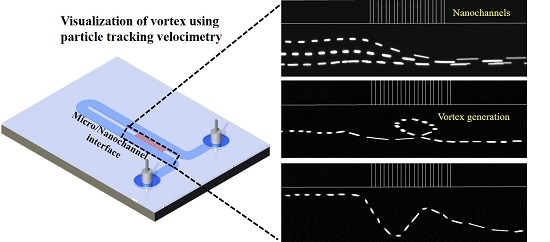
Quantification of Vortex Generation Due to Non-Equilibrium Electrokinetics at the Micro/Nanochannel Interface: Particle Tracking Velocimetry
Abstract
:1. Introduction
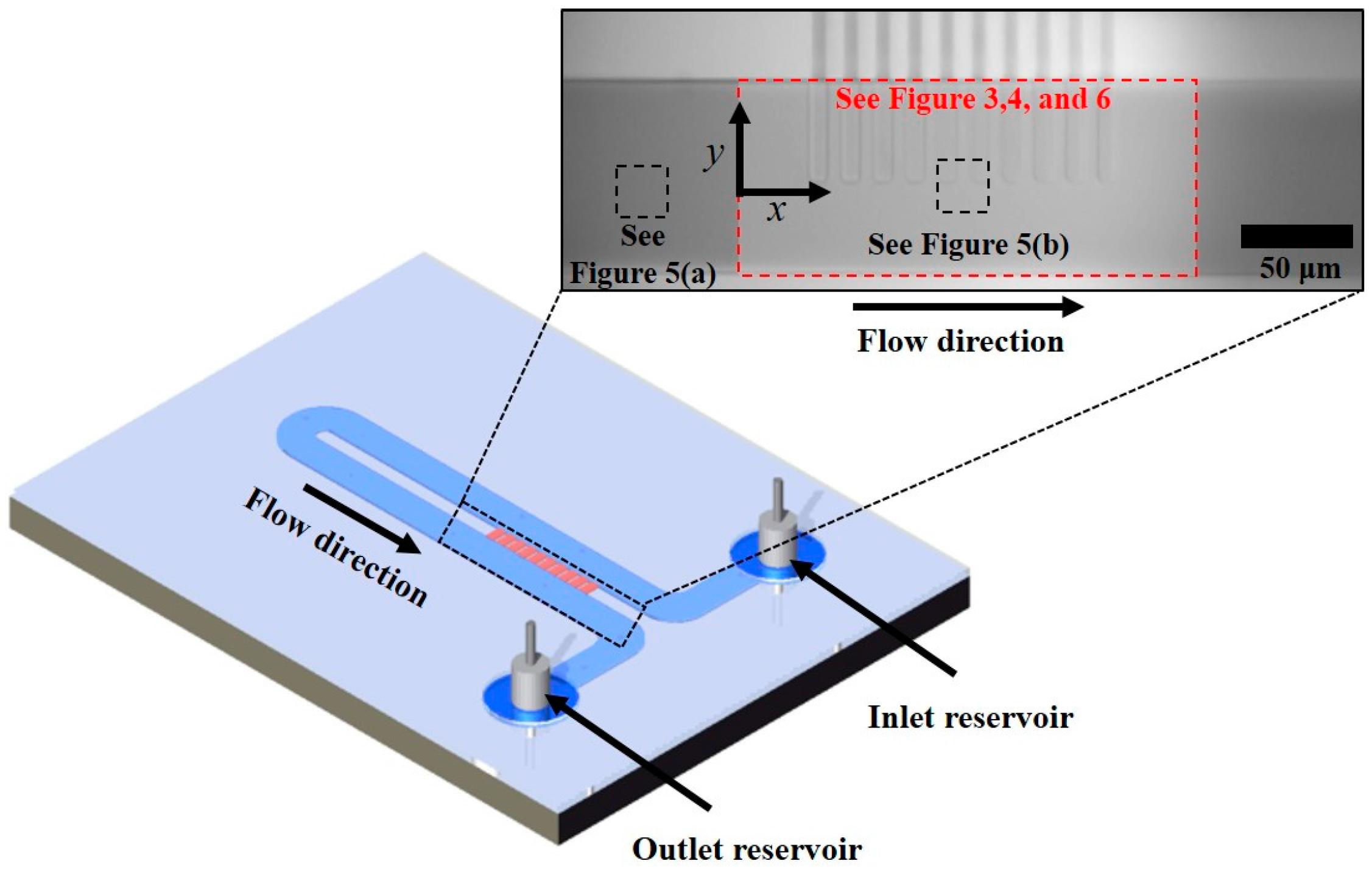
2. Experimental
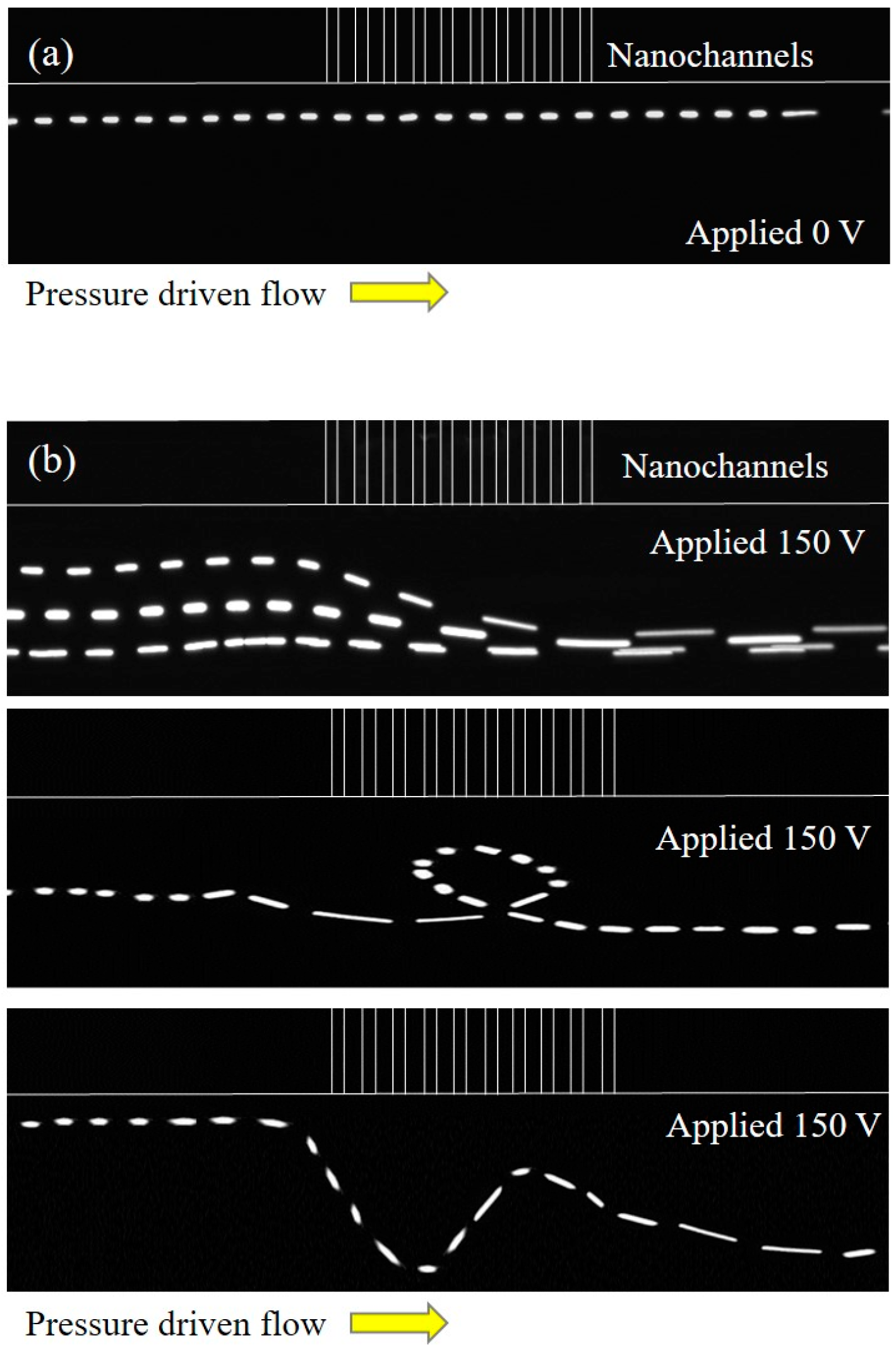
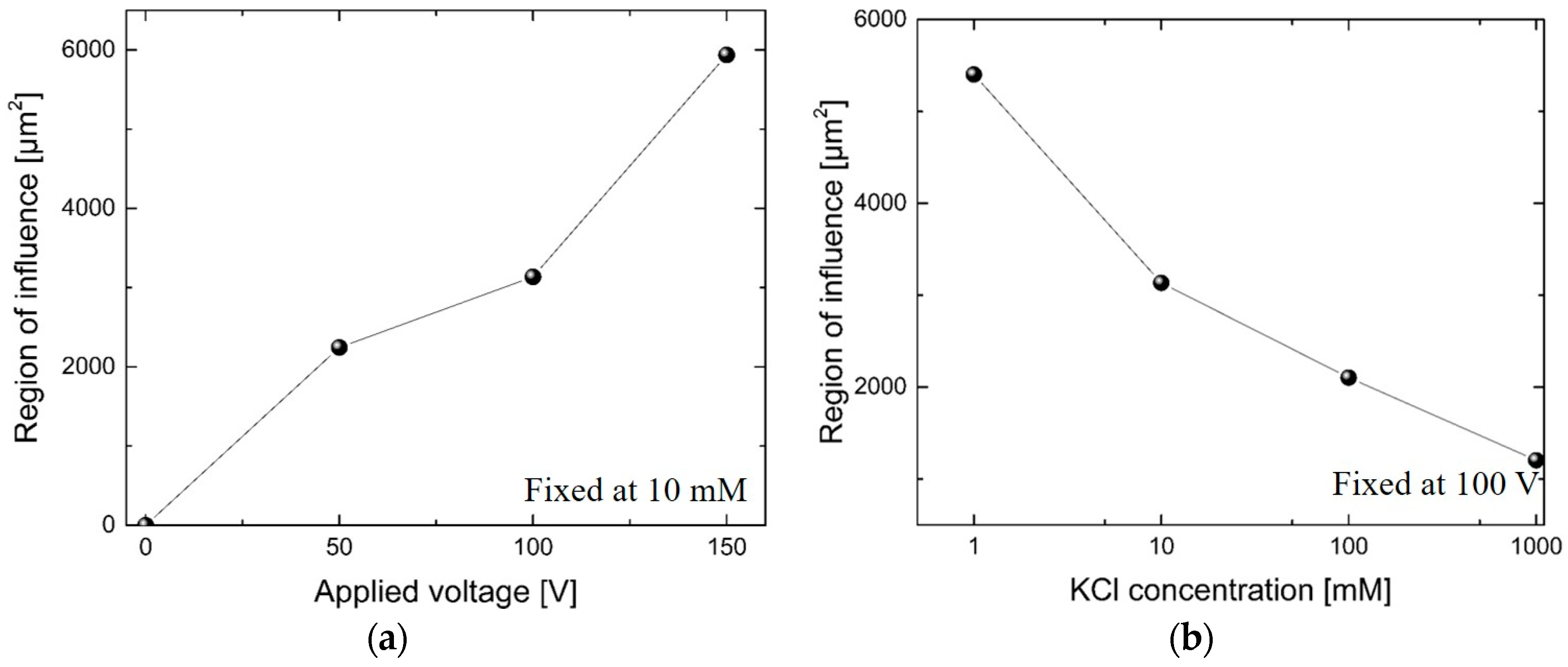
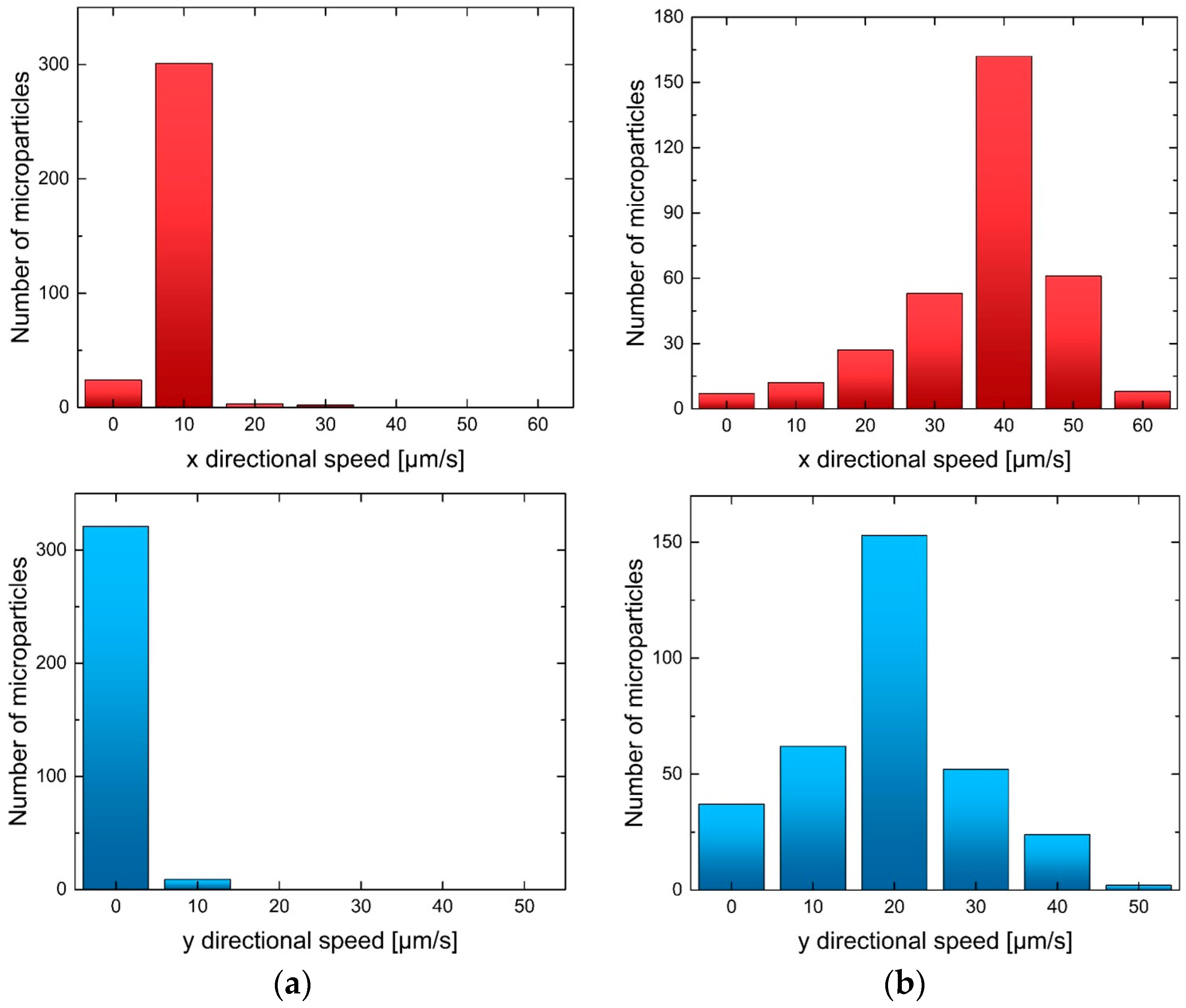
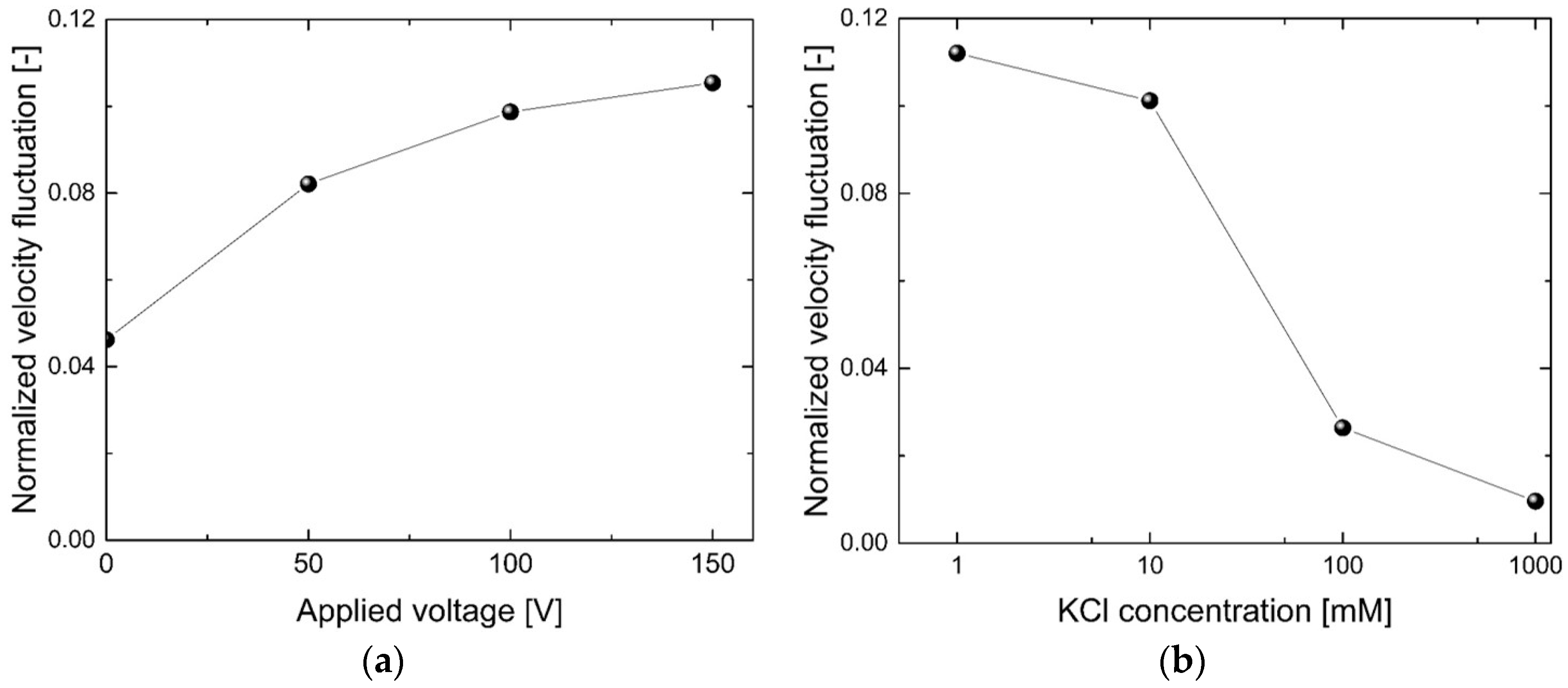
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bocquet, L.; Charlaix, E. Nanofluidics, from bulk to interfaces. Chem. Soc. Rev. 2010, 39, 1073–1095. [Google Scholar] [CrossRef] [PubMed]
- Eijkel, J.C.; van den Berg, A. Nanofluidics: What is it and what can we expect from it? Microfluid. Nanofluid. 2005, 1, 249–267. [Google Scholar] [CrossRef]
- Piruska, A.; Gong, M.; Sweedler, J.V.; Bohn, P.W. Nanofluidics in chemical analysis. Chem. Soc. Rev. 2010, 39, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Craighead, H. Future lab-on-a-chip technologies for interrogating individual molecules. Nature 2006, 442, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, P.S.; Manz, A. Lab-on-a-chip: Microfluidics in drug discovery. Nat. Rev. Drug Discov. 2006, 5, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Mark, D.; Haeberle, S.; Roth, G.; von Stetten, F.; Zengerle, R. Microfluidic lab-on-a-chip platforms: Requirements, characteristics and applications. Chem. Soc. Rev. 2010, 39, 1153–1182. [Google Scholar] [CrossRef] [PubMed]
