Structural and Performance Studies of Lanthanum–Nitrogen Co-Doped Titanium Dioxide Thin Films Under UV Aging
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Thin Films
2.2. Accelerated UV Aging of Thin Films
2.3. Characterization and Mechanical Testing of Thin Films
3. Results and Discussion
3.1. XRD Analysis
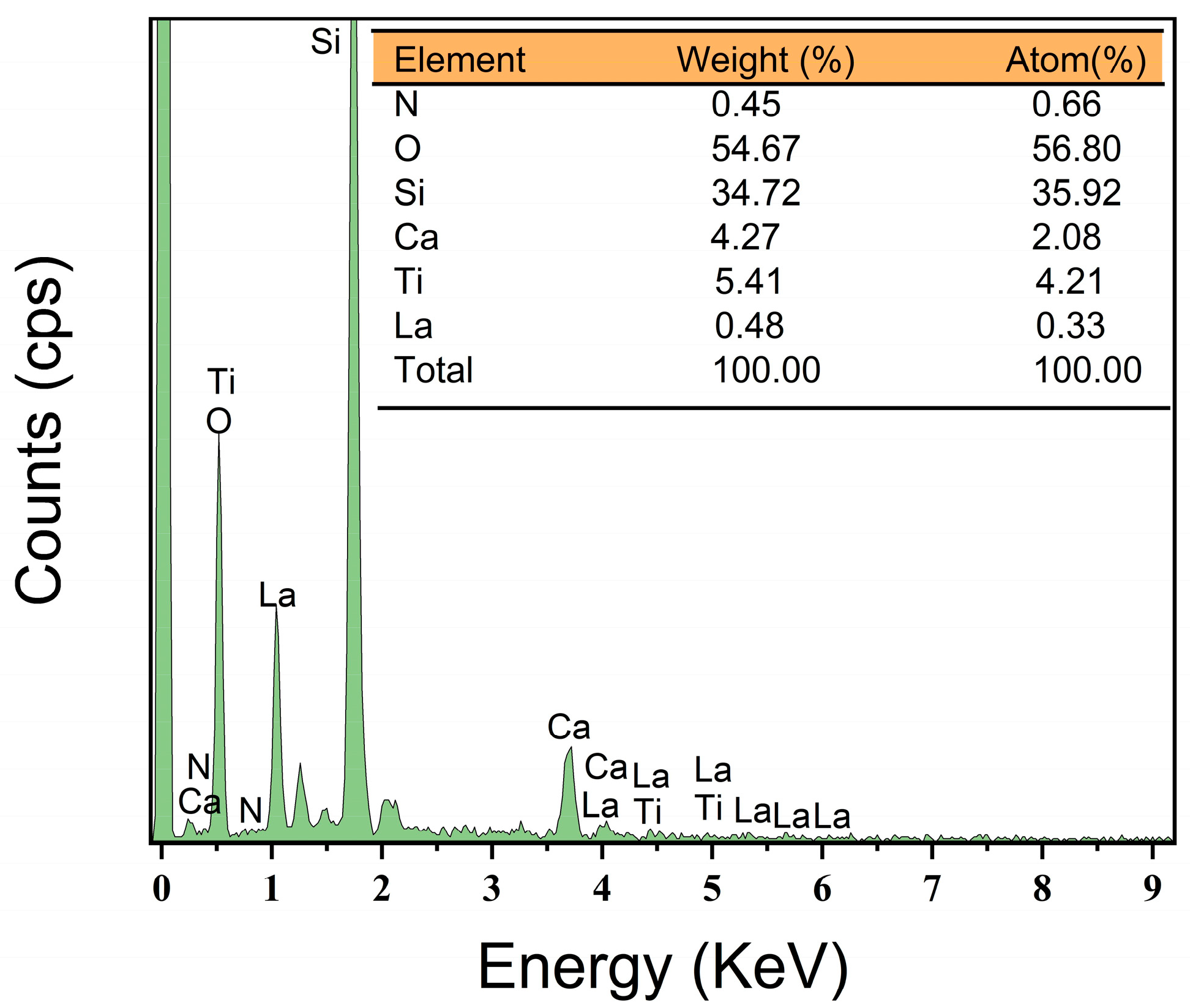
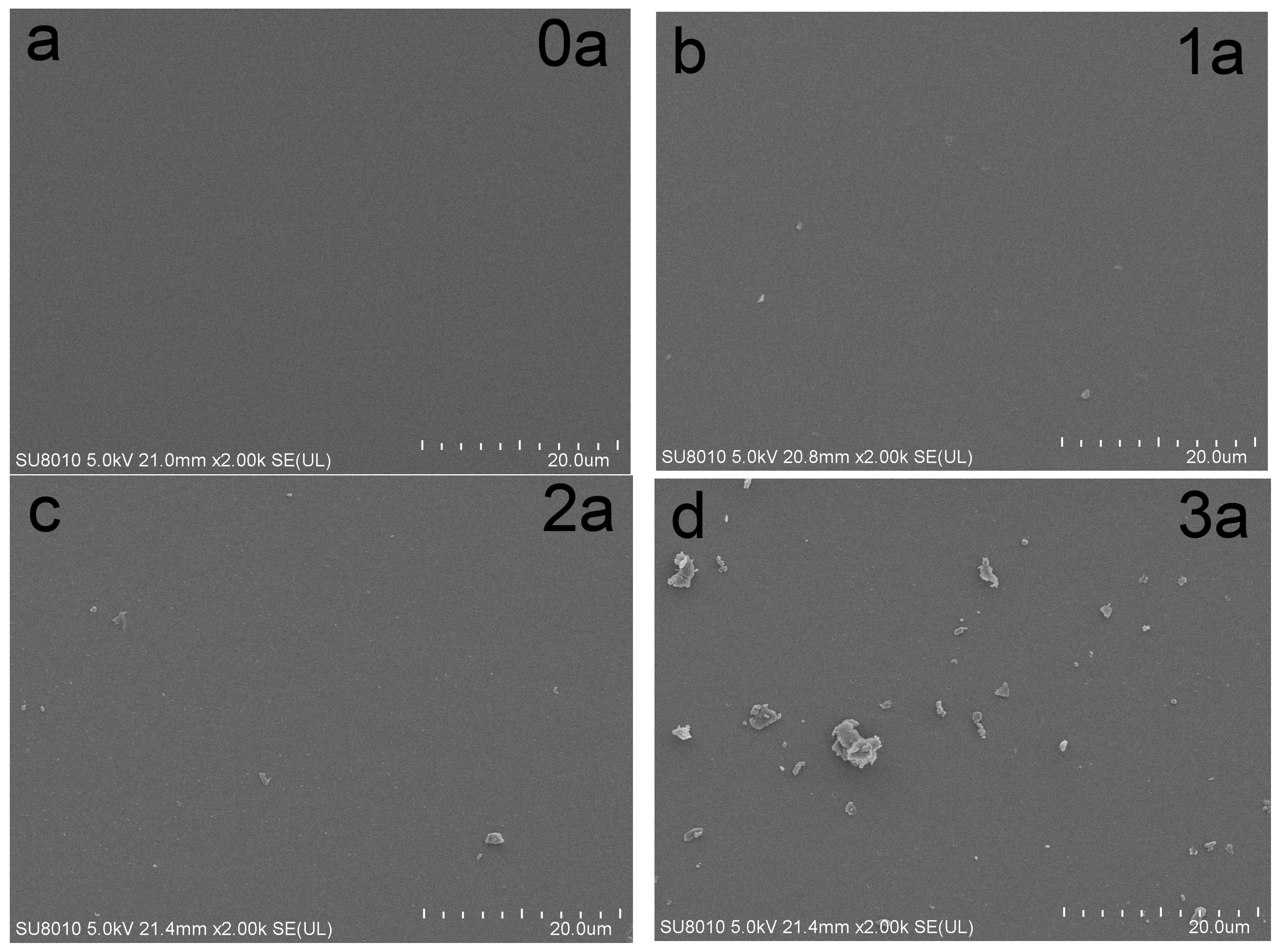
3.2. SEM and EDS Analysis
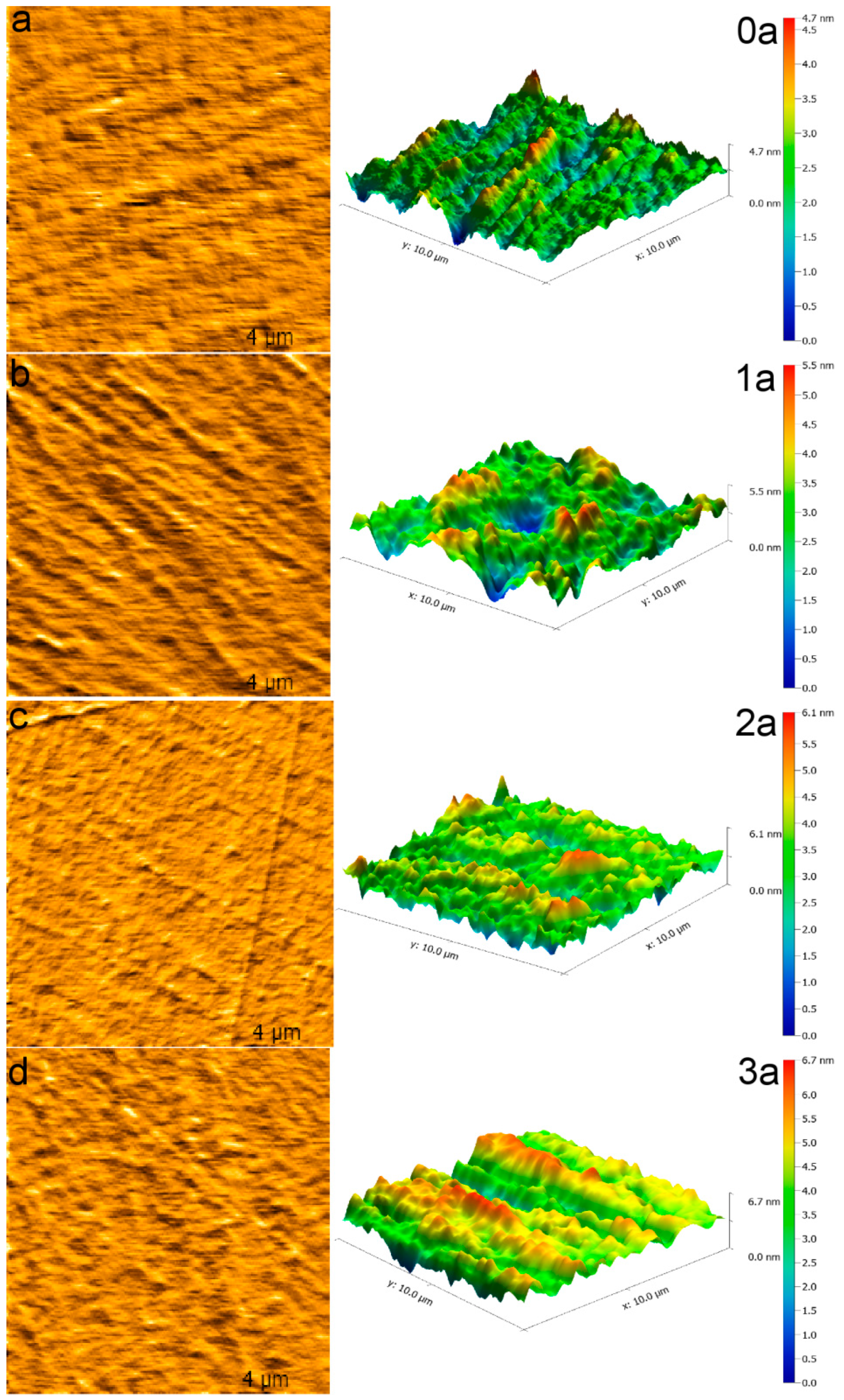
3.3. AFM Analysis
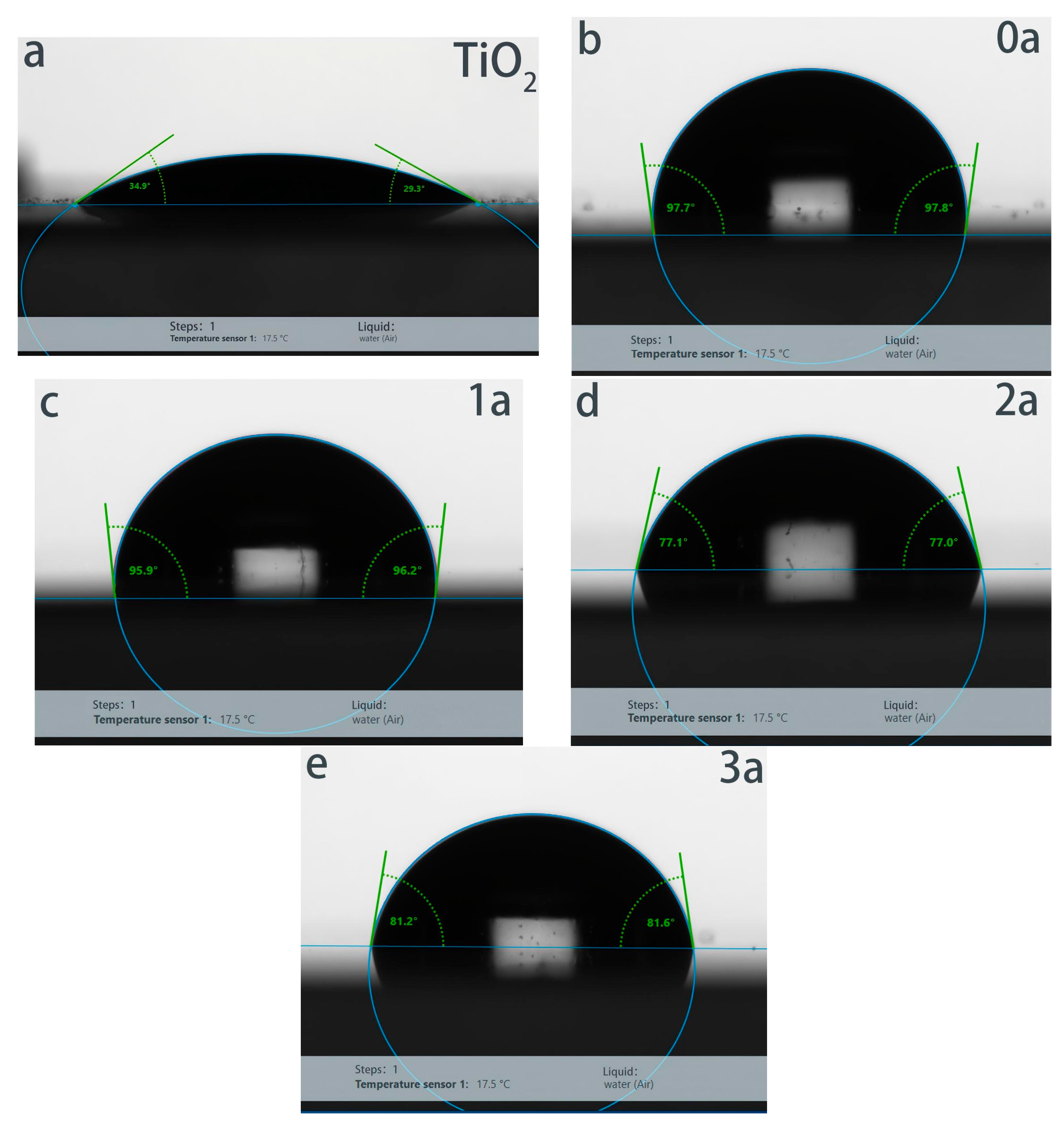
3.4. Contact Angle Analysis
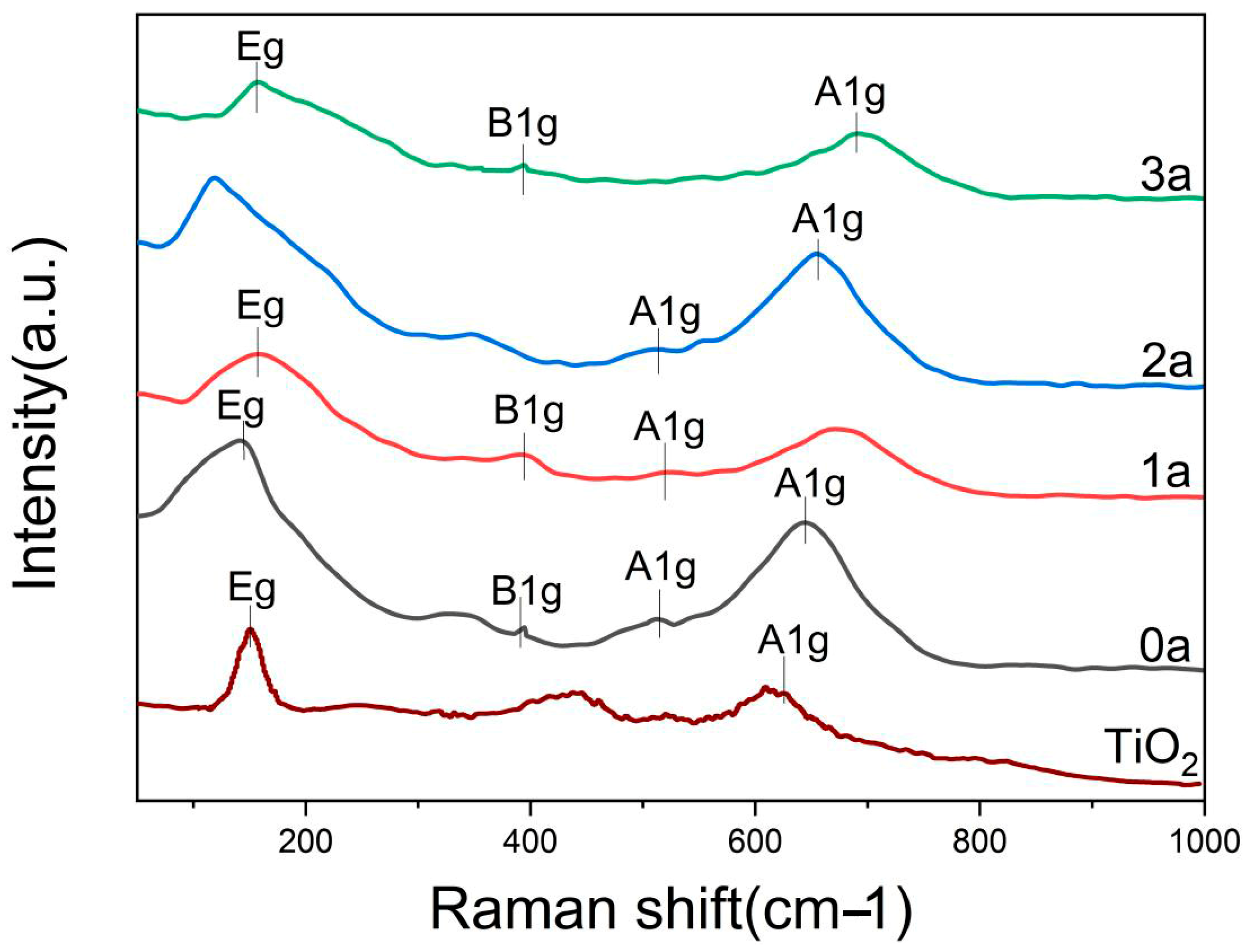
3.5. Raman Spectroscopy Analysis
3.6. Mechanical Property Testing
4. Conclusions
4.1. Structural Stability
4.2. Mechanical Properties
4.3. Water Resistance
4.4. Practical Implications and Future Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Soni, N.; Singh, P.K.; Mallick, S.; Pandey, Y.; Tiwari, S.; Mishra, A.; Tiwari, A. Advancing