An Efficient Manufacturing Method for Silicon Carbide Crystals in Polymers Based on a Multiscale Simulation-Driven Approach
Abstract
1. Introduction
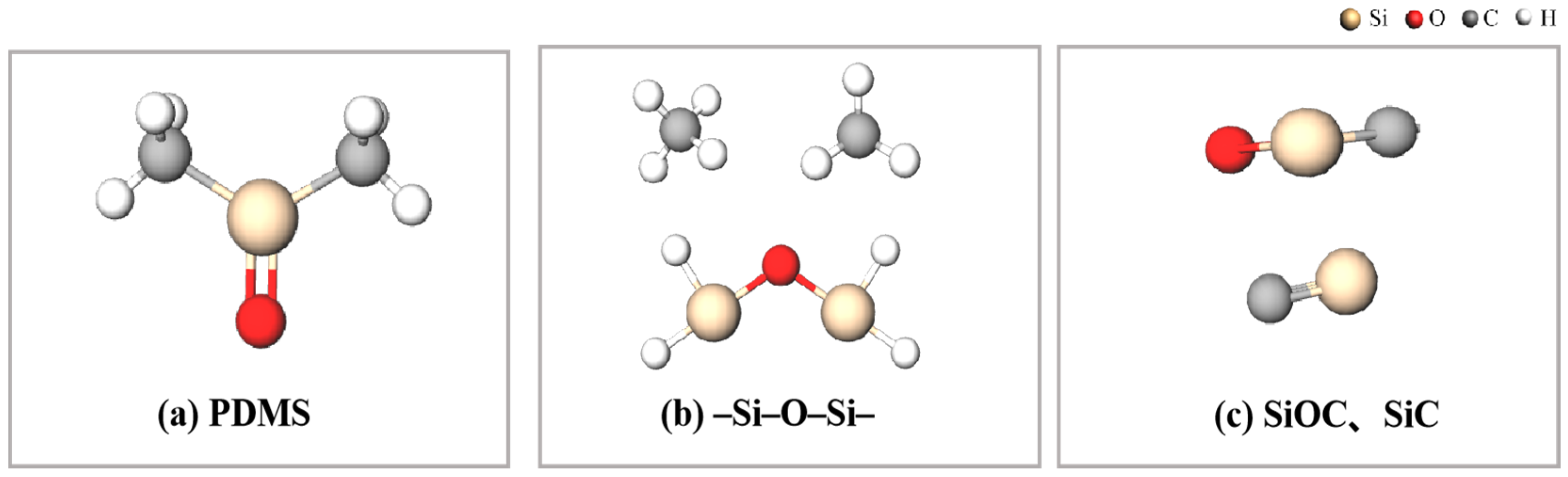
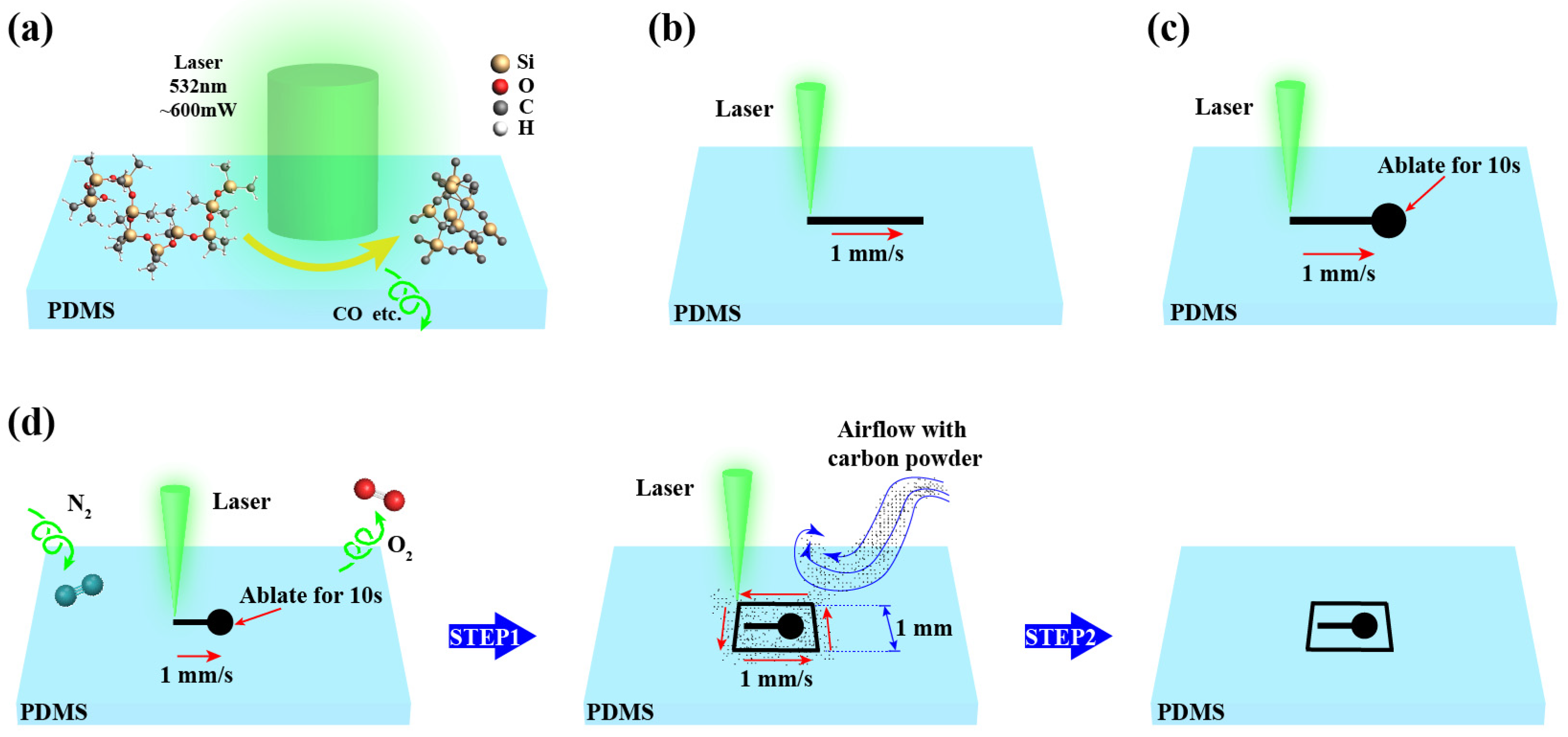
2. Mechanism of Laser Direct Writing PDMS Thermal Conversion to SiC
3. Multiscale Computational Simulation for Laser-Direct-Written Pyrolysis Processes
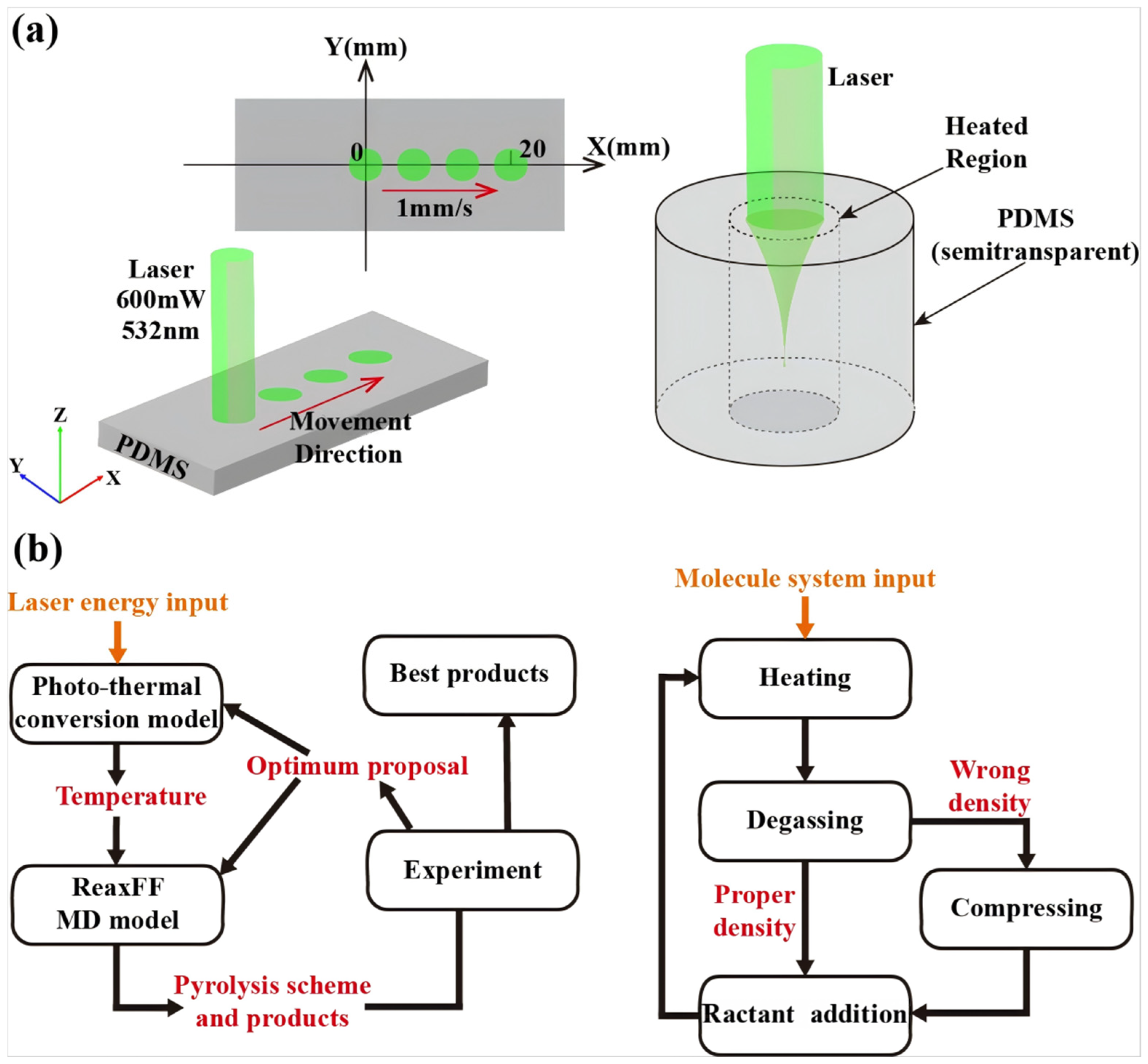
3.1. Establishment of a Multiscale Simulation Model
- (1)
- Heating: The system was heated from the initial ambient temperature (298 K) to the target pyrolysis temperature (values directly extracted from macroscopic FEA results) following a predefined thermal protocol (see Table 1). Temperature control was implemented using the NVT ensemble with the Berendsen thermostat, employing a time step of 0.12 fs.
- (2)
- Degassing: During heating and subsequent reactions, the Molecule Sink module was employed to automatically remove gaseous byproducts (including CO2, CO, H2, H2CO, C2H6, C2H4, and C2H2) every 50 timesteps, simulating the outgassing behavior of volatile species during actual pyrolysis.
- (3)
- Density Correction: Following each heating–degassing cycle, the system density was validated. If deviations from experimental values were detected, the simulation box volume was adjusted to restore the target density, ensuring physical consistency of the modeled system.
