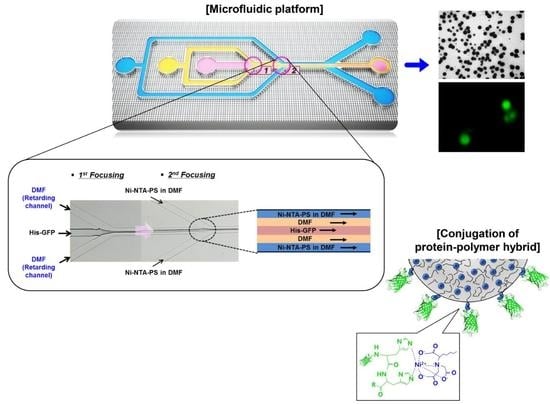
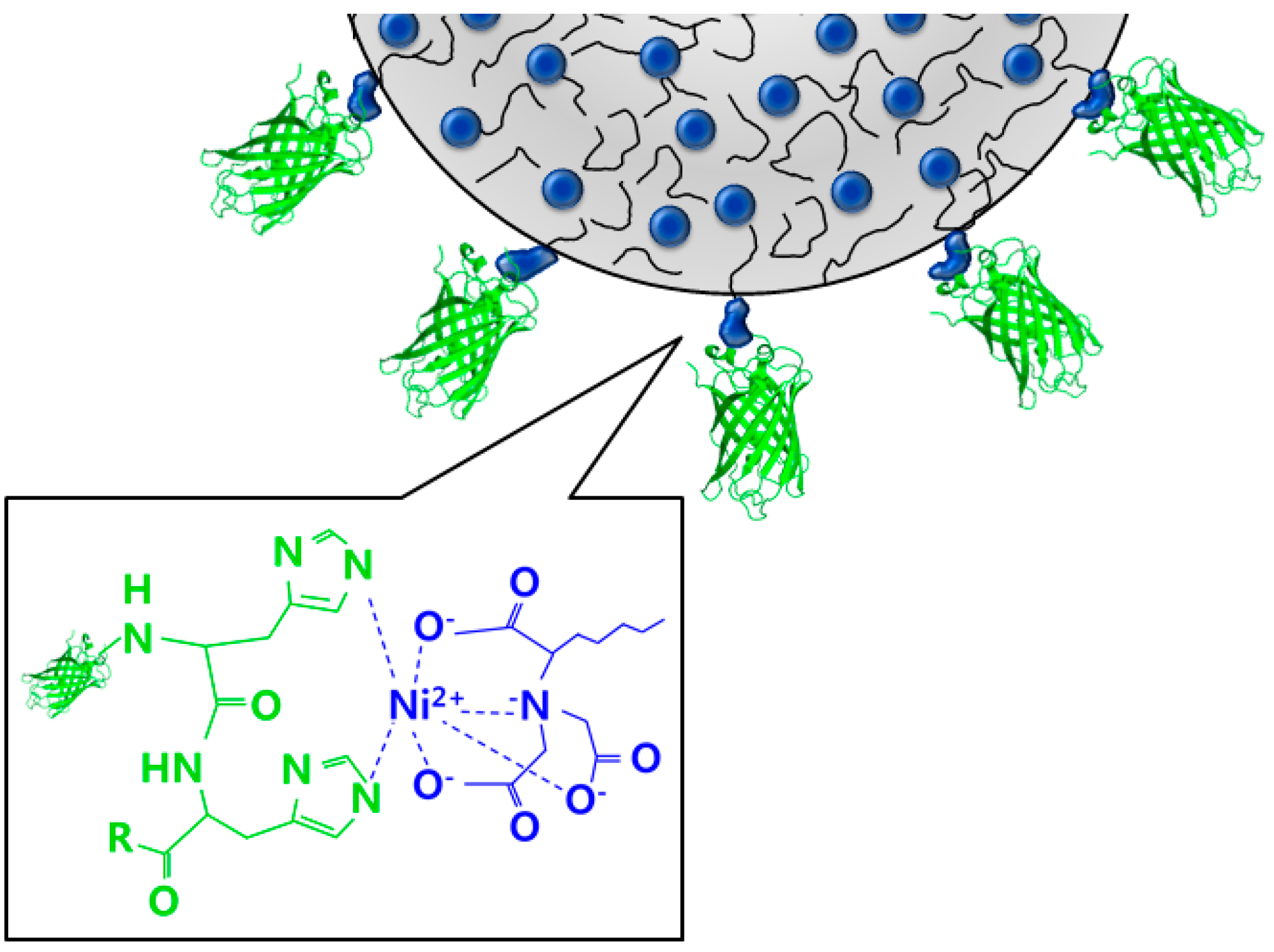
Preparation of Protein Nanoparticles Using NTA End Functionalized Polystyrenes on the Interface of a Multi-Laminated Flow Formed in a Microchannel
Abstract
:1. Introduction
2. Synthesis of Materials
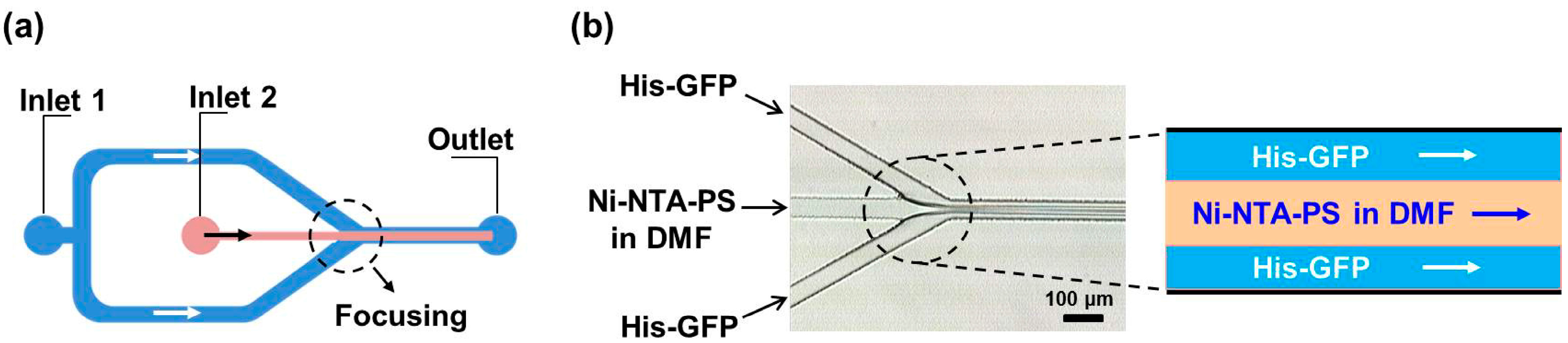
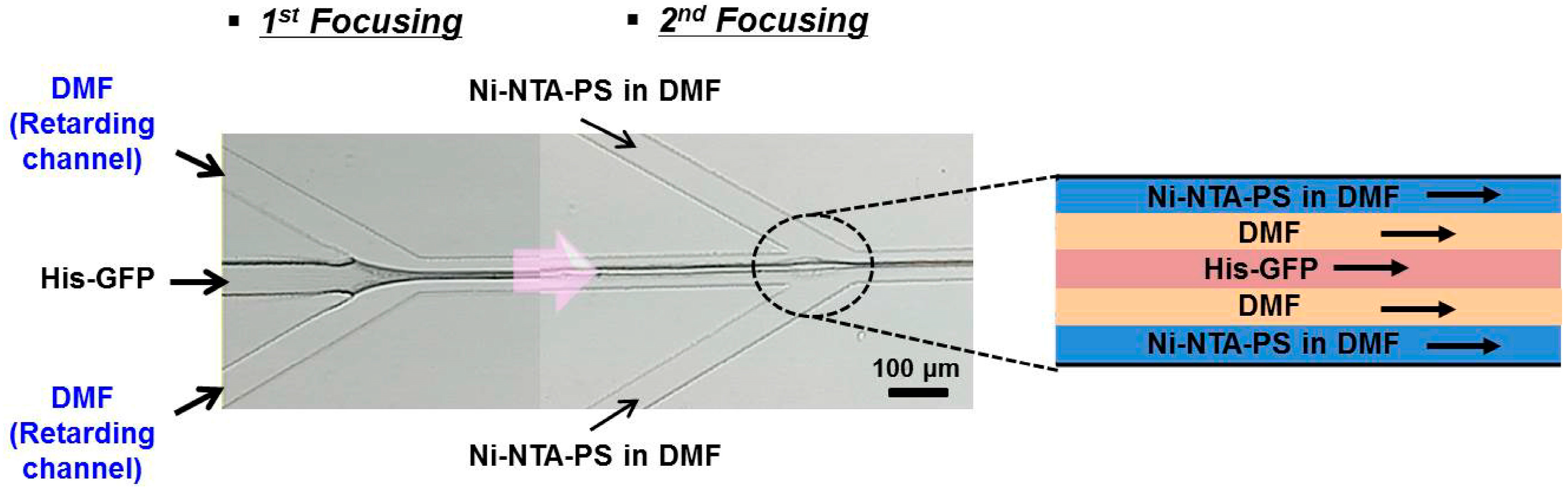
3. Microfluidic Preparation of Protein Nanoparticles
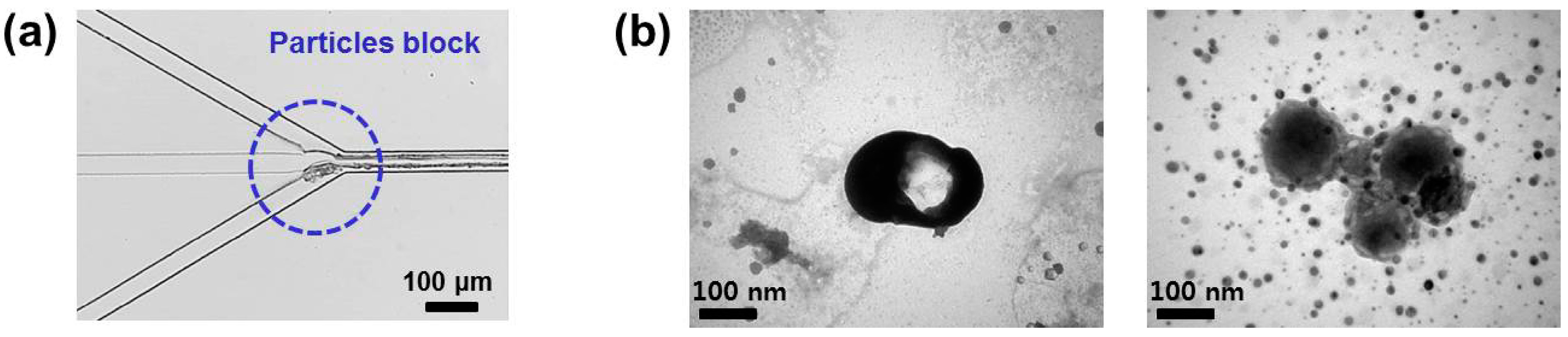
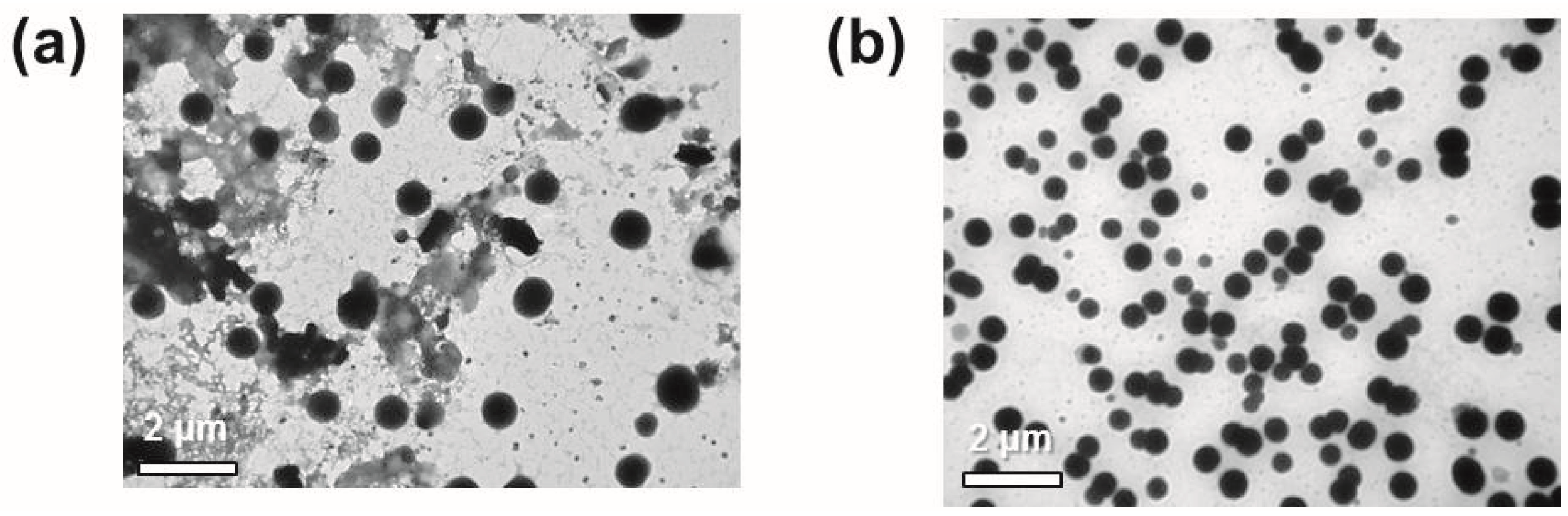
4. Evaluation of the Produced Protein Nanoparticles
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tiwari, G.; Tiwari, R.; Sriwastawa, S.; Bhati, L.; Pandey, S.; Pandey, P.; Bannerjee, S.K. Drug delivery systems: An updated review. Int. J. Pharm. Investig. 2012, 2, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Athar, M.; Das, A.J. Therapeutic nanoparticles: State-of-the-art of nanomedicine. Adv. Mater. Rev. 2014, 1, 25–37. [Google Scholar]
- Thakor, A.S.; Gambhir, S.S. Nanooncology: The future of cancer diagnosis and therapy. CA Cancer. J. Clin. 2013, 63, 395–418. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.M.; Valencia, P.M.; Zhang, L.; Langer, R.; Farokhzad, O.C. Polymeric nanoparticles for drug delivery. Methods Mol. Biol. 2010, 624, 163–175. [Google Scholar] [PubMed]
- Cryan, S.A. Carrier-based strategies for targeting protein and peptide drugs to the lungs. AAPS J. 2005, 7, E20–E41. [Google Scholar] [CrossRef] [PubMed]
- Marin, E.; Briceno, M.I.; Caballero-George, C. Critical evaluation of biodegradable polymers used in nanodrugs. Int. J. Nanomed. 2013, 8, 3071–3091. [Google Scholar]
