3D Microporous Scaffolds Manufactured via Combination of Fused Filament Fabrication and Direct Laser Writing Ablation
Abstract
:1. Introduction
2. Experimental Section
2.1. PLA Film Preparation and Surface Modifications
2.2. 3D Printing via Fused Filament Fabrication
2.3. Direct Laser Writing by Ablation
2.4. Cell Culture Assay
3. Results and Discussion
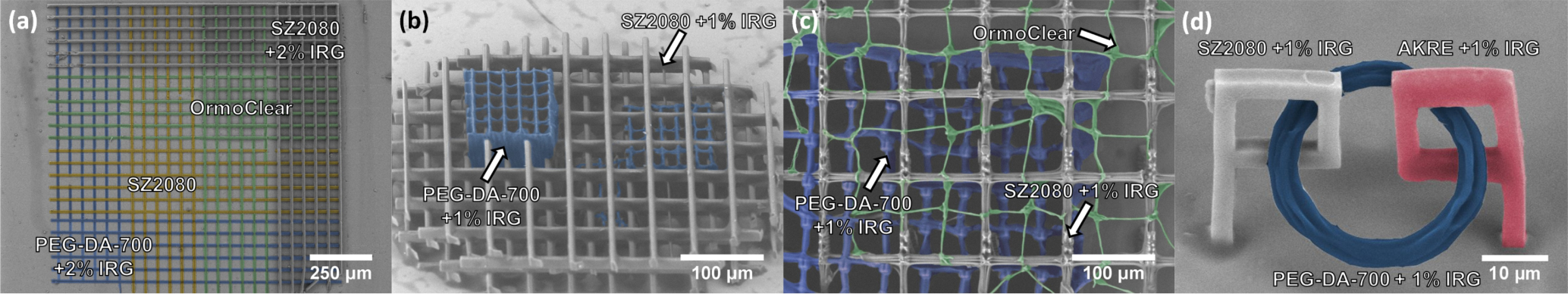
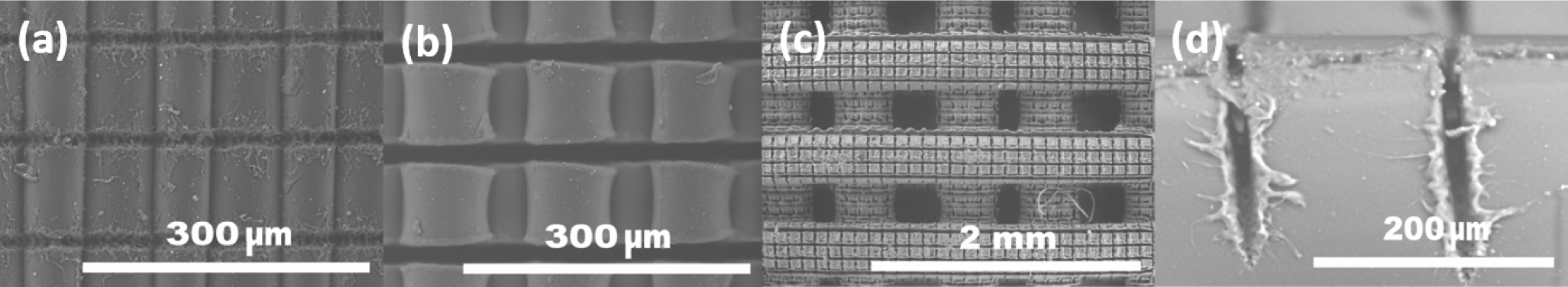
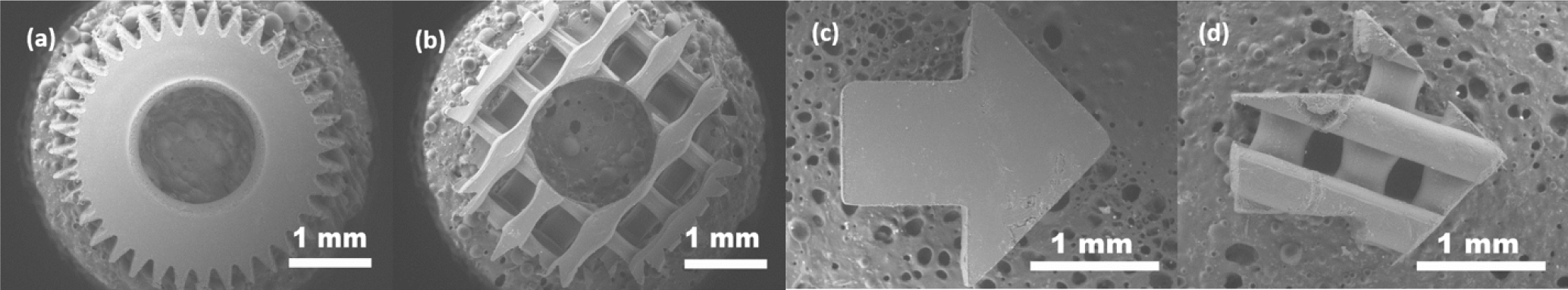
3.1. FFF 3D Microprinting
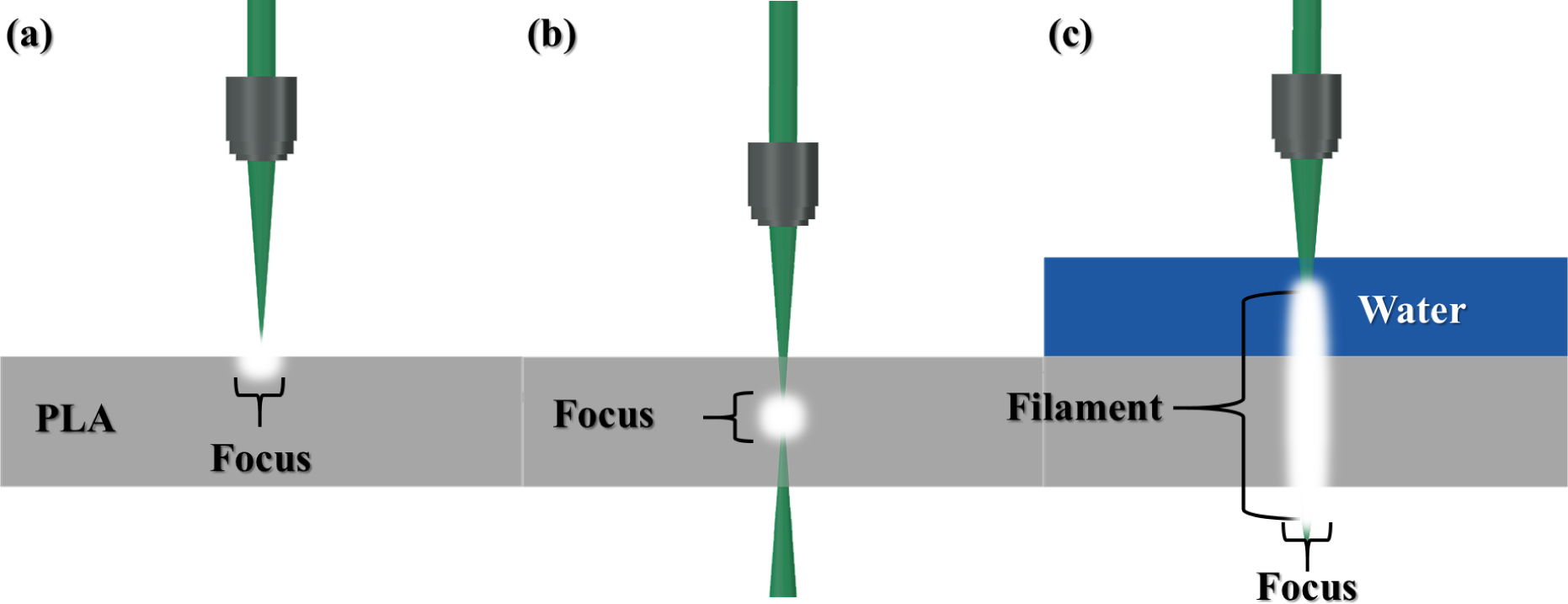
3.2. Characteristics of Light Filament-Assisted vs. Sharp Beam Focusing for 3D Microstructuring
