1. Introduction
Microfluidic devices for processing nano- and picolitre volumes of liquids is now a well established technology, of great benefit to molecular biology, diagnostic medicine [
1], and various branches of analytical chemistry [
2]. Closed-channel configurations, operating in a laminar flow regime, are the most common implementation of microscale fluid handling technology today, well suited for the small volume samples in pharmacology, proteomics, DNA analysis and other life science areas [
3]. The number of practical applications of microfluidics is rapidly growing, and the commercial availability of devices and methods is progressing accordingly. Recent technological developments, in particular droplet microfluidics [
4], are expected to further facilitate and advance this trend. Moreover, the drive to integrate various on-chip detection schemes with sample handling and analyte separation in the context of chemical and medical analytics led to a new class of microfluidic devices, the micro-total analysis systems (µTAS) [
5]. The fabrication of closed channel microfluidic devices is generally achieved through top-down microengineering techniques [
6,
7,
8]: lithography of quartz, glass and silicon substrates [
9], soft lithography employing various polymers [
10], and layered (laminate) technologies, for example utilizing paper or polymeric thin films [
11].
However, in many important instances a closed channel microfluidic device is not a practical solution, even though sample size, flow velocity and other requirements are in the optimal range [
6]. For example, it is difficult or often impossible to interact with selected surface regions surfaces or small surface adhered objects, such as tissue slices or single biological cells. In this context, perfusion of biological cells with chemical solutions is an important experimental procedure, often performed for the purpose of drug testing or screening, for studies of ion channel activity, chemical signaling and other properties and features of single cells. In order to perform these tasks in closed channel microfluidic devices, the cells have to be introduced into the channel structure, manipulated to the desired position, kept alive under spatially confined conditions, and often exchanged or removed from the channels [
12]. Growing cells in microscale channels is subject to limits imposed by the diffusion dominated material transport in confined volumes, which can have a detrimental effect on cell growth. Moreover, the spatially controlled delivery of small amounts of liquid to a larger surface area—for instance in order to create defined patterns on a surface, achieve localized surface functionalization, or perfuse regions of tissue slices—goes beyond the abilities of closed channel devices [
13].
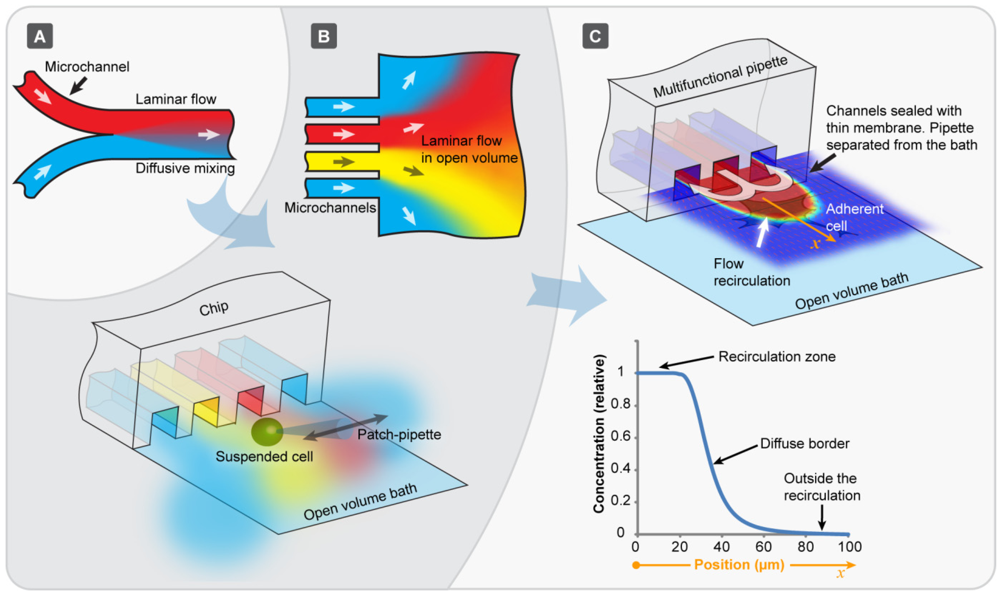
A fresh approach to the problem was introduced a few years back with the development of open channel microfluidic devices [
14], exploiting regions of coexisting flows at the exits of multiple parallel channels for cell perfusion. As microflows are typically laminar, several streams of fluid can flow in parallel in the same channel without converging into each other (
Figure 1(A)). Diffusion dominates the exchange of molecular species in those parallel streams, and equilibration is achieved on a short timescale of seconds to minutes. This feature allows for diffusional mixing as the sole mode of transport between flows, and can be used to control separation and the progress of chemical reactions. When such laminar microstreams exit into an open volume (
Figure 1(B), upper panel), the pattern remains parallel for a short distance before they diverge and merge. If a cell is manipulated through such a fluidic arrangement (