- Bocquet, L.; Tabeling, P. Physics and technological aspects of nanofluidics. Lab Chip 2014, 14, 3143–3158. [Google Scholar] [CrossRef] [PubMed]
- Mawatari, K.; Kazoe, Y.; Shimizu, H.; Pihosh, Y.; Kitamori, T. Extended-nanofluidics: Fundamental technologies, unique liquid properties, and application in chemical and bio analysis methods and devices. Anal. Chem. 2014, 86, 4068–4077. [Google Scholar] [CrossRef] [PubMed]
- Slouka, Z.; Senapati, S.; Chang, H.-C. Microfluidic systems with ion-selective membranes. Annu. Rev. Anal. Chem. 2014, 7, 317–335. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Dubsky, P.; van den Berg, A.; Eijkel, J. Concentration polarization in translocation of DNA through nanopores and nanochannels. Phys. Rev. Lett. 2012, 108, 138101. [Google Scholar] [CrossRef] [PubMed]
- Goet, G.; Baier, T.; Hardt, S. Micro contactor based on isotachophoretic sample transport. Lab Chip 2009, 9, 3586–3593. [Google Scholar] [CrossRef] [PubMed]
- Song, H.J.; Wang, Y.; Garson, C.; Pant, K. Nafion-film-based micro-nanofluidic device for concurrent DNA preconcentration and separation in free solution. Microfluid. Nanofluid. 