- Veronese, F.M.; Pasut, G. PEGylation successful approach to drug delivery. Drug Discov. Today 2005, 10, 1451–1458. [Google Scholar] [CrossRef]
- Vert, M. Aliphatic polyesters: Great degradable polymers that cannot do everything. Biomacromolecules 2005, 6, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Vriezema, D.M.; Garcia, P.M.L.; Sancho Oltra, N.; Hatzakis, N.S.; Kuiper, S.M.; Nolte, R.J.M.; Rowan, A.E.; Van Hest, J.C.M. Positional assembly of enzymes in polymersome nanoreactors for cascade reactions. Angew. Chem. Int. Ed. 2007, 46, 7378–7382. [Google Scholar] [CrossRef] [PubMed]
- Dirks, A.J.; Nolte, R.J.M.; Cornelissen, J.J.L.M. Protein–polymer hybrid amphiphiles. Adv. Mater. 2008, 20, 3953–3957. [Google Scholar] [CrossRef]
- Petrak, K. Essential properties of drug-targeting delivery systems. Drug Discov. Today 2005, 10, 1667. [Google Scholar] [CrossRef]
- Ge, J.; Neofytou, E.; Lei, J.; Beygui, R.E.; Zare, R.N. Protein-polymer hybrid nanoparticles for drug Delivery. Small 2012, 8, 3573. [Google Scholar] [CrossRef] [PubMed]
- Hubbell, J.A.; Chilkoti, A. Nanomaterials for drug delivery. Science 2012, 337, 303. [Google Scholar] [CrossRef] [PubMed]
- Mastrobattista, E.; Van der Aa, M.A.E.M.; Hennink, W.E.; Crommelin, D.J.A. Artificial viruses: A nanotechnological approach to gene delivery. Nat. Rev. Drug Discov. 2006, 5, 115. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P.; Lukyanov, A.N. Peptide and protein drug delivery to and into tumors: Challenges and solutions. Drug Discov. Today 2003, 8, 259. [Google Scholar] [CrossRef]
