Coupling of Ammonium Dihydrogen Phosphate Additives with LiPF6 Electrolytes for Improving Thermal Stability and Performance of Lithium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Liquid Electrolyte Preparation
2.2. Half-Cell Fabrication and Electrochemical Measurement
2.3. Characterizations
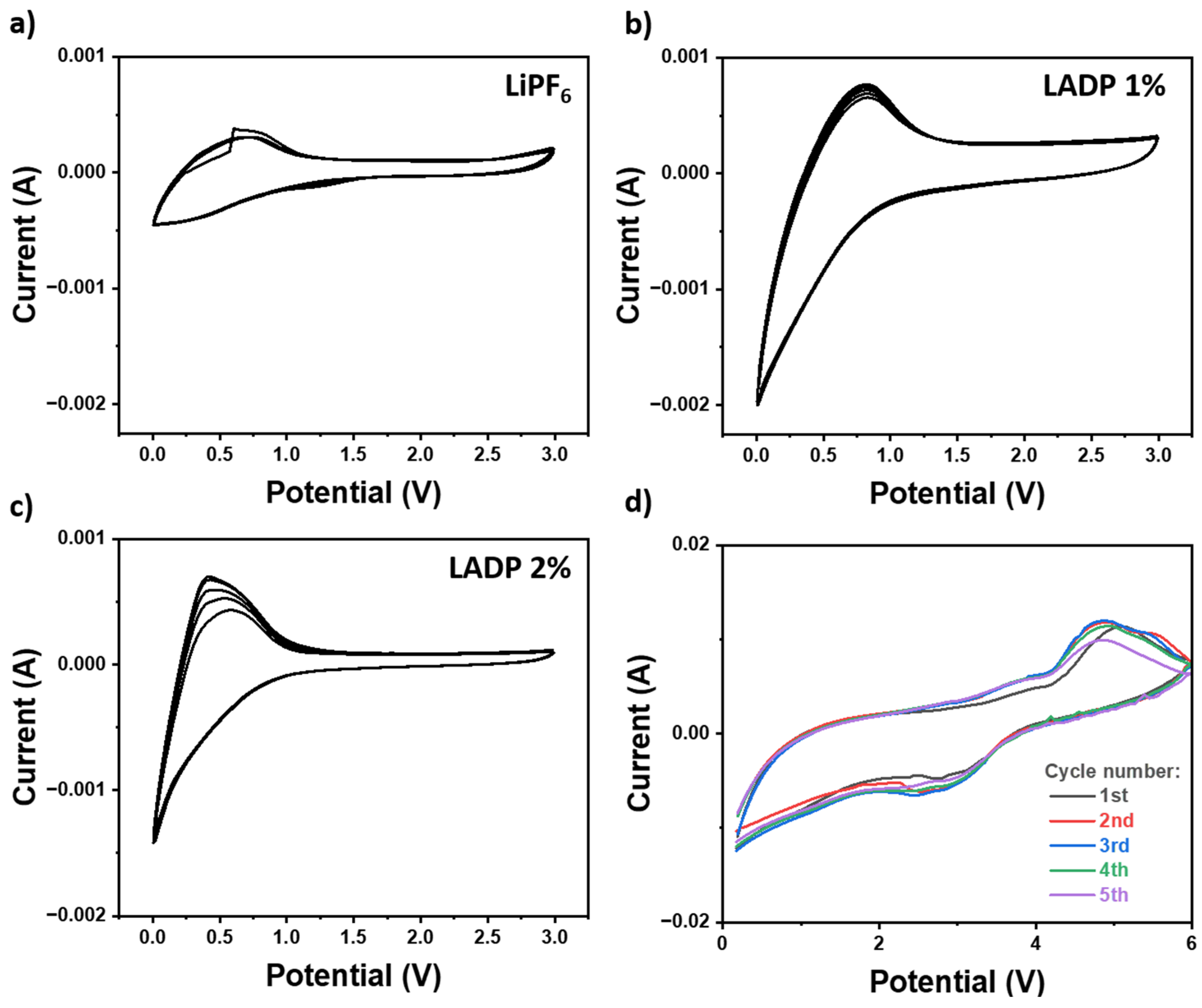
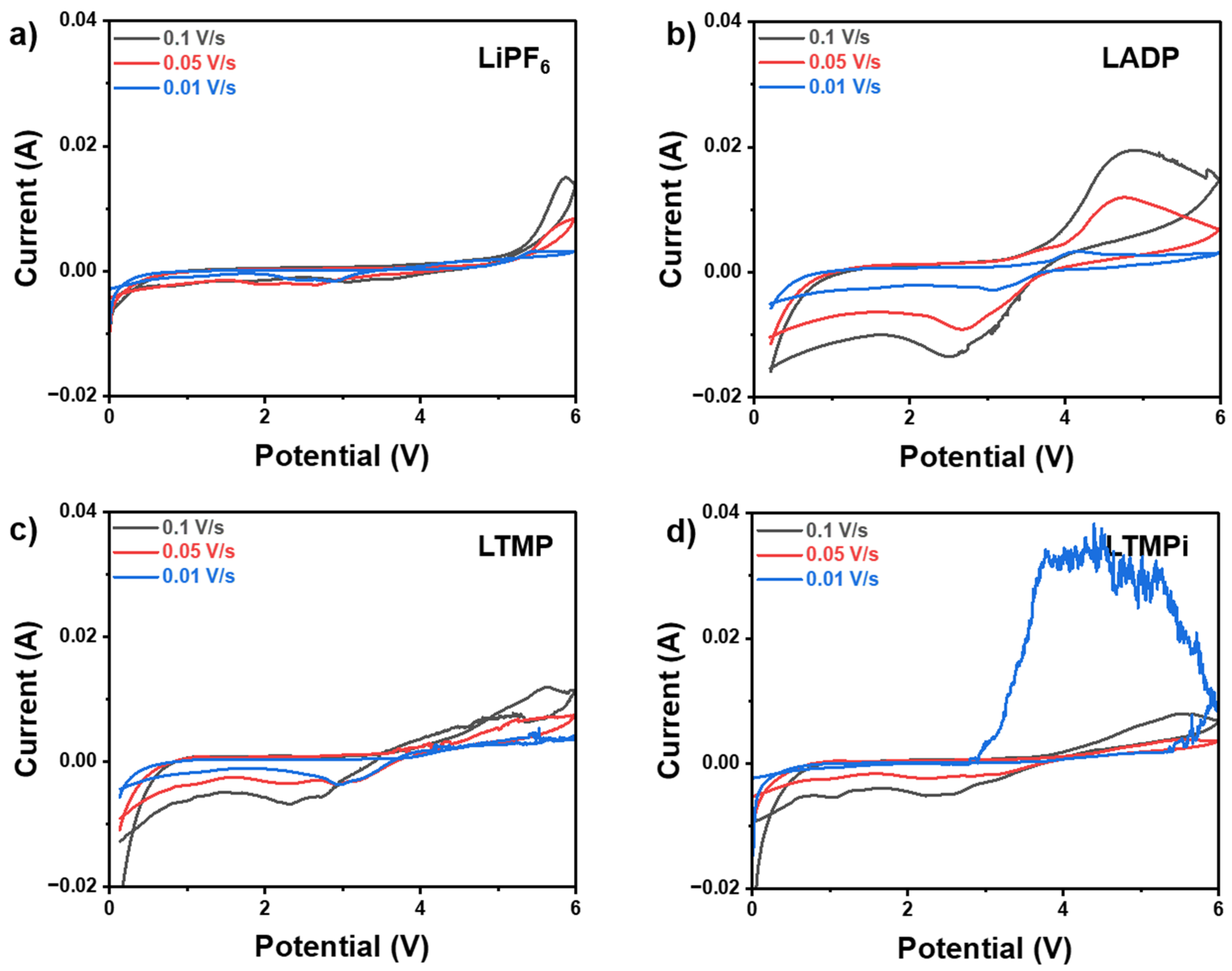
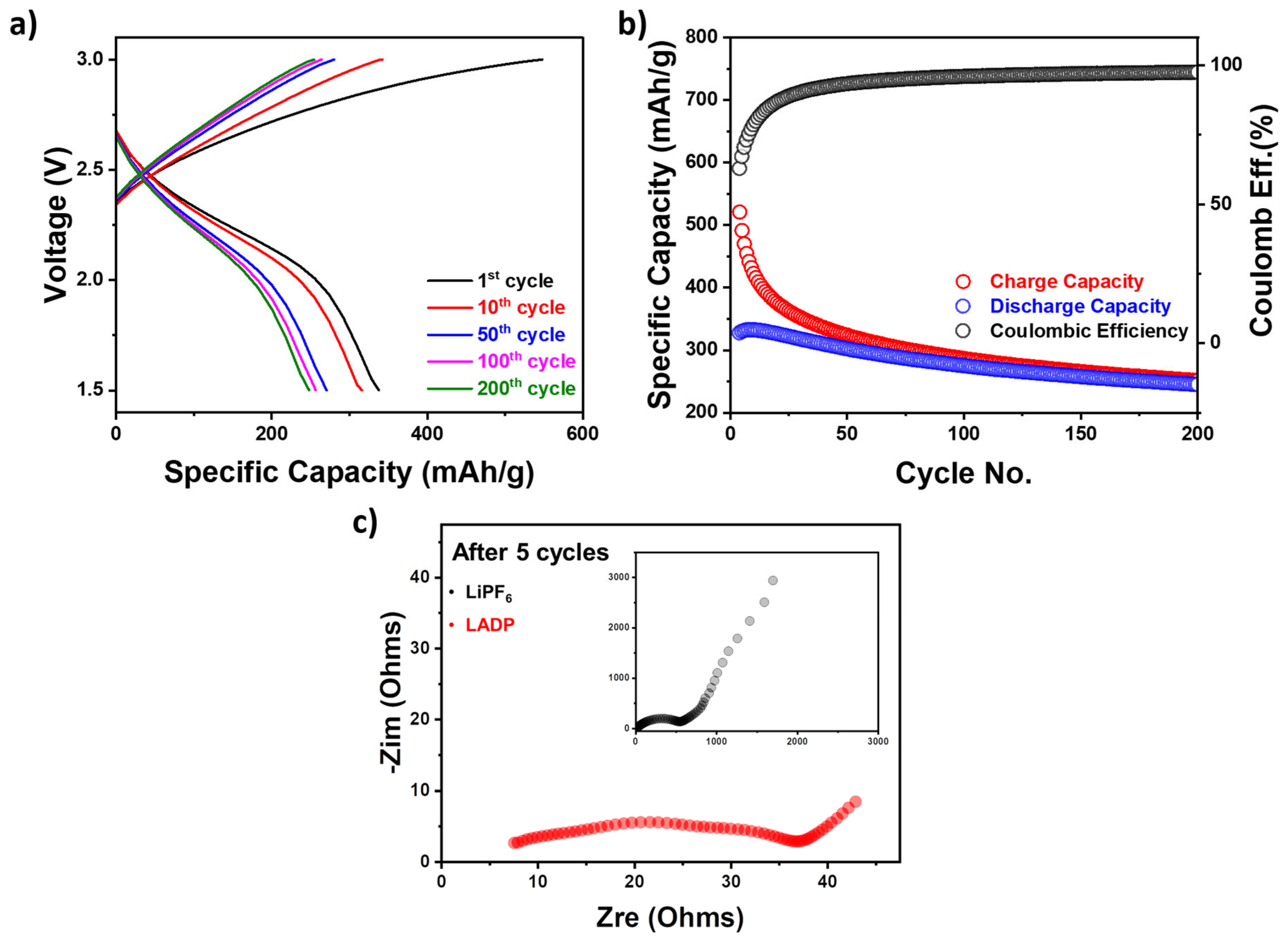
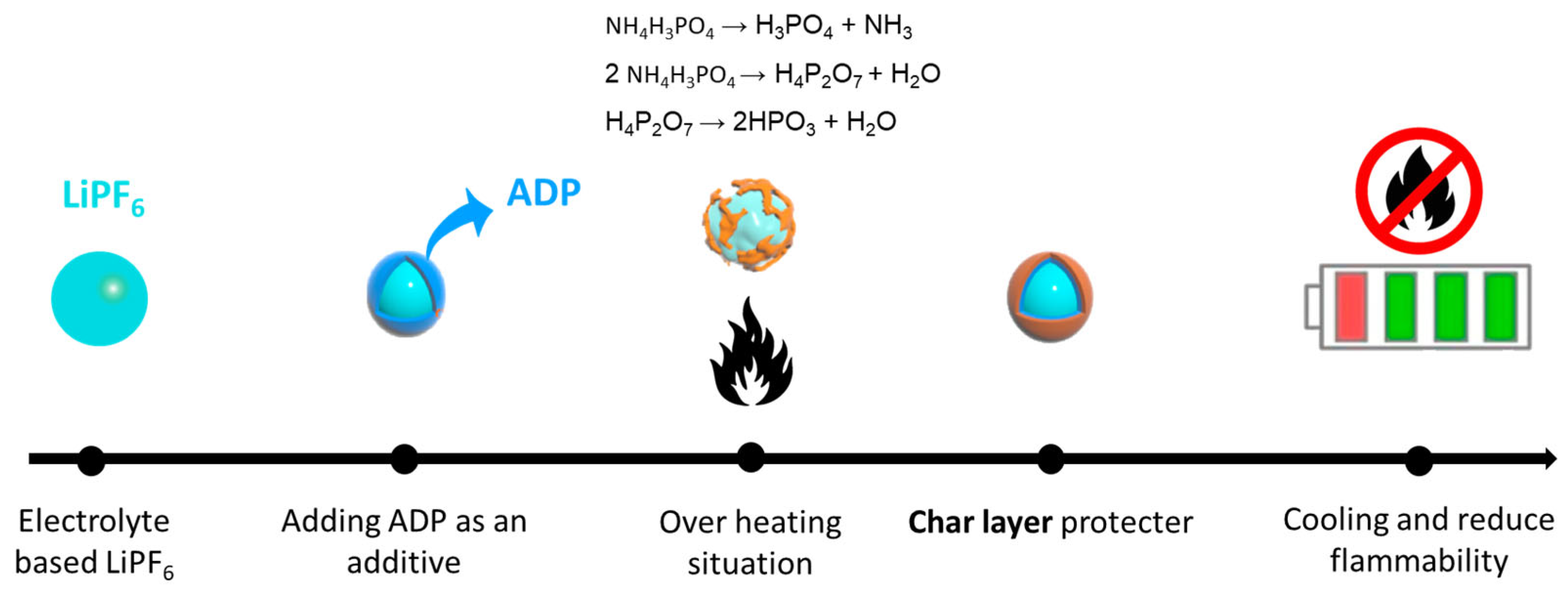
3. Results and Discussion
3.1. Thermal Properties
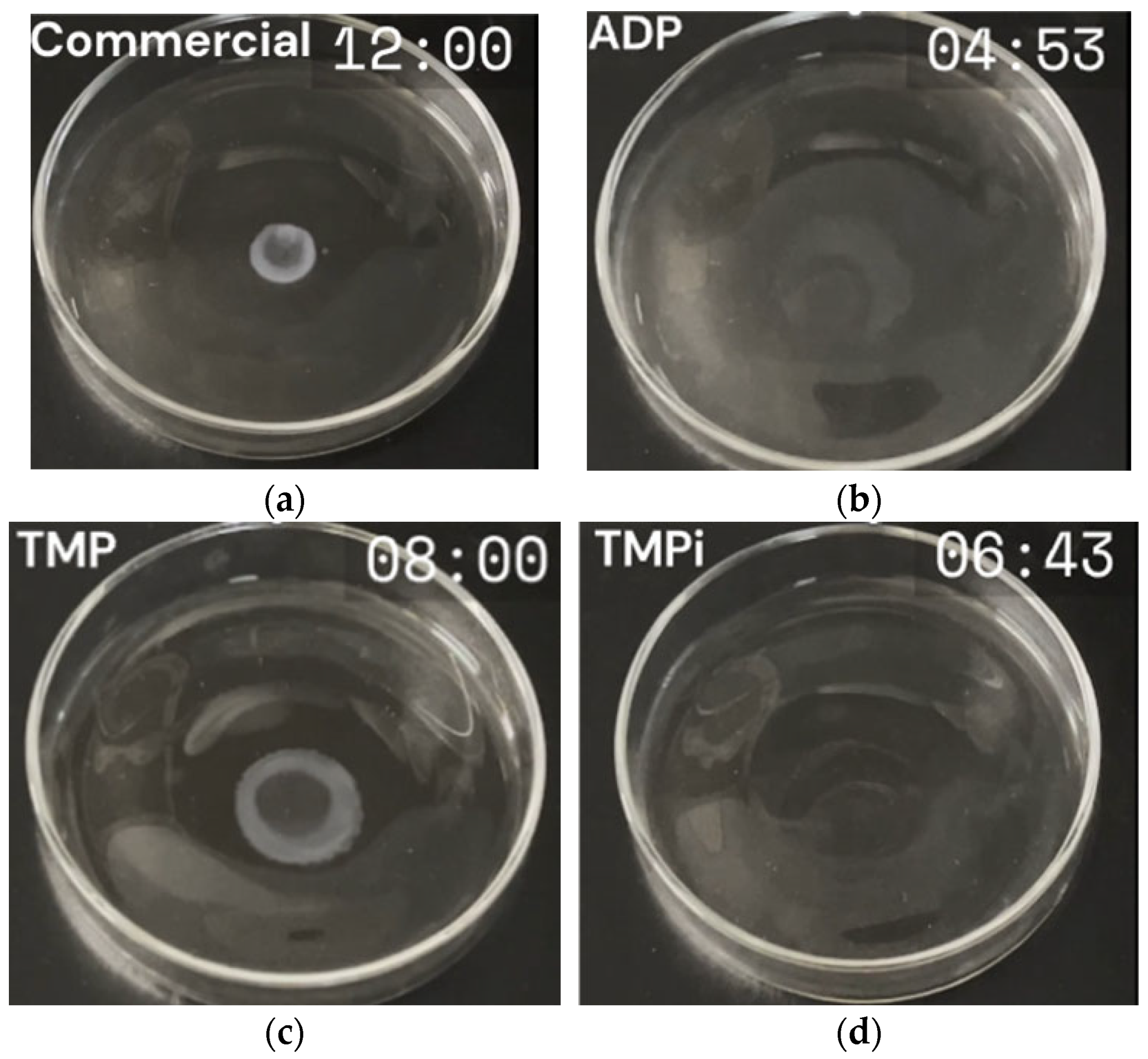
3.2. Flammability Test Results
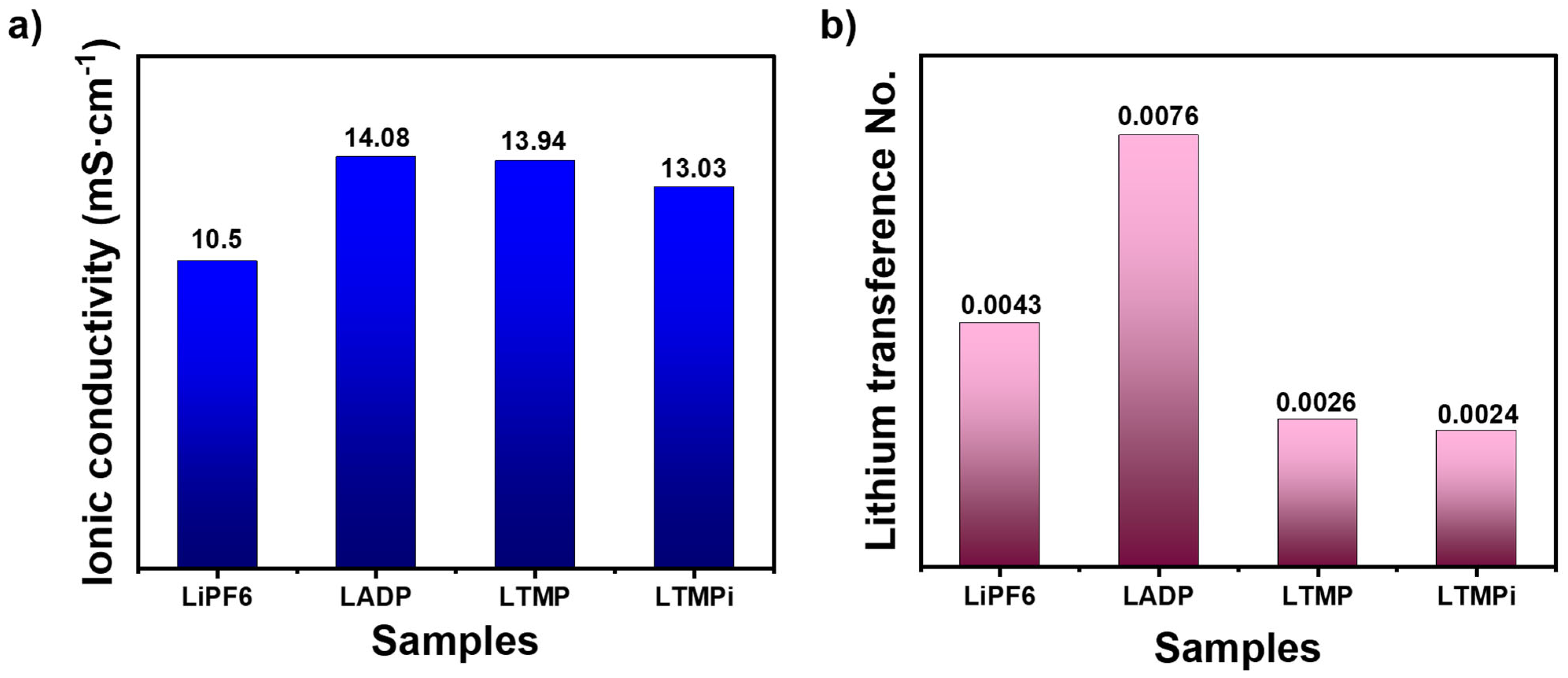
3.3. Electrochemical Measurements
3.4. ADP’s Role in Enhancing LIB Safety and Electrochemical Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Silva, O.; Bhurt, M.; Sajnani, S.; Maniama, S. Advancements in Battery Technology: Beyond Lithium-Lon Batteries. In Batteries; Jenny Stanford Publishing: Singapore, 2024; pp. 1–59. [Google Scholar]
- Ahmed, M.D.; Maraz, K.M. Revolutionizing energy storage: Overcoming challenges and unleashing the potential of next generation Lithium-ion battery technology. Mater. Eng. Res. 2023, 5, 265–278. [Google Scholar] [CrossRef]
- Xu, N.; Shi, J.; Liu, G.; Yang, X.; Zheng, J.; Zhang, Z.; Yang, Y. Research progress of fluorine-containing electrolyte additives for lithium ion batteries. J. Power Sources Adv. 2021, 7, 100043. [Google Scholar] [CrossRef]
- Cao, C.; Zhong, Y.; Shao, Z. Electrolyte Engineering for Safer Lithium-Ion Batteries: A Review. Chin. J. Chem. 2023, 41, 1119–1141. [Google Scholar] [CrossRef]
- Mohanty, D.; Hung, I.-M.; Hsieh, C.-T.; Pan, J.-P.; Liu, W.-R. Critical Review on High-Safety Lithium-Lon Batteries Modified by Self-Terminated Oligomers with Hyperbranched Architectures. Batteries 2024, 10, 65. [Google Scholar] [CrossRef]