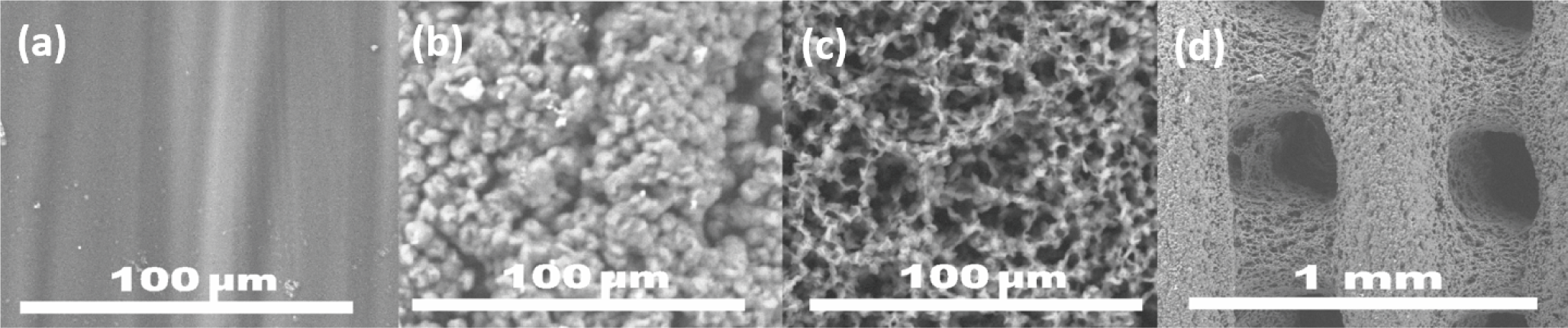
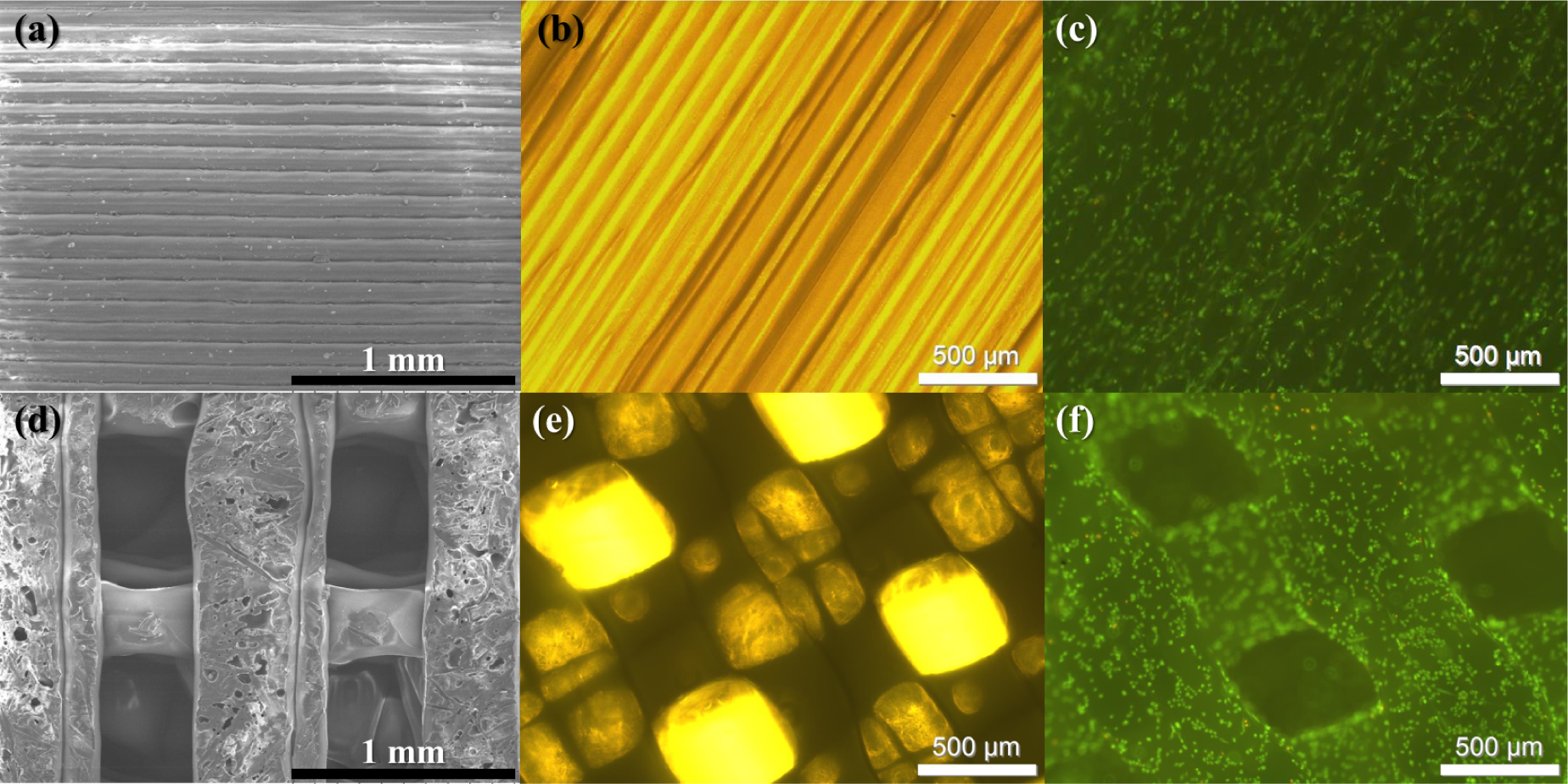
3.3. Surface Wettability via Roughness: Acetone Bath
3.4. Cell Growth on 3D PLA Scaffolds
3.5. Outlook
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lithuanian Laser Association Home Page. Available online: http://www.ltoptics.org/ accessed on 27 July 2014.
