From Micro to Marvel: Unleashing the Full Potential of Click Chemistry with Micromachine Integration
Abstract
1. Introduction
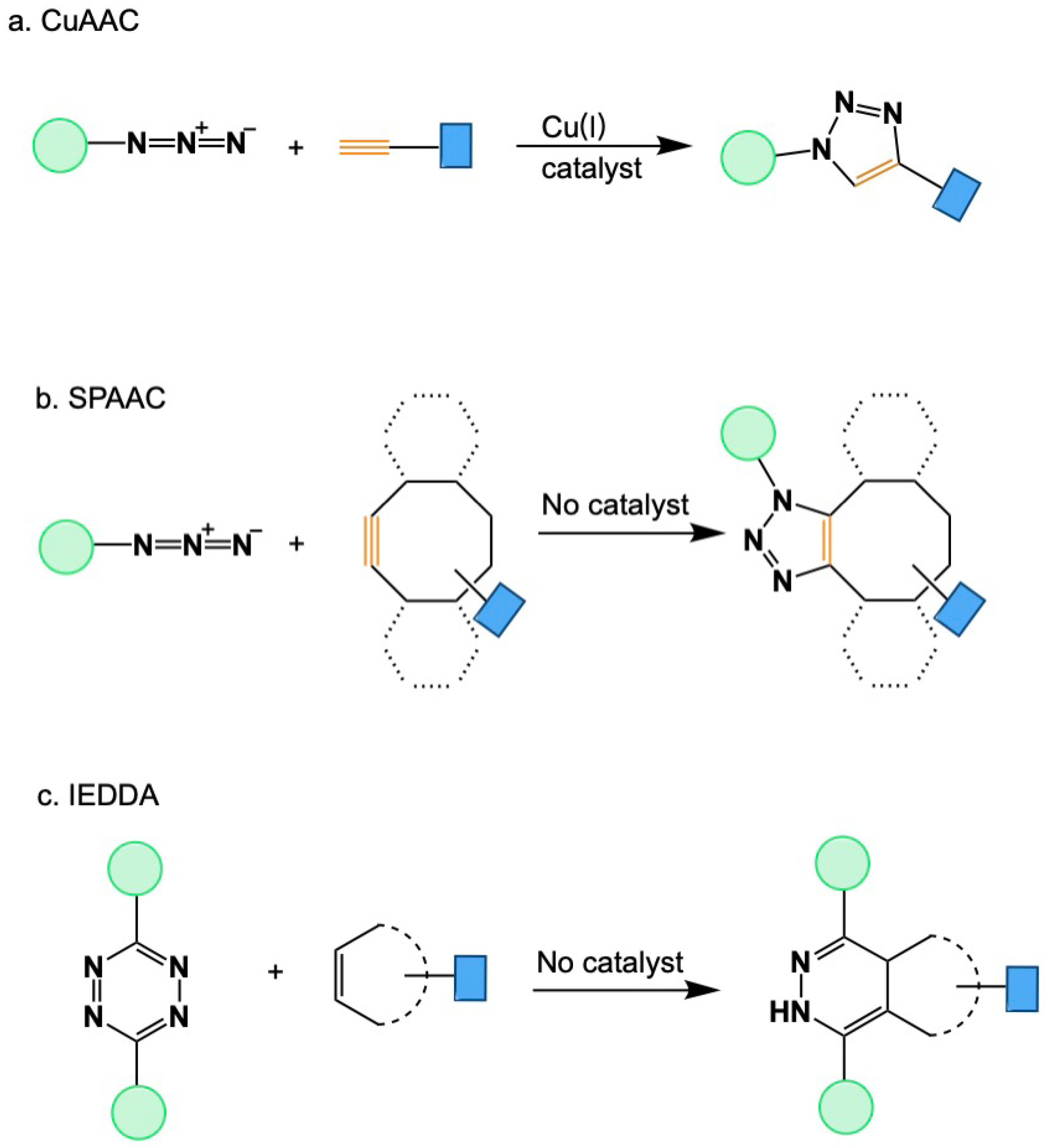
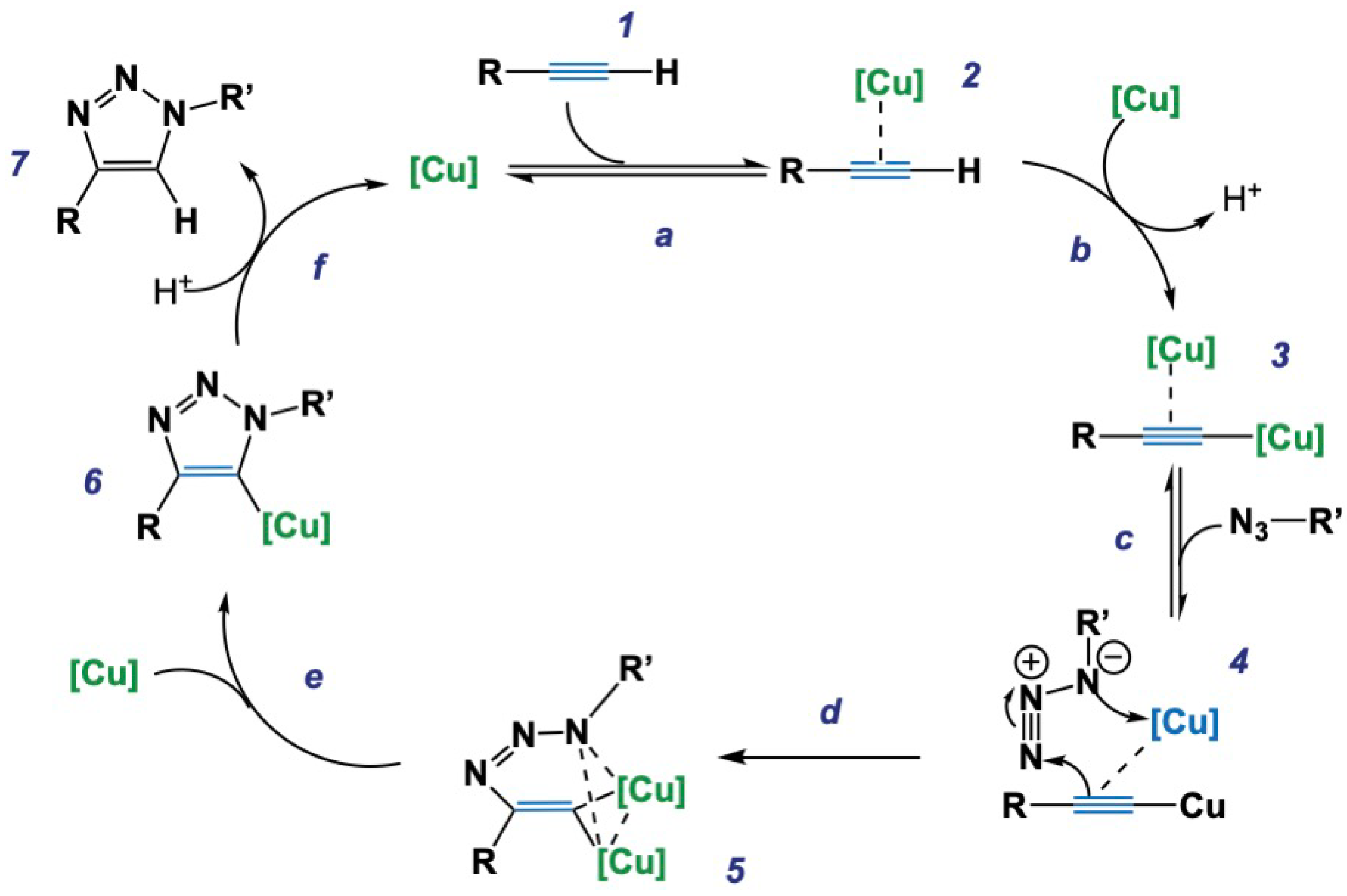
2. Fundamentals of Click Chemistry
3. Micromachines: Design and Functionality
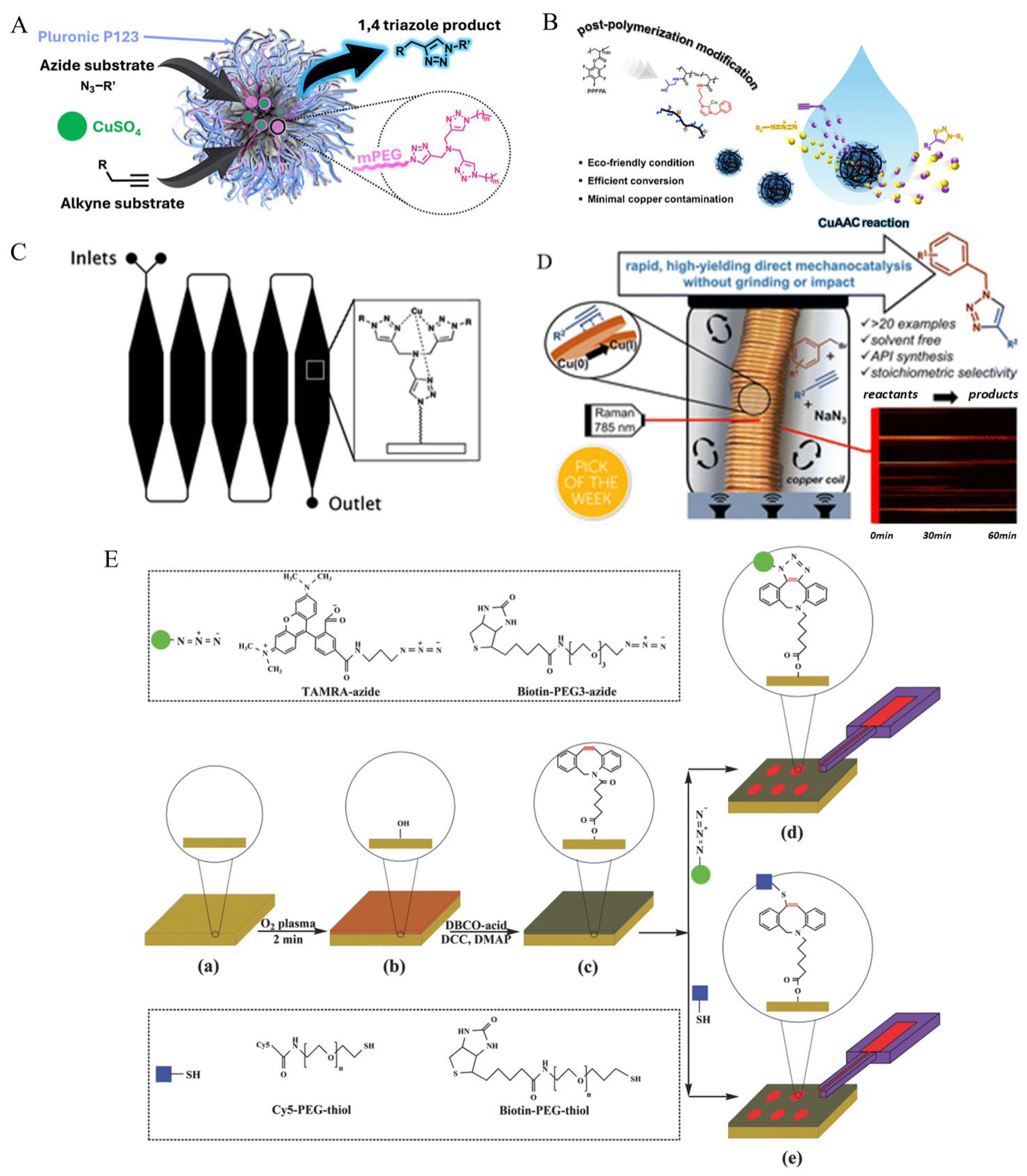
4. Integration of Micromachines with Click Chemistry
4.1. Enhanced Reaction Kinetics
4.2. Selective and Localized Reactions
4.3. Reagent Consumption and Environmental Impact
4.4. Automation and Programmability
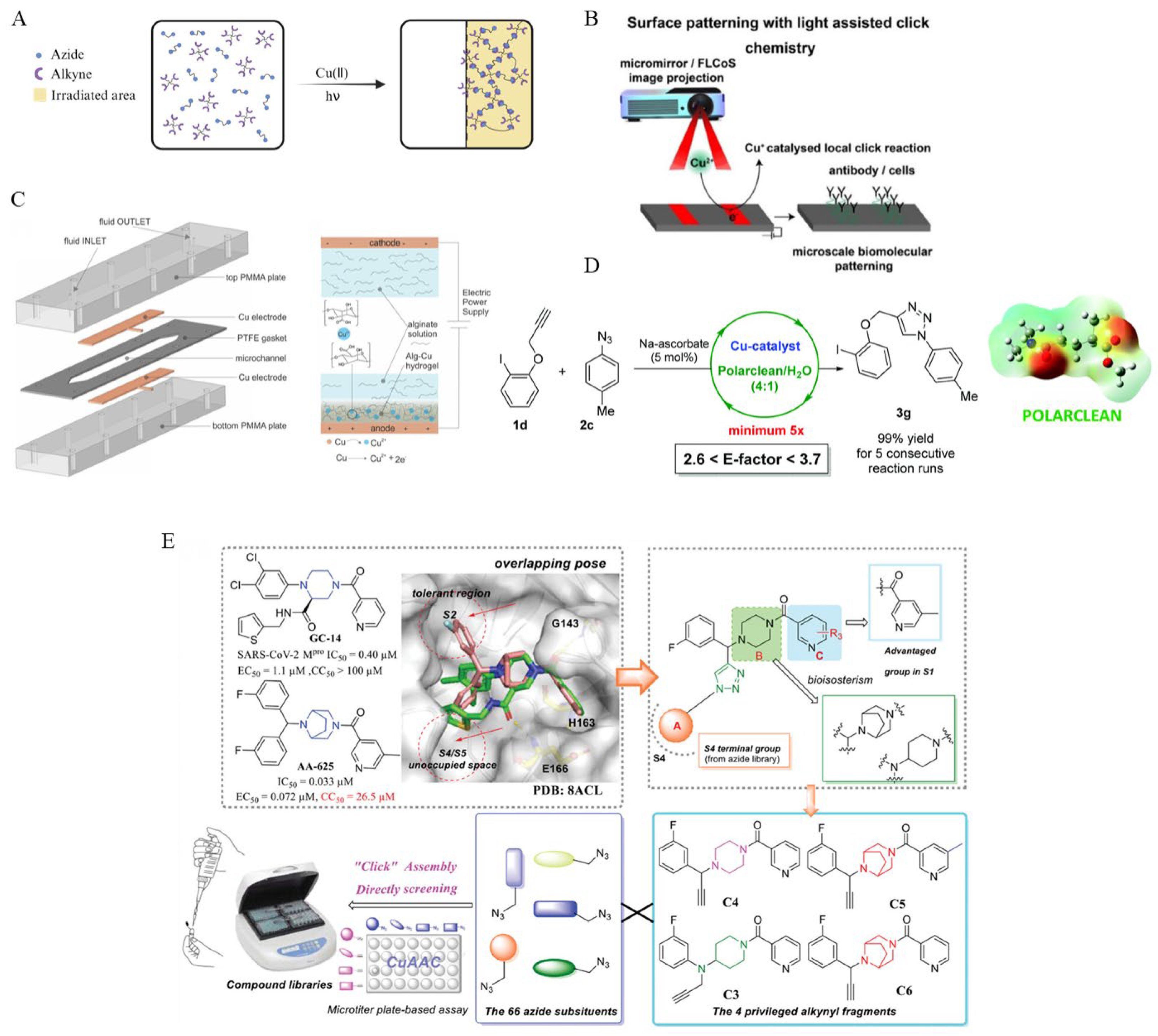
4.5. Micromachine-Assisted Click Chemistry in Complex Biological Environments
5. Recent Advances and Future Prospects of Micromachine-Assisted Click Chemistry in Biomedical Applications
5.1. Drug Delivery and Bioconjugation
5.2. Biosensing and Diagnostics
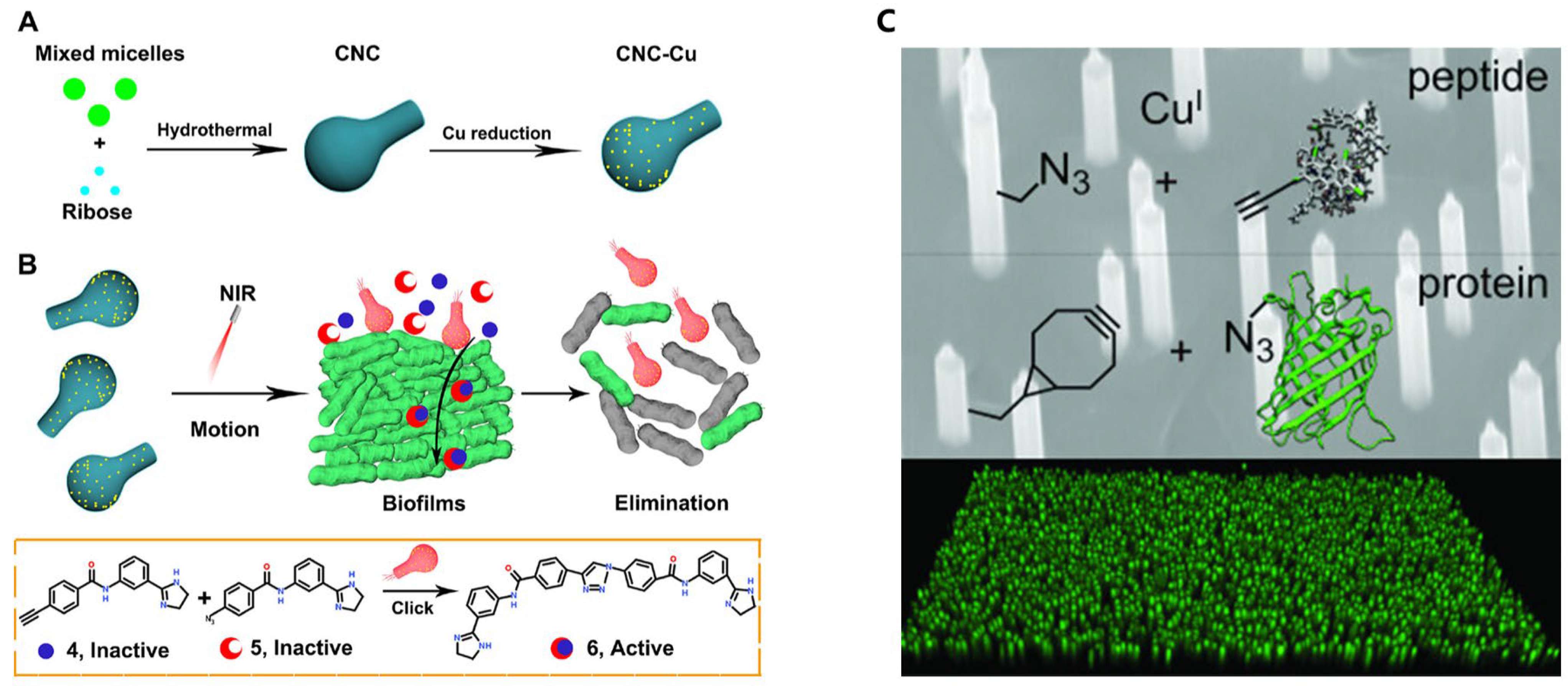
5.3. Smart Materials and Surface Functionalization
5.4. Environmental Remediation
6. Challenges and Limitations
6.1. Biocompatibility and Toxicity
6.2. Control and Stability
6.3. Scalability and Cost
7. Toward the Next Generation of Applications of Micromachines in Click Chemistry
7.1. AI-Driven Control and Machine Learning Approaches for Advanced Micromachines

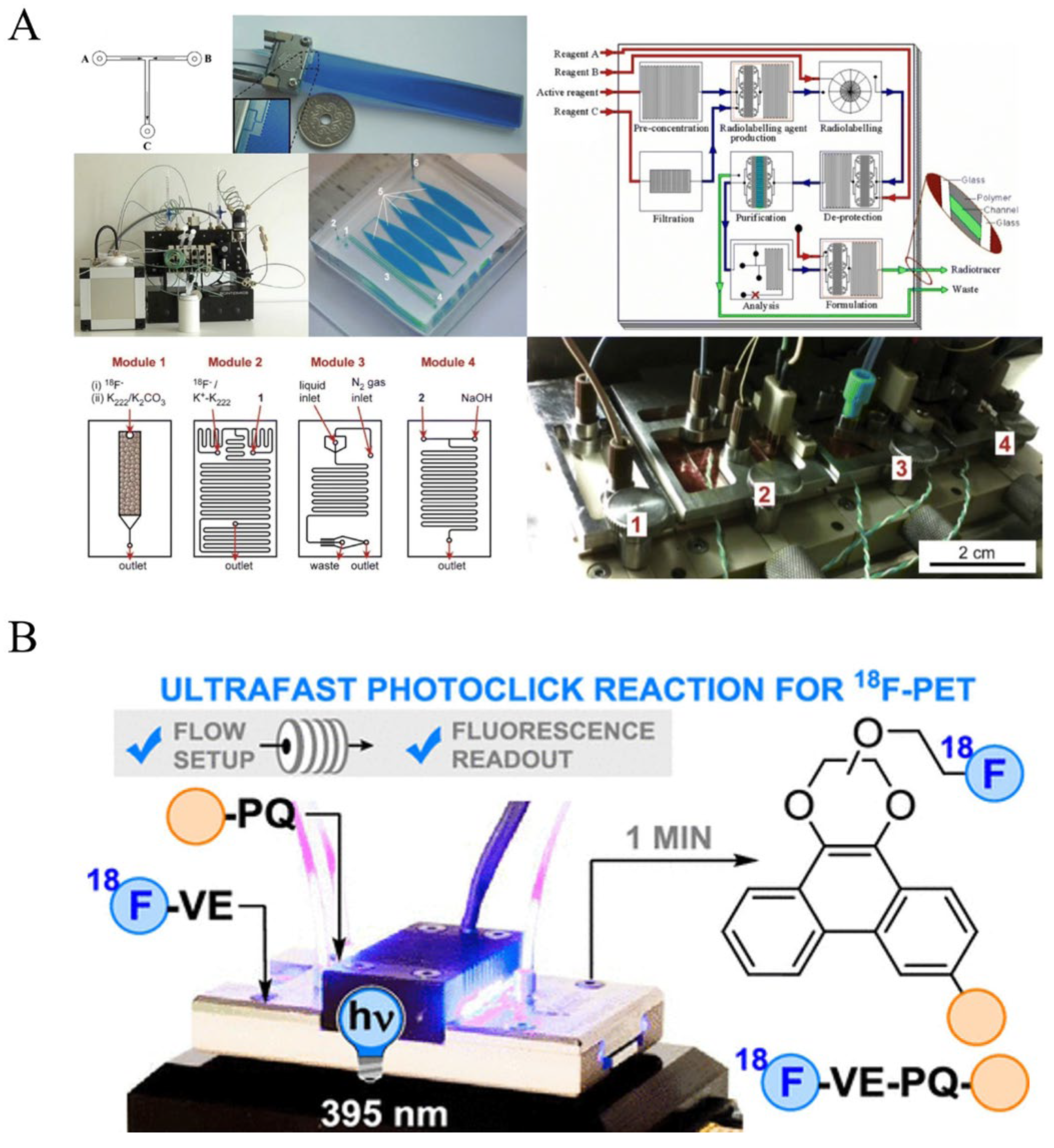
7.2. On-Chip Click Chemistry: Microfluidic Platforms for Next-Generation PET Tracer Development
7.3. Biohybrid Micro- and Nanorobot Systems
7.4. On-Demand Synthesis and Personalized Medicine
7.5. Self-Learning, Self-Healing, and Self-Evolving Microsystems
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MEMS | Microelectronic mechanical system |
| CuAAC | Copper-catalyzed azide–alkyne cycloaddition |
| SPAAC | Strain-promoted azide–alkyne cycloaddition |
| PDMS | Polydimethylsiloxane |
| MNRs | Micellar nanoreactors |
| DTAs | Dendritic amphiphiles |
| PPFPA | Poly(pentafluorophenyl) acrylate |
| NIR | Near-infrared radiation |
| CNC | Carbonaceous nanocalabash |
| AI | Artificial intelligence |
| PCL | Polycaprolactone |
| miRNA | MicroRNA |
| ddWalker | Digital DNA walker |
References
- Yuan, Y.; Liang, G. A biocompatible, highly efficient click reaction and its applications. Org. Biomol. Chem. 2014, 12, 865–871. [Google Scholar] [CrossRef]
- Wang, W.; Lorion, M.M.; Shah, J.; Kapdi, A.R.; Ackermann, L. Late-Stage Peptide Diversification by Position-Selective C-H Activation. Angew. Chem. Int. Ed. Engl. 2018, 57, 14700–14717. [Google Scholar] [CrossRef]
- Nicolas, J.; Mura, S.; Brambilla, D.; Mackiewicz, N.; Couvreur, P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem. Soc. Rev. 2013, 42, 1147–1235. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. Engl. 2001, 40, 2004–2021. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.Z.; Wang, C.Q.; Loh, T.P. Copper-Catalyzed Vicinal Oxyazidation and Diazidation of Styrenes under Mild Conditions: Access to Alkyl Azides. Org. Lett. 2015, 17, 6110–6113. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Saxena, M.; Rishi, N. An Overview of Recent Advances in Biomedical Applications of Click Chemistry. Bioconjug. Chem. 2021, 32, 1455–1471. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, M.K.; Sajadi, S.M.; Seidi, F.; Rabiee, N.; Fatahi, Y.; Rabiee, M.; Dominic, C.D.M.; Zarrintaj, P.; Formela, K.; Saeb, M.R.; et al. Clickable Polysaccharides for Biomedical Applications: A Comprehensive Review. Prog. Polym. Sci. 2022, 133, 101590. [Google Scholar] [CrossRef] [PubMed]
- Delplace, V. Rethinking Click and Bioorthogonal Chemistry for Biomedical Applications. ACS Mater. Lett. 2023, 6, 153–158. [Google Scholar] [CrossRef]
- Binder, W.H.; Sachsenhofer, R. ‘Click’ Chemistry in Polymer and Materials Science. Macromol. Rapid Commun. 2007, 28, 15–54. [Google Scholar] [CrossRef]
