Allergy Testing and Drug Screening on an ITO-Coated Lab-on-a-Disc
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture and Imaging
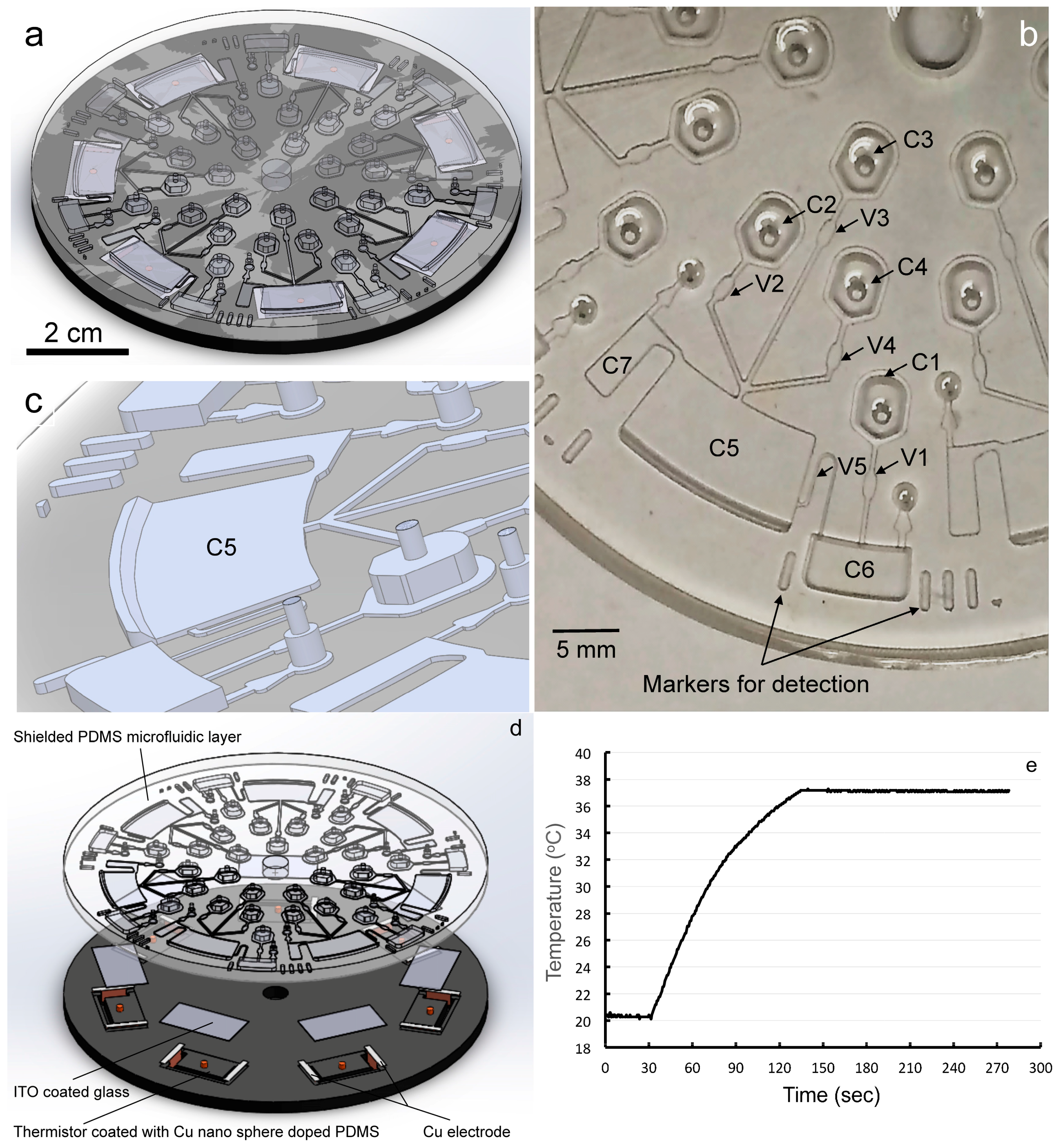