- (4)
- Reactant Introduction: To accurately simulate silicon SiC formation conditions following PDMS pyrolysis, additional carbon atoms were introduced into the system during the final stage, constructing a free carbon environment representative of pyrolytic byproducts.
| Step Range | Initial Temperature T0 (K) | Damping (fs) | Temperature Change per Step ∆T (K) |
|---|---|---|---|
| 0–5000 | 298 | 100 | 0 |
| 5001–70,000 | 298 | 100 | 0.0332 |
| 70,001–76,000 | 2000 | 100 | 0 |
| 76,001–202,000 | 2000 | 100 | −0.0166 |
| 202,001–240,000 | 298 | 100 | 0 |
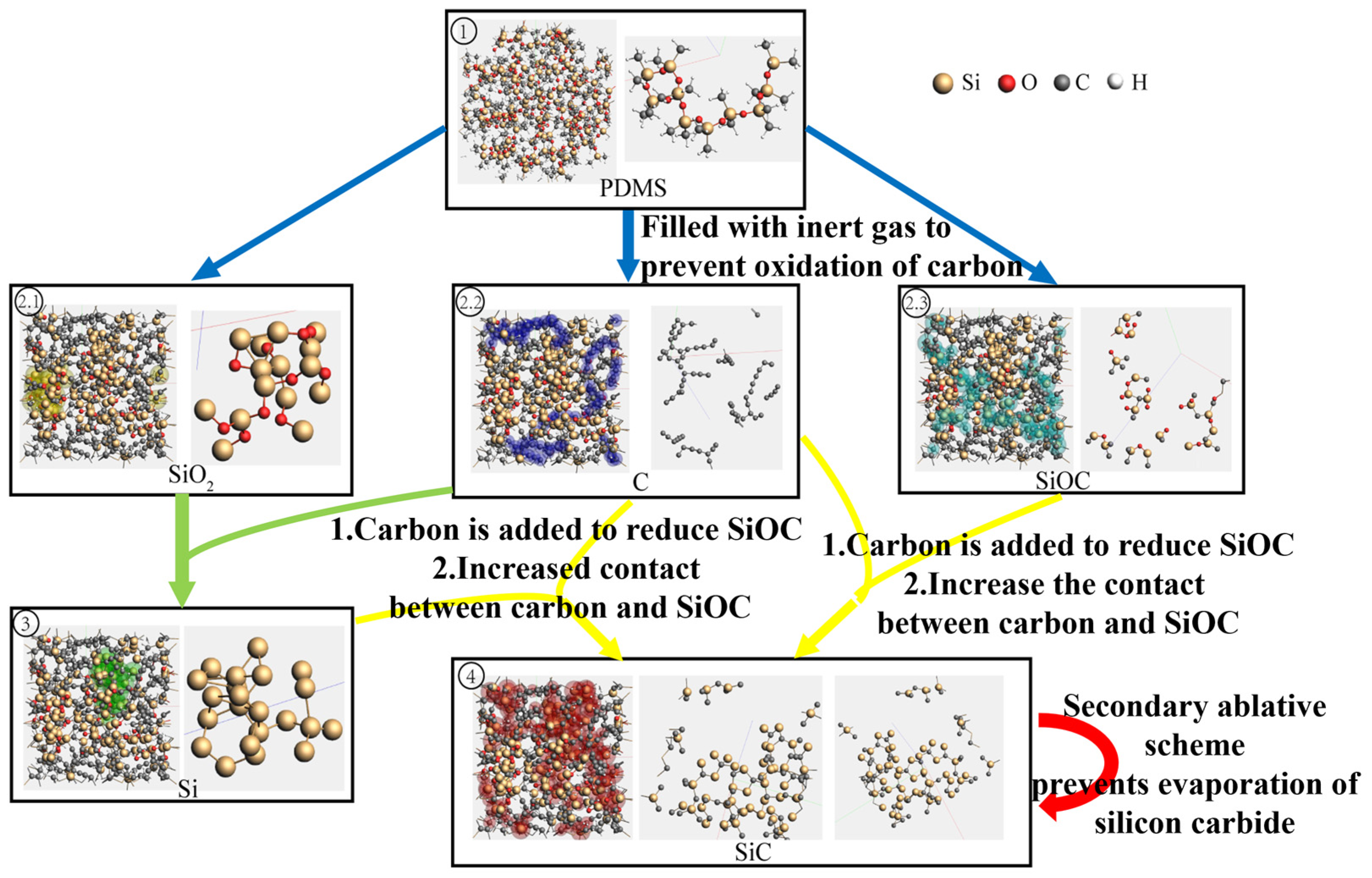
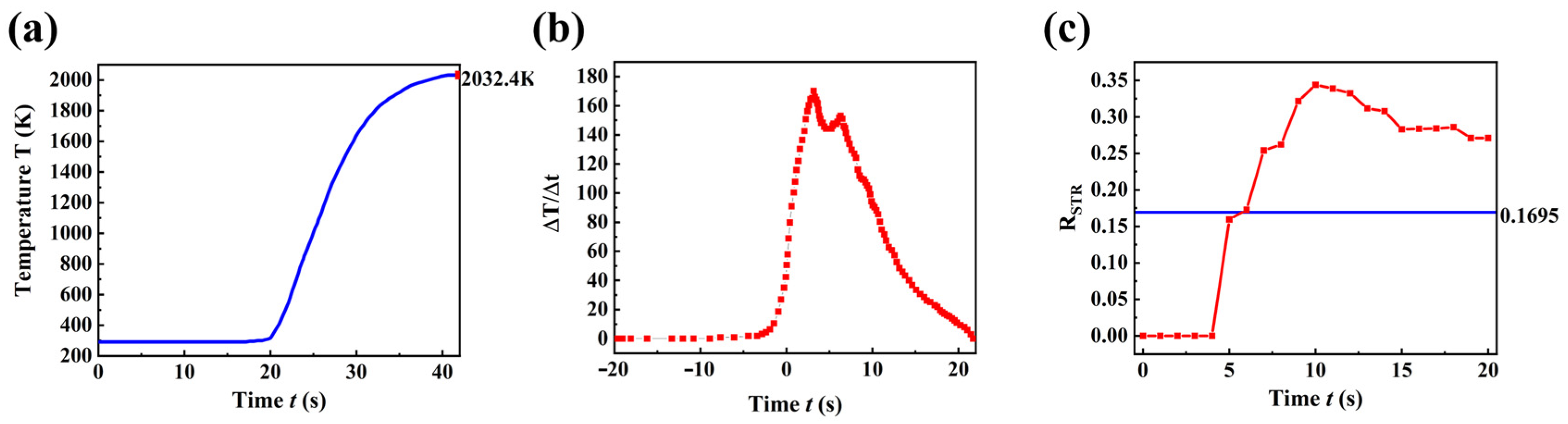
3.2. Multiscale Simulations Unravel the Pyrolytic Reaction Dynamics
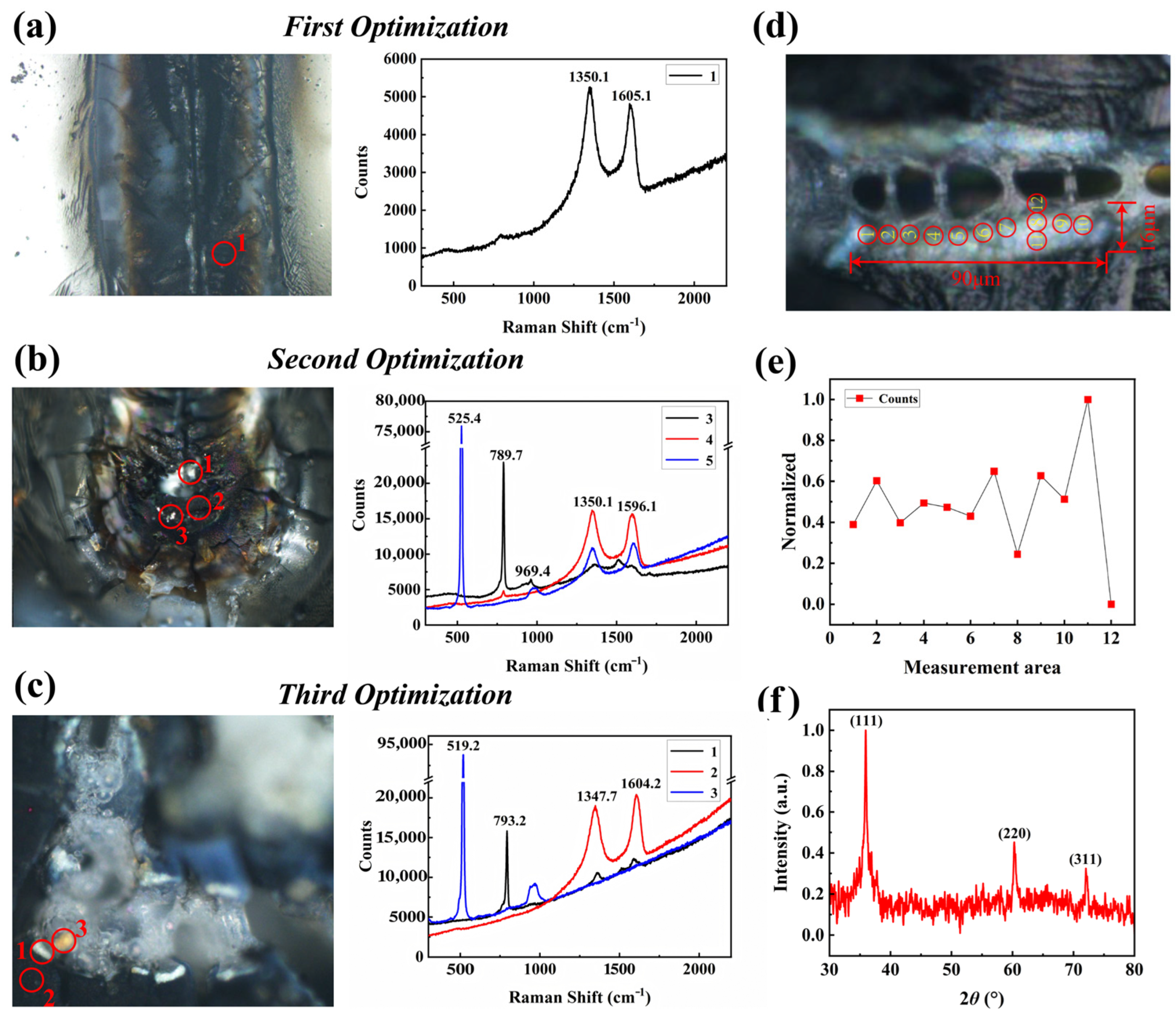
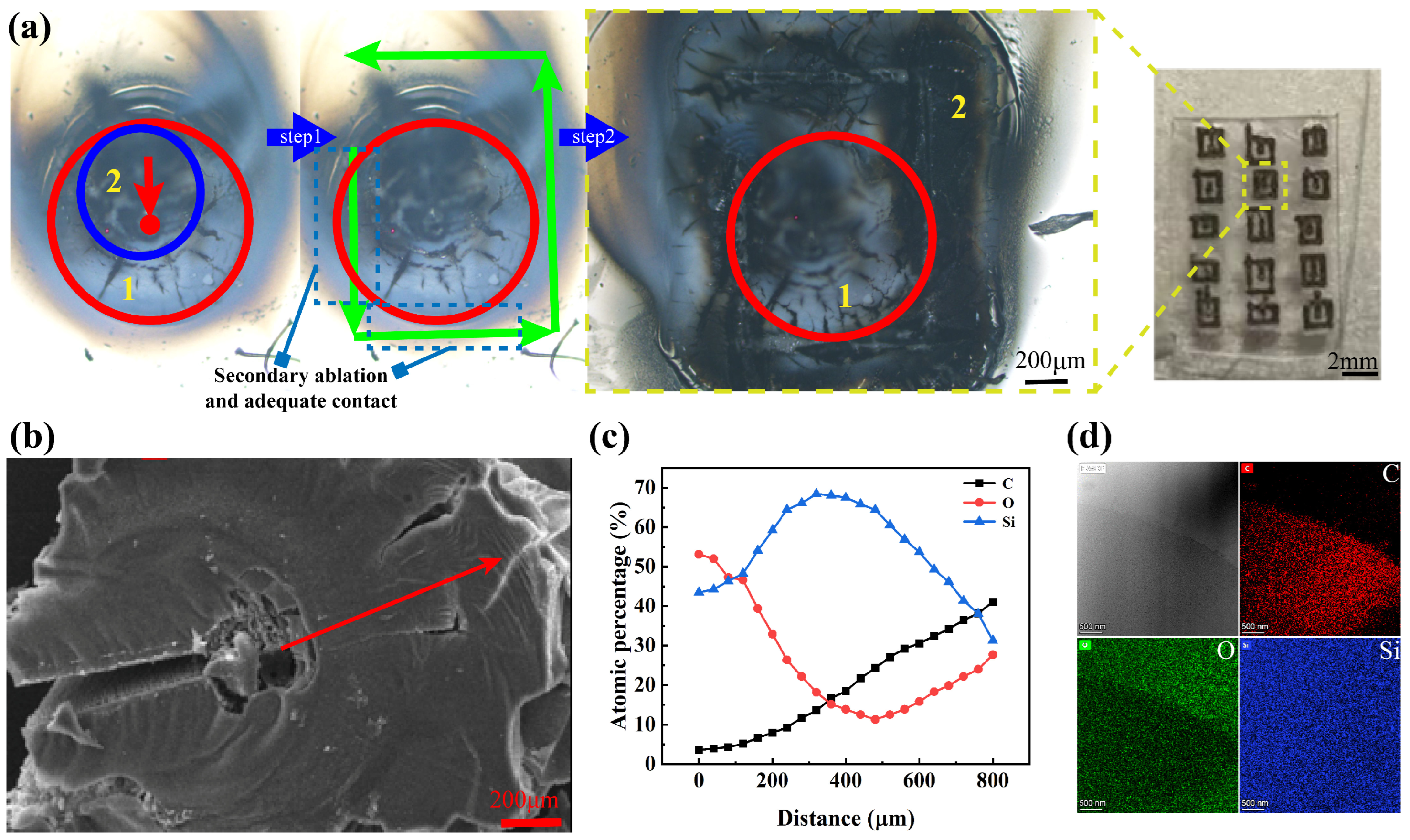
4. Secondary Pyrolysis for Enhanced SiC Yield
4.1. Dot-Frame Secondary Pyrolysis Processing Strategy
4.2. Analysis of Dot-Frame Secondary Pyrolysis Results
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, Y.-H.; Wang, H.; Shao, C.-W. Synthesis and Characterization of SiC Ceramic Precursor with High Boron Content. Chem. J. Chin. Univ. Chin. 2013, 34, 2420. [Google Scholar] [CrossRef]