- Hein, C.D.; Liu, X.M.; Wang, D. Click chemistry, a powerful tool for pharmaceutical sciences. Pharm. Res. 2008, 25, 2216–2230. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Coghi, P. Click chemistry in tuberculosis research: From drug design to therapeutic delivery—A systematic review. Eur. J. Chem. 2025, 16, 83–96. [Google Scholar] [CrossRef]
- Meldal, M.; Diness, F. Recent Fascinating Aspects of the CuAAC Click Reaction. Trends Chem. 2020, 2, 569–584. [Google Scholar] [CrossRef]
- Shen, Y.H.; Esper, A.M.; Ghiviriga, I.; Abboud, K.A.; Schanze, K.S.; Ehm, C.; Veige, A.S. SPAAC iClick: Progress towards a bioorthogonal reaction in-corporating metal ions. Dalton Trans. 2021, 50, 12681–12691. [Google Scholar] [CrossRef] [PubMed]
- Blackman, M.L.; Royzen, M.; Fox, J.M. Tetrazine ligation: Fast bioconjugation based on inverse-electron-demand Diels-Alder reactivity. J. Am. Chem. Soc. 2008, 130, 13518–13519. [Google Scholar] [CrossRef]
- Liang, T.; Chen, Z.; Li, H.; Gu, Z. Bioorthogonal catalysis for biomedical applications. Trends Chem. 2022, 4, 157–168. [Google Scholar] [CrossRef]
- Meldal, M. Polymer “Clicking” by CuAAC Reactions. Macromol. Rapid Commun. 2008, 29, 1016–1051. [Google Scholar] [CrossRef]
- Haldón, E.; Nicasio, M.C.; Pérez, P.J. Copper-catalysed azide–alkyne cycloadditions (CuAAC): An update. Org. Biomol. Chem. 2015, 13, 9528–9550. [Google Scholar] [CrossRef] [PubMed]
- Neumann, S.; Biewend, M.; Rana, S.; Binder, W.H. The CuAAC: Principles, Homogeneous and Heterogeneous Catalysts, and Novel Developments and Applications. Macromol. Rapid Commun. 2020, 41, e1900359. [Google Scholar] [CrossRef]
- Liang, L.; Astruc, D. The copper(I)-catalyzed alkyne-azide cycloaddition (CuAAC) “click” reaction and its applications. An overview. Coord. Chem. Rev. 2011, 255, 2933–2945. [Google Scholar] [CrossRef]
- Dudchak, R.; Podolak, M.; Holota, S.; Szewczyk-Roszczenko, O.; Roszczenko, P.; Bielawska, A.; Lesyk, R.; Bielawski, K. Click chemistry in the synthesis of antibody-drug conjugates. Bioorg. Chem. 2024, 143, 106982. [Google Scholar] [CrossRef]
- Devaraj, N.K.; Weissleder, R. Biomedical applications of tetrazine cycloadditions. Acc. Chem. Res. 2011, 44, 816–827. [Google Scholar] [CrossRef]
- Feng, Y.; Wu, Y.; Zuo, J.; Tu, L.; Que, I.; Chang, Y.; Cruz, L.J.; Chan, A.; Zhang, H. Assembly of upconversion nanophotosensitizer in vivo to achieve scatheless real-time imaging and selective photodynamic therapy. Biomaterials 2019, 201, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Hartung, K.M.; Sletten, E.M. Bioorthogonal chemistry: Bridging chemistry, biology, and medicine. Chem 2023, 9, 2095–2109. [Google Scholar] [CrossRef] [PubMed]
- Dommerholt, J.; Rutjes, F.; van Delft, F.L. Strain-Promoted 1,3-Dipolar Cycloaddition of Cycloalkynes and Organic Azides. Top. Curr. Chem. 2016, 374, 16. [Google Scholar] [CrossRef]
- Smeenk, M.L.W.J.; Agramunt, J.; Bonger, K.M. Recent developments in bioorthogonal chemistry and the orthogonality within. Curr. Opin. Chem. Biol. 2021, 60, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.; Chatterjee, S.; Kushwaha, A.; Nanda, S.; Dhilip Kumar, T.J.; Bandyopadhyay, A. Sulfonyl Diazaborine ‘Click’ Chemistry Enables Rapid and Efficient Bioorthogonal Labeling. Chemistry 2023, 29, e202300393. [Google Scholar] [CrossRef]
- Baskin, J.M.; Bertozzi, C.R. Bioorthogonal Click Chemistry: Covalent Labeling in Living Systems. QSAR Comb. Sci. 2007, 26, 1211–1219. [Google Scholar] [CrossRef]
- Mitry, M.M.A.; Greco, F.; Osborn, H.M.I. In Vivo Applications of Bioorthogonal Reactions: Chemistry and Targeting Mechanisms. Chem. Eur. J. 2023, 29, e202203942. [Google Scholar] [CrossRef]