2014, 17, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Yang, R.J. Effects of microchannel geometry on preconcentration intensity in microfluidic chips with straight or convergent–divergent microchannels. Electrophoresis 2012, 33, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Lee, J.H.; Moon, M.W.; Han, J.; Kamm, R.D. Non-lithographic wrinkle nanochannels for protein preconcentration. Adv. Mater. 2008, 20, 3011–3016. [Google Scholar] [CrossRef]
- Gadish, N.; Voldman, J. High-throughput positive-dielectrophoretic bioparticle microconcentrator. Anal. Chem. 2006, 78, 7870–7876. [Google Scholar] [CrossRef] [PubMed]
- Hlushkou, D.; Perry, J.M.; Jacobson, S.C.; Tallarek, U. Propagating concentration polarization and ionic current rectification in a nanochannel–nanofunnel device. Anal. Chem. 2011, 84, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Rhee, H.; Jeon, T.J.; Kim, D. Preconcentration of lipid vesicles using concentration polarization in a microfluidic chip. Sens. Actuators B Chem. 2016, 229, 276–280. [Google Scholar] [CrossRef]
- Kim, S.M.; Burns, M.A.; Hasselbrink, E.F. Electrokinetic protein preconcentration using a simple glass/poly (dimethylsiloxane) microfluidic chip. Anal. Chem. 2006, 78, 4779–4785. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.H.; Kim, S.J.; Cheow, L.F.; Li, L.D.; Kang, K.H.; Han, J. Massively parallel concentration device for multiplexed immunoassays. Lab Chip 2011, 11, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.H.; Song, Y.