Nanomaterial-Powered Biosensors: A Cutting-Edge Review of Their Versatile Applications
Abstract
1. Introduction
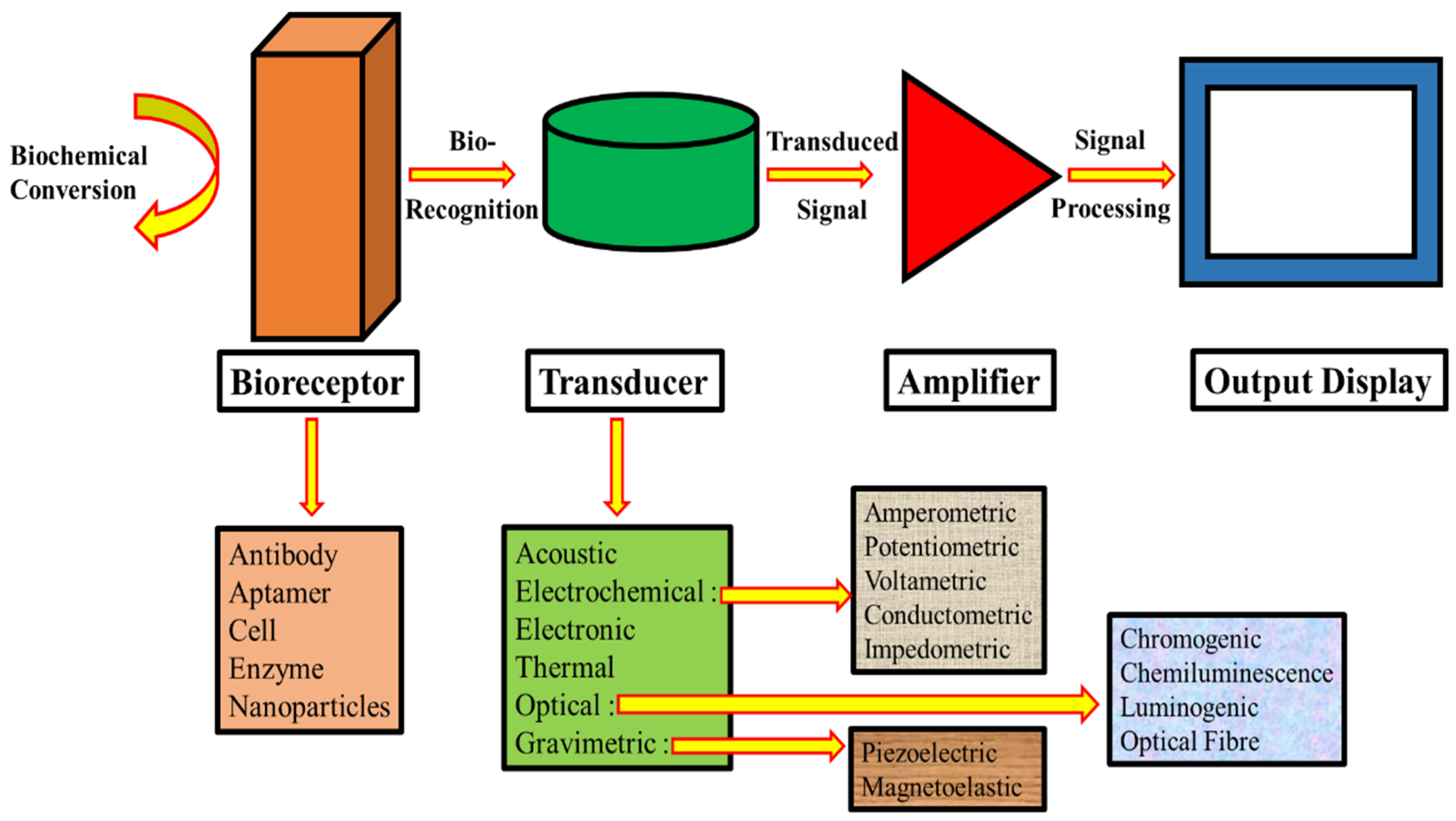
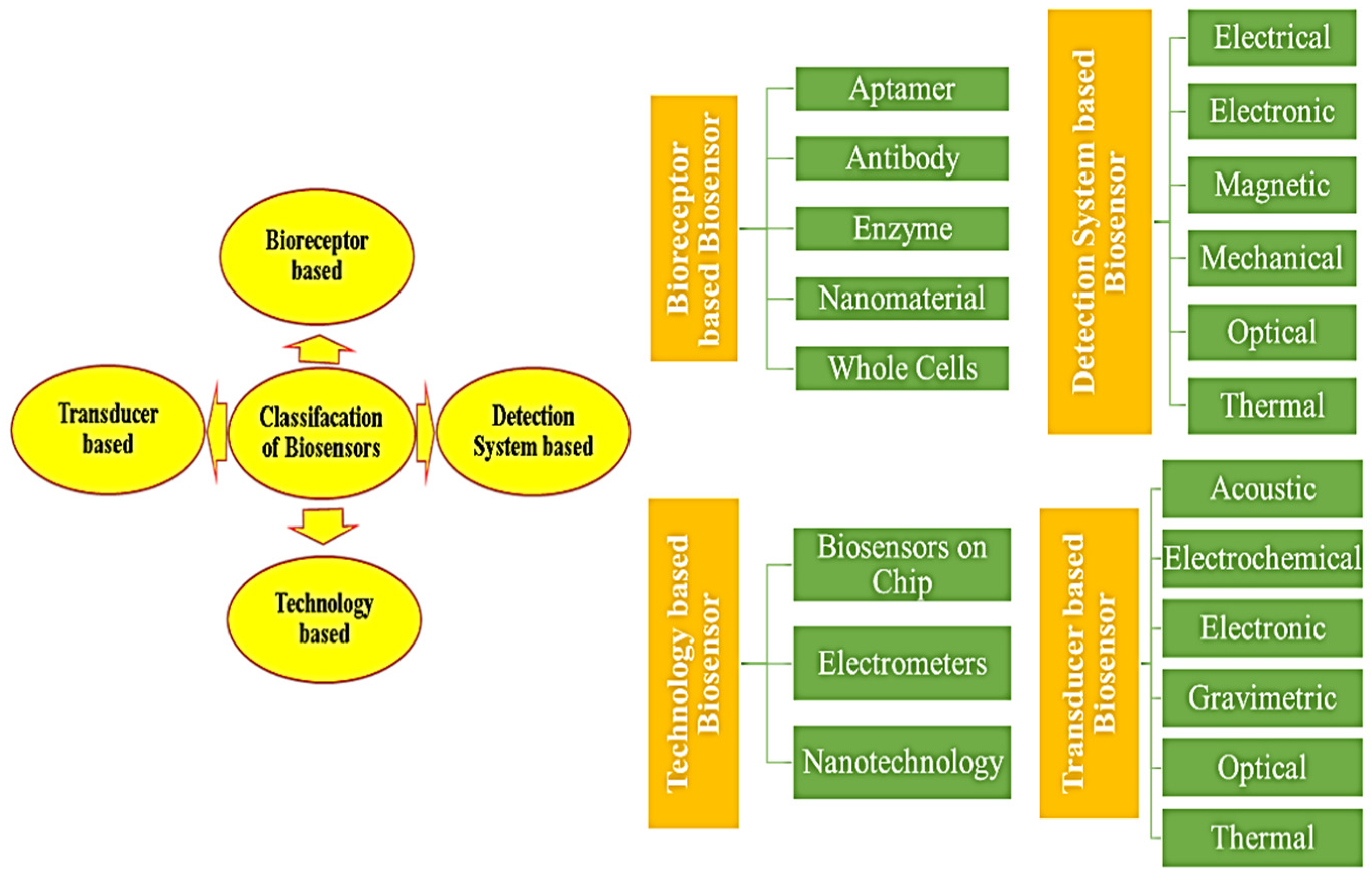
Features and Categories of Biological Sensing Systems
- Detection Limit and Response Linearity: Contemporary applications demand biosensors with exceptional sensitivity. Environmental monitoring requires detection capabilities in the parts-per-million range, while medical diagnostics often necessitate sensitivities from nanograms to femtograms per milliliter. Furthermore, the linear response of the device across varying analyte concentrations is essential for quantitative accuracy [9].
- Durability: The long-term stability of a biosensor is a crucial determinant of its commercial viability. A major challenge is signal attenuation with time, which calls for careful consideration throughout the design stage. Interestingly, the rate of deterioration is directly proportional to the temperature and increases at higher temperatures [10].
- Replicability: The capacity of a biosensor to produce consistent findings over several trials is a crucial performance indicator because of their dependability; devices that exhibit great replicability are exceptionally sought after. The biosensor’s overall reliability is enhanced by its capacity to replicate with great accuracy and precision, which make it a useful analytical tool [9].
- Specificity: The ability of a biosensor to discriminate between molecules is the most important factor in its design. The device must reliably identify the target analyte within a heterogeneous matrix containing structurally similar compounds or potential interferents. This selectivity is the cornerstone of biosensor functionality, ensuring accurate detection in complex biological or environmental samples [10].
- Bioreceptor type utilized in device construction.
- Transducer mechanism employed.
- Underlying technology driving the device design.
- Detection system implemented.