- Dong, J.; Krasnova, L.; Finn, M.G.; Sharpless, K.B. Sulfur(VI) fluoride exchange (SuFEx): Another good reaction for click chemistry. Angew. Chem. Int. Ed. Engl. 2014, 53, 9430–9448. [Google Scholar] [CrossRef]
- Geng, Z.; Shin, J.J.; Xi, Y.; Hawker, C.J. Click chemistry strategies for the accelerated synthesis of functional macromolecules. J. Polym. Sci. 2021, 59, 963–1042. [Google Scholar] [CrossRef]
- Nolan, M.D.; Scanlan, E.M. Applications of Thiol-Ene Chemistry for Peptide Science. Front. Chem. 2020, 8, 583272. [Google Scholar] [CrossRef]
- Sumpter, B.G.; Hong, K.; Vasudevan, R.K.; Ivanov, I.; Advincula, R. Autonomous continuous flow reactor synthesis for scalable atom-precision. Carbon. Trends 2023, 10, 100234. [Google Scholar] [CrossRef]
- Sticker, D.; Geczy, R.; Hafeli, U.O.; Kutter, J.P. Thiol-Ene Based Polymers as Versatile Materials for Microfluidic Devices for Life Sciences Applications. ACS Appl. Mater. Interfaces 2020, 12, 10080–10095. [Google Scholar] [CrossRef] [PubMed]
- Madou, M.J. Fundamentals of Microfabrication: The Science of Miniaturization, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Ahmad Fuaad, M.R.; Hasan, M.N.; Ahmad Asri, M.I.; Mohamed Ali, M.S. Microactuators technologies for biomedical applications. Microsyst. Technol. 2023, 29, 953–984. [Google Scholar] [CrossRef]
- Pelesko, J.A.; Bernstein, D.H. Modeling MEMS and NEMS, 1st ed.; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Ji, B.; Gao, K. Editorial for the Special Issue on Wearable and Implantable Bio-MEMS Devices and Applications. Micromachines 2024, 15, 955. [Google Scholar] [CrossRef]
- Hossain, N.; Mahmud, M.Z.A.; Hossain, A.; Rahman, M.K.; Islam, M.S.; Tasnim, R.; Mobarak, M.H. Advances of materials science in MEMS applications: A review. Results Eng. 2024, 22, 102115. [Google Scholar] [CrossRef]
- Karimi, K.; Fardoost, A.; Mhatre, N.; Rajan, J.; Boisvert, D.; Javanmard, M. A Thorough Review of Emerging Technologies in Micro- and Nanochannel Fabrication: Limitations, Applications, and Comparison. Micromachines 2024, 15, 1274. [Google Scholar] [CrossRef]
- Xu, J.; Harasek, M.; Gföhler, M. From Soft Lithography to 3D Printing: Current Status and Future of Microfluidic Device Fabrication. Polymers 2025, 17, 455. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Wang, F.; McAlpine, M.C. 3D printed microfluidics: Advances in strategies, integration, and applications. Lab Chip 2023, 23, 1279–1299. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, Z.; Zhao, Z.; Zhang, D.; Feng, J.; Yu, L.; Lin, Z.; Guo, Q.; Huang, J.; Mao, J.; et al. 3D printing of micro-nano devices and their applications. Microsyst. Nanoeng. 2025, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Sia, S.K.; Whitesides, G.M. Microfluidic devices fabricated in Poly(dimethylsiloxane) for biological studies. Electrophoresis 2003, 24, 3563–3576. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, J.; Ray, W.Z.; MacEwan, M.R. Implantable and Semi-Implantable Biosensors for Minimally Invasive Disease Diagnosis. Processes 2024, 12, 1535. [Google Scholar] [CrossRef]
- Kuan, H.; Xie, Y.; Guo, Y.; Gianoncelli, A.; Ribaudo, G.; Coghi, P. (2R, 4S, 5S) 1-(4-(4-(((7-Chloroquinolin-4-yl)amino)methyl)-1H-1,2,3-triazol-1-yl)-5-(hydroxymethyl)tetrahydrofuran-2-yl)-5-methylpyrimidine-2,4(1H,3H)-dione. Molbank 2023, 2023, M1681. [Google Scholar] [CrossRef]
- Galambos, P.; Eaton, W.P.; Shul, R.; Willison, C.G.; Sniegowski, J.J.; Miller, S.L.; Gutierrez, D. Surface Micromachine Microfluidics: Design, Fabrication, Packaging, and Characterization. In Proceedings of the ASME 1999 International Mechanical Engineering Congress and Exposition, Nashville, TN, USA, 14–19 November 1999. [Google Scholar]