-A.; Kim, S.J.; Kim, M.; Han, J.; Kang, K.H. Nanofluidic preconcentration device in a straight microchannel using ion concentration polarization. Lab Chip 2012, 12, 4472–4482. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Yang, H.; Sivagnanam, V.; Gijs, M.A.M. Microfluidic protein preconcentrator using a microchannel-integrated nafion strip: Experiment and modeling. Anal. Chem. 2010, 82, 9989–9997. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Raj, A.; Zhu, L.; Masel, R.I.; Shannon, M.A. Non-equilibrium electrokinetic micro/nanofluidic mixer. Lab Chip 2008, 8, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kim, D. Millisecond-order rapid micromixing with non-equilibrium electrokinetic phenomena. Microfluid. Nanofluid. 2012, 12, 897–906. [Google Scholar] [CrossRef]
- Stroock, A.D.; Dertinger, S.K.W.; Ajdari, A.; Mezic, I.; Stone, H.A.; Whitesides, G.M. Chaotic mixer for microchannels. Science 2002, 295, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Yang, C.; Miao, J.; Lam, Y.; Huang, X. Enhancement of electrokinetically driven microfluidic t-mixer using frequency modulated electric field and channel geometry effects. Electrophoresis 2009, 30, 3144–3152. [Google Scholar] [CrossRef] [PubMed]
- Chiu, P.-H.; Chang, C.-C.; Yang, R.-J. Electrokinetic Micromixing of Charged and Non-Charged Samples near Nano-/Microchannel Junction. Microfluid. Nanofluid. 2013, 14, 839–844. [Google Scholar] [CrossRef]