2.3. LOAD Design and Fabrication
2.4. Statistical Analysis
3. Results and Discussion
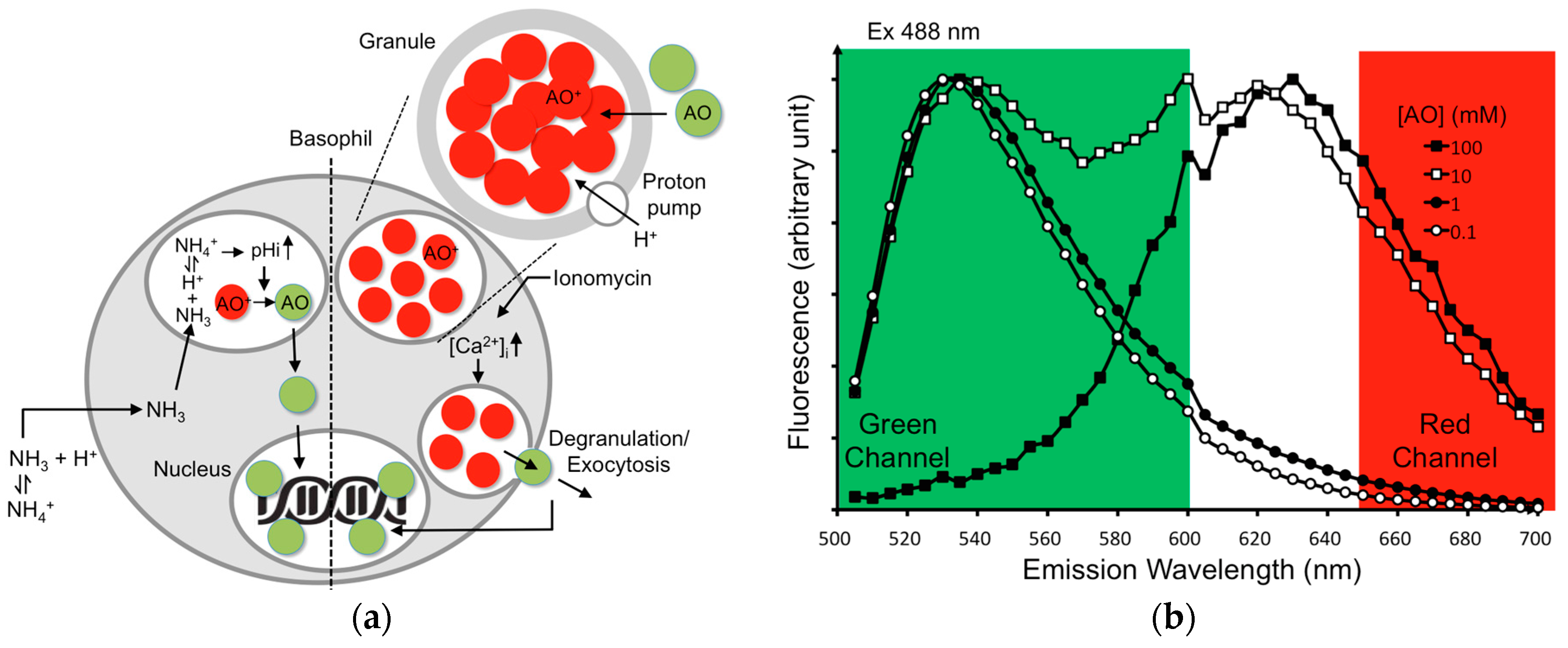
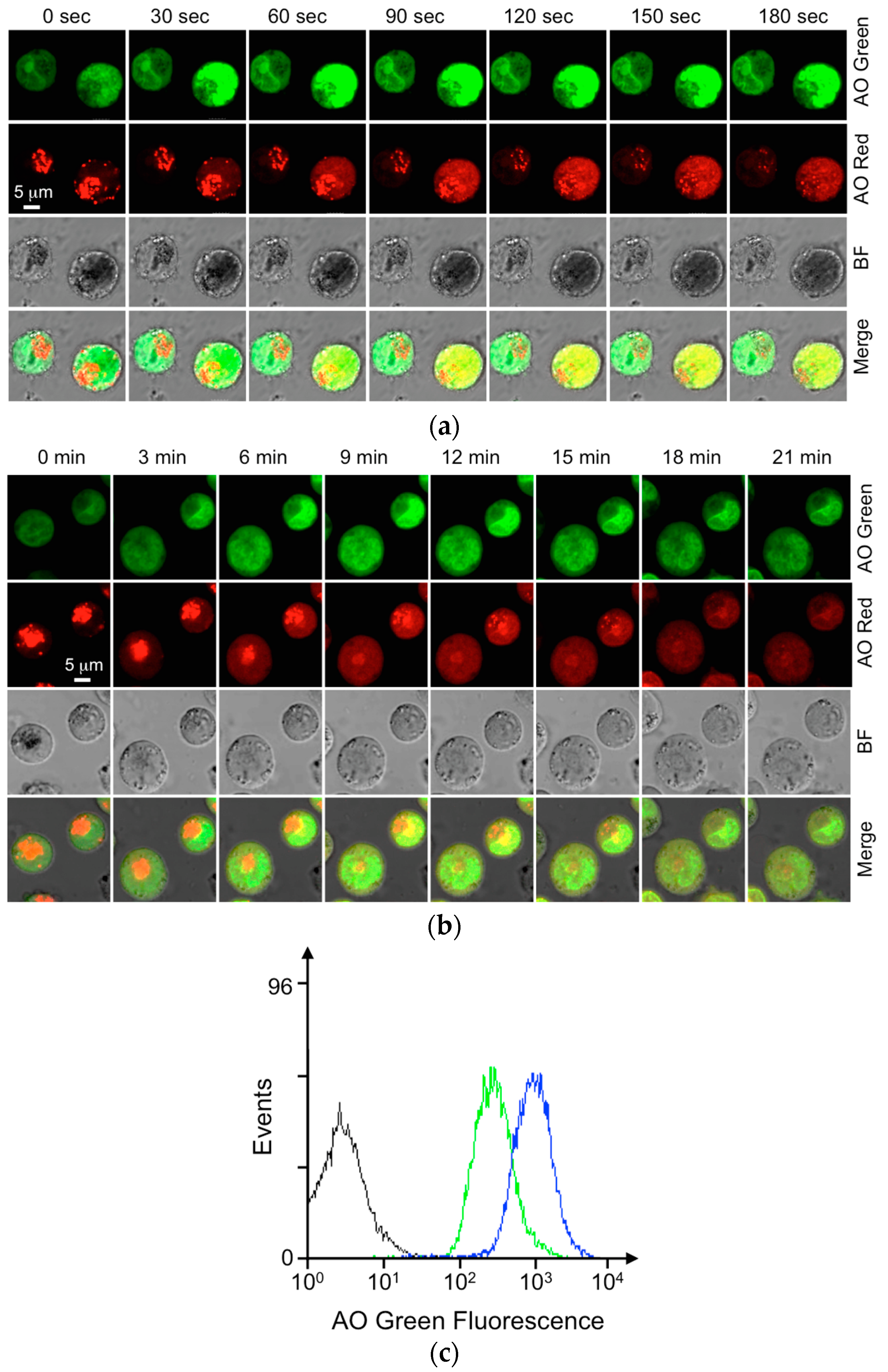
3.1. BAT Assay with AO-Loaded KU-812 Cells