- Cong, H.; Zhang, N. Perspectives in translating microfluidic devices from laboratory prototyping into scale-up production. Biomicrofluidics 2022, 16, 021301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Russell, M.G.; Jamison, T.F. Continuous Flow Total Synthesis of Rufinamide. Org. Process Res. Dev. 2014, 18, 1567–1570. [Google Scholar] [CrossRef]
- Arai, D.; Ogata, S.; Shimizu, T.; Yang, M. Enhancement of Convection and Molecular Transport into Film Stacked Structures by Introduction of Notch Shape for Micro-Immunoassay. Micromachines 2024, 15, 613. [Google Scholar] [CrossRef]
- Yun, X.; Xie, Y.; Ng, J.P.L.; Law, B.Y.K.; Wong, V.K.W.; Coghi, P. 2-Bromo-3-((1-(7-chloroquinolin-4-yl)-1H-1,2,3-triazol-4-yl)-methoxy)-benzaldehyde. Molbank 2022, 2022, M1351. [Google Scholar] [CrossRef]
- Lin, Z.; Jiang, T.; Shang, J. The emerging technology of biohybrid micro-robots: A review. Bio Des. Manuf. 2022, 5, 107–132. [Google Scholar] [CrossRef]
- Saladino, G.M.; Hamawandi, B.; Vogt, C.; Rajarao, G.K.; Toprak, M.S. Click chemical assembly and validation of bio-functionalized superparamagnetic hybrid microspheres. Appl. Nanosci. 2020, 10, 1861–1869. [Google Scholar] [CrossRef]
- Coghi, P.; Ng, J.P.L.; Nasim, A.A.; Wong, V.K.W. N-[7-Chloro-4-[4-(phenoxymethyl)-1H-1,2,3-triazol-1-yl]quinoline]-acetamide. Molbank 2021, 2021, M1213. [Google Scholar] [CrossRef]
- Fai, L.K.; Anyanwu, M.; Ai, J.; Xie, Y.; Gianoncelli, A.; Ribaudo, G.; Coghi, P. 4-(4-(((1H-Benzo[d][1,2,3]triazol-1-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)-7-chloroquinoline. Molbank 2022, 2022, M1404. [Google Scholar] [CrossRef]
- Luu, T.; Gristwood, K.; Knight, J.C.; Jörg, M. Click Chemistry: Reaction Rates and Their Suitability for Biomedical Applications. Bioconjug. Chem. 2024, 35, 715–731. [Google Scholar] [CrossRef]
- Vippala, K.; Wagle, S.S.; Rathee, P.; Mulamukkil, K.; Ayoub, Y.; Komlosh, A.; Gazal, S.; Avramovitch, B.; Amir, R.J. Micellar “Click” Nanoreactors: Spiking Pluronic-Based Micelles with Polymeric Ligands. Macromolecules 2024, 57, 10557–10566. [Google Scholar] [CrossRef]
- Sombat, W.; Padungros, P.; Hoven, V.P. Polymeric Micellar Nanocatalysts for CuAAC Click Reaction in Water. Langmuir 2025, 41, 6729–6739. [Google Scholar] [CrossRef]
- Li, H.; Whittenberg, J.J.; Zhou, H.; Ranganathan, D.; Desai, A.V.; Koziol, J.; Zeng, D.; Kenis, P.J.; Reichert, D.E. Development of a microfluidic “click chip” incorporating an immobilized Cu(I) catalyst. RSC Adv. 2015, 5, 6142–6150. [Google Scholar] [CrossRef] [PubMed]
- Lennox, C.B.; Borchers, T.H.; Gonnet, L.; Barrett, C.J.; Koenig, S.G.; Nagapudi, K.; Friščić, T. Direct mechanocatalysis by resonant acoustic mixing (RAM). Chem. Sci. 2023, 14, 7475–7481. [Google Scholar] [CrossRef]
- Dadfar, S.M.M.; Sekula-Neuner, S.; Bog, U.; Trouillet, V.; Hirtz, M. Site-Specific Surface Functionalization via Microchannel Cantilever Spotting (µCS): Comparison between Azide–Alkyne and Thiol–Alkyne Click Chemistry Reactions. Small 2018, 14, 1800131. [Google Scholar] [CrossRef] [PubMed]
- Thampi, S.P.; Doostmohammadi, A.; Shendruk, T.N.; Golestanian, R.; Yeomans, J.M. Active micromachines: Microfluidics powered by mesoscale turbulence. Sci. Adv. 2016, 2, e1501854. [Google Scholar] [CrossRef]
- Cai, G.; Xue, L.; Zhang, H.; Lin, J. A Review on Micromixers. Micromachines 2017, 8, 274. [Google Scholar] [CrossRef]
- Raza, W.; Hossain, S.; Kim, K.-Y. A Review of Passive Micromixers with a Comparative Analysis. Micromachines 2020, 11, 455. [Google Scholar] [CrossRef]
- Adzima, B.J.; Tao, Y.; Kloxin, C.J.; DeForest, C.A.; Anseth, K.S.; Bowman, C.N. Spatial and temporal control of the alkyne–azide cycloaddition by photoinitiated Cu(II) reduction. Nat. Chem. 2011, 3, 256–259. [Google Scholar] [CrossRef]