Figure 1(B), lower panel), very short solution exchange times, at the order of a few milliseconds, can be achieved. The flows are typically generated by means of applying air pressure to wells located at the opposite end of the channels. Open volume devices shortened the times necessary to perform dose-response determinations on single cells tremendously, but suffered from a number of drawbacks. Over time, the open volume becomes contaminated by the outflow from the channels. It is even more problematic that cells have to be picked up individually, and micromanipulated to the channel exits.
An elegant practical solution to these problems is offered by a fairly new microflow concept, which uses a dynamically defined open volume principle rather than pre-defined, closed or open channels for confinement and delivery of fluids (
Figure 1(C)). Hydrodynamically confined flow (HCF) devices are a modern class of microfluidic flow cells, where a small rapidly moving volume of fluid is spatially confined within another, significantly larger fluid volume. The two miscible or immiscible liquids are physically in contact, separated only by means of a dynamically created virtual boundary (
Figure 1(C) top panel). The transport of molecules across the boundary is only possible by diffusion. The boundary can be sharply defined (
Figure 1(C), bottom panel), and in practical terms be controlled by adjusting the ratio between the inflow and outflow rate.
Figure 1.
Evolution of chip-based perfusion microfluidics. (A) Conventional microchannel in a laminar flow regime. The different colors represent individual solutions of different compositions which coexist in the streams and mix only by diffusion; (B) Open volume microfluidic devices, where parallel microchannels lead into an open bath, where the individual streams coexist for a short distance. Arrays of densely packed chemical environments are formed by the flows from neighboring microchannels. When a suspended cell is translated through these environments very fast (10 ms range), rapid solution exchange around the single cell is achieved highly effectively; (C) The hydrodynamically confined flow device technology is a similar, but more recent concept, where the beneficial features of laminar flow are available outside the physical device boundaries for an extended period of time. A confined volume is created at the device tip, where active convection out-competes diffusion. Contamination of the open volume is prevented, and the device can be positioned close to surface adhered cells and tissues.
Figure 1.
Evolution of chip-based perfusion microfluidics. (A) Conventional microchannel in a laminar flow regime. The different colors represent individual solutions of different compositions which coexist in the streams and mix only by diffusion; (B) Open volume microfluidic devices, where parallel microchannels lead into an open bath, where the individual streams coexist for a short distance. Arrays of densely packed chemical environments are formed by the flows from neighboring microchannels. When a suspended cell is translated through these environments very fast (10 ms range), rapid solution exchange around the single cell is achieved highly effectively; (C) The hydrodynamically confined flow device technology is a similar, but more recent concept, where the beneficial features of laminar flow are available outside the physical device boundaries for an extended period of time. A confined volume is created at the device tip, where active convection out-competes diffusion. Contamination of the open volume is prevented, and the device can be positioned close to surface adhered cells and tissues.