- Zhao, J.; Feng, X.; Tran, M.-K.; Fowler, M.; Ouyang, M.; Burke, A.F. Battery safety: Fault diagnosis from laboratory to real world. J. Power Sources 2024, 598, 234111. [Google Scholar] [CrossRef]
- Lux, S.F.; Chevalier, J.; Lucas, I.T.; Kostecki, R. HF formation in LiPF6-based organic carbonate electrolytes. ECS Electrochem. Lett. 2013, 2, A121. [Google Scholar] [CrossRef]
- Hyung, Y.E.; Vissers, D.R.; Amine, K. Flame-retardant additives for lithium-ion batteries. J. Power Sources 2003, 119, 383–387. [Google Scholar] [CrossRef]
- Otsuki, M.; Ogino, T. Flame-retardant additives for lithium-ion batteries. In Lithium-Ion Batteries: Science and Technologies; Springer: New York, NY, USA, 2008; pp. 1–15. [Google Scholar]
- Zhou, D.; Li, W.; Tan, C.; Zuo, X.; Huang, Y. Cresyl diphenyl phosphate as flame retardant additive for lithium-ion batteries. J. Power Sources 2008, 184, 589–592. [Google Scholar] [CrossRef]
- Peng, H.-S.; Zheng, D.; Guo, Y.; Wang, P.; Zhang, J. Experimental investigation of both flame retardancy and electrochemical performance of PYR13TFSI for lithium battery electrolytes. J. Energy Storage 2025, 106, 114832. [Google Scholar] [CrossRef]
- Shaw, S. Halogenated flame retardants: Do the fire safety benefits justify the risks? Rev. Environ. Health 2010, 25, 261–306. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, Y.; Wang, N.; Fu, W. Recent progress in flame retardant technology of battery: A review. Resour. Chem. Mater. 2023, 2, 80–99. [Google Scholar] [CrossRef]
- Dagger, T.; Rad, B.R.; Schappacher, F.M.; Winter, M. Comparative performance evaluation of flame retardant additives for lithium ion batteries–I. Safety, chemical and electrochemical stabilities. Energy Technol. 2018, 6, 2011–2022. [Google Scholar] [CrossRef]
- Dalavi, S.; Xu, M.; Ravdel, B.; Zhou, L.; Lucht, B.L. Nonflammable electrolytes for lithium-ion batteries containing dimethyl methylphosphonate. J. Electrochem. Soc. 2010, 157, A1113. [Google Scholar] [CrossRef]
