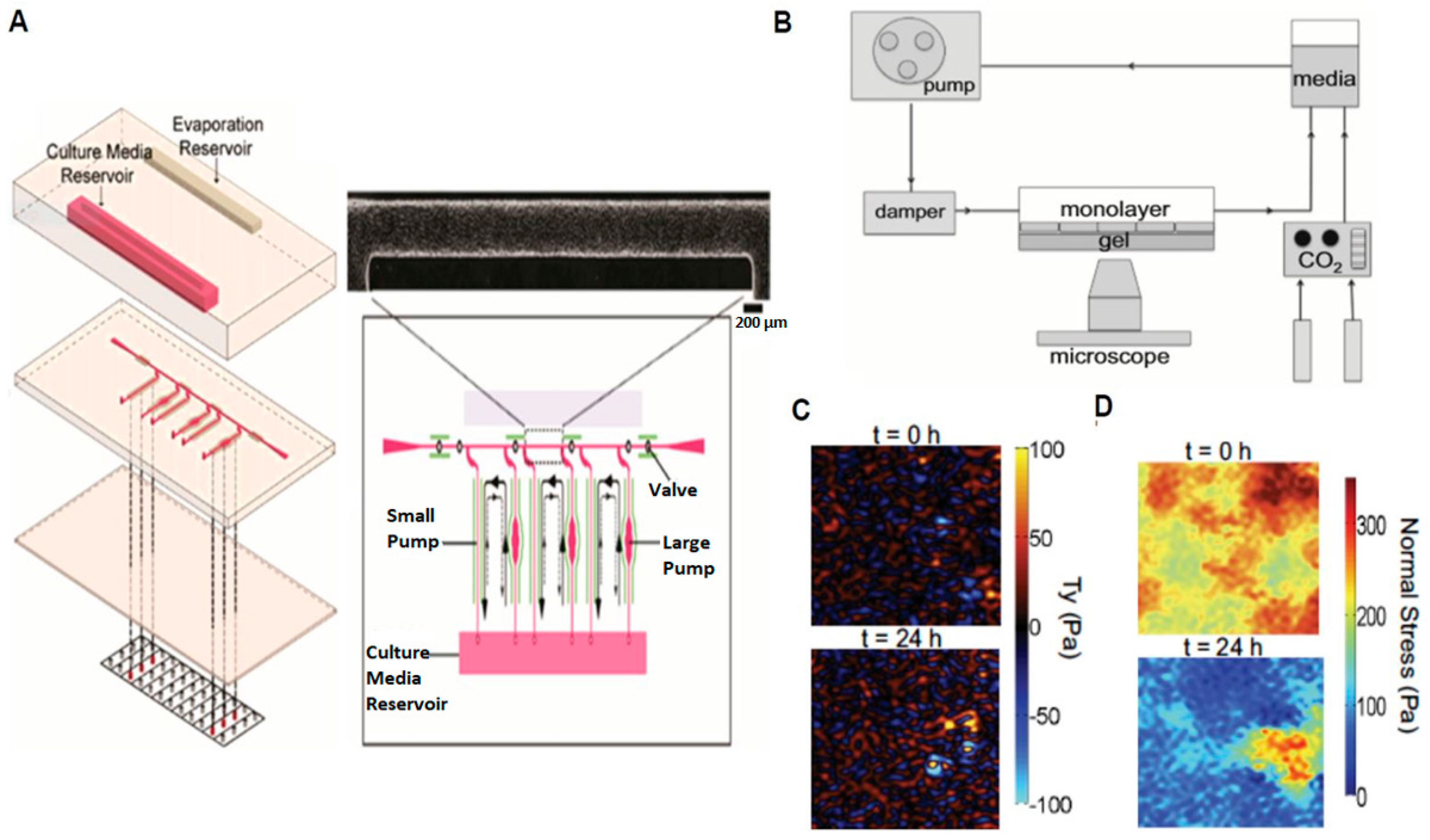
2.2. Probing Cellular Biomechanical Response
2.3. Probing Cellular Barrier Response
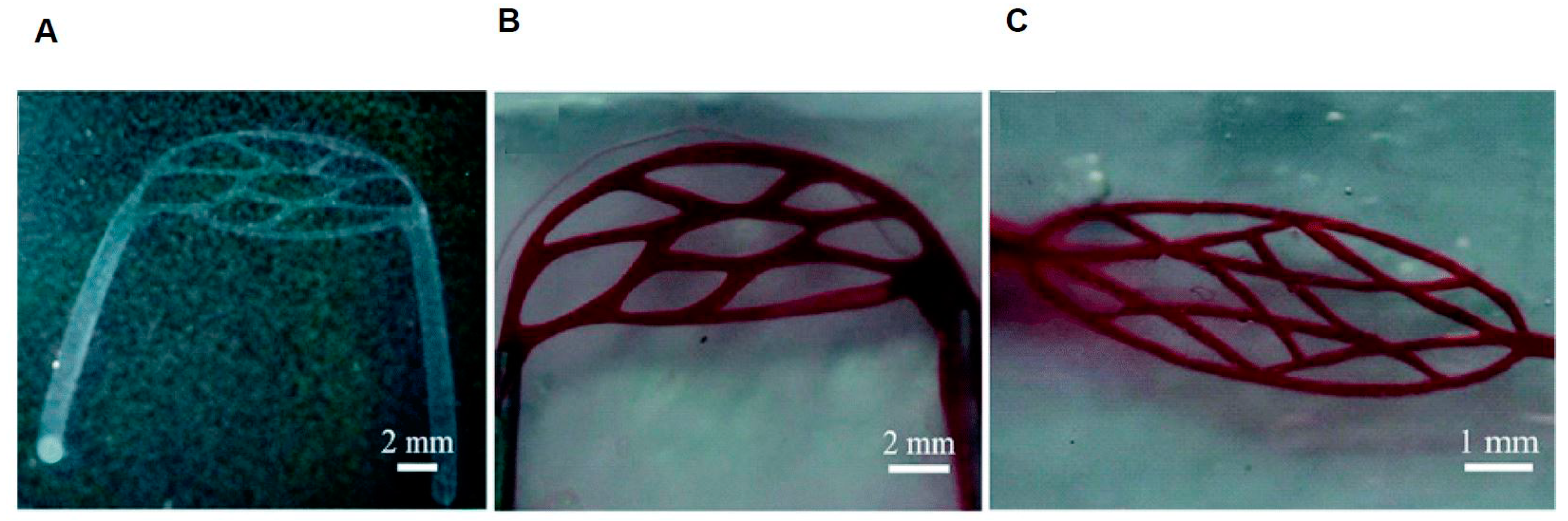
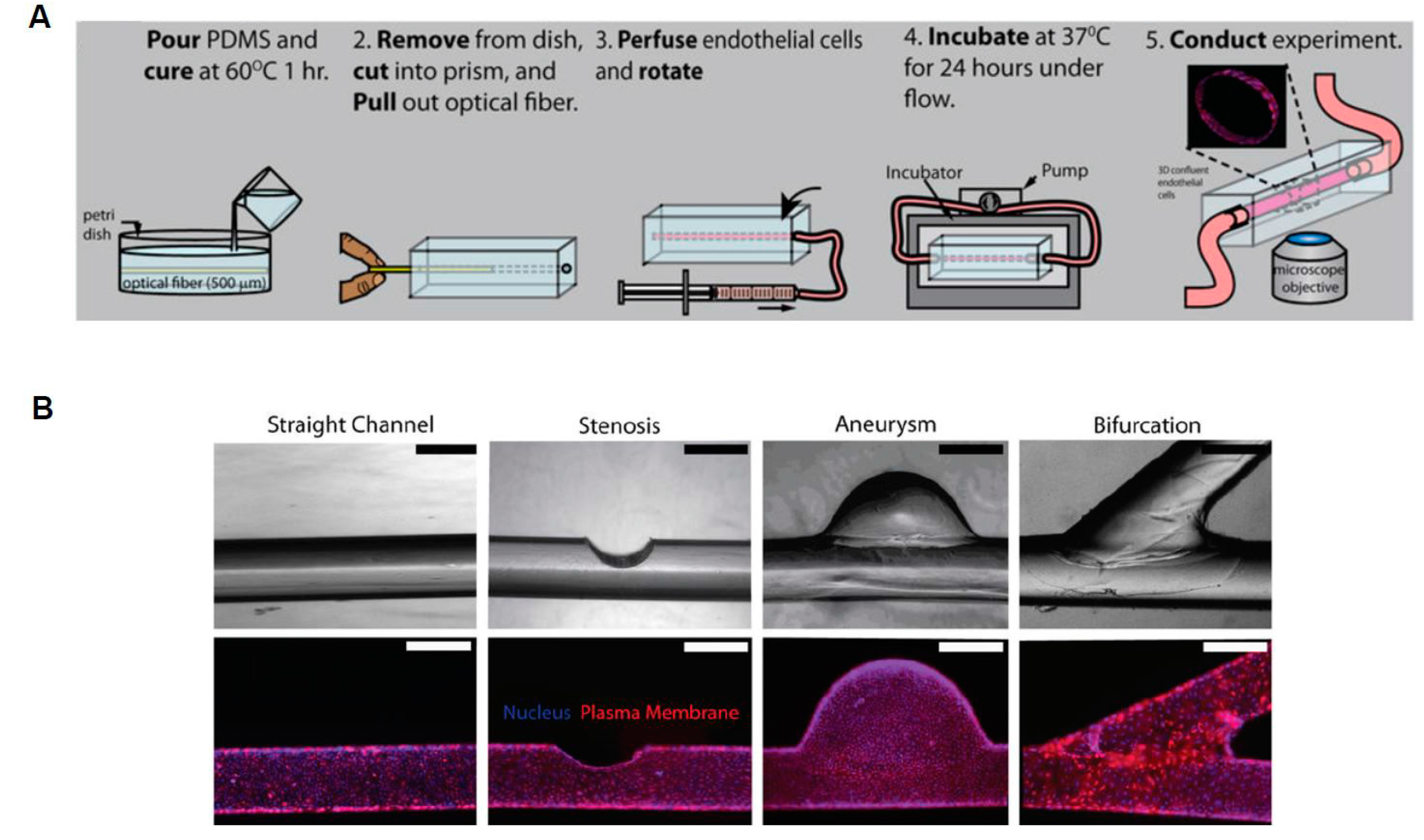
3. 3D Microdevices
4. 2D vs. 3D Microdevices: Advantages and Disadvantages
5. Future Directions
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Tortora, G.J.; Derrickson, B. Principles of Anatomy and Physiology, 14th ed.; Danvers, M.A., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; Volume 34, 1222p. [Google Scholar]
- Polacheck, W.J.; Li, R.; Uzel, S.G.; Kamm, R.D. Microfluidic platforms for mechanobiology. Lab Chip 2013, 13, 2252–2267. [Google Scholar] [PubMed]
- Gimbrone, M.A.; Anderson, K.R.; Topper, J.N. The Critical Role of Mechanical Forces in Blood Vessel Development, Physiology and Pathology. J. Vasc. Surg. 1999, 29, 1104–1151. [Google Scholar] [CrossRef]
- Dewey, C.F.; Bussolari, S.R.; Gimbrone, M.A.; Davies, P.F. The dynamic response of vascular endothelial cells to fluid shear stress. J. Biomech. Eng. 1981, 103, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; Haga, J.H.; Chien, S. Molecular basis of the effects of shear stress on vascular endothelial cells. J. Biomech. 2005, 38, 1949–1971. [Google Scholar]
- Fisher, A.B.; Chien, S.; Barakat, A.I.; Nerem, R.M. Endothelial cellular response to altered shear stress. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 281, L529–L533. [Google Scholar] [PubMed]
- Chau, L.; Doran, M.; Cooper-White, J. A novel multishear microdevice for studying cell mechanics. Lab Chip 2009, 9, 1897–1902. [Google Scholar] [CrossRef] [PubMed]
- Song, J.W.; Gu, W.; Futai, N.; Warner, K.A.; Nor, J.E.; Takayama, S. Computer-controlled microcirculatory support system for endothelial cell culture and shearing. Anal. Chem. 2005, 77, 3993–3999. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Lindken, R.; Hierck, B.P.; Westerweel, J. Tapered microfluidic chip for the study of biochemical and mechanical response at subcellular level of endothelial cells to shear flow. Lab Chip 2009, 9, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Steward, R.; Tambe, D.; Hardin, C.C.; Krishnan, R.; Fredberg, J.J. Fluid shear, intercellular stress, and endothelial cell alignment. Am. J. Physiol. Cell Physiol. 2015, 308, C657–C664. [Google Scholar] [PubMed]