- Ogawa, T.; Fukumoto, K.; Machida, H.; Norinaga, K. CFD simulation of CVD reactors in the CH3SiCl3(MTS)/H2 system using a two-step MTS decomposition and one-step SiC growth models. Heliyon 2023, 9, e15061. [Google Scholar] [CrossRef]
- Bouzat, F.; Graff, A.-R.; Lucas, R.; Foucaud, S. Preparation of C/SiC ceramics using a preceramic polycarbosilane synthesized via hydrosilylation. J. Eur. Ceram. Soc. 2016, 36, 2921. [Google Scholar] [CrossRef]
- Kousaalya, A.B.; Zeng, X.; Karakaya, M.; Trit, T.; Pilla, S.; Rao, A.M. Polymer-Derived Silicon Oxycarbide Ceramics as Promising Next-Generation Sustainable Thermoelectrics. ACS Appl. Mater. Interfaces 2018, 10, 2236–2241. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-L.; Zhou, X.-G.; Peng, S.-M.; Zhang, H.-B.; Zhou, X.-S. Fabrication, Microstructures and Properties of SiCf/SiC Composites Prepared With Two Kinds of SiC Fibers As Reinforcements. Carbon 2019, 150, 551. [Google Scholar] [CrossRef]
- Chehaidi, S.; Salles, V.; Foucaud, S.; Maitre, A.; Goursat, P. Influence of H2/NH3 mixtures on the composition of SiCNYO and SiCNAl(O) nanopowders. J. Eur. Ceram. Soc. 2011, 31, 1122. [Google Scholar] [CrossRef]