- Gao, Y.; Pan, Z.; Sun, J.; Liu, Z.; Wang, J. High-energy batteries: Beyond lithium-ion and their long road to commercialisation. Nano-Micro Lett. 2022, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- von Aspern, N.; Röser, S.; Rad, B.R.; Murmann, P.; Streipert, B.; Mönnighoff, X.; Tillmann, S.D.; Shevchuk, M.; Stubbmann-Kazakova, O.; Röschenthaler, G.-V. Phosphorus additives for improving high voltage stability and safety of lithium ion batteries. J. Fluor. Chem. 2017, 198, 24–33. [Google Scholar] [CrossRef]
- Feng, J.; Ma, P.; Yang, H.; Lu, L. Understanding the interactions of phosphonate-based flame-retarding additives with graphitic anode for lithium ion batteries. Electrochim. Acta 2013, 114, 688–692. [Google Scholar] [CrossRef]
- Lee, D.J.; Im, D.; Ryu, Y.-G.; Lee, S.; Yoon, J.; Lee, J.; Choi, W.; Jung, I.; Lee, S.; Doo, S.-G. Phosphorus derivatives as electrolyte additives for lithium-ion battery: The removal of O2 generated from lithium-rich layered oxide cathode. J. Power Sources 2013, 243, 831–835. [Google Scholar] [CrossRef]
- Han, Y.-K.; Yoo, J.; Yim, T. Why is tris (trimethylsilyl) phosphite effective as an additive for high-voltage lithium-ion batteries? J. Mater. Chem. A 2015, 3, 10900–10909. [Google Scholar] [CrossRef]
- Fan, C.; Gao, Y.; Li, Y.; Yan, L.; Zhu, D.; Guo, S.; Ou, C.; Wang, Z. A flame-retardant densified wood as robust and fire-safe structural material. Wood Sci. Technol. 2023, 57, 111–134. [Google Scholar] [CrossRef]
- Kong, L.; Guan, H.; Wang, X. In situ polymerization of furfuryl alcohol with ammonium dihydrogen phosphate in poplar wood for improved dimensional stability and flame retardancy. ACS Sustain. Chem. Eng. 2018, 6, 3349–3357. [Google Scholar] [CrossRef]
- Jaramillo, A.F.; Díaz-Gómez, A.; Ramirez, J.; Berrio, M.E.; Cornejo, V.; Rojas, D.; Montoya, L.F.; Mera, A.; Melendrez, M.F. Eco-friendly fire-resistant coatings containing dihydrogen ammonium phosphate microcapsules and tannins. Coatings 2021, 11, 280. [Google Scholar] [CrossRef]