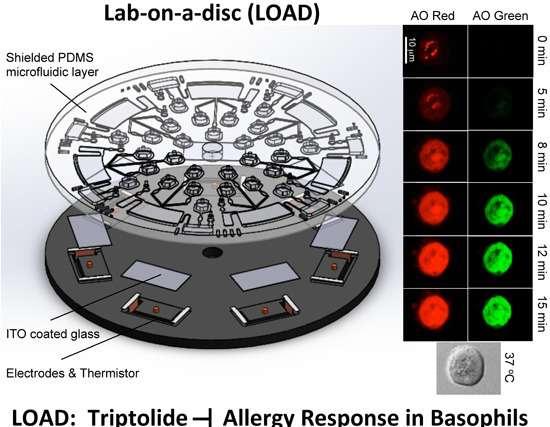
3.2. LOAD Design
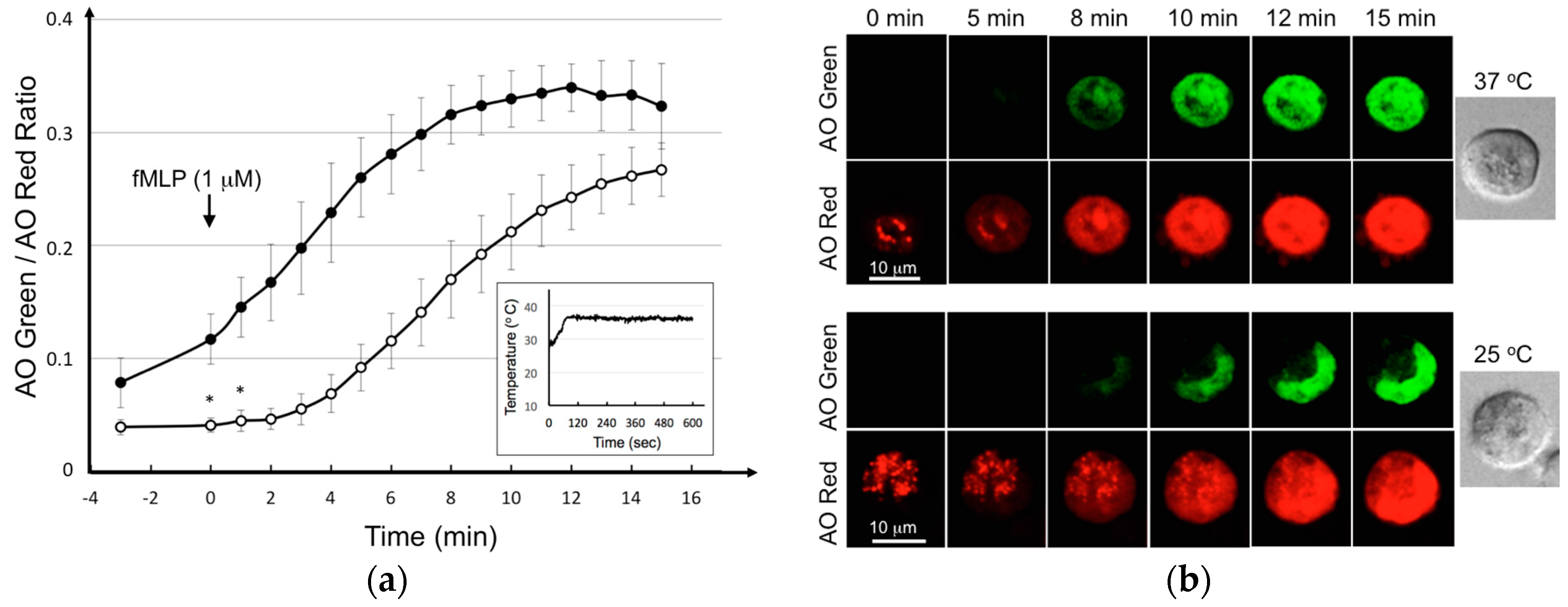
3.3. Triptolide Suppressed the fMLP-Mediated Degranulation in KU-812 Cells
4. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- WAO White Book 2013. Available online: http://www.worldallergy.org/definingthespecialty/white_book.php (accessed on 7 December 2015).