- Fukushima, Y.; Sato, N.; Funato, Y.; Sugiura, H.; Hotozuka, K.; Momose, T.; Shimogaki, Y. Multi-Scale Analysis and Elementary Reaction Simulation of SiC-CVD Using CH3SiCl3/H2. ECS J. Solid State Sci. Technol. 2013, 2, P497. [Google Scholar] [CrossRef]
- Liang, T.; Li, Y.-L.; Su, D.; Du, H.-B. Silicon oxycarbide ceramics with reduced carbon by pyrolysis of polysiloxanes in water vapor. J. Eur. Ceram. Soc. 2010, 30, 2682. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, Z.; Liu, W.; Wang, X.; Peng, Z.; Luo, Y.; Xu, C. The synthesis of SiCON ceramics through precursor method. J. Appl. Polym. Sci. 2012, 126, 859. [Google Scholar] [CrossRef]
- Cao, J.; Lu, Z.; Miao, K.; Li, S.; Wang, Z.; Li, D.; Lu, B. Investigation on microstructure control of in-situ synthesized high-performance Cf/SiC composites. J. Alloys Compd. 2019, 805, 308. [Google Scholar] [CrossRef]
- Deller, K.; Rieger, B. Synthesis of hydrocarbon-soluble, methyl-substituted highly branched polysilanes via the Wurtz-type reductive coupling of trifunctional trisilanes and their pyrolysis to silicon carbide. RSC Adv. 2015, 5, 87455. [Google Scholar] [CrossRef]
- Sun, H.; Fan, S.; Wang, L.; Ma, X.; Deng, J.; Cheng, L.; Zhang, L. Microstructure and tribological properties of PIP-SiC modified C/C–SiC brake materials. Ceram. Int. 2021, 47, 15568–15579. [Google Scholar] [CrossRef]