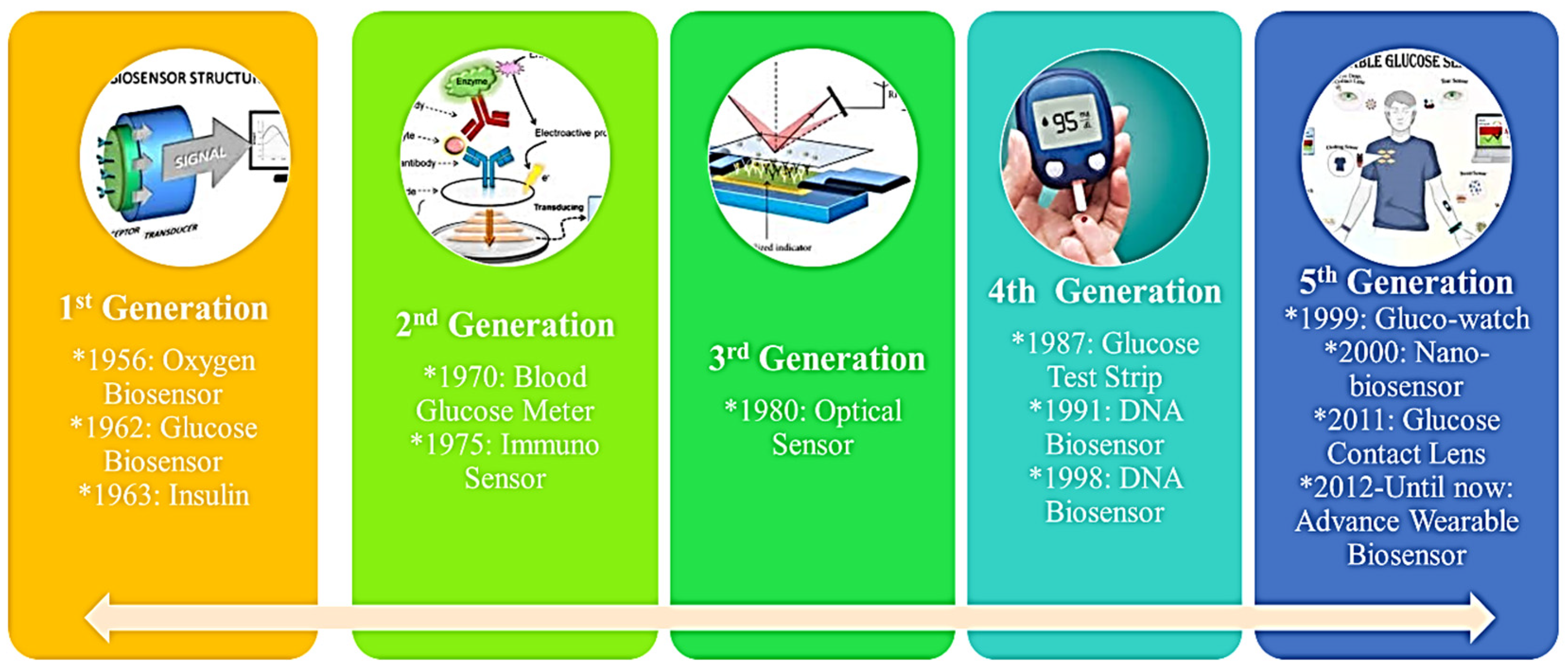
2. Development of NP-Based Biosensors and Nanotechnology
2.1. Biosensors Based on Carbon Nanotubes
2.2. Biosensors Based on Metal Oxides
2.2.1. Biosensor Based on Oxides of Copper
2.2.2. Biosensor Based on Oxides of Iron
2.2.3. Biosensor Based on Oxides of Manganese
2.2.4. Biosensor Based on Oxides of Zinc
2.3. Biosensors Based on Nanorods
2.4. Biosensors Based on Nanowires
| Sensor Type | Synthesis Method | Mechanism | Target Analyte | Detection Range | Ref |
|---|---|---|---|---|---|
| DNA-functionalized Au | Chemical vapor transport | SERS | Uranyl Ion | 10−7–10−12 M (1 pM) | [107] |
| Silver | Commercial source | Piezoresistive Sensing | Strain | 80–0% Strain (0.2%) | [108] |
| Pt and Pt Ox | E-beam fabrication | Chemical Resistance | Hydrogen | 1000–0.5 ppm (100 ppm) | [109] |
| Nickel-gold layered | Electrochemical deposition | Electrochemical | Glucose | 2–0.0025 mM (0.1 μM) | [110] |
| Nickel | Electrochemical deposition | Chemical Resistance | Hydrogen | 20–0.01 mM (0.8 μM) | [111] |
| Palladium-PAN composites | Electrospinning | Chemical Resistance | Hydrogen | 4–0.0001% (1 ppm) | [112] |
| Core–shell Pd@Ag | LPNE/GRR | Chemical Resistance | Hydrogen | 900–100 ppm (100 ppm) | [113] |
| Gold | Oriented attachment | Chemical Resistance | DNA | 1–0.001 nM (1 pM) | [114] |
| Copper phosphide | Hydrothermal synthesis | Electrochemical | Glucose | 1–0.005 mM (0.32 μM) | [115] |
| Graphene-gold hybrids | Hydrothermal synthesis | Cyclic Voltammetry | Tulobuterol | 7.6–0.076 μmol/L (0.01361) μmol/L | [116] |
| AuPt polydopamine | Hydrothermal synthesis | Voltammetry | Pesticides | 1000–0.5 ng/L (0.185 ng/L) | [117] |
| Au-decorated CoS2 | Hydrothermal/Lithography | Chemiluminescence | Hydrogen Peroxide | 100–1 μM (0.03 μM) | [118] |
| Gold | Nanoimprint lithography | Square wave Voltammetry | CRP | 220–5 fg/mL (2.25 fg/mL) | [119] |
| Jagged Pt Ni | Solvothermal method | Electrochemical | Caffeic Acid | 0.75–600 μM (0.05 μM) | [120] |
2.5. Biosensors Based on Quantum Dots
3. Applications of NMs Used for Biosensor Development
4. Adversities and Upcoming Patterns in Nanobiosensors
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCVD | Catalytic Chemical Vapor Deposition |
| CNT | Carbon Nanotubes |
| QD | Quantum Dots |
| NW | Nanowires |
| NR | Nanorods |
| NP | Nanoparticles |
| NM | Nanomaterials |
| NT | Nanotubes |
| CVD | Chemical Vapor Deposition |
| FET | Field Effect Transistor |
| CV | Cyclic Voltammetry |
| DPV | Differential Pulse Voltammetry |
References
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef]
- Heineman, W.R.; Jensen, W.B. Leland c. clark jr. (1918–2005). Biosens. Bioelectron. 2006, 21, 1403–1404. [Google Scholar] [CrossRef]
- Clark, L.C., Jr.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Updike, S.J.; Hicks, G.P. The enzyme electrode. Nature 1967, 214, 986–988. [Google Scholar] [CrossRef] [PubMed]
- Guilbault, G.G.; Montalvo, J.G., Jr. Urea-specific enzyme electrode. J. Am. Chem. Soc. 1969, 91, 2164–2165. [Google Scholar] [CrossRef]
- Lübbers, D.; Opitz, N. Die pCO2-/pO2-Optode: Eine neue p CO2- bzw. pO2-Meßsonde zur Messung des pCO2 oder pO2 von Gasen und Flüssigkeiten/The pCO2-/pO2-Optode: A New Probe for Measurement of pCO2 or pO2 in Fluids and Gases. Z. Naturforschung C 1975, 30, 532–533. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, A.; Ahmed, A.; Sundramoorthy, A.K.; Furukawa, H.; Arya, S.; Khosla, A. Recent advances in electrochemical biosensors: Applications, challenges, and future scope. Biosensors 2021, 11, 336. [Google Scholar] [CrossRef]
- Li, Y.; Guan, P.; Yu, F.; Li, W.; Xie, X. CeO2 nanorods embedded in Ni(OH)2 matrix for the non-enzymatic detection of glucose. Nanomaterials 2017, 7, 205. [Google Scholar] [CrossRef]
- Karunakaran, C.; Rajkumar, R.; Bhargava, K. Introduction to biosensors. In Biosensors and Bioelectronics; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–68. [Google Scholar]
- Naresh, V.; Lee, N. A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Patial, P.; Deshwal, M. Selectivity and sensitivity properties of metal oxide semiconductor-based gas sensor with dopants variation: A review. Trans. Electr. Electron. Mater. 2022, 23, 6–18. [Google Scholar] [CrossRef]
- Razlansari, M.; Ulucan-Karnak, F.; Kahrizi, M.; Mirinejad, S.; Sargazi, S.; Mishra, S.; Díez-Pascual, A.M. Nanobiosensors for detection of opioids: A review of the latest advancements. Eur. J. Pharm. Biopharm. 2022, 179, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Yuvaraj, G.; Ramesh, M.; Rajeshkumar, L. Carbon and cellulose-based nanoparticle-reinforced polymer nanocomposites: A critical review. Nanomaterials 2023, 13, 1803. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.A. Nanomaterials: Types, classifications, and sources. In Applications of Nanomaterials in Human Health; Springer: Singapore, 2020; pp. 1–13. [Google Scholar]
- Abdel-Karim, R.; Reda, Y.; Abdel-Fattah, A. Nanostructured materials-based nanosensors. J. Electrochem. Soc. 2020, 167, 037554. [Google Scholar] [CrossRef]
- Barabadi, H.; Najafi, M.; Samadian, H.; Azarnezhad, A.; Vahidi, H.; Mahjoub, M.A.; Ahmadi, A. A systematic review of the genotoxicity and antigenotoxicity of biologically synthesized metallic nanomaterials: Are green nanoparticles safe enough for clinical marketing? Medicina 2019, 55, 439. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.; Zhang, Y.; Yu, M.; Zhang, S.; Wu, S. Value of flexible nano-sensor with carbon nanotube and graphene in ultrasound screening of congenital heart malformations in early pregnancy. Sci. Adv. Mater. 2022, 14, 34–42. [Google Scholar] [CrossRef]
- Tang, X.; Bansaruntip, S.; Nakayama, N.; Yenilmez, E.; Chang, Y.L.; Wang, Q. Carbon nanotube DNA sensor and sensing mechanism. Nano Lett. 2006, 6, 1632–1636. [Google Scholar] [CrossRef]
- Li, W.S.; Hou, P.X.; Liu, C.; Sun, D.M.; Yuan, J.; Zhao, S.Y.; Cheng, H.M. High-quality, highly concentrated semiconducting single-wall carbon nanotubes for use in field effect transistors and biosensors. ACS Nano 2013, 7, 6831–6839. [Google Scholar] [CrossRef]
- Janssen, J.; Lambeta, M.; White, P.; Byagowi, A. Carbon nanotube-based electrochemical biosensor for label-free protein detection. Biosensors 2019, 9, 144. [Google Scholar] [CrossRef]
- Lee, B.Y.; Seo, S.M.; Lee, D.J.; Lee, M.; Lee, J.; Cheon, J.H.; Hong, S. Biosensor system-on-a-chip including CMOS-based signal processing circuits and 64 carbon nanotube-based sensors for the detection of a neurotransmitter. Lab Chip 2010, 10, 894–898. [Google Scholar] [CrossRef]
- Mann, F.A.; Herrmann, N.; Meyer, D.; Kruss, S. Tuning selectivity of fluorescent carbon nanotube-based neurotransmitter sensors. Sensors 2017, 17, 1521. [Google Scholar] [CrossRef]
- Kumar, D.; Chaturvedi, P.; Saho, P.; Jha, P.; Chouksey, A.; Lal, M.; Rawat, J.S.B.S.; Tandon, R.P.; Chaudhury, P.K. Effect of single wall carbon nanotube networks on gas sensor response and detection limit. Sens. Actuators B Chem. 2017, 240, 1134–1140. [Google Scholar] [CrossRef]
- Li, D.; Wang, C.; Sun, G.; Senapati, S.; Chang, H.C. A shear-enhanced CNT-assembly nanosensor platform for ultra-sensitive and selective protein detection. Biosens. Bioelectron. 2017, 97, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Romay, V.; Liu, Y.; Ibarlucea, B.; Baraban, L.; Khavrus, V.; Cuniberti, G. Chemiresistive biosensors based on carbon nanotubes for label-free detection of DNA sequences derived from avian influenza virus H5N1. Sens. Actuators B Chem. 2017, 249, 691–699. [Google Scholar] [CrossRef]