- Shiu, Y.T.; Li, S.; Marganski, W.A.; Usami, S.; Schwartz, M.A.; Wang, Y.L.; Dembo, M.; Chien, S. Rho mediates the shear-enhancement of endothelial cell migration and traction force generation. Biophys. J. 2004, 86, 2558–2565. [Google Scholar] [CrossRef]
- Lam, R.H.; Sun, Y.; Chen, W.; Fu, J. Elastomeric microposts integrated into microfluidics for flow-mediated endothelial mechanotransduction analysis. Lab Chip 2012, 12, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.L.; Tien, J.; Pirone, D.M.; Gray, D.S.; Bhadriraju, K.; Chen, C.S. Cells lying on a bed of microneedles: An approach to isolate mechanical force. Proc. Natl. Acad. Sci. USA 2003, 100, 1484–1489. [Google Scholar] [CrossRef] [PubMed]
- Estrada, R.; Giridharan, G.A.; Nguyen, M.D.; Prabhu, S.D.; Sethu, P. Microfluidic endothelial cell culture model to replicate disturbed flow conditions seen in atherosclerosis susceptible regions. Biomicrofluidics 2011, 5, 320060–3200611. [Google Scholar] [CrossRef] [PubMed]
- Sei, Y.J.; Ahn, S.I.; Virtue, T.; Kim, T.; Kim, Y. Detection of frequency-dependent endothelial response to oscillatory shear stress using a microfluidic transcellular monitor. Sci. Rep. 2017, 7, 10019. [Google Scholar] [CrossRef] [PubMed]
- Chin, L.K.; Yu, J.Q.; Fu, Y.; Yu, T.; Liu, A.Q.; Luo, K.Q. Production of reactive oxygen species in endothelial cells under different pulsatile shear stresses and glucose concentrations. Lab Chip 2011, 11, 1856–1863. [Google Scholar] [CrossRef] [PubMed]
- Booth, R.; Kim, H. Characterization of a microfluidic in vitro model of the blood-brain barrier (µBBB). Lab Chip 2012, 12, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Niklason, L.E. Microfluidic artificial “vessels” for dynamic mechanical stimulation of mesenchymal stem cells. Integr. Biol. 2012, 4, 1487–1497. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Jiang, B.; Wang, D.; Zhang, W.; Wang, Z.; Jiang, X. A microfluidic flow-stretch chip for investigating blood vessel biomechanics. Lab Chip 2012, 12, 3441–3450. [Google Scholar] [CrossRef] [PubMed]