- Zhu, C.; Feng, L.; Xu, B.; Lin, Q.; Cheng, X.; Chen, J.; He, G. Preparation of ultra-high temperature SiC–TiB2 nanocomposites from a single-source polymer precursor. Ceram. Int. 2020, 46, 19934. [Google Scholar] [CrossRef]
- Soundaraj, P.V.; Sembulingam, S.S.; Thiyagarajan, G.B.; Moharana, N.; Kumar, K.C.H.; Kumar, R. Microstructure dependent ablation behaviour of precursor derived SiOC ceramic foam for high temperature applications. J. Eur. Ceram. Soc. 2022, 42, 877–889. [Google Scholar] [CrossRef]
- Wan, C.; Xia, P.; Hua, J.; Chen, X.; Lang, W.; Xue, W.; Geng, Y.; Fang, T. Fabrication of SiC composites by selective laser sintering and reactive melt infiltration. Ceram. Int. 2025, 51, 14851. [Google Scholar] [CrossRef]
- Hong, J.; Cho, K.-Y.; Shin, D.-G.; Kim, S.H.; Riu, D.-H. Structural Evolution of Silicon Carbide Phase from the Polycarbosilane Cured with Iodine: NMR Study. J. Inorg. Organomet. Polym. Mater. 2018, 28, 2230. [Google Scholar] [CrossRef]
- Li, L.-B. In Situ Synchrotron Radiation Techniques: Watching Deformation-induced Structural Evolutions of Polymers. Chin. J. Polym. Sci. 2018, 36, 1093–1102. [Google Scholar] [CrossRef]
- Uene, N.; Mabuchi, T.; Zaitsu, M.; Yasuhara, S.; Tokumasu, T. Reactive force-field molecular dynamics simulation for the surface reaction of SiHx (x = 2–4) species on Si(100)-(2 × 1):H surfaces in chemical vapor deposition processes. Comput. Mater. Sci. 2022, 204, 111193. [Google Scholar] [CrossRef]