- Azagarsamy, M.A.; Anseth, K.S. Bioorthogonal Click Chemistry: An Indispensable Tool to Create Multifaceted Cell Culture Scaffolds. ACS Macro Lett. 2013, 2, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Lian, J.; Gonçales, V.R.; Vogel, Y.B.; Ciampi, S.; Tilley, R.D.; Gooding, J.J. Surface Patterning of Biomolecules Using Click Chemistry and Light-Activated Electrochemistry to Locally Generate Cu(I). ChemElectroChem 2020, 7, 4245–4250. [Google Scholar] [CrossRef]
- Coursari, D.; Efstathiou, S.; Al-Shok, L.; Hall, M.D.; Eissa, A.M.; Liarou, E.; Haddleton, D.M. Optimisation of azide–alkyne click reactions of polyacrylates using online monitoring and flow chemistry. Polym. Chem. 2025, 16, 1065–1071. [Google Scholar] [CrossRef]
- Huo, S.; Zhao, P.; Shi, Z.; Zou, M.; Yang, X.; Warszawik, E.; Loznik, M.; Gostl, R.; Herrmann, A. Mechanochemical bond scission for the activation of drugs. Nat. Chem. 2021, 13, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; An, M.; Liu, Y.; Sarwar, M.T.; Yang, H. Advances in Chemically Powered Micro/Nanorobots for Biological Applications: A Review. Adv. Funct. Mater. 2022, 33, 2209883. [Google Scholar] [CrossRef]
- Soler, L.; Sánchez, S. Catalytic nanomotors for environmental monitoring and water remediation. Nanoscale 2014, 6, 7175–7182. [Google Scholar] [CrossRef]
- Ramšak, A.; Gazvoda, M.; Plazl, I.; Ambrožič, R. Cu-alginate hydrogels in microfluidic systems: A sustainable catalytic approach for click chemistry. Front. Chem. Eng. 2024, 6, 1434131. [Google Scholar] [CrossRef]
- Luciani, L.; Goff, E.; Lanari, D.; Santoro, S.; Vaccaro, L. Waste-minimised copper-catalysed azide–alkyne cycloaddition in Polarclean as a reusable and safe reaction medium. Green Chem. 2018, 20, 183–187. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Hsiao, Y.W.; Baker-Fales, M.; Cameli, F.; Dimitrakellis, P.; Vlachos, D.G. Microflow chemistry and its electrification for sustainable chemical manufacturing. Chem. Sci. 2022, 13, 10644–10685. [Google Scholar] [CrossRef]
- Zhou, M.; Hou, T.; Li, J.; Yu, S.; Xu, Z.; Yin, M.; Wang, J.; Wang, X. Self-Propelled and Targeted Drug Delivery of Poly(aspartic acid)/Iron–Zinc Microrocket in the Stomach. ACS Nano 2019, 13, 1324–1332. [Google Scholar] [CrossRef]
- Heida, T.; Otto, O.; Biedenweg, D.; Hauck, N.; Thiele, J. Microfluidic Fabrication of Click Chemistry-Mediated Hyaluronic Acid Microgels: A Bottom-Up Material Guide to Tailor a Microgel’s Physicochemical and Mechanical Properties. Polymers 2020, 12, 1760. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Yang, M.; Lee, M.K.; Gao, S.; Song, L.; Jang, H.Y.; Jo, I.; Yang, C.C.; Sylvester, K.; Ko, C.; Wang, S.; et al. Miniaturized Modular Click Chemistry-enabled Rapid Discovery of Unique SARS-CoV-2 Mpro Inhibitors With Robust Potency and Drug-like Profile. Adv. Sci. 2024, 11, 2404884. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xia, B.; Hao, Z.; Kang, H.; Liu, W.; Chen, Y.; Jiang, Q.; Liu, J.; Gou, J.; Dong, B.; et al. A closed-loop catalytic nanoreactor system on a transistor. Sci. Adv. 2023, 9, eadj0839. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Liu, L.-Y.; Ju, X.-J.; Wang, W.; Xie, R.; Chu, L.-Y. Novel Smart Microreactors Equipped with Responsive Catalytic Nanoparticles on Microchannels. ACS Appl. Mater. Interfaces 2017, 9, 33137–33148. [Google Scholar] [CrossRef]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Mu, Q.; Wang, H.; Lin, G.; Zhang, M. Enzymatic and Cellular Degradation of Carbon-Based Biconcave Nanodisks. Micromachines 2022, 13, 1144. [Google Scholar] [CrossRef]
- Ghosal, K.; Bhattacharyya, S.K.; Mishra, V.; Zuilhof, H. Click Chemistry for Biofunctional Polymers: From Observing to Steering Cell Behavior. Chem. Rev. 2024, 124, 13216–13300. [Google Scholar] [CrossRef]