- Han, L.; Liao, C.; Liu, Y.; Yu, H.; Zhang, S.; Zhu, Y.; Li, Z.; Li, X.; Kan, Y.; Hu, Y. Non-flammable sandwich-structured TPU gel polymer electrolyte without flame retardant addition for high performance lithium ion batteries. Energy Storage Mater. 2022, 52, 562–572. [Google Scholar] [CrossRef]
- Agubra, V.A.; Fergus, J.W. The formation and stability of the solid electrolyte interface on the graphite anode. J. Power Sources 2014, 268, 153–162. [Google Scholar] [CrossRef]
- Mishra, R.; Anne, M.; Das, S.; Chavva, T.; Shelke, M.V.; Pol, V.G. Glory of Fire Retardants in Li-Lon Batteries: Could They Be Intrinsically Safer? Adv. Sustain. Syst. 2024, 8, 2400273. [Google Scholar] [CrossRef]
- Kim, J.H.; Hyun, J.H.; Kim, S.; Park, W.H.; Yu, S.H. Phosphorus-Based Flame-Retardant Electrolytes for Lithium Batteries. Adv. Energy Mater. 2025, 15, 2500587. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Xiang, H.; Ma, X.; Yuan, Q.; Wang, Q.; Chen, C. Trimethyl phosphite as an electrolyte additive for high-voltage lithium-ion batteries using lithium-rich layered oxide cathode. J. Power Sources 2013, 240, 471–475. [Google Scholar] [CrossRef]
- Yao, X.; Xie, S.; Chen, C.; Wang, Q.; Sun, J.; Li, Y.; Lu, S. Comparative study of trimethyl phosphite and trimethyl phosphate as electrolyte additives in lithium ion batteries. J. Power Sources 2005, 144, 170–175. [Google Scholar] [CrossRef]
- Jin, Z.; Gao, H.; Kong, C.; Zhan, H.; Li, Z. A novel phosphate-based flame retardant and film-forming electrolyte additive for lithium ion batteries. ECS Electrochem. Lett. 2013, 2, A66. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar] [CrossRef] [PubMed]