- 3D Printing Industry Home Page. Available online: http://3dprintingindustry.com/ accessed on 27 July 2014.
- Zhang, Y.L.; Chen, Q.D.; Xia, H.; Sun, H.B. Designable 3D nanofabrication by femtosecond laser direct writing. Nano Today 2010, 5, 435–448. [Google Scholar]
- Malinauskas, M.; Farsari, M.; Piskarskas, A.; Juodkazis, S. Ultrafast laser nanostructuring of photopolymers: A decade of advances. Phys. Rep. 2013, 533, 1–31. [Google Scholar]
- Sugioka, K.; Cheng, Y. Ultrafast lasers-reliable tools for advanced materials processing. Light Sci. Appl. 2014. [Google Scholar]
- Schell, A.; Kaschke, J.; Fischer, J.; Henze, R.; Wolters, J.; Wegener, M.; Benson, O. Three-dimensional quantum photonic elements based on single nitrogen vacancy-centres in laser-written microstructures. Sci. Rep. 2012, 3, 1577. [Google Scholar]
- Turner, M.; Saba, M.; Zhang, Q.; Cumming, B.; Schroder-Turk, G.; Gu, M. Miniature chiral beamsplitter based on gyroid photonic crystals. Nat. Photon. 2013, 7, 801–805. [Google Scholar]
- Žukauskas, A.; Malinauskas, M.; Brasselet, E. Monolithic generators of pseudo-nondiffracting optical vortex beams at the microscale. Appl. Phys. Lett. 2013, 103, 181122. [Google Scholar]
- Rajamanickam, V.; Ferrara, L.; Toma, A.; Zaccaria, R.P.; Das, G.; Fabrizio, E.D.; Liberale, C. Suitable photo-resists for two-photon polymerization using femtosecond fiber lasers. Microelectron. Eng. 2014, 121, 135–138. [Google Scholar]
- Weiss, T.; Hildebrand, G.; Schade, R.; Liefeith, K. Two-Photon polymerization for microfabrication of three-dimensional scaffolds for tissue engineering application. Eng. Life Sci. 2009, 9, 384–390. [Google Scholar]
- Raimondi, M.; Nava, M.; Eaton, S.; Bernasconi, A.; Vishnubhatla, K.; Cerullo, G.; Osellame, R. Optimization of Femtosecond Laser Polymerized Structural Niches to Control Mesenchymal Stromal Cell Fate in Culture. Micromachines 2014, 5, 341–358. [Google Scholar]
- Chen, X.; Su, Y.D.; Ajeti, V.; Chen, S.J.; Campagnola, P. Cell Adhesion on Micro-Structured Fibronectin Gradients Fabricated by Multiphoton Excited Photochemistry. Cellular Molecular Bioeng. 2012, 5, 307–319. [Google Scholar]
- Spivey, E.; Ritschdorff, E.; Connell, J.; McLennon, C.; Schmidt, C.; Shear, J. Multiphoton Lithography of Unconstrained Three-Dimensional Protein Microstructures. Adv. Func. Mater. 2013, 23, 333–339. [Google Scholar]
- Kufelt, O.; El-Tamer, A.; Sehring, C.; Schlie-Wolter, S.; Chichkov, B. Hyaluronic Acid Based Materials for Scaffolding via Two-Photon Polymerization. Biomacromolecules 2014, 15, 650–659. [Google Scholar]
- Dawood, F.; Qin, S.; Li, L.; Lina, E.; Fourkas, J. Simultaneous microscale optical manipulation, fabrication and immobilisation in aqueous media. Chem. Sci. 2012, 3, 2449–2456. [Google Scholar]
- Klein, F.; Richter, B.; Striebel, T.; Franz, C.; von Freymann, G.; Wegener, M.; Bastmeyer, M. Two-Component Polymer Scaffolds for Controlled Three-Dimensional Cell Culture. Adv. Mat. 2011, 23, 1341–1345. [Google Scholar]
- Rekštytė, S.; Kaziulionytė, E.; Balčiūnas, E.; Kaškelytė, D.; Malinauskas, M. Direct Laser Fabrication of Composite Material 3D Microstructured Scaffolds. J. Laser Micro/Nanoeng. 2014, 9, 25–30. [Google Scholar]
- Danilevičius, P.; Rekštytė, S.; Balčiuūas, E.; Kraniauskas, A.; Jarašien˙e, R.; Širmenis, R.; Baltriukienė, D.; Bukelskienė, V.; Gadonas, R.; Malinauskas, M. Micro-structured polymer scaffolds fabricated by direct laser writing for tissue engineerings. J. Biomed. Opt. 2012, 17, 081405. [Google Scholar]