- Kelkar, S.S.; Reineke, T.M. Theranostics: Combining imaging and therapy. Bioconjug. Chem. 2011, 22, 1897. [Google Scholar] [CrossRef] [PubMed]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Polymer-based nanocapsules for drug delivery. Int. J. Pharm. 2010, 385, 113–142. [Google Scholar] [CrossRef] [PubMed]
- Lertsutthiwong, P.; Noomun, K.; Jongaroonngamsang, N.; Rojsitthisak, P.; Nimmannit, U. Preparation of alginate nanocapsules containing turmeric oil. Carbohydr. Polym. 2008, 74, 209–214. [Google Scholar] [CrossRef]
- Pisani, E.; Fattal, E.; Paris, J.; Ringard, C.; Rosilio, V.; Tsapis, N. Surfactant dependent morphology of polymeric capsule of perfluorooctyl bromide: Influence of polymer adsorption at the dichrloromethane-water interface. J. Colloid Interface Sci. 2008, 326, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Lutter, S.; Koetz, J.; Tiersch, B.; Boschetti de Fierro, A.; Abetz, V. Formation of gold nanoparticles in triblock terpolymer-modified inverse microemulsions. Colloid Surf. A. 2008, 329, 160–176. [Google Scholar] [CrossRef]
- Guinebretiere, S.; Briancon, S.; Fessi, H.; Teodorescu, V.S.; Blanchin, M.G. Nanocapsules of biodegradable polymers: Preparation and characterization by direct high resolution elctron microscopy. Mater. Sci. Eng. C. 2002, 21, 137–142. [Google Scholar] [CrossRef]
- Limayem, I.; Charcosset, C.; Fessi, H. Purification of nanoparticle suspensions by a concentration/diafiltration process. Sep. Purif. Technol. 2004, 38, 1–9. [Google Scholar] [CrossRef]
- Choi, M.J.; Soottutantawat, A.; Nuchuchua, O.; Min, S.G.; Ruktanonchai, U. Physical and light oxidative properties of eugenol encapsulated by molecular inclusion and emulsion-diffusion method. Food Res. Int. 2009, 42, 148–156. [Google Scholar] [CrossRef]