Sustainable Energy: Exploring New Frontiers and Opportunities in the Green Transition. Adv. Sustain. Syst. 2024, 8, 2400160. [Google Scholar] [CrossRef]
- Dong, W.; Ma, M. Recent developments and advanced applications of promising functional nanocomposites for green buildings: A review. J. Build. Eng. 2025, 102, 111905. [Google Scholar] [CrossRef]
- Yakubu, S.; Samikannu, R.; Gawusu, S.; Wetajega, S.D.; Okai, V.; Shaibu, A.-K.S.; Workneh, G.A. A holistic review of the effects of dust buildup on solar photovoltaic panel efficiency. Sol. Compass 2025, 13, 100101. [Google Scholar] [CrossRef]
- Bora, B.; Rai, S.; Dhar, A. Effect of UV irradiation on PV modules and their simulation in newly designed site-specific accelerated ageing tests. Sol. Energy 2023, 253, 309–320. [Google Scholar] [CrossRef]
- Abdullah, M.; Hosain, M.M.; Parvez, M.M.H.; Motayed, M.S.H. Prospects and challenges of thin film coating materials and their applications. Inorg. Chem. Commun. 2025, 175, 114117. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Rahman, K.H.; Wu, C.-C.; Chen, K.-C. A Review on the Pathways of the Improved Structural Characteristics and Photocatalytic Performance of Titanium Dioxide (TiO2) Thin Films Fabricated by the Magnetron-Sputtering Technique. Catalysts 2020, 10, 598. [Google Scholar] [CrossRef]
- Dai, L.; Fu, P.; Chen, J.; Sun, F. Nitrogen doping mediated oxygen vacancy and Ti valence regulation to enhance photocatalytic H2 generation. Int. J. Hydrogen Energy 2023, 48, 26187–26199. [Google Scholar] [CrossRef]
- Chu, J.; Sun, Y.; Han, X.; Zhang, B.; Du, Y.; Song, B.; Xu, P. Mixed titanium oxide strategy for enhanced photocatalytic hydrogen evolution. ACS Appl. Mater. Interfaces 2019, 11, 18475–18482. [Google Scholar] [CrossRef] [PubMed]