![Micromachines 03 00442 g001]()
The idea of a dynamic fluid confinement was first published in 2003 by Feinerman and Moses [
15], who described the use of a picoliter “fountain-pen” constructed from co-axial dual glass pipettes. This inspiring device allowed delivery of a liquid to an arbitrary location within the open bath, and contamination of the surrounding bath by the inflowing reagents is effectively avoided. This early device has its drawbacks in practical use. For example, upscaling of fabrication is impractical, and small errors in positioning in close proximity to the surface will instantly break the delicate glass assembly. The first report of a microfabricated device of this kind, which overcomes these limitations, arrived soon thereafter. It appeared as recently as 2005 in a publication [
16], where the term “hydrodynamic flow confinement” was also coined.
The operation principle of a hydrodynamically confined flow device is illustrated on the minimal two-channel configuration in
Figure 2. One channel serves as injection port or outlet (positive pressure), where liquid is introduced into an open volume, and one as aspiration port or inlet (negative pressure), through which liquid is removed from it.
Figure 2 shows a micrograph of the exits of two adjacent channels into an open volume, overlaid with arrows depicting the flow lines. The inflow into the aspiration port consists mainly of the open bath liquid (black lines in
Figure 2), and partially of the fluid injected through the outlet channel into the flow field of the aspiration channel (white lines in
Figure 2). The stream exiting from the outlet channel is confined and directed into the aspiration channel.
Figure 2.
Fluorescence micrograph, providing the view through an open volume onto the two quadratic channels of a microfluidic device which generates a hydrodynamically confined flow. The aqueous flow from the injection channel (outflow) contains fluorescein and red fluorescent beads to directly visualize the flow lines. The open volume appears dark. White arrows depict the flow lines of the injection channel outflow, black arrows the flow lines of the open volume medium. INJ: injection channel (outflow), ASP: aspiration channel (inflow). The image was adapted with permission from [
16]. 2005 Nature Publishing Group.
Figure 2.
Fluorescence micrograph, providing the view through an open volume onto the two quadratic channels of a microfluidic device which generates a hydrodynamically confined flow. The aqueous flow from the injection channel (outflow) contains fluorescein and red fluorescent beads to directly visualize the flow lines. The open volume appears dark. White arrows depict the flow lines of the injection channel outflow, black arrows the flow lines of the open volume medium. INJ: injection channel (outflow), ASP: aspiration channel (inflow). The image was adapted with permission from [
16]. 2005 Nature Publishing Group.
In order to achieve hydrodynamic confinement, the flow rate of the aspiration flow must be higher than the flow rate of the injection flow, and the outflow to inflow ratio must be smaller than a critical value. The ratio for the device in
Figure 2, featuring 20 × 20 µm channels with 30 µm separation, and an injection flow rate of 0.44 nL/s, is ~0.4. The boundary between the confined and the open volume is diffusive; its width is determined by the solution exchange time,
i.e., the time a molecule stays in the confined volume, and its diffusivity. The experimental conditions necessary to achieve efficient confinement, while inhibiting diffusion across the virtual boundary, are characterized by low Reynolds and high Péclet numbers.
There is currently only a relatively small number of HCF devices presented in the literature, as this novel class of device has just started to progress into a research area [
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27]. The pronounced advantages of the technology, being highly localized confinement, the ability to work on adherent cell cultures, and the possibility to use familiar and well-established culture protocols, come at the cost of low throughput. HCF devices are limited to relatively slow movements inside the open volume, as rapid shifts in position cause turbulences and distortions of the confined volume, which causes loss of confinement and contamination of the open volume. HCF devices are currently best suited for adherent single cell handling [
16,
19], some excel in surface processing [
24,
27] and analysis [
23], and others can be advantageously applied to tissue cultures [
18], which are typically too large to be introduced into closed channel devices. Some devices can be used in conjunction with other probes, such as patch clamp needles or external electrodes [
26]. Despite the low throughput characteristics, the high spatial resolution and contamination-free fluid delivery renders HCF devices as valuable research instruments for many applications that require localized perfusion.