- Villa, K.; Pumera, M. Fuel-free light-driven micro/nanomachines: Artificial active matter mimicking nature. Chem. Soc. Rev. 2019, 48, 4966–4978. [Google Scholar] [CrossRef] [PubMed]
- Puttaraksa, N.; Sada, S.; Kosumsupamala, K.; Seki, H.; Whitlow, H.J.; Nishikawa, H. Precise Fabrication of Elongated Janus Microparticles. Part. Part. Syst. Charact. 2025, 42, 2400210. [Google Scholar] [CrossRef]
- Gao, W.; Wang, J. Synthetic micro/nanomotors in drug delivery. Nanoscale 2014, 6, 10486–10494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Kuang, G.; Wang, L.; Fan, L.; Zhou, Y.; Shang, L.; Zhao, Y.; Sun, W. Bio-inspired biorthogonal compartmental microparticles for tumor chemotherapy and photothermal therapy. J. Nanobiotechnol. 2024, 22, 498. [Google Scholar] [CrossRef] [PubMed]
- Mallick, S.; Abouomar, R.; Rivas, D.; Sokolich, M.; Kirmizitas, F.C.; Dutta, A.; Das, S. Doxorubicin-Loaded Microrobots for Targeted Drug Delivery and Anticancer Therapy. Adv. Heal. Healthc. Mater. 2023, 12, e2300939. [Google Scholar] [CrossRef]
- Rastmanesh, A.; Tavakkoli Yaraki, M.; Wu, J.; Wang, Z.; Ghoderao, P.; Gao, Y.; Tan, Y.N. Bioinspired micro/nanomotors towards a self-propelled noninvasive diagnosis and treatment of cancer. Mol. Syst. Des. Eng. 2021, 6, 566–593. [Google Scholar] [CrossRef]
- Liu, C.; Niu, J.; Cui, T.; Ren, J.; Qu, X. A Motor-Based Carbonaceous Nanocalabash Catalyst for Deep-Layered Bioorthogonal Chemistry. J. Am. Chem. Soc. 2022, 144, 19611–19618. [Google Scholar] [CrossRef]
- Aatkar, A.; Vuorinen, A.; Longfield, O.E.; Gilbert, K.; Peltier-Heap, R.; Wagner, C.D.; Zappacosta, F.; Rittinger, K.; Chung, C.-w.; House, D.; et al. Efficient Ligand Discovery Using Sulfur(VI) Fluoride Reactive Fragments. ACS Chem. Biol. 2023, 18, 1926–1937. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Dong, J. Applications of SuFEx Click Chemistry in Polymer Science. In Click Chemistry in Polymer Science: Designs to Applications; Singha, N.K., Mondal, P., Hoogenboom, R., Eds.; Royal Society of Chemistry: Cambridge, UK, 2024; pp. 156–176. [Google Scholar]
- Li, J.; Esteban-Fernández de Ávila, B.; Gao, W.; Zhang, L.; Wang, J. Micro/Nanorobots for Biomedicine: Delivery, Surgery, Sensing, and Detoxification. Sci. Robot. 2017, 2, eaam6431. [Google Scholar] [CrossRef]
- Vutti, S.; Schoffelen, S.; Bolinsson, J.; Buch-Månson, N.; Bovet, N.; Nygård, J.; Martinez, K.L.; Meldal, M. Click Chemistry Mediated Functionalization of Vertical Nanowires for Biological Applications. Chem. Eur. J. 2016, 22, 496–500. [Google Scholar] [CrossRef]
- Lee, Y.-W.; Ceylan, H.; Yasa, I.C.; Kilic, U.; Sitti, M. 3D-Printed Multi-Stimuli-Responsive Mobile Micromachines. ACS Appl. Mater. Interfaces 2021, 13, 12759–12766. [Google Scholar] [CrossRef]
- Kumar, R.; Yang, B.; Barton, J.; Stejfova, M.; Schäfer, A.; Koenig, M.; Knittel, P.; Cigler, P.; Hirtz, M. Diamond Surfaces with Clickable Antifouling Polymer Coating for Microarray-Based Biosensing. Adv. Mater. Interfaces 2022, 9, 2201453. [Google Scholar] [CrossRef]
- Chen, Y.; Xianyu, Y.; Wu, J.; Yin, B.; Jiang, X. Click Chemistry-Mediated Nanosensors for Biochemical Assays. Theranostics 2016, 6, 969–985. [Google Scholar] [CrossRef] [PubMed]
- Hvilsted, S. Facile design of biomaterials by ‘click’ chemistry. Polym. Int. 2012, 61, 485–494. [Google Scholar] [CrossRef]
- Zhao, C.; Li, L.-Y.; Guo, M.-M.; Zheng, J. Functional polymer thin films designed for antifouling materials and biosensors. Chem. Pap. 2012, 66, 323–339. [Google Scholar] [CrossRef]
- Vilela, D.; Parmar, J.; Zeng, Y.; Zhao, Y.; Sánchez, S. Graphene-Based Microbots for Toxic Heavy Metal Removal and Recovery from Water. Nano Lett. 2016, 16, 2860–2866. [Google Scholar] [CrossRef]