- Steward, R.L.; Tan, C.; Cheng, C.M.; LeDuc, P.R. Cellular force signal integration through vector logic gates. J. Biomech. 2015, 48, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, E.; Petrich, B.G.; Shattil, S.J.; Ginsberg, M.H.; Groisman, A.; Kasirer-Friede, A. Microfluidic devices for studies of shear-dependent platelet adhesion. Lab Chip 2008, 8, 1486–1495. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Yim, Y.; Ahn, K.H.; Lee, S.J. Extensional flow-based assessment of red blood cell deformability using hyperbolic converging microchannel. Biomed. Microdevices 2009, 11, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Yaginuma, T.; Oliveira, M.S.N.; Lima, R.; Ishikawa, T.; Yamaguchi, T. Human red blood cell behavior under homogeneous extensional flow in a hyperbolic-shaped microchannel. Biomicrofluidics 2014, 7, 54110. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.O.; Pinho, D.; Faustino, V.; Lima, R. A simple microfluidic device for the deformability assessment of blood cells in a continuous flow. Biomed. Microdevices 2015, 17, 108. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Ristenpart, W.D.; Stone, H.A. Dynamics of shear-induced ATP release from red blood cells. Proc. Natl. Acad. Sci. USA 2008, 105, 16432–16437. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, A.M.; Wan, J.; Ristenpart, W.D.; Stone, H.A. The dynamic behavior of chemically “stiffened” red blood cells in microchannel flows. Microvasc. Res. 2010, 80, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Yap, B.; Kamm, R.D. Mechanical deformation of neutrophils into narrow channels induces pseudopod projection and changes in biomechanical properties. J. Appl. Physiol. 2005, 98, 1930–1939. [Google Scholar] [CrossRef] [PubMed]