- Huang, K.-D.; Yang, R.-J. Formation of Ionic Depletion/Enrichment Zones in a Hybrid Micro-/Nano-channel. Microfluid. Nanofluid. 2008, 5, 631–638. [Google Scholar] [CrossRef]
- Huang, K.-D.; Yang, R.-J. A Nanochannel-Based Concentrator Utilizing Concentration Polarization Effect. Electrophoresis 2008, 29, 4862–4870. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Yang, R.-J. Electrokinetic Mixing in Microfluidic Systems (Invited Review Paper). Microfluid. Nanofluid. 2007, 3, 501–525. [Google Scholar] [CrossRef]
- Zaltzman, B.; Rubinstein, I. Electro-osmotic slip and electroconvective instability. J. Fluid Mech. 2007, 579, 173–226. [Google Scholar] [CrossRef]
- Green, Y.; Park, S.; Yossifon, G. Bridging the gap between an isolated nanochannel and a communicating multipore heterogeneous membrane. Phys. Rev. E 2015, 91, 011002. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Yeh, C.P.; Yang, R.J. Ion concentration polarization near microchannel–nanochannel interfaces: Effect of ph value. Electrophoresis 2012, 33, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Li, L.D.; Han, J. Amplified electrokinetic response by concentration polarization near nanofluidic channel. Langmuir 2009, 25, 7759–7765. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Song, Y.