- Rao, J.P.; Geckeler, K.E. Polymer nanoparticles: Preparation techniques and size-control parameters. Prog. Polym. Sci. 2011, 36, 887–913. [Google Scholar] [CrossRef]
- Shimanovich, U.; Bernardes, G.J.L.; Knowles, T.P.J.; Cavaco-Paulo, A. Protein micro- and nano-capsules for biomedical applications. Chem. Soc. Rev. 2014, 43, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Hung, L.H.; Lee, A.P. Microfluidic devices for the synthesis of nanoparticles and biomaterials. J. Med. Biol. Eng. 2007, 27, 1. [Google Scholar]
- Karnik, R.; Gu, F.; Basto, P.; Cannizzaro, C.; Dean, L.; Kyei-Manu, W.; Farokhzad, O.C. Microfluidic platform for controlled synthesis of polymeric nanoparticles. Nano Lett. 2008, 8, 2906–2912. [Google Scholar] [CrossRef] [PubMed]
- Jahn, A.; Vreeland, W.N.; Gaitan, M.; Locascio, L.E. Controlled vesicle self-assembly in microfluidic channels with hydrodynamic focusing. J. Am. Chem. Soc. 2004, 126, 2674–2675. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.; Jeon, H.J.; Kwon, B.H.; Kim, H.H.; Tran, D.L.; Morten, K.; Go, J.S. Synthesis fluorescent magnetic nanoparticles in a microchannel using the La Mer process and the characterization of their properties. J. Mater. Sci. 2014, 49, 4583–4589. [Google Scholar] [CrossRef]
- Cho, H.Y.; Kadir, M.A.; Kim, B.S.; Han, H.S.; Nagasundarapadian, S.; Kim, Y.R.; Ko, S.B.; Lee, S.G.; Paik, H.J. Synthesis of well-defined (nitrilotriacetic acid)-end-functionalized polystyrenes and their bioconjugation with histidine-tagged green fluorescent proteins. Macromolecules 2011, 44, 4672–4680. [Google Scholar] [CrossRef]