- Pardo, A.; Romero, J.; Ortiz, E. High-temperature behaviour of ammonium dihydrogen phosphate. J. Phys. Conf. Ser. 2017, 935, 012050. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, F.; Li, C.; Li, Z.; Li, R. Fire extinguishing performance and mechanism for several typical dry water extinguishing agents. RSC Adv. 2021, 11, 9827–9836. [Google Scholar] [CrossRef]
- Septiana, A.; Honggowiranto, W.; Kartini, E.; Hidayat, R. Comparative study on the ionic conductivities and redox properties of LiPF6 and LiTFSI electrolytes and the characteristics of their rechargeable lithium ion batteries. IOP Conf. Ser. Mater. Sci. Eng. 2018, 432, 012061. [Google Scholar] [CrossRef]
- Shim, E.-G.; Nam, T.-H.; Kim, J.-G.; Kim, H.-S.; Moon, S.-I. Effects of functional electrolyte additives for Li-ion batteries. J. Power Sources 2007, 172, 901–907. [Google Scholar] [CrossRef]
- Wohde, F.; Balabajew, M.; Roling, B. Li+ transference numbers in liquid electrolytes obtained by very-low-frequency impedance spectroscopy at variable electrode distances. J. Electrochem. Soc. 2016, 163, A714. [Google Scholar] [CrossRef]
- Adenusi, H.; Chass, G.A.; Passerini, S.; Tian, K.V.; Chen, G. Lithium batteries and the solid electrolyte interphase (SEI)—Progress and outlook. Adv. Energy Mater. 2023, 13, 2203307. [Google Scholar] [CrossRef]
- Wang, Y.; Nakamura, S.; Ue, M.; Balbuena, P.B. Theoretical studies to understand surface chemistry on carbon anodes for lithium-ion batteries: Reduction mechanisms of ethylene carbonate. J. Am. Chem. Soc. 2001, 123, 11708–11718. [Google Scholar] [CrossRef]
- Webber, A. Conductivity and viscosity of Solutions of LiCF3SO3, Li(CF3SO2)2N, and their mixtures. J. Electrochem. Soc. 1991, 138, 2586. [Google Scholar] [CrossRef]
- Takami, N.; Ohsaki, T.; Inada, K. The impedance of lithium electrodes in LiPF6-based electrolytes. J. Electrochem. Soc. 1992, 139, 1849. [Google Scholar] [CrossRef]
- Misiewicz, C.; Edström, K.; Berg, E.J. Formation of a Cathode Electrolyte Interphase on High-Voltage Li-Ion Cathodes. Chem. Mater. 2024, 36, 9729–9740. [Google Scholar] [CrossRef]