- He, T.; Liu, S.; Yang, Y.; Chen, X. Application of Micro/Nanomotors in Environmental Remediation: A Review. Micromachines 2024, 15, 1443. [Google Scholar] [CrossRef]
- Li, Z. Nature-Inspired Biohybrid Microrobots: Advancing In Vivo Biomedical Innovations Toward Clinical Translation. Ph.D. Thesis, University of California, San Diego, CA, USA, 2025. [Google Scholar]
- Li, Z.; Zou, J.; Chen, X. In Response to Precision Medicine: Current Subcellular Targeting Strategies for Cancer Therapy. Adv. Mater. 2023, 35, e2209529. [Google Scholar] [CrossRef]
- Shaukat, U.; Rossegger, E.; Schlogl, S. A Review of Multi-Material 3D Printing of Functional Materials via Vat Photopolymerization. Polymers 2022, 14, 2449. [Google Scholar] [CrossRef]
- Wang, S.; Xu, J.; Zhou, Q.; Geng, P.; Wang, B.; Zhou, Y.; Liu, K.; Peng, F.; Tu, Y. Biodegradability of Micro/Nanomotors: Challenges and Opportunities. Adv. Healthc. Mater. 2021, 10, 2100335. [Google Scholar] [CrossRef]
- Hortelão, A.C.; Patiño, T.; Perez-Jiménez, A.; Blanco, À.; Sánchez, S. Enzyme-Powered Nanobots Enhance Anticancer Drug Delivery. Adv. Funct. Mater. 2018, 28, 1705086. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, D.; Liang, S.; Dai, Y.; Bai, X.; Song, B.; Zhang, D.; Chen, H.; Feng, L. Recent Advances in Field-Controlled Micro–Nano Manipulations and Micro–Nano Robots. Adv. Intell. Syst. 2022, 4, 2100116. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, L.; Zhong, W.; Yan, Q.; Gao, Y.; Hong, W.; She, Y.; Yang, G. Recent Advances in Motion Control of Micro/Nanomotors. Adv. Intell. Syst. 2020, 2, 2000049. [Google Scholar] [CrossRef]
- Dolev, A.; Kaynak, M.; Sakar, M.S. On-Board Mechanical Control Systems for Untethered Microrobots. Adv. Intell. Syst. 2021, 3, 2000233. [Google Scholar] [CrossRef]
- Fan, Y. Low-cost microfluidics: Materials and methods. Micro Nano Lett. 2018, 13, 1367–1372. [Google Scholar] [CrossRef]
- Tsao, C.-W. Polymer Microfluidics: Simple, Low-Cost Fabrication Process Bridging Academic Lab Research to Commercialized Production. Micromachines 2016, 7, 225. [Google Scholar] [CrossRef]
- Lu, J.M.; Wang, H.F.; Guo, Q.H.; Wang, J.W.; Li, T.T.; Chen, K.X.; Zhang, M.T.; Chen, J.B.; Shi, Q.N.; Huang, Y.; et al. Roboticized AI-assisted microfluidic photocatalytic synthesis and screening up to 10,000 reactions per day. Nat. Commun. 2024, 15, 8826. [Google Scholar] [CrossRef] [PubMed]
- Luttens, A.; Cabeza de Vaca, I.; Sparring, L.; Brea, J.; Martínez, A.L.; Kahlous, N.A.; Radchenko, D.S.; Moroz, Y.S.; Loza, M.I.; Norinder, U.; et al. Rapid traversal of vast chemical space using machine learning-guided docking screens. Nat. Comput. Sci. 2025, 5, 301–312. [Google Scholar] [CrossRef]
- Ouassaf, M.; Mazri, R.; Khan, S.U.; Rengasamy, K.R.R.; Alhatlani, B.Y. Machine Learning-Guided Screening and Molecular Docking for Proposing Naturally Derived Drug Candidates Against MERS-CoV 3CL Protease. Int. J. Mol. Sci. 2025, 26, 3047. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, J.; Ji, F.; Li, Y.; Yung, K.-L.; Ferreira, A.; Zhang, L. Machine learning for micro- and nanorobots. Nat. Mach. Intell. 2024, 6, 605–618. [Google Scholar] [CrossRef]
- Josephson, J.D.; Pezacki, J.P.; Nakajima, M. Machine Learning Enabling the Prediction of Activation Energies of SPAAC. J. Phys. Org. Chem. 2025, 38, e4679. [Google Scholar] [CrossRef]
- Wang, M.; Li, S.; Wang, J.; Zhang, O.; Du, H.; Jiang, D.; Wu, Z.; Deng, Y.; Kang, Y.; Pan, P.; et al. ClickGen: Directed exploration of synthesizable chemical space via modular reactions and reinforcement learning. Nat. Commun. 2024, 15, 10127. [Google Scholar] [CrossRef] [PubMed]
- Stuyver, T.; Coley, C.W. Machine Learning-Guided Computational Screening of New Candidate Reactions with High Bioorthogonal Click Potential. Chemistry 2023, 29, e202300387. [Google Scholar] [CrossRef]