- Lambert, C.; Sarrat, A.; Bienvenu, F.; Brabant, S.; Nicaise-Roland, P.; Alyanakian, M.A.; Apoil, P.A.; Capron, C.; Couderc, R.; Evrard, B.; et al. The importance of EN ISO 15189 accreditation of allergen-specific IgE determination for reliable in vitro allergy diagnosis. Allergy 2015, 70, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Novembre, E.; Bernardini, R.; Bertini, G.; Massai, G.; Vierucci, A. Skin-prick-test-induced anaphylaxis. Allergy 1995, 50, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Pien, G.C.; Orange, J.S. Evaluation and clinical interpretation of hypergammaglobulinemia E: differentiating atopy from immunodeficiency. Ann Allergy Asthma Immunol. 2008, 100, 392–395. [Google Scholar] [CrossRef]
- Thyssen, J.P.; Menné, T.; Elberling, J.; Plaschke, P.; Johansen, J.D. Hypersensitivity to local anaesthetics—Update and proposal of evaluation algorithm. Contact Dermat. 2008, 59, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Narita, S.; Goldblum, R.M.; Watson, C.S.; Brooks, E.G.; Estes, D.M.; Curran, E.M.; Midoro-Horiuti, T. Environmental estrogens induce mast cell degranulation and enhance IgE-mediated release of allergic mediators. Environ. Health Perspect. 2007, 115, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M.; Hauser, R. Bisphenol A and children’s health. Curr. Opin. Pediatr. 2011, 23, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Menard, S.; Guzylack-Piriou, L.; Leveque, M.; Braniste, V.; Lencina, C.; Naturel, M.; Moussa, L.; Sekkal, S.; Harkat, C.; Gaultier, E.; et al. Food intolerance at adulthood after perinatal exposure to the endocrine disruptor bisphenol A. FASEB J. 2014, 28, 4893–4900. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.; Miller, R. The Impact of Bisphenol A and Phthalates on Allergy, Asthma, and Immune Function: A Review of Latest Findings. Curr. Environ. Health Rep. 2015, 2, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Ebo, D.G.; Bridts, C.H.; Hagendorens, M.M.; Aerts, N.E.; de Clerck, L.S.; Stevens, W.J. Basophil activation test by flow cytometry: Present and future applications in allergology. Cytom. B Clin. Cytom. 2008, 74, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Chirumbolo, S. Basophil activation test in allergy: Time for an update? Int. Arch. Allergy Immunol. 2012, 158, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Kleine-Tebbe, J.; Erdmann, S.; Knol, E.F.; MacGlashan, D.W., Jr.; Poulsen, L.K.; Gibbs, B.F. Diagnostic tests based on human basophils: Potentials, pitfalls and perspectives. Int. Arch. Allergy Immunol. 2006, 141, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Casado, C.; Garrido-Arandia, M.; Gamboa, P.; Blanca-López, N.; Canto, G.; Varela, J.; Cuesta-Herranz, J.; Pacios, L.F.; Díaz-Perales, A.; Tordesillas, L. Allergenic characterization of new mutant forms of Pru p 3 as new immunotherapy vaccines. Clin. Dev. Immunol. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Sturm, G.J.; Kranzelbinder, B.; Sturm, E.M.; Heinemann, A.; Groselj-Strele, A.; Aberer, W. The basophil activation test in the diagnosis of allergy: Technical issues and critical factors. Allergy 2009, 64, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Almlöf, I.; Nilsson, K.; Johansson, V.; Akerblom, E.; Slotte, H.; Ahlstedt, S.; Matsson, P. Induction of basophilic differentiation in the human basophilic cell line KU812. Scand. J. Immunol. 