- Park, M.; Kim, H.S.; Kim, T.; Kim, J.; Seo, S.; Lee, B.Y. Real-time monitoring of microbial activity using hydrogel-hybridized carbon nanotube transistors. Sens. Actuators B Chem. 2018, 263, 486–492. [Google Scholar] [CrossRef]
- Li, X.; Le Thai, M.; Dutta, R.K.; Qiao, S.; Chandran, G.T.; Penner, R.M. Sub-6 nm palladium nanoparticles for faster, more sensitive H2 detection using carbon nanotube ropes. ACS Sens. 2017, 2, 282–289. [Google Scholar] [CrossRef]
- Ishihara, S.; Labuta, J.; Nakanishi, T.; Tanaka, T.; Kataura, H. Amperometric detection of sub-ppm formaldehyde using single-walled carbon nanotubes and hydroxylamines: A referenced chemiresistive system. ACS Sens. 2017, 2, 1405–1409. [Google Scholar] [CrossRef]
- Panes-Ruiz, L.A.; Shaygan, M.; Fu, Y.; Liu, Y.; Khavrus, V.; Oswald, S.; Cuniberti, G. Toward highly sensitive and energy efficient ammonia gas detection with modified single-walled carbon nanotubes at room temperature. ACS Sens. 2018, 3, 79–86. [Google Scholar] [CrossRef]
- He, M.; Croy, R.G.; Essigmann, J.M.; Swager, T.M. Chemiresistive carbon nanotube sensors for N-nitrosodialkylamines. ACS Sens. 2019, 4, 2819–2824. [Google Scholar] [CrossRef]
- Xiao, M.; Liang, S.; Han, J.; Zhong, D.; Liu, J.; Zhang, Z.; Peng, L. Batch fabrication of ultrasensitive carbon nanotube hydrogen sensors with sub-ppm detection limit. ACS Sens. 2018, 3, 749–756. [Google Scholar] [CrossRef]
- Falco, A.; Loghin, F.C.; Becherer, M.; Lugli, P.; Salmerón, J.F.; Rivadeneyra, A. Low-cost gas sensing: Dynamic self-compensation of humidity in cnt-based devices. ACS Sens. 2019, 4, 3141–3146. [Google Scholar] [CrossRef]
- Seekaew, Y.; Wisitsoraat, A.; Phokharatkul, D.; Wongchoosuk, C. Room temperature toluene gas sensor based on TiO2 nanoparticles decorated 3D graphene-carbon nanotube nanostructures. Sens. Actuators B Chem. 2019, 279, 69–78. [Google Scholar] [CrossRef]
- Hwang, S.I.; Franconi, N.G.; Rothfuss, M.A.; Bocan, K.N.; Bian, L.; White, D.L.; Burkert, S.C.; Euler, R.W.; Sopher, B.J.; Vinay, M.L.; et al. Tetrahydrocannabinol detection using semiconductor-enriched single-walled carbon nanotube chemiresistors. ACS Sens. 2019, 4, 2084–2093. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lotfi, A.; Hesketh, P.J.; Kumar, S. Carbon nanotube thin-film-transistors for gas identification. Sens. Actuators B Chem. 2019, 281, 1080–1087. [Google Scholar] [CrossRef]
- Ji, S.; Lee, M.; Kim, D. Detection of early stage prostate cancer by using a simple carbon nanotube@paper biosensor. Biosens. Bioelectron. 2018, 102, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Clément, P.; Eklöf-Österberg, J.; Kelley-Loughnane, N.; Moth-Poulsen, K.; Chávez, J.L.; Palma, M. Reconfigurable carbon nanotube multiplexed sensing devices. Nano Lett. 2018, 18, 4130–4135. [Google Scholar] [CrossRef]
- Bushmaker, A.W.; Oklejas, V.; Walker, D.; Hopkins, A.R.; Chen, J.; Cronin, S.B. Single-ion adsorption and switching in carbon nanotubes. Nat. Commun. 2016, 7, 10475. [Google Scholar] [CrossRef]
- Liang, Y.; Xiao, M.; Wu, D.; Lin, Y.; Liu, L.; He, J.; Zhang, G.; Peng, L.M.; Zhang, Z. Wafer-scale uniform carbon nanotube transistors for ultrasensitive and label-free detection of disease biomarkers. ACS Nano 2020, 14, 8866–8874. [Google Scholar] [CrossRef]
- Li, W.; Gao, Y.; Zhang, J.; Wang, X.; Yin, F.; Li, Z.; Zhang, M. Universal DNA detection realized by peptide based carbon nanotube biosensors. Nanoscale Adv. 2020, 2, 717–723. [Google Scholar] [CrossRef]
- Patial, P.; Deshwal, M. Systematic Review on Design and Development of Efficient Semiconductor Based Surface Acoustic Wave Gas Sensor. Trans. Electr. Electron. Mater. 2021, 22, 385–393. [Google Scholar] [CrossRef]
- Ji, X.; Yang, Y.; Wang, A.; Zhao, Q. One-step hydrothermal synthesis of CuO micro-crystals for non-enzymatic glucose sensors. Sci. Adv. Mater. 2022, 14, 638–643. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q. Design and infrared spectral modulation properties of Cu/CuO one-dimensional photonic crystals. Sci. Adv. Mater. 2022, 14, 372–382. [Google Scholar] [CrossRef]
- Ping, J.; Ru, S.; Fan, K.; Wu, J.; Ying, Y. Copper oxide nanoparticles and ionic liquid modified carbon electrode for the non-enzymatic electrochemical sensing of hydrogen peroxide. Microchim. Acta 2010, 171, 117–123. [Google Scholar] [CrossRef]
- Dhara, K.; Thiagarajan, R.; Nair, B.G.; Thekkedath, G.S.B. Highly sensitive and wide-range nonenzymatic disposable glucose sensor based on a screen-printed carbon electrode modified with reduced graphene oxide and Pd-CuO nanoparticles. Microchim. Acta 2015, 182, 2183–2192. [Google Scholar] [CrossRef]
- Patial, P.; Deshwal, M. An Analysis of Applications of Nanotechnology in Science and Engineering. In Proceedings of the 2021 2nd Global Conference for Advancement in Technology (GCAT), Bangalore, India, 1–3 October 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 1–6. [Google Scholar]
- Khoshhesab, Z.M. Simultaneous electrochemical determination of acetaminophen, caffeine and ascorbic acid using a new electrochemical sensor based on CuO-graphene nanocomposite. RSC Adv. 2015, 5, 95140–95148. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, J.; Zhang, S.; Wang, W.; Chen, Z. A highly sensitive nonenzymatic glucose sensor based on CuO nanoparticles decorated carbon spheres. Sens. Actuators B Chem. 2015, 211, 385–391. [Google Scholar] [CrossRef]
- Cheng, D.; Qin, J.; Feng, Y.; Wei, J. Synthesis of mesoporous CuO hollow sphere nanozyme for paper-based hydrogen peroxide sensor. Biosensors 2021, 11, 258. [Google Scholar] [CrossRef] [PubMed]
- Doğan, H.Ö.; Urhan, B.K.; Cepni, E.; Eryiğit, M. Simultaneous electrochemical detection of ascorbic acid and dopamine on Cu2O/CuO/electrochemically reduced graphene oxide (CuxO/ERGO)-nanocomposite-modified electrode. Microchem. J. 2019, 150, 104157. [Google Scholar] [CrossRef]
- Zou, J.; Wu, S.; Liu, Y.; Sun, Y.; Cao, Y.; Hsu, J.P.; Jiang, J. An ultra-sensitive electrochemical sensor based on 2D g-C3N4/CuO nanocomposites for dopamine detection. Carbon 2018, 130, 652–663. [Google Scholar] [CrossRef]
- Kang, S.Z.; Liu, H.; Li, X.; Sun, M.; Mu, J. Electrochemical behavior of eugenol on TiO2 nanotubes improved with Cu2O clusters. RSC Adv. 2014, 4, 538–543. [Google Scholar] [CrossRef]
- Dai, Z.; Yang, A.; Bao, X.; Yang, R. Facile non-enzymatic electrochemical sensing for glucose based on Cu2O-BSA nanoparticles modified GCE. Sensors 2019, 19, 2824. [Google Scholar] [CrossRef]
- Song, J.; Xu, L.; Zhou, C.; Xing, R.; Dai, Q.; Liu, D.; Song, H. Synthesis of graphene oxide based CuO nanoparticles composite electrode for highly enhanced nonenzymatic glucose detection. ACS Appl. Mater. Interfaces 2013, 5, 12928–12934. [Google Scholar] [CrossRef]
- Wang, X.; Liu, E.; Zhang, X. Non-enzymatic glucose biosensor based on copper oxide-reduced graphene oxide nanocomposites synthesized from water-isopropanol solution. Electrochim. Acta 2014, 130, 253–260. [Google Scholar] [CrossRef]