- Torgersen, J.; Qin, X.H.; Li, Z.; Ovsianikov, A.; Liska, R.; Stampfl, J. Hydrogels for Two-Photon Polymerization: A Toolbox for Mimicking the Extracellular Matrix. Adv. Func. Mat. 2013, 23, 4542–4554. [Google Scholar]
- Ovsianikov, A.; Muhleder, S.; Torgersen, J.; Li, Z.; Qin, X.H.; Vlierberghe, S.; Dubruel, P.; Holnthoner, W.; Redl, H.; Liska, R.; Stampfl, J. Laser Photofabrication of Cell-Containing Hydrogel Constructs. Langmuir 2014, 30, 3787–3794. [Google Scholar]
- Malinauskas, M.; Baltriukienė, D.; Kraniauskas, A.; Danilevičius, P.; Jarašienė, R.; Širmenis, R.; Žukauskas, A.; Balčiūnas, E.; Purlys, V.; Gadonas, R.; Bukelskienė, V.; Sirvydis, V.; Piskarskas, A. In vitro and in vivo biocompatibility study on laser 3D microstructurable polymers. Appl. Phys. A 2012, 108, 751–759. [Google Scholar]
- Žukauskas, A.; Batavičiūtė, G.; Ščiuka, M.; Jukna, T.; Melninkaitis, A.; Malinauskas, M. Characterization of photopolymers used in laser 3D micro/nanolithography bymeans of laser-induced damage threshold (LIDT). Opt. Mat. Express 2014, 4, 1601–1616. [Google Scholar]
- Gittard, S.; Nguyen, A.; Obata, K.; Koroleva, A.; Narayan, R.; Chichkov, B. Fabrication of microscale medical devices by two-photon polymerization with multiple foci via a spatial light modulator. Biomed. Opt. Express 2011, 2, 3167–3178. [Google Scholar]
- Stankevičius, E.; Gertus, T.; Rutkauskas, M.; Gedvilas, M.; Račiukaitis, G.; Gadonas, R.; Smilgevičius, V.; Malinauskas, M. Fabrication of micro-tube arrays in photopolymer SZ2080 by using three different methods of a direct laser polymerization technique. J. Micromech. Microeng. 2012, 22, 065022. [Google Scholar]
- Beke, S.; Barenghi, R.; Farkas, B.; Romano, I.; Korosic, L.; Scaglione, S.; Brandi, F. Improved cell activity on biodegradable photopolymer scaffolds using titanate nanotube coatings. Mat. Sci. Eng. C 2014, 44, 38–43. [Google Scholar]
- Beke, S.; Anjum, F.; Ceseracciu, L.; Romano, I.; Athanassiou, A.; Diaspro, A.; Brandi, F. Rapid fabrication of rigid biodegradable scaffolds by excimer laser mask projection technique: A comparison between 248 and 308 nm. Laser Phys. 2013, 23, 035602. [Google Scholar]
- Buividas, R.; Rekštytė, S.; Malinauskas, M.; Juodkazis, S. Nano-groove and 3D fabrication by controlled avalanche using femtosecond laser pulses. Opt. Mater. Express 2013, 3, 1674–1686. [Google Scholar]
- Rekštytė, S.; Malinauskas, M.; Juodkazis, S. Three-dimensional laser micro-sculpturing of silicone: Towards bio-compatible scaffolds. Opt. Express 2013, 21, 17028–17041. [Google Scholar]
- Williams, J.; Adewunmi, A.; Schek, R.; Flanagan, C.; Krebsbach, P.H.; Feinberg, S.; Hollister, S.; Das, S. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials 2005, 26, 4817–4827. [Google Scholar]
- Yadroitsev, I.; Thivillon, L.; Bertrand, P.; Smurov, I. Strategy of manufacturing components with designed internal structure by selective laser melting of metallic powder. Appl. Surf. Sci. 2007, 254, 980–983. [Google Scholar]
- Heinl, P.; Rottmair, A.; Korner, C.; Singer, R. Cellular Titanium by Selective Electron Beam Melting. Adv. Eng. Mat. 2007, 9, 360–364. [Google Scholar]
- Harrysson, O.; Cansizoglu, O.; Marcellin-Little, D.; Cormier, D.; West, H. Direct metal fabrication of titanium implants with tailored materials and mechanical properties using electron beam melting technology. Mat. Sci. Eng. C 2008, 28, 366–373. [Google Scholar]
- Schleifenbaum, H.; Meiners, W.; Wissenbach, K.; Hinke, C. Individualized production by means of high power Selective Laser Melting. CIRP J. Manuf. Sci. Technol. 2010, 2, 161–169. [Google Scholar]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar]