- Yih, P.H. Reactive ion etching of crystalline silicon carbide and fabrication of silicon carbide devices. Vacuum 1994. [Google Scholar]
- Mullins, V.C.; Wiggins, J.S. Evaluating Cure Environment Effect in the Formation of Silicon Oxycarbide Polymer Derived Ceramics. Sampe J. 2025, 61, 6–11. [Google Scholar] [CrossRef]
- Naserifar, S.; Goddard, W.A., III; Tsotsis, T.T.; Sahimi, M. First principles-based multiparadigm, multiscale strategy for simulating complex materials processes with applications to amorphous SiC films. J. Chem. Phys. 2015, 142, 174703. [Google Scholar] [CrossRef] [PubMed]
- Samieegohar, M. Molecular Understanding of Biointerfacial Behavior for Biosensing and High-Temperature Interfacial Chemical Reactions in Pyrolysis; Lamar University-Beaumont: Beaumont, TX, USA, 2017. [Google Scholar]
- Kang, C.-Y.; Tang, J.; Li, L.-M.; Pan, H.-B.; Yan, W.-S.; Xu, P.-S.; Wei, S.-Q.; Chen, X.-F.; Xu, X.-G. Preparation of graphene on different-polarity 6H-SiC substrates and the study of their electronic structures. ACTA Phys. Sin. 2011, 60, 047302. [Google Scholar] [CrossRef]