The developments reported by several different research groups are summarized in
Table 1. Technologically closely related flow devices, which do not employ hydrodynamic confinement, but feature similar channel arrangements and in/out-flow concepts with an exposed liquid volume, are included for comparison. These can be considered milestones in the development of the HCF devices, and have their own interesting set of applications, for example in electrochemical surface analysis and in parallel assay technology. Highlighted in the table are typical materials and fabrication as well as application parameters, working distances and applications as reported in the original publication.
Figure 3.
Hydrodynamically confined flow devices. (
A) The microfluidic multipurpose probe [
16,
17], fabricated from a planar silicon dice. It features one injection port and one aspiration port and is positioned in parallel orientation to the surface;(
B) The vertical microfluidic probe [
24], constructed from a bonded silicon-glass quadrilateral, held in place by a holding clamp. It is identical in channel arrangement and function to the microfluidic multipurpose probe (MFP), but fabrication has been facilitated. The channel outlet face of the chip is also oriented parallel to the surface of interest, such that the surface is in full contact with the confined volume; (
C) The multipurpose pipette [
26], fabricated as a bonded PDMS-glass composite. This device creates a hydrodynamically confined volume at the tip of a pipette-shaped device, which is pressurized via on-chip wells confined within a holding interface. This device can be positioned at an angle α to the surface, since the three channel design with one injection port and two adjacent aspiration ports is supported by a thin bottom membrane, which allows close surface proximity. The fabrication procedure for the multilayered PDMS device by Queval
et al. [
18] is similar to the one depicted here, but requires an alignment of the channels prior to bonding;(
D–
F) Photographic images of the devices of (A–C), as shown in the original publications.
Figure 3.
Hydrodynamically confined flow devices. (
A) The microfluidic multipurpose probe [
16,
17], fabricated from a planar silicon dice. It features one injection port and one aspiration port and is positioned in parallel orientation to the surface;(
B) The vertical microfluidic probe [
24], constructed from a bonded silicon-glass quadrilateral, held in place by a holding clamp. It is identical in channel arrangement and function to the microfluidic multipurpose probe (MFP), but fabrication has been facilitated. The channel outlet face of the chip is also oriented parallel to the surface of interest, such that the surface is in full contact with the confined volume; (
C) The multipurpose pipette [
26], fabricated as a bonded PDMS-glass composite. This device creates a hydrodynamically confined volume at the tip of a pipette-shaped device, which is pressurized via on-chip wells confined within a holding interface. This device can be positioned at an angle α to the surface, since the three channel design with one injection port and two adjacent aspiration ports is supported by a thin bottom membrane, which allows close surface proximity. The fabrication procedure for the multilayered PDMS device by Queval
et al. [
18] is similar to the one depicted here, but requires an alignment of the channels prior to bonding;(
D–
F) Photographic images of the devices of (A–C), as shown in the original publications.
![Micromachines 03 00442 g003]()
Figure 3 summarizes three current concepts of hydrodynamically confined flow devices, the microfluidic multipurpose probe (MFP), the vertical microfluidic probe (vMFP), and the multifunctional micropipette (MFπ), pioneered by different research groups. They represent chronologically major steps in the development of the concept from the earliest reported device [
16], via a considerable conceptual improvement, in particular with respect to fabrication complexity [
24], to the most recent HCF pipette [
26]. The three different devices are also representative of typical material choices, ranging from silicon, via a silicon glass hybrid to PDMS elastomer. The fabrication strategies and applications are very briefly discussed further in the text.
Figure 3(A), depicts schematically the microfluidic probe developed by Junker
et al. from IBM. From design and idea, but not necessarily from principle, it may be considered a remote descendant of IBM’s classic ink jet printing technology [
16].