- Xu, F.; Deng, M.; Li, G.; Chen, S.; Wang, L. Electrochemical behavior of cuprous oxide-reduced graphene oxide nanocomposites and their application in nonenzymatic hydrogen peroxide sensing. Electrochim. Acta 2013, 88, 59–65. [Google Scholar] [CrossRef]
- Razmi, H.; Nasiri, H.; Mohammad-Rezaei, R. Amperometric determination of L-tyrosine by an enzymeless sensor based on a carbon ceramic electrode modified with copper oxide nanoparticles. Microchim. Acta 2011, 173, 59–64. [Google Scholar] [CrossRef]
- Kaushik, A.; Solanki, P.R.; Ansari, A.A.; Sumana, G.; Ahmad, S.; Malhotra, B.D. Iron oxide-chitosan nanobiocomposite for urea sensor. Sens. Actuators B Chem. 2009, 138, 572–580. [Google Scholar] [CrossRef]
- Li, B.Q.; Nie, F.; Sheng, Q.L.; Zheng, J.B. An electrochemical sensor for sensitive determination of nitrites based on Ag-Fe3O4-graphene oxide magnetic nanocomposites. Chem. Pap. 2015, 69, 911–920. [Google Scholar] [CrossRef]
- Lee, S.; Oh, J.; Kim, D.; Piao, Y. A sensitive electrochemical sensor using an iron oxide/graphene composite for the simultaneous detection of heavy metal ions. Talanta 2016, 160, 528–536. [Google Scholar] [CrossRef]
- Ran, G.; Chen, X.; Xia, Y. Electrochemical detection of serotonin based on a poly (bromocresol green) film and Fe3O4 nanoparticles in a chitosan matrix. RSC Adv. 2017, 7, 1847–1851. [Google Scholar] [CrossRef]
- Cai, Z.; Ye, Y.; Wan, X.; Liu, J.; Yang, S.; Xia, Y.; He, Q. Morphology-dependent electrochemical sensing properties of iron oxide-graphene oxide nanohybrids for dopamine and uric acid. Nanomaterials 2019, 9, 835. [Google Scholar] [CrossRef]
- Peik-See, T.; Pandikumar, A.; Nay-Ming, H.; Hong-Ngee, L.; Sulaiman, Y. Simultaneous electrochemical detection of dopamine and ascorbic acid using an iron oxide/reduced graphene oxide modified glassy carbon electrode. Sensors 2014, 14, 15227–15243. [Google Scholar] [CrossRef]
- Hou, C.; Tang, W.; Zhang, C.; Wang, Y.; Zhu, N. A novel and sensitive electrochemical sensor for bisphenol A determination based on carbon black supporting ferroferric oxide nanoparticles. Electrochim. Acta 2014, 144, 324–331. [Google Scholar] [CrossRef]
- Rani, G.J.; Babu, K.J.; Rajan, M.J. Watsonia meriana flower like Fe3O4/reduced graphene oxide nanocomposite for the highly sensitive and selective electrochemical sensing of dopamine. J. Alloys Compd. 2016, 688, 500–512. [Google Scholar] [CrossRef]
- Naghib, S.M.; Rahmanian, M.; Majidzadeh-A, K.; Asiaei, S.; Vahidi, O. Novel magnetic nanocomposites comprising reduced graphene oxide/Fe3O4/gelatin utilized in ultrasensitive non-enzymatic biosensing. Int. J. Electrochem. Sci. 2016, 11, 10256–10269. [Google Scholar] [CrossRef]
- Al-Mokaram, A.M.A.A.; Yahya, R.; Abdi, M.M.; Mahmud, H.N.M.E. One-step electrochemical deposition of Polypyrrole-Chitosan-Iron oxide nanocomposite films for non-enzymatic glucose biosensor. Mater. Lett. 2016, 183, 90–93. [Google Scholar] [CrossRef]
- Ali, M.; Barman, K.; Jasimuddin, S.; Ghosh, S.K. Fluid interface-mediated nanoparticle membrane as an electrochemical sensor. RSC Adv. 2014, 4, 61404–61408. [Google Scholar] [CrossRef]
- Bonyani, M.; Mirzaei, A.; Leonardi, S.G.; Bonavita, A.; Neri, G. Electrochemical properties of Ag@ iron oxide nanocomposite for application as nitrate sensor. Electroanalysis 2015, 27, 2654–2662. [Google Scholar] [CrossRef]
- Suresh, R.; Vijayaraj, A.; Giribabu, K.; Manigandan, R.; Prabu, R.; Stephen, A.; Narayanan, V. Fabrication of iron oxide nanoparticles: Magnetic and electrochemical sensing property. J. Mater. Sci. Mater. Electron. 2013, 24, 1256–1263. [Google Scholar] [CrossRef]
- Arani, N.H.; Ghoreishi, S.M.; Khoobi, A. Increasing the electrochemical system performance using a magnetic nanostructured sensor for simultaneous determination of l-tyrosine and epinephrine. Anal. Methods 2019, 11, 1192–1198. [Google Scholar] [CrossRef]
- Chauhan, N.; Balayan, S.; Jain, U. Sensitive biosensing of neurotransmitter: 2D material wrapped nanotubes and MnO2 composites for the detection of acetylcholine. Synth. Met. 2020, 263, 116354. [Google Scholar] [CrossRef]
- Wang, M.Y.; Zhu, W.; Ma, L.; Ma, J.J.; Zhang, D.E.; Tong, Z.W.; Chen, J. Enhanced simultaneous detection of ractopamine and salbutamol-Via electrochemical-facial deposition of MnO2 nanoflowers onto 3D RGO/Ni foam templates. Biosens. Bioelectron. 2016, 78, 259–266. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, D.; Lou, X.W. Shape-controlled synthesis of MnO2 nanostructures with enhanced electrocatalytic activity for oxygen reduction. J. Phys. Chem. C 2010, 114, 1694–1700. [Google Scholar] [CrossRef]
- Tehseen, B.; Rehman, A.; Rahmat, M.; Bhatti, H.N.; Wu, A.; Butt, F.K.; Bajwa, S.Z. Solution growth of 3D MnO2 mesh comprising 1D nanofibres as a novel sensor for selective and sensitive detection of biomolecules. Biosens. Bioelectron. 2018, 117, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yu, A.; Ahmed, R.; Wang, H.; Li, H.; Chen, Z. Manganese dioxide nanotube and nitrogen-doped carbon nanotube based composite bifunctional catalyst for rechargeable zinc-air battery. Electrochim. Acta 2012, 69, 295–300. [Google Scholar] [CrossRef]
- Devaraj, S.; Munichandraiah, N. Effect of crystallographic structure of MnO2 on its electrochemical capacitance properties. J. Phys. Chem. C 2008, 112, 4406–4417. [Google Scholar] [CrossRef]
- Vijayalakshmi, K.; Renitta, A.; Alagusundaram, K.; Monamary, A. Novel two-step process for the fabrication of MnO2 nanostructures on tantalum for enhanced electrochemical H2O2 detection. Mater. Chem. Phys. 2018, 214, 431–439. [Google Scholar] [CrossRef]
- Vukojević, V.; Djurdjić, S.; Ognjanović, M.; Fabian, M.; Samphao, A.; Kalcher, K.; Stanković, D.M. Enzymatic glucose biosensor based on manganese dioxide nanoparticles decorated on graphene nanoribbons. J. Electroanal. Chem. 2018, 823, 610–616. [Google Scholar] [CrossRef]
- Shoja, Y.; Rafati, A.A.; Ghodsi, J. Polythiophene supported MnO2 nanoparticles as nano-stabilizer for simultaneously electrostatically immobilization of d-amino acid oxidase and hemoglobin as an efficient bio-nanocomposite in fabrication of dopamine bi-enzyme biosensor. Mater. Sci. Eng. C 2017, 76, 637–645. [Google Scholar] [CrossRef]
- Knežević, S.; Ognjanović, M.; Nedić, N.; Mariano, J.F.; Milanović, Z.; Petković, B.; Antić, B.; Djurić, S.V.; Stanković, D. A single drop histamine sensor based on AuNPs/MnO2 modified screen-printed electrode. Microchem. J. 2020, 155, 104778. [Google Scholar] [CrossRef]
- Abd El-Haleem, H.S.; Hefnawy, A.; Hassan, R.Y.; Badawi, A.H.; El-Sherbiny, I.M. Manganese dioxide-core-shell hyperbranched chitosan (MnO2-HBCs) nano-structured screen printed electrode for enzymatic glucose biosensors. RSC Adv. 2016, 6, 109185–109191. [Google Scholar] [CrossRef]
- Han, L.; Shao, C.; Liang, B.; Liu, A. Genetically engineered phage-templated MnO2 nanowires: Synthesis and their application in electrochemical glucose biosensor operated at neutral pH condition. ACS Appl. Mater. Interfaces 2016, 8, 13768–13776. [Google Scholar] [CrossRef]
- Shu, Y.; Xu, J.; Chen, J.; Xu, Q.; Xiao, X.; Jin, D.; Hu, X. Ultrasensitive electrochemical detection of H2O2 in living cells based on ultrathin MnO2 nanosheets. Sens. Actuators B: Chem. 2017, 252, 72–78. [Google Scholar] [CrossRef]