- Wang, S.; Zong, Q.; Yang, H.; Tan, C.; Huang, Q.; Liu, X.; Zhang, G.; French, P.; Ye, H. Rapid Fabrication of High-Performance Flexible Pressure Sensors Using Laser Pyrolysis Direct Writing. ACS Appl. Mater. Interfaces 2023, 15, 41055–41066. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hogan, T. Characterization of SiC Selective Laser Fabrication. In Proceedings of the 2024 IEEE Nanotechnology Materials and Devices Conference (NMDC), Salt Lake City, UT, USA, 21–24 October 2024; pp. 141–143. [Google Scholar] [CrossRef]
- Fuller, M.E.; Goldsmith, C.F. Shock Tube Laser Schlieren Study of the Pyrolysis of Isopropyl Nitrate. J. Phys. Chem. A 2019, 123, 5876. [Google Scholar] [CrossRef]
- Zhong, H.; Wang, Z.; Zhou, H.; Ni, D.; Kan, Y.; Ding, Y.; Dong, S. Properties and microstructure evolution of Cf/SiC composites fabricated by polymer impregnation and pyrolysis (PIP) with liquid polycarbosilane. Ceram. Int. 2017, 43, 7387–7392. [Google Scholar] [CrossRef]
- Duan, S.; Wei, Y.; Wang, Y.; Zhai, L.; Qin, Y.; Guo, Z.; Li, D.; Hou, W.; Liu, S.; Li, X.; et al. Giant gauge factors in an anchored sandwich structure with a soft break mechanism. Cell Rep. Phys. Sci. 2024, 5, 101893. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, F.; An, P.; Ye, L.; Qiu, W.; Zhao, T. Polymer precursor synthesis of TaC–SiC ultrahigh temperature ceramic nanocomposites. RSC Adv. 2016, 6, 88776. [Google Scholar] [CrossRef]