- Solchenbach, S.; Metzger, M.; Egawa, M.; Beyer, H.; Gasteiger, H.A. Quantification of PF5 and POF3 from side reactions of LiPF6 in Li-ion batteries. J. Electrochem. Soc. 2018, 165, A3022. [Google Scholar] [CrossRef]
- Lennartz, P.; Paren, B.A.; Herzog-Arbeitman, A.; Chen, X.C.; Johnson, J.A.; Winter, M.; Shao-Horn, Y.; Brunklaus, G. Practical considerations for enabling Li|polymer electrolyte batteries. Joule 2023, 7, 1471–1495. [Google Scholar] [CrossRef]
- Sabaghi, D.; Wang, G.; Mikhailova, D.; Morag, A.; Omar, A.; Li, D.; Vand, S.K.H.; Yu, M.; Feng, X.; Nia, A.S. High energy density and durable pouch-cell graphite-based dual ion battery using concentrated hybrid electrolytes. J. Power Sources 2023, 588, 233685. [Google Scholar] [CrossRef]
- Du, H.; Wang, Y.; Kang, Y.; Zhao, Y.; Tian, Y.; Wang, X.; Tan, Y.; Liang, Z.; Wozny, J.; Li, T. Side reactions/changes in lithium-ion batteries: Mechanisms and strategies for creating safer and better batteries. Adv. Mater. 2024, 36, 2401482. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, M.S.; Xu, K.; Allen, J.; Jow, T.R. Understanding solid electrolyte interface film formation on graphite electrodes. Electrochem. Solid-State Lett. 2001, 4, A206. [Google Scholar] [CrossRef]
- Li, X.; Sun, X.; Hu, X.; Fan, F.; Cai, S.; Zheng, C.; Stucky, G.D. Review on comprehending and enhancing the initial Coulombic efficiency of anode materials in lithium-ion/sodium-ion batteries. Nano Energy 2020, 77, 105143. [Google Scholar] [CrossRef]
- Han, L.; Liao, C.; Mu, X.; Wu, N.; Xu, Z.; Wang, J.; Song, L.; Kan, Y.; Hu, Y. Flame-retardant ADP/PEO solid polymer electrolyte for dendrite-free and long-life lithium battery by generating Al, P-rich SEI layer. Nano Lett. 2021, 21, 4447–4453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S. A review on electrolyte additives for lithium-ion batteries. J. Power Sources 2006, 162, 1379–1394. [Google Scholar] [CrossRef]