- Gao, W.; Liu, Z.; Qi, L.; Lai, J.; Kitte, S.A.; Xu, G. Ultrasensitive glutathione detection based on lucigenin cathodic electrochemiluminescence in the presence of MnO2 nanosheets. Anal. Chem. 2016, 88, 7654–7659. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Yang, S.; Cai, Z.; He, Q.; Ye, Y.; Xia, Y.; Liu, J. Facile synthesis of MnO2 nanoflowers/N-doped reduced graphene oxide composite and its application for simultaneous determination of dopamine and uric acid. Nanomaterials 2019, 9, 847. [Google Scholar] [CrossRef] [PubMed]
- Li, P.X.; Yang, A.Y.; Xin, L.; Xue, B.; Yin, C.H. Photocatalytic activity and mechanism of Cu2+ doped ZnO nanomaterials. Sci. Adv. Mater. 2022, 14, 1599–1604. [Google Scholar] [CrossRef]
- Arshi, N.; Ahmed, F.; Kumar, S.; Umar, A.; Aljaafari, A.; Alshoaibi, A.; Melaibari, A. Construction of dye-sensitized solar cells using coffee as a natural dye and ZnO nanorods based photoanode. Sci. Adv. Mater. 2022, 14, 1388–1393. [Google Scholar] [CrossRef]
- Tashkhourian, J.; Hemmateenejad, B.; Beigizadeh, H.; Hosseini-Sarvari, M.; Razmi, Z. ZnO nanoparticles and multiwalled carbon nanotubes modified carbon paste electrode for determination of naproxen using electrochemical techniques. J. Electroanal. Chem. 2014, 714, 103–108. [Google Scholar] [CrossRef]
- Bashami, R.M.; Hameed, A.; Aslam, M.; Ismail, I.M.; Soomro, M.T. The suitability of ZnO film-coated glassy carbon electrode for the sensitive detection of 4-nitrophenol in aqueous medium. Anal. Methods 2015, 7, 1794–1801. [Google Scholar] [CrossRef]
- Fang, L.; Liu, B.; Liu, L.; Li, Y.; Huang, K.; Zhang, Q. Direct electrochemistry of glucose oxidase immobilized on Au nanoparticles-functionalized 3D hierarchically ZnO nanostructures and its application to bioelectrochemical glucose sensor. Sens. Actuators B Chem. 2016, 222, 1096–1102. [Google Scholar] [CrossRef]
- Fallatah, A.; Kuperus, N.; Almomtan, M.; Padalkar, S. Sensitive biosensor based on shape-controlled ZnO nanostructures grown on flexible porous substrate for pesticide detection. Sensors 2022, 22, 3522. [Google Scholar] [CrossRef]
- Cao, J.; Sun, T.; Grattan, K.T. Gold nanorod-based localized surface plasmon resonance biosensors: A review. Sens. Actuators B Chem. 2014, 195, 332–351. [Google Scholar] [CrossRef]
- Ibupoto, Z.H.; Ali, S.M.U.; Khun, K.; Chey, C.O.; Nur, O.; Willander, M. ZnO nanorods based enzymatic biosensor for selective determination of penicillin. Biosensors 2011, 1, 153–163. [Google Scholar] [CrossRef]
- Zhang, H.; Song, D.; Gao, S.; Zhang, H.; Zhang, J.; Sun, Y. Enhanced wavelength modulation SPR biosensor based on gold nanorods for immunoglobulin detection. Talanta 2013, 115, 857–862. [Google Scholar] [CrossRef]
- Pabbi, M.; Kaur, A.; Mittal, S.K.; Jindal, R. A surface expressed alkaline phosphatase biosensor modified with flower shaped ZnO for the detection of chlorpyrifos. Sens. Actuators B Chem. 2018, 258, 215–227. [Google Scholar] [CrossRef]
- Zong, X.; Zhu, R. ZnO nanorod-based FET biosensor for continuous glucose monitoring. Sens. Actuators B Chem. 2018, 255, 2448–2453. [Google Scholar] [CrossRef]
- Liu, G.; Feng, D.Q.; Qian, Y.; Wang, W.; Zhu, J.J. Construction of FRET biosensor for off-on detection of lead ions based on carbon dots and gold nanorods. Talanta 2019, 201, 90–95. [Google Scholar] [CrossRef]
- Bagyalakshmi, S.; Sivakami, A.; Balamurugan, K.S. A ZnO nanorods based enzymatic glucose biosensor by immobilization of glucose oxidase on a chitosan film. Obes. Med. 2020, 18, 100229. [Google Scholar] [CrossRef]
- Swargiary, K.; Metem, P.; Kulatumyotin, C.; Thaneerat, S.; Ajchareeyasoontorn, N.; Jitpratak, P.; Viphavakit, C. ZnO nanorods coated single-mode-multimode-single-mode optical fiber sensor for VOC biomarker detection. Sensors 2022, 22, 6273. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, K.; Bangar, M.A.; Yun, M.; Chen, W.; Myung, N.V.; Mulchandani, A. Bioaffinity sensing using biologically functionalized conducting-polymer nanowire. J. Am. Chem. Soc. 2005, 127, 496–497. [Google Scholar] [CrossRef] [PubMed]
- Patolsky, F.; Zheng, G.; Lieber, C.M. Nanowire-based biosensors. Anal. Chem. 2006, 78, 4260–4269. [Google Scholar] [CrossRef] [PubMed]
- Hakim, M.M.; Lombardini, M.; Sun, K.; Giustiniano, F.; Roach, P.L.; Davies, D.E.; Ashburn, P. Thin film polycrystalline silicon nanowire biosensors. Nano Lett. 2012, 12, 1868–1872. [Google Scholar] [CrossRef]
- Irrera, A.; Leonardi, A.A.; Di Franco, C.; Lo Faro, M.J.; Palazzo, G.; D’Andrea, C.; Priolo, F. New generation of ultrasensitive label-free optical Si nanowire-based biosensors. ACS Photonics 2018, 5, 471–479. [Google Scholar] [CrossRef]
- Leonardi, A.A.; Lo Faro, M.J.; Petralia, S.; Fazio, B.; Musumeci, P.; Conoci, S.; Priolo, F. Ultrasensitive label-and PCR-free genome detection based on cooperative hybridization of silicon nanowires optical biosensors. ACS Sens. 2018, 3, 1690–1697. [Google Scholar] [CrossRef]
- Ivanov, Y.D.; Romanova, T.S.; Malsagova, K.A.; Pleshakova, T.O.; Archakov, A.I. Use of silicon nanowire sensors for early cancer diagnosis. Molecules 2021, 26, 3734. [Google Scholar] [CrossRef]
- Gwak, R.; Kim, H.; Yoo, S.M.; Lee, S.Y.; Lee, G.J.; Lee, M.K.; Kim, B. Precisely determining ultralow level UO22+ in natural water with plasmonic nanowire interstice sensor. Sci. Rep. 2016, 6, 19646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, H.; Yang, S.; Shi, X.; Zhang, D.; Shan, C.; Guo, Z. Ultrasensitive and highly compressible piezoresistive sensor based on polyurethane sponge coated with a cracked cellulose nanofibril/silver nanowire layer. ACS Appl. Mater. Interfaces 2019, 11, 10922–10932. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, C.S.; Bhat, N. Self-heating oxidized suspended Pt nanowire for high performance hydrogen sensor. Sens. Actuators B Chem. 2018, 260, 236–242. [Google Scholar] [CrossRef]
- Qin, L.; He, L.; Zhao, J.; Zhao, B.; Yin, Y.; Yang, Y. Synthesis of Ni/Au multilayer nanowire arrays for ultrasensitive non-enzymatic sensing of glucose. Sens. Actuators B Chem. 2017, 240, 779–784. [Google Scholar] [CrossRef]
- Trafela, Š.; Zavašnik, J.; Šturm, S.; Rožman, K.Ž. Formation of a Ni(OH)2/NiOOH active redox couple on nickel nanowires for formaldehyde detection in alkaline media. Electrochim. Acta 2019, 309, 346–353. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.J.; Shin, H.; Koo, W.T.; Jang, J.S.; Kang, J.Y.; Kim, I.D. High-resolution, fast, and shape-conformable hydrogen sensor platform: Polymer nanofiber yarn coupled with nanograined Pd@ Pt. ACS Nano 2019, 13, 6071–6082. [Google Scholar] [CrossRef]
- Jang, J.S.; Qiao, S.; Choi, S.J.; Jha, G.; Ogata, A.F.; Koo, W.T.; Kim, D.-H.; Kim, I.-D.; Penner, R.M. Hollow Pd-Ag composite nanowires for fast responding and transparent hydrogen sensors. ACS Appl. Mater. Interfaces 2017, 9, 39464–39474. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, Q.Y.; Xiang, F.; Hu, Y.; Zhang, L.; Xu, X.; Liu, N.; Huang, S. Applying AuNPs/SWCNT to fabricate electrical nanogap device for DNA hybridization detection. Carbon 2020, 157, 40–46. [Google Scholar] [CrossRef]
- Xie, L.; Asiri, A.M.; Sun, X. Monolithically integrated copper phosphide nanowire: An efficient electrocatalyst for sensitive and selective nonenzymatic glucose detection. Sens. Actuators B Chem. 2017, 244, 11–16. [Google Scholar] [CrossRef]