- Chastek, T.; Wadajkar, A.; Nguyen, K.; Hudson, S.; Chastek, T. Polyglycol-templated synthesis of poly(N-isopropyl acrylamide) microgels with improved biocompatibility. Colloid Polym. Sci. 2010, 288, 105–114. [Google Scholar]
- Ferraro, P.; Coppola, S.; Grilli, S.; Paturzo, M.; Vespini, V. Dispensing nano-pico droplets and liquid patterning by pyroelectrodynamic shooting. Nat. Nanotechnol. 2010, 5, 429–435. [Google Scholar]
- Knoll, A.W.; Zientek, M.; Cheong, L.; Rawlings, C.; Paul, P.; Holzner, F.; Hedrick, J.; Coady, D.; Allen, R.; Durig, U. Closed-loop high-speed 3D thermal probe nanolithography. In SPIE Proc.; 2014; Volume 9049, p. 90490B. [Google Scholar]
- RepRap. Available online: http://reprap.org/wiki/Fused_filament_fabrication accessed on 27 July 2014.
- Wang, H.W.; Cheng, C.W.; Li, C.W.; Chang, H.W.; Wu, P.H.; Wang, G.J. Fabrication of pillared PLGA microvessel scaffold using femtosecond laser ablation. Int. J. Nanomed. 2012, 7, 1865–1873. [Google Scholar]
- Danilevicius, P.; Georgiadi, L.; Pateman, C.; Claeyssens, F.; Chatzinikolaidou, M.; Farsari, M. The effect of porosity on cell ingrowth into accurately defined, laser-made,polylactide-based 3D scaffolds. Appl. Surf. Sci. 2014. [Google Scholar] [CrossRef]
- Melissinaki, V.; Gill, A.; Ortega, I.; Vamvakaki, M.; Ranella, A.; Haycock, J.; Fotakis, C.; Farsari, M.; Claeyssens, F. Direct laser writing of 3D scaffolds for neural tissue engineering applications. Biofabrication 2011, 3, 045005. [Google Scholar]
- Van Manen, E.; Zhang, W.; Walboomers, X.; Vazquez, B.; Yang, F.; Ji, W.; Yu, N.; Spear, D.; Jansen, J.; Yelick, P. The influence of electrospun fibre scaffold orientation and nano-hydroxyapatite content on the development of tooth bud stem cells in vitro. Odontology 2014, 102, 14–21. [Google Scholar]
- Ivanova, O.; Williams, C.; Campbell, T. Additive manufacturing (AM) and nanotechnology: Promises and challenges. Rapid Prototyp. J. 2013, 19, 353–364. [Google Scholar]
- Xiong, W.; Zhou, Y.; He, X.; Gao, Y.; Mahjouri-Samani, M.; Jiang, L.; Baldacchini, T.; Lu, Y. Simultaneous additive and subtractive three-dimensional nanofabrication using integrated two-photon polymerization and multiphoton ablation. Light Sci. Appl. 2012. [Google Scholar]
- Eschenbaum, C.; Grossmann, D.; Dopf, K.; Kettlitz, S.; Bocksrocker, T.; Valouch, S.; Lemmer, U. Hybrid lithography: Combining UV-exposure and two photon direct laser writing. Opt. Express 2013, 21, 29921–29926. [Google Scholar]
- Hengsbach, S.; Lantada, A. Rapid prototyping of multi-scale biomedical microdevices by combining additive manufacturing technologies. Biomed. Microdevices 2014, 16, 617–627. [Google Scholar]
- Butkus, S.; Gaižauskas, E.; Paipulas, D.; Viburys, Z.; Kaškelytė, D.; Barkauskas, M.; Alesenkov, A.; Sirutkaitis, V. Rapid microfabrication of transparent materials using filamented femtosecond laser pulses. Appl. Phys. A 2014, 114, 81–90. [Google Scholar]
- Juodkazis, S.; Misawa, H.; Louchev, O.; Kitamura, K. Femtosecond laser ablation of chalcogenide glass: Explosive formation of nano-fibres against thermo-capillary growth of micro-spheres. Nanotechnology 2006, 17, 4802–4805. [Google Scholar]
- Tan, D.; Li, Y.; Qi, F.; Yang, H.; Gong, Q.; Dong, X.; Duan, X. Reduction in feature size of two-photon polymerization using SCR500. Appl. Phys. Lett. 2007, 90, 071106. [Google Scholar]