- Griep, L.M.; Wolbers, F.; De Wagenaar, B.; ter Braak, P.M.; Weksler, B.B.; Romero, I.A.; Couraud, P.O.; Vermes, I.; van der Meer, A.D.; van den Berg, A. BBB on chip: microfluidic platform to mechanically and biochemically modulate blood-brain barrier function. Biomed. Microdevices 2013, 15, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Yeon, J.H.; Na, D.; Choi, K.; Ryu, S.W.; Choi, C.; Park, J.K. Reliable permeability assay system in a microfluidic device mimicking cerebral vasculatures. Biomed. Microdevices 2012, 14, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Huang, S.; Yu, K.J.; Clyne, A.M. Dextran and Polymer Polyethylene Glycol (PEG) Coating Reduce Both 5 and 30 nm Iron Oxide Nanoparticle Cytotoxicity in 2D and 3D Cell Culture. Int. J. Mol. Sci. 2012, 13, 5554–5570. [Google Scholar] [CrossRef] [PubMed]
- Trkov, S.; Eng, G.; Di Liddo, R.; Parnigotto, P.P.; Vunjak-Novakovic, G. Micropatterned three-dimensional hydrogel system to study human endothelial—Mesenchymal stem cell interactions. J. Tissue Eng. Regen. Med. 2010, 4, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Jin, Z.H.; Gan, B.W.; Lv, S.W.; Xie, M.; Huang, W.H. Engineering interconnected 3D vascular networks in hydrogels using molded sodium alginate lattice as the sacrificial template. Lab Chip 2014, 14, 3109–3116. [Google Scholar] [CrossRef] [PubMed]
- Mannino, R.G.; Myers, D.R.; Ahn, B.; Wang, Y.; Rollins, M.; Gole, H.; Lin, A.S.; Guldberg, R.E.; Giddens, D.P.; Timmins, L.H.; et al. Do-it-yourself in vitro vasculature that recapitulates in vivo geometries for investigating endothelial-blood cell interactions. Sci. Rep. 2015, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Gershlak, J.R.; Hernandez, S.; Fontana, G.; Perreault, L.R.; Hansen, K.J.; Larson, S.A.; Binder, B.Y.; Dolivo, D.M.; Yang, T.; Dominko, T.; et al. Crossing kingdoms: Using decellularized plants as perfusable tissue engineering scaffolds. Biomaterials 2017, 125, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Abdal-hay, A.; Khalil, K.A.; Al-Jassir, F.F.; Gamal-Eldeen, A.M. Biocompatibility properties of polyamide 6/PCL blends composite textile scaffold using EA.hy926 human endothelial cells. Biomed. Mater. 2017, 12, 035002. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.M.M.; Salerno, S.; Morelli, S.; Giorno, L.; De Bartolo, L. 3D liver membrane system by co-culturing human hepatocytes, sinusoidal endothelial and stellate cells. Biofabrication 2017, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.E.; Kota, N.; Kim, Y.; Wang, Y.; Stolz, D.B.; LeDuc, P.R.; Ozdoganlar, O.B. Fabrication of circular microfluidic channels by combining mechanical micromilling and soft lithography. Lab Chip 2011, 11, 1550–1555. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.H.; Moya, M.L.; Hughes, C.C.; George, S.C.; Lee, A.P. A microfluidic platform for generating large-scale nearly identical human microphysiological vascularized tissue arrays. Lab Chip 2013, 13, 2990–2998. [Google Scholar] [CrossRef] [PubMed]
- Pullens, R.A.A.; Stekelenburg, M.; Baaijens, F.; Post, M.J. The influence of endothelial cells on the ECM composition of 3D engineered cardiovascular constructs. J. Tissue Eng. Regen. Med. 2009, 3, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Bogorad, M.I.; DeStefano, J.; Wong, A.D.; Searson, P.C. Tissue-engineered 3D microvessel and capillary network models for the study of vascular phenomena. Microcirculation 2017, 24, 12. [Google Scholar] [CrossRef] [PubMed]
- Stähli, C.; James-Bhasin, M.; Hoppe, A.; Boccaccini, A.R.; Nazhat, S.N. Effect of ion release from Cu-doped 45S5 Bioglass (R) on 3D endothelial cell morphogenesis. Acta Biomater. 2015, 19, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.D.; Searson, P.C. Live-Cell Imaging of Invasion and Intravasation in an Artificial Microvessel Platform. Cancer Res. 2014, 74, 4937–4945. [Google Scholar] [CrossRef] [PubMed]
- Faustino, V.; Catarino, S.O.; Lima, R.; Minas, G. Biomedical microfluidic devices by using low-cost fabrication techniques: A review. J. Biomech. 2016, 49, 2280–2292. [Google Scholar] [CrossRef] [PubMed]
- Smith, Q.; Gerecht, S. Going with the flow: microfluidic platforms in vascular tissue engineering. Curr. Opin. Chem. Eng. 2014, 3, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Tien, J. Microfluidic approaches for engineering vasculature. Curr. Opin. Chem. Eng. 2014, 3, 36–41. [Google Scholar] [CrossRef]