- Cao, B.; Tian, Y.; Wen, H.F.; Guo, H.; Wu, X.; Li, L.; Zhang, Z.; Liu, L.; Zhu, Q.; Tang, J.; et al. Recent progress on fabrication, spectroscopy properties, and device applications in Sn-doped CdS micro-nano structures. J. Semicond. 2024, 45, 091101. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Jia, C.; Sun, H.; Tian, Y. An Efficient Manufacturing Method for Silicon Carbide Crystals in Polymers Based on a Multiscale Simulation-Driven Approach. Micromachines 2025, 16, 946. https://doi.org/10.3390/mi16080946
Wang J, Jia C, Sun H, Tian Y. An Efficient Manufacturing Method for Silicon Carbide Crystals in Polymers Based on a Multiscale Simulation-Driven Approach. Micromachines. 2025; 16(8):946. https://doi.org/10.3390/mi16080946
Chicago/Turabian StyleWang, Jia, Caiqin Jia, Heming Sun, and Ye Tian. 2025. "An Efficient Manufacturing Method for Silicon Carbide Crystals in Polymers Based on a Multiscale Simulation-Driven Approach" Micromachines 16, no. 8: 946. https://doi.org/10.3390/mi16080946
APA StyleWang, J., Jia, C., Sun, H., & Tian, Y. (2025). An Efficient Manufacturing Method for Silicon Carbide Crystals in Polymers Based on a Multiscale Simulation-Driven Approach. Micromachines, 16(8), 946. https://doi.org/10.3390/mi16080946