- Vijayarangan, R.; Bharathkumar, S.; Mohan, S.; Valdes, H.; Ilangovan, R.; Amin, M.A.; Vyas, S.; El-Bahy, Z.M. Rationalizing Fe-Modified TiO2 through doping, composite formation, and single-phase structuring for enhanced photocatalysis via inter- and intra-charge transfers. Mater. Sci. Eng. B 2024, 309, 117652. [Google Scholar] [CrossRef]
- Wan, B.; Liu, X.; Qi, L. Research progress of TiO2-based photocatalytic CO2 reduction. Chin. J. Appl. Chem. 2024, 41, 637–658. [Google Scholar]
- Yang, Z.; Ma, Z.; Ren, J.; Xiong, Y.; Ren, F. Theoretical and experimental studies on the optical properties of La-doped TiO2 with oxygen vacancies. Opt. Mater. 2025, 159, 116521. [Google Scholar] [CrossRef]
- Zhu, X.; Pei, L.; Zhu, R.; Jiao, Y.; Tang, R.; Feng, W. Preparation and characterization of Sn/La co-doped TiO2 nanomaterials and their phase transformation and photocatalytic activity. Sci. Rep. 2018, 8, 12387. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.K.; Ganguli, S.; Sabur, M.A. Nitrogen doped titanium dioxide (N-TiO2): Electronic band structure, visible light harvesting and photocatalytic applications. J. Water Process Eng. 2023, 55, 104183. [Google Scholar] [CrossRef]
- Xu, J.; Wu, Y.; Zhang, S. Effects of rare earth element doping on the structure and photocatalytic performance of TiO2 thin films. J. Ceram. 2019, 40, 14–17. [Google Scholar]
- Xiong, J.; Liu, Y.; Xia, L.; Jiang, G.; Xiao, D.; Mishra, Y.K. Electrochromic properties of cobalt-doped titanium dioxide films. Ionics 2025, 31, 743–756. [Google Scholar] [CrossRef]
- Guner, B.; Safikhani-Mahmoudi, M.; Li, F.; Zou, K.; Dagdeviren, O.E. Ultraviolet irradiation penetration depth on TiO2. Commun. Chem. 2025, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, H.; Shi, B.; Wang, J.; Wu, C.; Wang, C.; Wang, X.; Liu, W.; Dai, C.; Wang, D. Fast and controllable anatase-to-rutile phase transition irradiated by NIR light. Chin. Chem. Lett. 2025, 110815. [Google Scholar] [CrossRef]
- Pan, Y.; Bai, T.; Tian, Y. Effects of UV Irradiation on the Optical Properties of TiO2 Films. J. Appl. Opt. 2013, 34, 128–132. [Google Scholar]
- Luo, X.; Wang, J.; Wang, C.; Zhu, S.; Li, Z.; Tang, X.; Wu, M. Degradation and mineralization of benzohydroxamic acid by synthesized mesoporous La/TiO2. Int. J. Environ. Res. Public Health 2016, 13, 997. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Li, Y.; Ding, Y. Ground Simulation Methods for the Effects of UV Radiation on Aerospace Materials. Spacecr. Environ. Eng. 2015, 32, 43–48. [Google Scholar]
- ISO 4892-2:2013; Plastics—Methods of Exposure to Laboratory Light Sources—Part 2: Xenon-Arc Lamps. ISO: Geneva, Switzerland, 2013.