- Kim, S.; Kim, W.; Lim, S.; Jeon, J.S. Vasculature-On-A-Chip for In Vitro Disease Models. Bioengineering. 2017, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.; Ishikawa, T.; Imai, Y.; Takeda, M.; Wada, S.; Yamaguchi, T. Radial dispersion of red blood cells in blood flowing through glass capillaries: the role of hematocrit and geometry. J. Biomech. 2008, 41, 2188–2196. [Google Scholar] [CrossRef] [PubMed]

| Fabrication Method | Advantages | Disadvantages |
|---|---|---|
| Lithography | Can create complex vascular networks [32]. | Traditionally has squared geometries. |
| Micro Milling | Can be used with lithography [37]. | Resolution depended on milling machine [37]. |
| Angiogenesis | Cellular action created channels [38]. | Difficulty creating consistent geometry [38]. |
| Rod/Wire Template. | Can Create simple anatomically abnormal vessels [33,42]. | Unable to create complex networks [42]. |
| Model Dimensions | Advantages | Disadvantages |
|---|---|---|
| 2D | Lab on chip technology [2,33,38]. Fast data processing times and simulations. | Lacks 3D geometric cell considerations [30]. |
| 3D | Able to more accurately model 3D cell interactions [30]. Able to model anatomical abnormalities [33]. | May require stacked images for analyses. More complex data analysis [33,40]. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.M.; Beverung, S.; Steward Jr., R. Bio-Inspired Microdevices that Mimic the Human Vasculature. Micromachines 2017, 8, 299. https://doi.org/10.3390/mi8100299
Islam MM, Beverung S, Steward Jr. R. Bio-Inspired Microdevices that Mimic the Human Vasculature. Micromachines. 2017; 8(10):299. https://doi.org/10.3390/mi8100299
Chicago/Turabian StyleIslam, Md. Mydul, Sean Beverung, and Robert Steward Jr. 2017. "Bio-Inspired Microdevices that Mimic the Human Vasculature" Micromachines 8, no. 10: 299. https://doi.org/10.3390/mi8100299
APA StyleIslam, M. M., Beverung, S., & Steward Jr., R. (2017). Bio-Inspired Microdevices that Mimic the Human Vasculature. Micromachines, 8(10), 299. https://doi.org/10.3390/mi8100299