-A.; Han, J. Nanofluidic concentration devices for biomolecules utilizing ion concentration polarization: Theory, fabrication, and applications. Chem. Soc. Rev. 2010, 39, 912–922. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Wang, Y.-C.; Lee, J.H.; Jang, H.; Han, J. Concentration polarization and nonlinear electrokinetic flow near a nanofluidic channel. Phys. Rev. Lett. 2007, 99, 044501. [Google Scholar] [CrossRef] [PubMed]
- Mani, A.; Zangle, T.A.; Santiago, J.G. On the propagation of concentration polarization from microchannel-nanochannel interfaces part I: Analytical model and characteristic analysis. Langmuir 2009, 25, 3898–3908. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, I.; Zaltzman, B. Extended space charge in concentration polarization. Adv. Colloid Interface Sci. 2010, 159, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Zangle, T.A.; Mani, A.; Santiago, J.G. Theory and experiments of concentration polarization and ion focusing at microchannel and nanochannel interfaces. Chem. Soc. Rev. 2010, 39, 1014–1035. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Jeon, T.-J.; Kim, S.M.; Kim, D. Quantification of vortex generation due to non-equilibrium electrokinetics at the micro/nanochannel interface: Spectral analysis. Micromachines 2016, 7, 109. [Google Scholar] [CrossRef]