- Vittadini, A.; Casarin, M.; Selloni, A. Chemistry of and on TiO2-anatase surfaces by DFT calculations: A partial review. Theor. Chem. Acc. 2006, 117, 663–671. [Google Scholar] [CrossRef]
- Li, H.; Wan, J.; Dang, W.H. Preparation and Characterization of Nano-TiO2/PE Antibacterial Films. Adv. Mater. Res. 2011, 284, 1790–1793. [Google Scholar] [CrossRef]
- Singh, K.; Harish, S.; Hayakawa, Y.; Shimomura, M. Experimental and theoretical study of oxygen vacancy induced La-doped mesoporous TiO2 for enhanced thermal stability. AIP Adv. 2023, 13, 055107. [Google Scholar] [CrossRef]
- Sikam, P.; Thirayatorn, R.; Kaewmaraya, T.; Thongbai, P.; Moontragoon, P.; Ikonic, Z. Improved Thermoelectric Properties of SrTiO3 via (La, Dy and N) Co-Doping: DFT Approach. Molecules 2022, 27, 7923. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhao, X.; Cheng, X.; Sun, H.; Li, Y.; Li, P.; Fan, W. Synergistic Effects in La/N Codoped TiO2 Anatase (101) Surface Correlated with Enhanced Visible-Light Photocatalytic Activity. Langmuir 2012, 28, 5882–5891. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Huang, G.; Liu, X.; Nie, X.; Qi, C.; Wang, H.; Hu, J.; Fang, H.; Gao, Y.; Liu, W.-T. Room temperature bilayer water structures on a rutile TiO2(110) surface: Hydrophobic or hydrophilic? Chem. Sci. 2022, 13, 10546–10554. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Cao, J.-J.; Kang, F.; You, S.-J.; Chang, C.-W.; Wang, Y.-F. High Selectivity of Visible-Light-Driven La-doped TiO2 Photocatalysts for NO Removal. Aerosol Air Qual. Res. 2017, 17, 2555–2565. [Google Scholar] [CrossRef]




| Aging Time/Years | Irradiation Duration/Hours | Simulated Time/Days |
|---|---|---|
| 0 years | 0 h | 0 days |
| 1 year | 48 h | 2 days |
| 2 years | 96 h | 4 days |
| 3 years | 144 h | 6 days |
| 5 years | 240 h | 10 days |
| UV Aging Time | Average Grain Diameter (nm) | Average Roughness (pm) | Root Mean Square Roughness (pm) |
|---|---|---|---|
| 0 years | 2.462 | 422.6 | 541.9 |
| 1 year | 2.893 | 623.4 | 801.7 |
| 2 years | 3.199 | 648.5 | 818.8 |
| 3 years | 4.288 | 683.3 | 865.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, P.; Zhang, L.; Yuan, Y. Structural and Performance Studies of Lanthanum–Nitrogen Co-Doped Titanium Dioxide Thin Films Under UV Aging. Micromachines 2025, 16, 842. https://doi.org/10.3390/mi16080842
Cao P, Zhang L, Yuan Y. Structural and Performance Studies of Lanthanum–Nitrogen Co-Doped Titanium Dioxide Thin Films Under UV Aging. Micromachines. 2025; 16(8):842. https://doi.org/10.3390/mi16080842
Chicago/Turabian StyleCao, Pengcheng, Li Zhang, and Yanbo Yuan. 2025. "Structural and Performance Studies of Lanthanum–Nitrogen Co-Doped Titanium Dioxide Thin Films Under UV Aging" Micromachines 16, no. 8: 842. https://doi.org/10.3390/mi16080842
APA StyleCao, P., Zhang, L., & Yuan, Y. (2025). Structural and Performance Studies of Lanthanum–Nitrogen Co-Doped Titanium Dioxide Thin Films Under UV Aging. Micromachines, 16(8), 842. https://doi.org/10.3390/mi16080842