- Huang, H.; Nie, R.; Song, Y.; Ji, Y.; Guo, R.; Liu, Z. Highly sensitive electrochemical sensor for tulobuterol detection based on facile graphene/Au nanowires modified glassy carbon electrode. Sens. Actuators B Chem. 2016, 230, 422–426. [Google Scholar] [CrossRef]
- Wu, Y.; Jiao, L.; Xu, W.; Gu, W.; Zhu, C.; Du, D.; Lin, Y. Polydopamine-capped bimetallic AuPt hydrogels enable robust biosensor for organophosphorus pesticide detection. Small 2019, 15, 1900632. [Google Scholar] [CrossRef]
- Zhu, Q.; Huang, J.; Yan, M.; Ye, J.; Wang, D.; Lu, Q.; Yang, X. N-(Aminobutyl)-N-(ethylisoluminol)-functionalized gold nanoparticles on cobalt disulfide nanowire hybrids for the non-enzymatic chemiluminescence detection of H2O2. Nanoscale 2018, 10, 14847–14851. [Google Scholar] [CrossRef]
- Vilian, A.E.; Kim, W.; Park, B.; Oh, S.Y.; Kim, T.; Huh, Y.S.; Han, Y.K. Efficient electron-mediated electrochemical biosensor of gold wire for the rapid detection of C-reactive protein: A predictive strategy for heart failure. Biosens. Bioelectron. 2019, 142, 111549. [Google Scholar] [CrossRef]
- Wang, J.; Yang, B.; Gao, F.; Song, P.; Li, L.; Zhang, Y.; Du, Y. Ultra-stable electrochemical sensor for detection of caffeic acid based on platinum and nickel jagged-like nanowires. Nanoscale Res. Lett. 2019, 14, 11. [Google Scholar] [CrossRef]
- Ramesh, M.; Janani, R.; Deepa, C.; Rajeshkumar, L. Nanotechnology-enabled biosensors: A review of fundamentals, design principles, materials, and applications. Biosensors 2022, 13, 40. [Google Scholar] [CrossRef]
- Ma, F.; Li, C.C.; Zhang, C.Y. Development of quantum dot-based biosensors: Principles and applications. J. Mater. Chem. B 2018, 6, 6173–6190. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, W. Nitrogen-doped carbon quantum dots: Facile synthesis and application as a “turn-off” fluorescent probe for detection of Hg2+ ions. Biosens. Bioelectron. 2014, 55, 83–90. [Google Scholar] [CrossRef]
- Pooja, D.; Saini, S.; Thakur, A.; Kumar, B.; Tyagi, S.; Nayak, M.K. A “Turn-On” thiol functionalized fluorescent carbon quantum dot based chemosensory system for arsenite detection. J. Hazard. Mater. 2017, 328, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Hallaj, T.; Amjadi, M.; Manzoori, J.L.; Azizi, N. A novel chemiluminescence sensor for the determination of indomethacin based on sulfur and nitrogen co-doped carbon quantum dot-KMnO4 reaction. Luminescence 2017, 32, 1174–1179. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, Y.; Xu, L.; Han, Z.; Yin, H.; Ai, S. Photoelectrochemical apta-biosensor for zeatin detection based on graphene quantum dots improved photoactivity of graphite-like carbon nitride and streptavidin induced signal inhibition. Sens. Actuators B Chem. 2018, 257, 237–244. [Google Scholar] [CrossRef]
- Savas, S.; Altintas, Z. Graphene quantum dots as nanozymes for electrochemical sensing of Yersinia enterocolitica in milk and human serum. Materials 2019, 12, 2189. [Google Scholar] [CrossRef]
- Wei, X.H.; Qiao, X.; Fan, J.; Hao, Y.Q.; Zhang, Y.T.; Zhou, Y.L.; Xu, M.T. A label-free ECL aptasensor for sensitive detection of carcinoembryonic antigen based on CdS QDs@ MOF and TEOA@ Au as bi-coreactants of Ru (bpy)32+. Microchem. J. 2022, 173, 106910. [Google Scholar] [CrossRef]
- Liu, Y.; Li, B.; Yao, Y.; Yang, B.; Tian, T.; Miao, Y.; Liu, B. An electrochemiluminescence sensor for 17β-estradiol detection based on resonance energy transfer in α-FeOOH@ CdS/Ag NCs. Talanta 2021, 221, 121479. [Google Scholar] [CrossRef]
- Vasilescu, I.; Eremia, S.A.; Kusko, M.; Radoi, A.; Vasile, E.; Radu, G.L. Molybdenum disulphide and graphene quantum dots as electrode modifiers for laccase biosensor. Biosens. Bioelectron. 2016, 75, 232–237. [Google Scholar] [CrossRef]
- Pourghobadi, Z.; Mirahmadpour, P.; Zare, H. Fluorescent biosensor for the selective determination of dopamine by TGA-capped CdTe quantum dots in human plasma samples. Opt. Mater. 2018, 84, 757–762. [Google Scholar] [CrossRef]
- Yu, M.; Zhao, K.; Zhu, X.; Tang, S.; Nie, Z.; Huang, Y.; Yao, S. Development of near-infrared ratiometric fluorescent probe based on cationic conjugated polymer and CdTe/CdS QDs for label-free determination of glucose in human body fluids. Biosens. Bioelectron. 2017, 95, 41–47. [Google Scholar] [CrossRef]
- Liang, N.; Hu, X.; Li, W.; Wang, Y.; Guo, Z.; Huang, X.; Shi, J. A dual-signal fluorescent sensor based on MoS2 and CdTe quantum dots for tetracycline detection in milk. Food Chem. 2022, 378, 132076. [Google Scholar] [CrossRef]
- Safari, M.; Najafi, S.; Arkan, E.; Amani, S.; Shahlaei, M. Facile aqueous synthesis of Ni-doped CdTe quantum dots as fluorescent probes for detecting pyrazinamide in plasma. Microchem. J. 2019, 146, 293–299. [Google Scholar] [CrossRef]
- Yang, M.; Wang, C.; Liu, E.; Hu, X.; Hao, H.; Fan, J. A novel ascorbic acid ratiometric fluorescent sensor based on ZnCdS quantum dots embedded molecularly imprinted polymer and silica-coated CdTeS quantum dots. J. Mol. Liq. 2021, 337, 116438. [Google Scholar] [CrossRef]
- Yang, M.; Wang, C.; Yan, Y.; Liu, E.; Hu, X.; Hao, H.; Fan, J. Visual detection of folic acid based on silica coated CdTeS quantum dots in serum samples. Mater. Res. Bull. 2021, 144, 111509. [Google Scholar] [CrossRef]
- Jamei, H.R.; Rezaei, B.; Ensafi, A.A. Ultra-sensitive and selective electrochemical biosensor with aptamer recognition surface based on polymer quantum dots and C60/MWCNTs-polyethylenimine nanocomposites for analysis of thrombin protein. Bioelectrochemistry 2021, 138, 107701. [Google Scholar] [CrossRef] [PubMed]
- de Castro, A.C.H.; Alves, L.M.; Siquieroli, A.C.S.; Madurro, J.M.; Brito-Madurro, A.G. Label-free electrochemical immunosensor for detection of oncomarker CA125 in serum. Microchem. J. 2020, 155, 104746. [Google Scholar] [CrossRef]
- Shi, S.; Wu, H.; Zhang, L.; Wang, S.; Xiong, P.; Qin, Z.; Liao, J. Gold nanoparticles based electrochemical sensor for sensitive detection of uranyl in natural water. J. Electroanal. Chem. 2021, 880, 114884. [Google Scholar] [CrossRef]
- Niu, X.; Zhong, Y.; Chen, R.; Wang, F.; Liu, Y.; Luo, D. A “turn-on” fluorescence sensor for Pb2+ detection based on graphene quantum dots and gold nanoparticles. Sens. Actuators B Chem. 2018, 255, 1577–1581. [Google Scholar] [CrossRef]
- Khaliq, N.; Rasheed, M.A.; Khan, M.; Maqbool, M.; Ahmad, M.; Karim, S.; Ali, G. Voltage-switchable biosensor with gold nanoparticles on TiO2 nanotubes decorated with CdS quantum dots for the detection of cholesterol and H2O2. ACS Appl. Mater. Interfaces 2021, 13, 3653–3668. [Google Scholar] [CrossRef]
- Jandas, P.J.; Luo, J.; Prabakaran, K.; Chen, F.; Fu, Y.Q. Highly stable, love-mode surface acoustic wave biosensor using Au nanoparticle-MoS2-rGO nano-cluster doped polyimide nanocomposite for the selective detection of carcinoembryonic antigen. Mater. Chem. Phys. 2020, 246, 122800. [Google Scholar] [CrossRef]
- Kasturi, S.; Eom, Y.; Torati, S.R.; Kim, C. Highly sensitive electrochemical biosensor based on naturally reduced rGO/Au nanocomposite for the detection of miRNA-122 biomarker. J. Ind. Eng. Chem. 2021, 93, 186–195. [Google Scholar] [CrossRef]
- Basiri, S.; Mehdinia, A.; Jabbari, A. A sensitive triple colorimetric sensor based on plasmonic response quenching of green synthesized silver nanoparticles for determination of Fe2+, hydrogen peroxide, and glucose. Colloids Surf. A Physicochem. Eng. Asp. 2018, 545, 138–146. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Li, H.; Du, B.; Ma, H.; Wu, D.; Wei, Q. A silver-palladium alloy nanoparticle-based electrochemical biosensor for simultaneous detection of ractopamine, clenbuterol and salbutamol. Biosens. Bioelectron. 2013, 49, 14–19. [Google Scholar] [CrossRef]