The device consists of a flat silicon plate of cm
2 dimensions with two central microscale orifices on a central mesa-like structure, one for solution inlet and one for outlet. For operation, it is arranged parallel to the surface of interest, and submerged in a shallow bath of fluid. It is held at a fixed distance of a few micrometers (
cf. Table 1) by four protruding corner posts. The plate is interfaced by supply tubes, and can be positioned by a micromanipulation device. When positive pressure is applied to the injection port and a moderate vacuum to the aspiration port, a stream of liquid moves through the bath and creates a defined volume of fluid, which is spatially confined to the region between surface and silicon mesa. The red color represents the inflow (injection) into and the blue color the outflow (aspiration) from the open volume. A part of the open volume is simultaneously drawn into the outflow channel. This open volume component is typically the major share of the total inflow volume, which is fundamental to define the hydrodynamic confinement. In this two-cannel device, the close proximity of the surface is preferred to prevent fluid from escaping the confinement. The lower panel in
Figure 3(A) displays the bottom plate of the device together with an enlarged view of the mesa.
Figure 3(D) shows a photograph of this device, as presented in the original publication.
Figure 3(B) schematically displays a design modification of the MFP, termed the vertical microfluidic probe [
24]. The large silicon bottom plate of the earlier design has been replaced by a tetragonal Si/glass composite with a flat, polished apex, which can be clamped and interfaced by a sealing holder. In order to achieve efficient interaction of the out-flowing liquid with the surface, this design still requires parallel alignment of the channel outlet plane with the surface. The generation of the confined volume is commensurate to the MFP, fluid circulation is also achieved through tubing and syringe pumps. The photograph in
Figure 3(E) shows the device protruding from its holder, which has in- and outflow tubing attached. Both MFP and vMFP are fabricated from silicon or bonded silicon/glass. Somewhat disadvantageous for use with upright microscopes is the vertical positioning. The vertical probe eliminates some of the problems, since it is rather small and at least partly transparent. Inverse microscopes are accessible, even though a means of precise positioning might be advisable, such as a motorized stage. The multifunctional pipette (MFπ), depicted schematically in
Figure 3(C) and as a photograph in its interface/holder in
Figure 3(F), overcomes these problems by both using a transparent material combination and a design which allows positioning at an angle to the surface. The MFπ is a three-channel device, with one central injection, and two adjacent aspiration ports in the same plane. This arrangement improves the confinement around the injection channel, and reduces the danger of device failure due to aspiration channel clogging. An important functional feature of the device is the 10–30 µm thin transparent bottom membrane, which allows it, just like the coaxial pipette [
15], to be manipulated close to an object of interest on the surface. In contrast to the coaxial pipette, the MFπ tip is entirely fabricated from PDMS elastomer, and can be repeatedly brought in contact with the surface without loss of integrity. The flow profile generated by the three-channel arrangement is similar to the one provided by the coaxial fountain pen. It allows free-standing operation, since no fluid can escape the hydrodynamic confinement within the frontal recirculation zone. The lower panel in
Figure 3(A) shows a side view (FEM simulation of the concentration profile at the channel outlet) and a top view. The channels are filled with a red colored liquid for visualization. The device further features on-chip fluid reservoirs and microfluidic circuitry and requires only positive and negative pressure supplied through an interface/holder. This pipette opens interesting opportunities in biosciences, pharmacology and clinical research, since it can be co-located with additional probing equipment under most microscopes, and allows highly localized interaction of a chemical or biochemical stimulant with surface-adhered cells and tissue in dish cultures.
The three devices have individual design strengths, which make them attractive research instruments in particular application areas (
cf. Table 1). Each of the concepts requires a different set of microfabrication techniques for fabrication and assembly, owing to the materials requirements and, most likely, to the availability of processing equipment and expertise.
3. Application Examples
Each of the HCF devices discussed above was designed to provide one or more solution(s) to technological challenges, and in some instances to make microscale processes or scientific experiments feasible which were previously difficult or impossible to perform. The applications, which were reported for each device in the respective original publication, are summarized in
Table 1. While similar in principle and operation, the differences in design and implementation give each device its own range of particularly suitable applications. While the silicon and silicon-glass composite probes are useful mainly for chemical surface processing, such as staining, etching, labeling or decorating surface areas or surface-attached objects, the MFπ has its strong side in those single cell experiments, where superfusion and direct or indirect support of other probing techniques is desired. In particular, electrophysiology pipettes (patch clamp), intracellular recording probes, and electroporation electrodes can be used together with the pipette.