- Wang, X.; Cai, J.; Sun, L.; Zhang, S.; Gong, D.; Li, X.; Yue, S.; Feng, L.; Zhang, D. Facile Fabrication of Magnetic Microrobots Based on Spirulina Templates for Targeted Delivery and Synergistic Chemo-Photothermal Therapy. ACS Appl. Mater. Interfaces 2019, 11, 4745–4756. [Google Scholar] [CrossRef]
- Li, J.; Yu, J. Biodegradable Microrobots and Their Biomedical Applications: A Review. Nanomaterials 2023, 13, 1590. [Google Scholar] [CrossRef]
- Phelps, M.E. Positron emission tomography provides molecular imaging of biological processes. Proc. Natl. Acad. Sci. USA 2000, 97, 9226–9233. [Google Scholar] [CrossRef] [PubMed]
- Mc Veigh, M.; Bellan, L.M. Microfluidic synthesis of radiotracers: Recent developments and commercialization prospects. Lab Chip 2024, 24, 1226–1243. [Google Scholar] [CrossRef]
- Bauer, D.; Cornejo, M.A.; Hoang, T.T.; Lewis, J.S.; Zeglis, B.M. Click Chemistry and Radiochemistry: An Update. Bioconjug. Chem. 2023, 34, 1925–1950. [Google Scholar] [CrossRef]
- Fu, Y.; Helbert, H.; Simeth, N.A.; Crespi, S.; Spoelstra, G.B.; van Dijl, J.M.; van Oosten, M.; Nazario, L.R.; van der Born, D.; Luurtsema, G.; et al. Ultrafast Photoclick Reaction for Selective 18F-Positron Emission Tomography Tracer Synthesis in Flow. J. Am. Chem. Soc. 2021, 143, 10041–10047. [Google Scholar] [CrossRef]
- Zhang, F.; Zhuang, J.; Li, Z.; Gong, H.; de Ávila, B.E.; Duan, Y.; Zhang, Q.; Zhou, J.; Yin, L.; Karshalev, E.; et al. Nanoparticle-modified microrobots for in vivo antibiotic delivery to treat acute bacterial pneumonia. Nat. Mater. 2022, 21, 1324–1332. [Google Scholar] [CrossRef]
- Li, J.; Dekanovsky, L.; Khezri, B.; Wu, B.; Zhou, H.; Sofer, Z. Biohybrid Micro- and Nanorobots for Intelligent Drug Delivery. Cyborg Bionic Syst. 2022, 2022, 9824057. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Guo, Z.; Li, Z.; Luan, H.; Yu, Y.; Zhu, A.T.; Ding, S.; Gao, W.; Fang, R.H.; Zhang, L.; et al. Biohybrid microrobots locally and actively deliver drug-loaded nanoparticles to inhibit the progression of lung metastasis. Sci. Adv. 2024, 10, eadn6157. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Zhai, Y.; Fan, W.; Ren, W.; Li, Z.; Liu, C. Click Chemistry-Actuated Digital DNA Walker Confined on a Single Particle toward Absolute MicroRNA Quantification. Anal. Chem. 2021, 93, 1620–1626. [Google Scholar] [CrossRef] [PubMed]
- Parvin, N.; Joo, S.W.; Jung, J.H.; Mandal, T.K. Innovative Micro- and Nano-Architectures in Biomedical Engineering for Therapeutic and Diagnostic Applications. Micromachines 2025, 16, 419. [Google Scholar] [CrossRef]
- Tsai, H.-F.; Podder, S.; Chen, P.-Y. Microsystem Advances through Integration with Artificial Intelligence. Micromachines 2023, 14, 826. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Ren, Z.; Coluccini, C.; Coghi, P. From Micro to Marvel: Unleashing the Full Potential of Click Chemistry with Micromachine Integration. Micromachines 2025, 16, 712. https://doi.org/10.3390/mi16060712
Chen Z, Ren Z, Coluccini C, Coghi P. From Micro to Marvel: Unleashing the Full Potential of Click Chemistry with Micromachine Integration. Micromachines. 2025; 16(6):712. https://doi.org/10.3390/mi16060712
Chicago/Turabian StyleChen, Zihan, Zimo Ren, Carmine Coluccini, and Paolo Coghi. 2025. "From Micro to Marvel: Unleashing the Full Potential of Click Chemistry with Micromachine Integration" Micromachines 16, no. 6: 712. https://doi.org/10.3390/mi16060712
APA StyleChen, Z., Ren, Z., Coluccini, C., & Coghi, P. (2025). From Micro to Marvel: Unleashing the Full Potential of Click Chemistry with Micromachine Integration. Micromachines, 16(6), 712. https://doi.org/10.3390/mi16060712