- Cummins, Z.; Probst, R.; Shapiro, B. Electrokinetic tweezing: Three-dimensional manipulation of microparticles by real-time imaging and flow control. Lab Chip 2013, 13, 4040–4046. [Google Scholar] [CrossRef] [PubMed]
- Pope, S.B. Turbulent Flows; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]

© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.J.; Kwon, K.; Jeon, T.-J.; Kim, S.M.; Kim, D. Quantification of Vortex Generation Due to Non-Equilibrium Electrokinetics at the Micro/Nanochannel Interface: Particle Tracking Velocimetry. Micromachines 2016, 7, 127. https://doi.org/10.3390/mi7070127
Lee SJ, Kwon K, Jeon T-J, Kim SM, Kim D. Quantification of Vortex Generation Due to Non-Equilibrium Electrokinetics at the Micro/Nanochannel Interface: Particle Tracking Velocimetry. Micromachines. 2016; 7(7):127. https://doi.org/10.3390/mi7070127
Chicago/Turabian StyleLee, Seung Jun, Kilsung Kwon, Tae-Joon Jeon, Sun Min Kim, and Daejoong Kim. 2016. "Quantification of Vortex Generation Due to Non-Equilibrium Electrokinetics at the Micro/Nanochannel Interface: Particle Tracking Velocimetry" Micromachines 7, no. 7: 127. https://doi.org/10.3390/mi7070127
APA StyleLee, S. J., Kwon, K., Jeon, T.-J., Kim, S. M., & Kim, D. (2016). Quantification of Vortex Generation Due to Non-Equilibrium Electrokinetics at the Micro/Nanochannel Interface: Particle Tracking Velocimetry. Micromachines, 7(7), 127. https://doi.org/10.3390/mi7070127