Figure 5 shows application examples for four different HCF devices. In the original publication of the MFP [
16] (
cf. Figure 2(A)), several application examples were provided. In one example, continuous variation of the scanning velocity of the MFP was utilized to create local concentration gradients, useful for example for patterning surfaces with biomolecules, such as proteins. A MFP with 24 µm separated 40 × 40 µm apertures was positioned 15–20 µm above a substrate covered with fixed NIH3T3 fibroblast cells, and the cells were exposed to a solution of a membrane soluble fluorescent dye, which selectively stained the targeted cells.
Figure 5(A) shows another reported example, the selective detachment and collection of a single living cell from a surface, exploiting the shear forces exerted by the moving liquid within the hydrodynamic confinement region. This is on one hand an interesting method for harvesting single adherent cells, but on the other hand illustrates that the HCF technique also poses a certain risk of involuntarily removing cells from the culture plate. The rapid solution exchange achieved by the MFP also suggests applications in chemistry, micro fabrication and surface processing. Mask-less lithography, where photoresistance is developed directly on the size scale of the desired structures, is a promising technique for patterning or modifying planar surfaces. The MFP was reported to be able to write a pattern into an AZ4562 positive photoresist layer of 3 µm thickness, dispensing AZ400K developer as process liquid. The shape of the spots is here determined by the geometry of the HCF region.
Figure 5(B) shows the application of a multi-channel version of the MFP for the localized microperfusion of hippocampal organotypic slice from a L15 transgenic mouse. This MFP was made in poly(dimethylsiloxane) and features six micrometre-scale apertures. Each aperture can be used deliberately for either injection or aspiration of solutions, allowing many possible combinations. The MFP used for the perfusion of a small number of cells in a brain slice with concurrent multicolor confocal imaging of the perfused fluorescent dye and fluorescently labeled sub-cellular structures within the tissue. This HCF device offers opportunities for, e.g., studies of neuron interactions where high spatial resolution is required [
18]. In
Figure 5(C), the application of the MFπ [
19] (
cf. Figure 2(C)) for a single cell dose-response determination is depicted. This device was designed for carrying out a variety of on-chip fluid processing operations, such as mixing, multiplexing, or gradient generation. Using a fluorescence uptake assay, the generation of dose-response curves
in situ from adherent Chinese Hamster Ovary (CHO) cells expressing proton-activated human transient receptor potential vanilloid (hTRPV1) receptors was demonstrated. The pipette served as an automatic solution switching and dilution device in order to supply a sequence of buffer solutions of decreasing pH. A device integrated mixer [
37] generated the dilution sequence, which was used to perfuse the targeted cells. Reduced pH (5.4) under calcium-free conditions causes TRPV1 pore dilation, and thus allows for entry of a fluorescent dye into the cell. Further application examples for the MFπ were reported, including membrane bleb formation by exposing selected groups of cells to formaldehyde/dithiothreitol-containing solutions, and the sequential delivery of several active compounds to selected cells by valve-less switching between reagent streams originating from on-chip wells [
26]. In
Figure 5(D), a recently reported application of the vMFP (
cf. Figure 2(B)), termed micro-immunohistochemistry, is shown [
27]. By means of this HCF device, nanolitre amounts of antibody solutions were applied to micrometre-sized regions of tissue for their incubation with primary antibodies, which is typical in conventional IHC.
Figure 5.