- Kardir, M.A.; Kim, S.J.; Ha, E.J.; Cho, H.Y.; Kim, B.S.; Choi, D.H.; Lee, S.G.; Kim, B.G.; Kim, S.W.; Paik, H.J. Encapsulation of nanoparticles using nitrilotriacetic acid end-functionalized polystyrenes and their application for the separation of proteins. Adv. Funct. Mater. 2012, 22, 4032–4037. [Google Scholar] [CrossRef]
- Kadir, M.A.; Lee, C.Y.; Han, H.S.; Kim, B.S.; Ha, E.J.; Jeong, J.H.; Song, J.K.; Lee, S.G.; Ahn, S.S.; Paik, H.J. In situ formation of polymer-protein hybrid spherical aggregates from (nitrilotriacetic acid)-end-functionalized polystyrenes and His-tagged proteins. Polym. Chem. 2013, 4, 2286–2292. [Google Scholar] [CrossRef]
- Toedorescu, M.; Matyjaszewski, K. Atom transfer radical polymerization of (meth)acrylamides. Macromolecules 1999, 32, 4826–4831. [Google Scholar] [CrossRef]
- Jahn, A.; Stavis, S.M.; Hong, J.S.; Vreeland, W.N.; DeVoe, D.L.; Gaitan, M. Microfluidic mixing and the formation of nanoscale lipid vesicles. ACS Nano 2010, 4, 2077–2087. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Kim, E.Y.; Park, J.C.; Chang, S.C.; Park, Y.J.; Morten, K.; Go, J.S. Continuous and surfactant-free preparation of nanocapsulized proteins. Microfluid. Nanofluid. 2012, 12, 141–149. [Google Scholar] [CrossRef]
- Oh, M.C.; Park, J.H.; Jeon, H.J.; Go, J.S. Hollow-core polymeric nanoparticles for the enhancement of OLED outcoupling efficiency. Displays 2015, 37, 72–78. [Google Scholar] [CrossRef]
- Kolishetti, N.; Dhar, S.; Valencia, P.M.; Lin, L.Q.; Kamik, R.; Lippard, S.J.; Langer, R.; Farokhzad, O.C. Engineering of self-assembled nanoparticle platform for precisely controlled combination drug therapy. Proc. Natl. Acad. Sci. USA 2010, 107, 17939–17944. [Google Scholar] [CrossRef] [PubMed]