- Malinauskas, M.; Bičkauskaitė, G.; Rutkauskas, M.; Paipulas, D.; Purlys, V.; Gadonas, R. Self-polymerization of nano-fibres and nano-membranes induced by two-photon absorbtion. Lith. J. Phys. 2010, 50, 135–140. [Google Scholar]
- Minardi, S.; Majus, D.; Gopal, A.; Tamošauskas, G.; Milian, C.; Couairon, A.; Pertsch, T.; Dubietis, A. Energy deposition dynamics of femtosecond pulses in water. ArXiv E-Prints 2014. arXiv:1405.5378. [Google Scholar]
- Ertl, P.; Sticker, D.; Charwat, V.; Kasper, C.; Lepperdinger, G. Lab-on-a-chip technologies for stem cell analysis. Trends Biotechnol. 2014, 32, 245–253. [Google Scholar]
- Hasan, A.; Paul, A.; Vrana, N.; Zhao, X.; Memic, A.; Hwang, Y.S.; Dokmeci, M.; Khademhosseini, A. Microfluidic techniques for development of 3D vascularized tissue. Biomaterials 2014, 35, 7308–7325. [Google Scholar]
- Monzon, M.; Gibson, I.; Benitez, A.; Lorenzo, L.; Hernandez, P.; Marrero, M. Process and material behavior modeling for a new design of micro-additive fused deposition. Int. J. Adv. Manuf. Tech. 2013, 67, 2717–2726. [Google Scholar]
- Ohl, C.D.; Arora, M.; Dijkink, R.; Janve, V.; Lohse, D. Surface cleaning from laser-induced cavitation bubbles. Appl. Phys. Lett. 2006, 89, 074102. [Google Scholar]
- Song, W.; Hong, M.; Lukyanchuk, B.; Chong, T. Laser-induced cavitation bubbles for cleaning of solid surfaces. J. Appl. Phys. 2003. [Google Scholar]
- Hawkins, A.; Milbrandt, T.; Puleo, D.; Hilt, J. Composite hydrogel scaffolds with controlled pore opening via biodegradable hydrogel porogen degradation. J. Biomed. Mater. Res. A. 2014, 102, 400–412. [Google Scholar]
- Murphy, C.; Haugh, M.; O’Brien, F. The effect of mean pore size on cell attachment, proliferation and migrationin collagen–glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar]
- Simitzi, C.; Stratakis, E.; Fotakis, C.; Athanassakis, I.; Ranella, A. Microconical silicon structures influence NGF-induced PC12 cell morphology. J. Tissue Eng. Regen. Med. 2014. [Google Scholar] [CrossRef]
- Murphy, C.; Haugh, M.; O’Brien, F. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar]
- Murphy, C.; O’Brien, F.; Little, D.; Schindeler, A. Cell-scaffold interactions in the bone tissue engineering triad. Eur. Cells Mater. 2013, 26, 24052425. [Google Scholar]
- Nooeaid, P.; Salih, V.; Beier, J.; Boccaccin, A. Osteochondral tissue engineering: Scaffolds, stem cells and applications. J. Cell. Mol. Med. 2012, 16, 2247–2270. [Google Scholar]
- Juodkazis, S.; Mizeikis, V.; Seet, K.; Misawa, H.; Wegst, U. Mechanical properties and tuning of three-dimensional polymeric photonic crystals. Appl. Phys. Lett. 2007, 91, 241904. [Google Scholar]
- Wagers, A.; Weissman, I. Plasticity of Adult Stem Cells Review. Cell 2004, 116, 639–648. [Google Scholar]
- Donoso, M.; Mendez-Vilas, A.; Bruque, J.; Gonzalez-Martin, M. On the relationship between common amplitude surface roughness parameters and surface area: Implications for the study of cell–material interactions. Int. Biodet. Biodegr. 2007, 59, 245–251. [Google Scholar]
- Chang, H.; Wang, Y. Cell Responses to Surface and Architecture of Tissue Engineering Scaffolds. In Regenerative Medicine and Tissue Engineering—Cells and Biomaterials; Eberli, D., Ed.; InTech: Rijeka, Croatia, 2011; pp. 569–588. [Google Scholar]
- Hatano, K.; Inoue, H.; Kojo, T.; Tsujisawa, T.; Uchiyama, C.; Uchida, Y. Effect of Surface Roughness on Proliferation and Alkaline Phosphatase Expression of Rat Calvarial Cells Cultured on Polystyrene. Bone 1999, 25, 439–445. [Google Scholar]