- Su, C.C.; He, M.; Shi, J.; Amine, R.; Zhang, J.; Amine, K. Solvation rule for solid-electrolyte interphase enabler in lithium-metal batteries. Angew. Chem. Int. Ed. 2020, 59, 18229–18233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S. A review on the separators of liquid electrolyte Li-ion batteries. J. Power Sources 2007, 164, 351–364. [Google Scholar] [CrossRef]
- Miao, X.; Wang, H.; Sun, R.; Wang, C.; Zhang, Z.; Li, Z.; Yin, L. Interface engineering of inorganic solid-state electrolytes for high-performance lithium metal batteries. Energy Environ. Sci. 2020, 13, 3780–3822. [Google Scholar] [CrossRef]
- Xu, K. Electrolytes and interphases in Li-ion batteries and beyond. Chem. Rev. 2014, 114, 11503–11618. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shen, X.; Guo, X.; Li, S.; Zhang, H.; Zhang, C.; Hua, M.; Pan, X. High efficiency of the NH4H2PO4/Mg(OH)2 composite for guaranteeing safety of wood production. J. Loss Prev. Process Ind. 2021, 69, 104364. [Google Scholar]
- Qi, J.; Li, H.; Han, K.; Zuo, Q.; Gao, J.; Wang, Q.; Lu, C. Influence of ammonium dihydrogen phosphate on potassium retention and ash melting characteristics during combustion of biomass. Energy 2016, 102, 244–251. [Google Scholar] [CrossRef]
- Li, K.; Wang, B.; Bolatibieke, D.; Nan, D.-H.; Lu, Q. Pyrolysis of biomass impregnated with ammonium dihydrogen phosphate for polygeneration of phenol and supercapacitor electrode material. Front. Chem. 2020, 8, 436. [Google Scholar] [CrossRef]
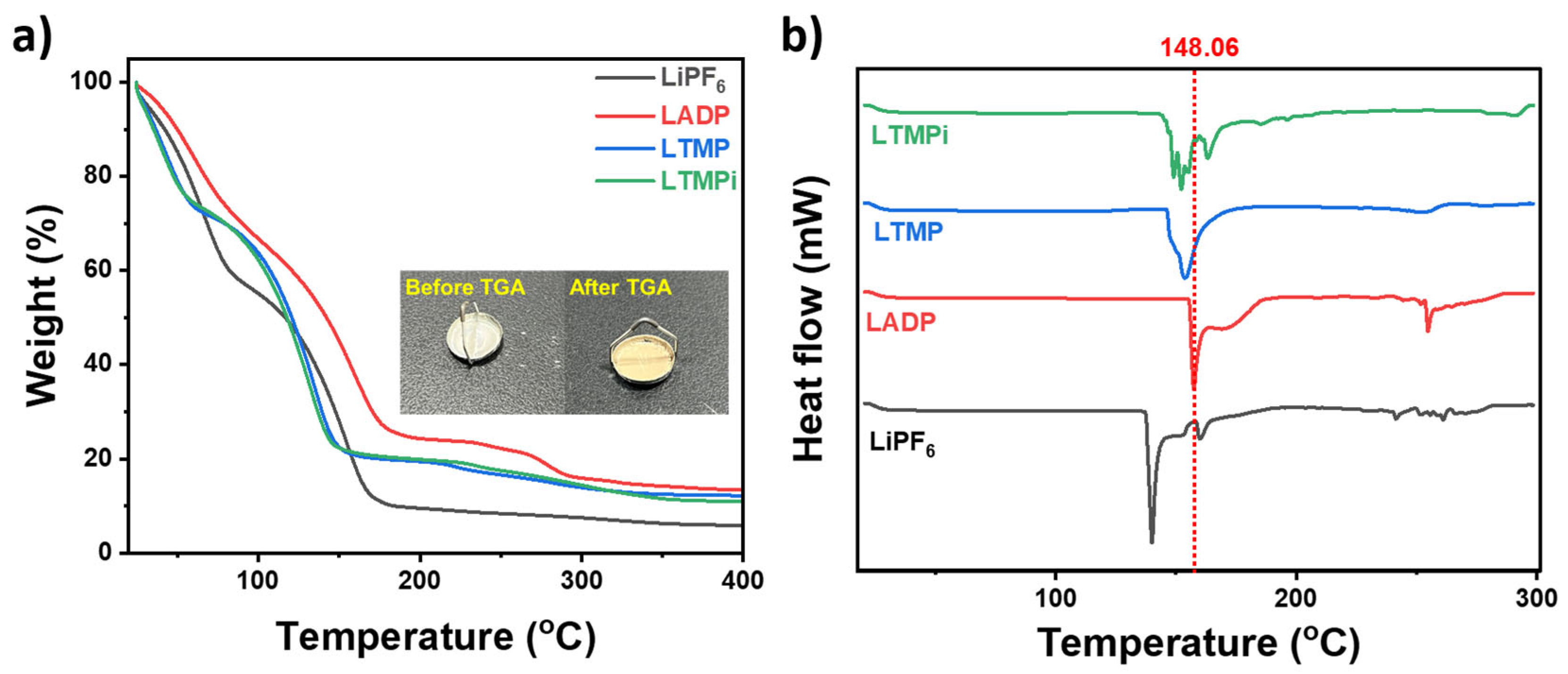

| Samples | Td1 (°C) | Td2 (°C) | Td3 (°C) |
|---|---|---|---|
| LiPF6 | 54.89 | 138.78 | 221.00 |
| LADP | 61.06 | 160.05 | 278.60 |
| LTMP | 40.71 | 131.54 | 254.56 |
| LTMPi | 40.25 | 131.78 | 255.10 |
| Sample | Rbulk or Rb (Ω) | Rin (Ω) | Rdiffusion (Ω) |