Application examples of HCF superfusion devices. (
A) Single Cell Selective Detachment and Aspiration of a Multipurpose Microfluidic Probe (MFP)[
16]. Reprinted with permission from [
16]. Copyright 2005 Nature Publishing Group;(
B) Perfusion of Organotypic Tissue Slices with a Microfluidic Probe [
18]. Confocal cross-sections (x-y and z scan) of a hippocampal organotypic slice from a L15 transgenic mouse, recorded during application of the MFP [
18]. The cell nuclei (blue), mGFP–labeled pyramidal cells (green) and 10 kDa dextran conjugated to Alexa 647 (red), which is used to superfuse the tissue, are visualized simultaneously. Reprinted with permission from [
18]. Copyright 2009 Royal Society of Great Britain;(
C) Single Cell Dose Response Measurement using a Microfluidic Pipette (MFπ)[
19]. Graphic time series of the dependency of intracellular fluorescence intensity on the pH, obtained by selective exposure of CHO cells with overexpressed TRPV1 ion channels to a pH dilution series generated by means of the pipette. The decreasing pH causes pore dilation and uptake of the fluorescent dye YO-PRO-1. The two traces represent fluorescence intensities measured from two independent cells. Arrows: concentrationswitching times; Inset: illustration of the device application. Reprinted with permission from [
19]. Copyright 2009 Royal Society of Great Britain;(
D) Immunohistochemistry using a Vertical Microfluidic Probe (vMFP)[
27]. Staining of a human ductal carcinoma breast tissue section for the presence of tumor suppressor protein p53 (antibody: monoclonal mouse anti-human α-p53) and human progesterone receptor (antibody: anti progesterone receptor α-PR), with an additional hematoxylin (Natural Black 1) nuclear counterstain. Reprinted with permission from [
27]. Copyright 2012 Royal Society of Great Britain.
Figure 5.
Application examples of HCF superfusion devices. (
A) Single Cell Selective Detachment and Aspiration of a Multipurpose Microfluidic Probe (MFP)[
16]. Reprinted with permission from [
16]. Copyright 2005 Nature Publishing Group;(
B) Perfusion of Organotypic Tissue Slices with a Microfluidic Probe [
18]. Confocal cross-sections (x-y and z scan) of a hippocampal organotypic slice from a L15 transgenic mouse, recorded during application of the MFP [
18]. The cell nuclei (blue), mGFP–labeled pyramidal cells (green) and 10 kDa dextran conjugated to Alexa 647 (red), which is used to superfuse the tissue, are visualized simultaneously. Reprinted with permission from [
18]. Copyright 2009 Royal Society of Great Britain;(
C) Single Cell Dose Response Measurement using a Microfluidic Pipette (MFπ)[
19]. Graphic time series of the dependency of intracellular fluorescence intensity on the pH, obtained by selective exposure of CHO cells with overexpressed TRPV1 ion channels to a pH dilution series generated by means of the pipette. The decreasing pH causes pore dilation and uptake of the fluorescent dye YO-PRO-1. The two traces represent fluorescence intensities measured from two independent cells. Arrows: concentrationswitching times; Inset: illustration of the device application. Reprinted with permission from [
19]. Copyright 2009 Royal Society of Great Britain;(
D) Immunohistochemistry using a Vertical Microfluidic Probe (vMFP)[
27]. Staining of a human ductal carcinoma breast tissue section for the presence of tumor suppressor protein p53 (antibody: monoclonal mouse anti-human α-p53) and human progesterone receptor (antibody: anti progesterone receptor α-PR), with an additional hematoxylin (Natural Black 1) nuclear counterstain. Reprinted with permission from [
27]. Copyright 2012 Royal Society of Great Britain.
![Micromachines 03 00442 g005]()
By exchanging the fluids in the supply tubing of the device (
cf. Figure 2(B)) the probe was used to stain individual cores of tissue microarrays with multiple antigens. The use of this technology is more preservative of tissue samples and reagents than the conventional technique, and addresses antibody cross-reactivity issues. Different staining conditions can be conveniently applied to a single tissue section.
The reported application examples from a range of fields within biology and microtechnology (
cf. Table 1) demonstrate clearly the high potential of the different HCF devices, as they address experimental problems that could not, or only with difficulties, be solved by traditional glass pipette methods, closed channel microfluidic chips or other kinds of traditional or microdevice technology.