- Han, G.; Cai, J.; Liu, C.; Ren, J.; Wang, X.; Yang, J.; Wang, X. Highly sensitive electrochemical sensor based on xylan-based Ag@ CQDs-rGO nanocomposite for dopamine detection. Appl. Surf. Sci. 2021, 541, 148566. [Google Scholar] [CrossRef]
- Brondani, D.; Scheeren, C.W.; Dupont, J.; Vieira, I.C. Biosensor based on platinum nanoparticles dispersed in ionic liquid and laccase for determination of adrenaline. Sens. Actuators B Chem. 2009, 140, 252–259. [Google Scholar] [CrossRef]
- Yang, Q.; Li, N.; Li, Q.; Chen, S.; Wang, H.L.; Yang, H. Amperometric sarcosine biosensor based on hollow magnetic Pt-Fe3O4@ C nanospheres. Anal. Chim. Acta 2019, 1078, 161–167. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, X.; Chang, W.; Zhang, Y.; Maruyama, T.; Luo, L.; Zhao, X. Green fabrication of Cu/rGO decorated SWCNT buckypaper as a flexible electrode for glucose detection. Mater. Sci. Eng. C 2021, 120, 111757. [Google Scholar] [CrossRef]
- Wang, B.; Luo, Y.; Gao, L.; Liu, B.; Duan, G. High-performance field-effect transistor glucose biosensors based on bimetallic Ni/Cu metal-organic frameworks. Biosens. Bioelectron. 2021, 171, 112736. [Google Scholar] [CrossRef]
- Hu, F.; Liu, T.; Pang, J.; Chu, Z.; Jin, W. Facile preparation of porous Co3O4 nanocubes for directly screen-printing an ultrasensitive glutamate biosensor microchip. Sens. Actuators B Chem. 2020, 306, 127587. [Google Scholar] [CrossRef]
- Li, Y.; Tang, L.; Deng, D.; He, H.; Yan, X.; Wang, J.; Luo, L. Hetero-structured MnO-Mn3O4@ rGO composites: Synthesis and nonenzymatic detection of H2O2. Mater. Sci. Eng. C 2021, 118, 111443. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, S.; Li, M.; Li, W.; Zhao, Y.; Qi, J.; Cui, X. Graphene quantum dots decorated graphene as an enhanced sensing platform for sensitive and selective detection of copper (II). J. Electroanal. Chem. 2017, 797, 113–120. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahn, M.S.; Hahn, Y.B. ZnO nanorods array based field-effect transistor biosensor for phosphate detection. J. Colloid Interface Sci. 2017, 498, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhu, F.; Xiao, Q.; Su, W.; Sheng, J.; Huang, C.; Hu, B. A CdTe/CdS/ZnS core/shell/shell QDs-based “OFF-ON” fluorescent biosensor for sensitive and specific determination of L-ascorbic acid. RSC Adv. 2014, 4, 46751–46761. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Ma, Q. A novel amplified electrochemiluminescence biosensor based on Au NPs@ PDA@ CuInZnS QDs nanocomposites for ultrasensitive detection of p53 gene. Biosens. Bioelectron. 2018, 117, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Nuzaihan, M.; Hashim, U.; Arshad, M.M.; Kasjoo, S.R.; Rahman, S.F.A.; Ruslinda, A.R.; Shahimin, M.M. Electrical detection of dengue virus (DENV) DNA oligomer using silicon nanowire biosensor with novel molecular gate control. Biosens. Bioelectron. 2016, 83, 106–114. [Google Scholar] [CrossRef]
- Li, L.; Lu, H.; Deng, L. A sensitive NADH and ethanol biosensor based on graphene-Au nanorods nanocomposites. Talanta 2013, 113, 1–6. [Google Scholar] [CrossRef]
- Çakıroğlu, B.; Özacar, M. A self-powered photoelectrochemical glucose biosensor based on supercapacitor Co3O4-CNT hybrid on TiO2. Biosens. Bioelectron. 2018, 119, 34–41. [Google Scholar] [CrossRef]
- Huang, Q.; Lin, X.; Tong, L.; Tong, Q.X. Graphene quantum dots/multiwalled carbon nanotubes composite-based electrochemical sensor for detecting dopamine release from living cells. ACS Sustain. Chem. Eng. 2020, 8, 1644–1650. [Google Scholar] [CrossRef]
- Mustapha Kamil, Y.; Al-Rekabi, S.H.; Yaacob, M.H.; Syahir, A.; Chee, H.Y.; Mahdi, M.A.; Abu Bakar, M.H. Detection of dengue using PAMAM dendrimer integrated tapered optical fiber sensor. Sci. Rep. 2019, 9, 13483. [Google Scholar] [CrossRef]
- Ferrier, D.C.; Honeychurch, K.C. Carbon nanotube (CNT)-based biosensors. Biosensors 2021, 11, 486. [Google Scholar] [CrossRef]
- Krishnan, S.K.; Nataraj, N.; Meyyappan, M.; Pal, U. Graphene-based field-effect transistors in biosensing and neural interfacing applications: Recent advances and prospects. Anal. Chem. 2023, 95, 2590–2622. [Google Scholar] [CrossRef]
- Gazzato, L.; Frasconi, M. Carbon nanotubes and their composites for flexible electrochemical biosensors. Anal. Sens. 2025, 5, e202400038. [Google Scholar] [CrossRef]
- Kny, E.; Hasler, R.; Luczak, W.; Knoll, W.; Szunerits, S.; Kleber, C. State of the art and future research directions of materials science applied to electrochemical biosensor developments. Anal. Bioanal. Chem. 2024, 416, 2247–2259. [Google Scholar] [CrossRef] [PubMed]
- Khonina, S.N.; Kazanskiy, N.L. Trends and advances in wearable plasmonic sensors utilizing surface-enhanced Raman spectroscopy (SERS): A Comprehensive Review. Sensors 2025, 25, 1367. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, T.; Li, Y.; Yang, R.; Zhang, G. 2D-materials-based wearable biosensor systems. Biosensors 2022, 12, 936. [Google Scholar] [CrossRef]
- Orts Mercadillo, V.; Chan, K.C.; Caironi, M.; Athanassiou, A.; Kinloch, I.A.; Bissett, M.; Cataldi, P. Electrically conductive 2D material coatings for flexible and stretchable electronics: A comparative review of graphenes and MXenes. Adv. Funct. Mater. 2022, 32, 2204772. [Google Scholar] [CrossRef]
- Keles, G.; Sifa Ataman, E.; Taskin, S.B.; Polatoglu, İ.; Kurbanoglu, S. Nanostructured metal oxide-based electrochemical biosensors in medical diagnosis. Biosensors 2024, 14, 238. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, N.; Chan, V. Recent advances in silicon quantum dot-based fluorescent biosensors. Biosensors 2023, 13, 311. [Google Scholar] [CrossRef]

| Type of Sensors | Device Configuration | Synthesis Methods | Target Analyte | Detection Limit | Ref |
|---|---|---|---|---|---|
| CNTs | Amperometric | Dielectrophoresis | Streptavidin | 100.0 aM | [22] |
| Dielectrophoresis | HER2 antibody | 10.0 fM | [22] | ||
| Drop–Coat (Paper Filter) | Formaldehyde | 0.016 ppm | [23] | ||
| Chemoresistive | Direct Contact Printing | H5N1 DNA | 2.0 pM | [24] | |
| Drop Coating | PSA | 1.18 ng/mL | [25] | ||
| Dieletrophoresis | H2 | 10 ppm | [26] | ||
| Dieletrophoresis | NO2 | 0.5–20 ppm | [27] | ||
| Drop Coat | NH3 | 100 ppb | [28] | ||
| Drop Coat | N-nitroso dialkylamine | 1 ppb | [29] | ||
| Immersed in a solution of Carbon Nanotubes | H2 | 0.89 ppm | [30] | ||
| Spray Deposition | NH3 | 10 ppm | [31] | ||
| Spray Deposition | CO2 | 600 ppm | [31] | ||
| Spray Deposition | CO | 3 ppm | [31] | ||
| Spray Deposition | Ethanol | 17 ppm | [31] | ||
| CCVD | Toulene | 50 ppm | [32] | ||
| Dieletrophoresis | Tetrahydrocannabinol | 0.163 ng | [33] | ||
| Drop Coat | NH3 | 2 ppm | [34] | ||
| Drop Coat | NO2 | 2 ppm | [34] | ||
| Spin Coat | FET | DNA | 880 ng/L | [35] | |
| FET | Dielectrophoresis | Cortisol | 50 nm | [36] | |
| Dielectrophoresis | NPY | 500 pm | [36] | ||
| Dielectrophoresis | DHEAS | 10 nM | [36] | ||
| OTS Masking | Aspergillus niger | - | [37] | ||
| CCVD | N2+ ion | Single ion | [24] | ||
| Immersed in CNT Solution | DNA | 60 aM | [38] | ||