|---|---|---|---|
| LiPF6 | 10.39 | 568.13 | 2406.49 |
| LADP | 7.75 | 61.06 | 1005.94 |
| LTMP | 7.82 | 108.33 | 2988.98 |
| LTMPi | 8.37 | 102.28 | 3487.46 |
| Sample | Electrolyte | Coulombic Efficiency |
|---|---|---|
| Graphite|Li cell (our work) | 1 M LiFP6-EC/DEC/DMC 1/1/1 (v/v) | ≥98% |
| Li|Li symmetric cell [50] | 1.1 M LiPF6-FEC/EMC 1/19 (v/v) | ~62.5% |
| 1.1 M LiPF6-FEC/EMC 1/14 (v/v) | ~78% | |
| 1.1 M LiPF6-FEC/EMC 1/9 (v/v) | ~88% | |
| 1.1 M LiPF6-FEC/EMC 1/6 (v/v) | ~92% | |
| 1.1 M LiPF6-FEC/EMC 1/4 (v/v) | ~98% | |
| 1.1 M LiPF6-FEC/EMC 3/7 (v/v) | ~97% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phung, M.T.; Vu, T.T.P.; Lee, S.B.; Kong, I.; Kim, M.; Akhtar, M.S.; Yang, O.-B. Coupling of Ammonium Dihydrogen Phosphate Additives with LiPF6 Electrolytes for Improving Thermal Stability and Performance of Lithium-Ion Batteries. Micromachines 2025, 16, 966. https://doi.org/10.3390/mi16090966
Phung MT, Vu TTP, Lee SB, Kong I, Kim M, Akhtar MS, Yang O-B. Coupling of Ammonium Dihydrogen Phosphate Additives with LiPF6 Electrolytes for Improving Thermal Stability and Performance of Lithium-Ion Batteries. Micromachines. 2025; 16(9):966. https://doi.org/10.3390/mi16090966
Chicago/Turabian StylePhung, M. Thien, T. Thu Phuong Vu, Seung Beop Lee, Ing Kong, Min Kim, Mohammad Shaheer Akhtar, and O-Bong Yang. 2025. "Coupling of Ammonium Dihydrogen Phosphate Additives with LiPF6 Electrolytes for Improving Thermal Stability and Performance of Lithium-Ion Batteries" Micromachines 16, no. 9: 966. https://doi.org/10.3390/mi16090966
APA StylePhung, M. T., Vu, T. T. P., Lee, S. B., Kong, I., Kim, M., Akhtar, M. S., & Yang, O.-B. (2025). Coupling of Ammonium Dihydrogen Phosphate Additives with LiPF6 Electrolytes for Improving Thermal Stability and Performance of Lithium-Ion Batteries. Micromachines, 16(9), 966. https://doi.org/10.3390/mi16090966