| Entry | Flow Rate of His-GFP (μL/min) | Lamination Width of Second Focusing (μm) | Mean Diameter of Nanoparticle (nm) |
|---|---|---|---|
| 1 | 50 | 8.06 | 49 3 |
| 2 | 100 | 11.29 | 237 13 |
| 3 | 125 | 12.90 | 350 16 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, H.J.; Lee, C.Y.; Kim, M.J.; Nguyen, X.D.; Park, D.H.; Kim, H.H.; Go, J.S.; Paik, H.-j. Preparation of Protein Nanoparticles Using NTA End Functionalized Polystyrenes on the Interface of a Multi-Laminated Flow Formed in a Microchannel. Micromachines 2017, 8, 10. https://doi.org/10.3390/mi8010010
Jeon HJ, Lee CY, Kim MJ, Nguyen XD, Park DH, Kim HH, Go JS, Paik H-j. Preparation of Protein Nanoparticles Using NTA End Functionalized Polystyrenes on the Interface of a Multi-Laminated Flow Formed in a Microchannel. Micromachines. 2017; 8(1):10. https://doi.org/10.3390/mi8010010
Chicago/Turabian StyleJeon, Hyeong Jin, Chae Yeon Lee, Moon Jeong Kim, Xuan Don Nguyen, Dong Hyeok Park, Hyung Hoon Kim, Jeung Sang Go, and Hyun-jong Paik. 2017. "Preparation of Protein Nanoparticles Using NTA End Functionalized Polystyrenes on the Interface of a Multi-Laminated Flow Formed in a Microchannel" Micromachines 8, no. 1: 10. https://doi.org/10.3390/mi8010010
APA StyleJeon, H. J., Lee, C. Y., Kim, M. J., Nguyen, X. D., Park, D. H., Kim, H. H., Go, J. S., & Paik, H.-j. (2017). Preparation of Protein Nanoparticles Using NTA End Functionalized Polystyrenes on the Interface of a Multi-Laminated Flow Formed in a Microchannel. Micromachines, 8(1), 10. https://doi.org/10.3390/mi8010010