1988, 28, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.L.; Cheung, K.L.; Kong, S.K.; Zhou, J.Q.; Kwan, Y.W.; Wong, C.K.; Ho, H.P. An integrated lab-on-a-disc for automated cell-based allergen screening bioassays. Talanta 2012, 97, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.P.; Lau, P.M.; Kwok, H.C.; Wu, S.Y.; Gao, M.; Cheung, A.K.; Chen, Q.; Wang, G.; Kwan, Y.W.; Wong, C.K.; et al. Allergen screening bioassays: Recent developments in lab-on-a-chip and lab-on-a-disc systems. Bioanalysis 2014, 6, 2005–2018. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Ho, H.P.; Chen, Q.; Yang, A.K.; Kwok, H.C.; Wu, S.Y.; Kong, S.K.; Kwan, Y.W.; Zhang, X. A lab-in-a-droplet bioassay strategy for centrifugal microfluidics with density difference pumping, power to disc and bidirectional flow control. Lab Chip 2013, 13, 3698–3706. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Corwith, K.; Holowka, D.; Baird, B. Spatiotemporal resolution of mast cell granule exocytosis reveals correlation with Ca2+ wave initiation. J. Cell Sci. 2012, 125, 2986–2994. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.M.; Shear, J.B.; Zipfel, W.R.; Maiti, S.; Webb, W.W. Mucosal mast cell secretion processes imaged using three-photon microscopy of 5-hydroxytryptamine autofluorescence. Biophys. J. 1999, 76, 1835–1846. [Google Scholar] [CrossRef]
- Williams, R.M.; Webb, W.W. Single granule pH cycling in antigen induced mast cell secretion. J. Cell Sci. 2000, 113, 3839–3850. [Google Scholar] [PubMed]
- Jaiswal, J.K.; Fix, M.; Takano, T.; Nedergaard, M.; Simon, S.M. Resolving vesicle fusion from lysis to monitor calcium-triggered lysosomal exocytosis in astrocytes. Proc. Natl. Acad. Sci. USA 2007, 104, 14151–14156. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.L.; Wu, M.H.; Kuo, C.Y.; Lee, K.D.; Shen, Y.L. Application of indium tin oxide (ITO)-based microheater chip with uniform thermal distribution for perfusion cell culture outside a cell incubator. Biomed. Microdevices 2010, 12, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Aranda, A.; Mayorga, C.; Ariza, A.; Doña, I.; Rosado, A.; Blanca-Lopez, N.; Andreu, I.; Torres, M.J. In vitro evaluation of IgE-mediated hypersensitivity reactions to quinolones. Allergy 2011, 66, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Rostami-Yazdi, M.; Gerdes, S.; Mrowietz, U. Triptolide in the treatment of psoriasis and other immune-mediated inflammatory diseases. Br. J. Clin. Pharmacol. 2012, 74, 424–436. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwok, H.C.; Lau, P.M.; Wu, S.Y.; HO, H.P.; Gao, M.; Kwan, Y.W.; Wong, C.K.; Kong, S.K. Allergy Testing and Drug Screening on an ITO-Coated Lab-on-a-Disc. Micromachines 2016, 7, 38. https://doi.org/10.3390/mi7030038
Kwok HC, Lau PM, Wu SY, HO HP, Gao M, Kwan YW, Wong CK, Kong SK. Allergy Testing and Drug Screening on an ITO-Coated Lab-on-a-Disc. Micromachines. 2016; 7(3):38. https://doi.org/10.3390/mi7030038
Chicago/Turabian StyleKwok, Ho Chin, Pui Man Lau, Shu Yuen Wu, Ho Pui HO, Minghui Gao, Yiu Wa Kwan, Chun Kwok Wong, and Siu Kai Kong. 2016. "Allergy Testing and Drug Screening on an ITO-Coated Lab-on-a-Disc" Micromachines 7, no. 3: 38. https://doi.org/10.3390/mi7030038
APA StyleKwok, H. C., Lau, P. M., Wu, S. Y., HO, H. P., Gao, M., Kwan, Y. W., Wong, C. K., & Kong, S. K. (2016). Allergy Testing and Drug Screening on an ITO-Coated Lab-on-a-Disc. Micromachines, 7(3), 38. https://doi.org/10.3390/mi7030038