| Immersed in CNT Solution | Micro vesicle | 1 ppml | [38] | ||
| OTS Masking | Aquaporin-4 | 1 ng | [39] |
| Copper Oxide System | Target Analyte | Detection Method | Detection Limit | Ref |
|---|---|---|---|---|
| CuO-Graphene/CPE | Acetaminophen | Differential Pulse Voltammetry | 0.006 μM | [49] |
| CuO-Graphene/CPE | Caffeine | Differential Pulse Voltammetry | 0.011 μM | [49] |
| CuxO/ERGO | Dopamine | Electrochemical | 11.0 nM | [50] |
| CuO/g-C3N4 composites | Dopamine | Electrochemical | 1 × 10−10 mol/L | [51] |
| Cu2O-TiNTs | Eugenol | CV | 1.32 μM | [52] |
| Cu2O-BSA NPs | Glucose | CV | 0.41 μM | [53] |
| CuO/GO | Glucose | Electrochemical | 0.67 μM | [54] |
| CuO-rGO | Glucose | Amperometry | 0.12 μM | [55] |
| Cu2O-rGO/GCE | H2O2 | Amperometry | 21.8 μM | [56] |
| CuO NPs/CCE | Tyrosine | Amperometry | 160.0 nM | [57] |
| Iron Oxide Composites | Target Molecule | Detection Technique | Detection Limit | Ref |
|---|---|---|---|---|
| Fe3O4/rGO composite | Ascorbic Acid | DPV | 0.44 μM | [63] |
| Fe3O4 NPs with CB/GCE | Bisphenol A | DPV | 0.032 nM | [64] |
| Fe3O4/rGO composite | Dopamine | Amperometric | 5.0 nM | [65] |
| rGO/Fe3O4/Gelatin CPE | Glucose | CV | 0.022 μM | [66] |
| P Py-chitosan-Iron oxide | Glucose | Electrochemical | 225.0 μM | [67] |
| PEG-Fe3O4on GE | L-Dopa | DPV | 9.6 nM | [68] |
| Ag@Fe2O3/SPCE | Nitrate | Amperometric | 30.0 μM | [69] |
| Fe2O3/GCE | Pyrocatechol | Chronoamperometry | - | [70] |
| Fe3O4-modified | Tyrosine | DPV | 50.0 nM | [71] |
| Fe2O3 NPs | Uric Acid | Electrochemical | 2.4 nM | [62] |
| Dimensions | Improved Electrode | Detection Method | Sample | Detection Range | Ref |
|---|---|---|---|---|---|
| 0-D | MnO2 NPs on Ta MnO2 NSPs-GNR/SPCE | CV & Amperometric | Milk Honey | 1–2 μM 0.1–1.4 mM | [78,79] |
| 0-D | MnO2 NSPs-GNR composites MnO2 NPs-Polythiophene/GCE | Electrochemical | Honey Human serum | 0.1–1.4 mM 0.04–9 μM | [79,80] |
| 1-D | Au/MnO2 NNDs/SPCE MnO2 NRs-HBCs/SPE M13-E4@MnO2NWs | Amperometric CV and Chrono amp. Electrochemical | Blood Plasma Blood Serum, Peach Juice | 0.3–5.1 μM 28–93 μg/ML 5 μM–2 nM | [81,82,83] |
| 2-D | MWCNT-MnO2/rGO/Au MnO2 NSs/GCE Lucigenin/MnO2 NSs/GCE | CV Electrochemical ECL | Serum SP2/0 cells Human Serum | 0.1–100 μM 2–10 μM 10–2000 nM | [72,84,85] |
| 3-D | MnO2 nanomesh/GCE MnO2 NFs/N-rGO MnO2 NFs/3D-RGO/Ni | Electrochemical | Blood, Urine Human Serum Pork | 0.2–10 mM 6–10 μM 17–962 nM | [75,86,87] |
| Quantum Dot Material | Detection Method | Matrix | Target Molecule | Detection Range | Ref |
|---|---|---|---|---|---|
| CdS MOF structure | ECL | Human serum | Carcinoembryonic antigen | - | [128] |
| α-FeOOH with CdS/Ag | ECL | - | 17β-estradiol | 0.01–10.0 pg/mL | [129] |
| MoS2 coupled with GQDs | Electrochemical | Wine Matrices | Caffeic acid | 0.38–100.0 μM | [130] |
| CdTe | Fluorometric | Bio fluids | Dopamine | 0.5–10.0 μM | [131] |
| Polymer-CdTe/CdS | Fluorometric | Human fluids | Glucose | 0.2–5.0 mM | [132] |
| MoS2 integrated CdTe | Fluorometric | Milk | Tetracycline | 0.1–1 μM | [133] |
| Nickel-doped CdTe | Fluorometric | Plasma | Pyrazinamide | 2.0–100.0 μM | [134] |
| ZnCdS MIP coating | Fluorometric | Vitamin C formulations | Ascorbic acid | 1.0–500.0 μM | [135] |
| CdTeS coated with SiO2 | Image analysis | Serum | Folic acid | 5.0–80.0 μM | [136] |
| CdTe | PET | Synthetic media | ds DNA | 0.0874–20 μg/mL | [137] |
| Nanomaterial | Transducer | Target Analyte | Detectable Amount | Ref |
|---|---|---|---|---|
| Gold nanobipyramids | SPR | Aflatoxin B1 | 0.4 nanomolar | [138] |
| Gold NPs | Electrochemical | Uranyl ions | 0.3 μg/L | [139] |
| Gold NPs | Fluorescence | Lead ions | 16.7 nanomolar | [140] |
| Gold/CdS QDs on titanate NTs | Electrochemical | Cholesterol | 0.012 micromolar | [141] |
| Gold NP-MoS2-rGO | SAW | Carcinoembryonic antigen | 0.084 ng/mL | [142] |
| Gold/rGO | Electrochemical | miRNA-122 | 1.73 picomolar | [143] |
| Silver NPs | Colorimetric | Hydrogen peroxide | 0.032 micromolar | [144] |
| Silver/palladium NPs | Electrochemical | Ractopamine | 1.52 pg/mL | [145] |
| Silver@carbon QDs-rGO | Electrochemical | Dopamine | 0.59 nanomolar | [146] |
| Platinum NPs | Voltammetric | Adrenaline | 2.93 × 10−4 mol/L | [147] |
| Platinum-iron oxide@carbon | Amperometric | Sarcosine | 0.43 micromolar | [148] |
| Copper/reduced graphene oxide-black phosphorus | Electrochemical | Glucose | 11 micromolar | [149] |
| Nickel/copper metal–organic framework | Field-effect transistor | Glucose | 0.51 micromolar | [150] |
| Cobalt oxide nanocubes | Electrochemical chip | Glutamate | 10 micromolar | [151] |
| Manganese oxide-Mn3O4@reduced graphene oxide | Impedimetric | Hydrogen peroxide | 0.1 micromolar | [152] |
| Zinc oxide NRs | Field-effect transistor | Phosphate | 0.5 millimolar | [153] |
| GQDs | Electrochemical | Copper ions | 1.34 nanomolar | [154] |
| CdS/CdTe/ZnS QDs | Fluorescence | L-ascorbic acid | 1.8 × 10−9 molar | [155] |
| Gold NPs@polydopamine@CuInZnS QDs | Electrochemiluminescence | P53 gene | 0.03 nmol/L | [156] |
| Silicon NWs | Field-effect transistor | Virus of Dengue | 2 femtomolar | [157] |
| Gold NPs@polydopamine@CuInZnS QDs | Electrochemiluminescence | P53 gene | 0.03 nmol/L | [156] |
| Silicon NWs | Field-effect transistor | Virus of Dengue | 2 femtomolar | [157] |
| Graphene-gold NRs | Amperometric | NADH | 6 micromolar | [158] |
| Cobalt oxide-carbon nanotube/titanium dioxide | Photoelectrochemical | Glucose | 0.16 micromolar | [159] |
| Graphene QDs-multi-walled CNTs | Electrochemical | Dopamine | 0.87 nanomolar | [160] |
| PAMAM dendrimer | Optical fiber | Dengue virus envelope protein | 19.53 nm/nM | [161] |
| Nanomaterial | Electrical Conductivity | Sensitivity | Mechanical Flexibility | Environmental Stability | Applications | Ref |
|---|---|---|---|---|---|---|
| CNTs | Excellent | High | Very high | Good | Neurotransmitter detection, strain sensor | [162,163,164,165] |
| Graphene | Superior | High | Excellent | Moderate | pH sensor, FET-based detector | [166,167,168] |
| Metal Oxides (ZnO, CuO, Fe2O3) | Moderate | Moderate | Moderate | Excellent | Electrochemical detection of H2O2, glucose, urea | [169] |
| Quantum Dots (QDs) | Moderate | Extremely high | Low | Low | Optical biosensor, cancer biomarker | [170] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patial, P.; Deshwal, M.; Bansal, S.; Sharma, A.; Kaur, K.; Prakash, K. Nanomaterial-Powered Biosensors: A Cutting-Edge Review of Their Versatile Applications. Micromachines 2025, 16, 1042. https://doi.org/10.3390/mi16091042
Patial P, Deshwal M, Bansal S, Sharma A, Kaur K, Prakash K. Nanomaterial-Powered Biosensors: A Cutting-Edge Review of Their Versatile Applications. Micromachines. 2025; 16(9):1042. https://doi.org/10.3390/mi16091042
Chicago/Turabian StylePatial, Payal, Manish Deshwal, Shonak Bansal, Anjana Sharma, Kamaldeep Kaur, and Krishna Prakash. 2025. "Nanomaterial-Powered Biosensors: A Cutting-Edge Review of Their Versatile Applications" Micromachines 16, no. 9: 1042. https://doi.org/10.3390/mi16091042
APA StylePatial, P., Deshwal, M., Bansal, S., Sharma, A., Kaur, K., & Prakash, K. (2025). Nanomaterial-Powered Biosensors: A Cutting-Edge Review of Their Versatile Applications. Micromachines, 16(9), 1042. https://doi.org/10.3390/mi16091042