- Ivanova, E.; Hasan, J.; Webb, H.; Gervinskas, G.; Juodkazis, S.; Truong, V.; Wu, A.; Lamb, R.; Baulin, V.; Watson, G.; Watson, J.; Mainwaring, D.; Crawford, R. Bactericidal activity of black silicon. Nat. Commun. 2013, 4, 2838. [Google Scholar]
- Ovsianikov, A.; Gruene, M.; Pflaum, M.; Koch, L.; Maiorana, F.; Wilhelmi, M.; Haverich, A.; Chichkov, B. Laser printing of cells into 3D scaffolds. Biofabrication 2010, 2, 014104. [Google Scholar]
- Hallo, L.; Mezel, C.; Bourgeade, A.; Hebert, D.; Gamaly, E.; Juodkazis, S. Laser-matter interaction in transparent materials: Confined microexplosion and jet formation. In Extreme Photonics and Applications, NATO Science for Peace and Security Series B: Physics and Biophysics; Springer: Heidelberg, Germany, 2010; pp. 121–146. [Google Scholar]
- Wei, G.; Ma, P. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials 2004, 25, 4749–4757. [Google Scholar]
- Galanopoulos, S.; Chatzidai, N.; Melissinaki, V.; Selimis, A.; Schizas, C.; Farsari, M.; Karalekas, D. Design, fabrication and computational characterization of a 3D micro-valve built by multi-photon polymerization. Micromachines 2014, 5, 505–514. [Google Scholar]
- Juodkazis, S. Writing 3D patterns of microvessels. Int. J. Nanomedicine 2012, 7, 3701–3702. [Google Scholar]
- Bejan, A.; Lorente, S. Constructal law of design and evolution: Physics, biology, technology, and society. J. Appl. Phys. 2013, 113, 151301. [Google Scholar]
- Comina, G.; Suska, A.; Filippini, D. PDMS lab-on-a-chip fabrication using 3D printed templates. Lab Chip 2014, 14, 424–430. [Google Scholar]
- Gloag, E.; Javed, M.; Wang, H.; Gee, M.; Wade, S.; Turnbull, L.; Whitchurch, C. Stigmergy: A key driver of self-organization in bacterial biofilms. Commun. Integr. Biol. 2013. [Google Scholar]
- Loo, C.; Lee, W.; Cavaliere, R.; Whitchurch, C.; Rohanizadeh, R. Superhydrophobic, Nanotextured Polyvinyl Chloride Films for Delaying Pseudomonas Aeruginosa Attachment to Intubation Tubes and Medical Plastics. Acta Biomater 2012, 8, 1881–1890. [Google Scholar]



© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malinauskas, M.; Rekštytė, S.; Lukoševičius, L.; Butkus, S.; Balčiūnas, E.; Pečiukaitytė, M.; Baltriukienė, D.; Bukelskienė, V.; Butkevičius, A.; Kucevičius, P.; et al. 3D Microporous Scaffolds Manufactured via Combination of Fused Filament Fabrication and Direct Laser Writing Ablation. Micromachines 2014, 5, 839-858. https://doi.org/10.3390/mi5040839
Malinauskas M, Rekštytė S, Lukoševičius L, Butkus S, Balčiūnas E, Pečiukaitytė M, Baltriukienė D, Bukelskienė V, Butkevičius A, Kucevičius P, et al. 3D Microporous Scaffolds Manufactured via Combination of Fused Filament Fabrication and Direct Laser Writing Ablation. Micromachines. 2014; 5(4):839-858. https://doi.org/10.3390/mi5040839
Chicago/Turabian StyleMalinauskas, Mangirdas, Sima Rekštytė, Laurynas Lukoševičius, Simas Butkus, Evaldas Balčiūnas, Milda Pečiukaitytė, Daiva Baltriukienė, Virginija Bukelskienė, Arūnas Butkevičius, Povilas Kucevičius, and et al. 2014. "3D Microporous Scaffolds Manufactured via Combination of Fused Filament Fabrication and Direct Laser Writing Ablation" Micromachines 5, no. 4: 839-858. https://doi.org/10.3390/mi5040839
APA StyleMalinauskas, M., Rekštytė, S., Lukoševičius, L., Butkus, S., Balčiūnas, E., Pečiukaitytė, M., Baltriukienė, D., Bukelskienė, V., Butkevičius, A., Kucevičius, P., Rutkūnas, V., & Juodkazis, S. (2014). 3D Microporous Scaffolds Manufactured via Combination of Fused Filament Fabrication and Direct Laser Writing Ablation. Micromachines, 5(4), 839-858. https://doi.org/10.3390/mi5040839






