Roll-to-Roll (R2R) High-Throughput Manufacturing of Foil-Based Microfluidic Chips for Neurite Outgrowth Studies
Abstract
1. Introduction
2. Materials and Methods
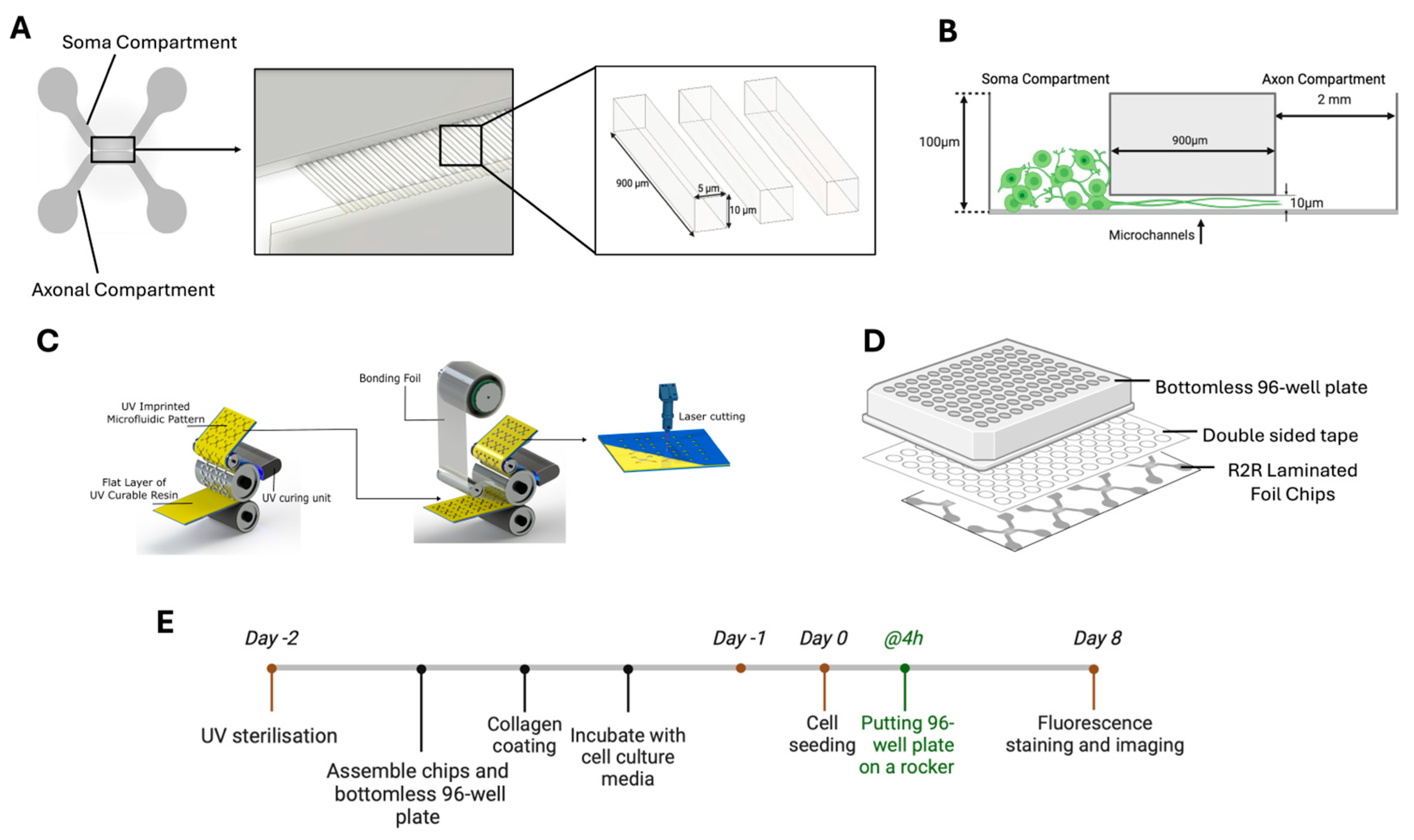
2.1. Microfluidic Chip Fabrication: Roll-to-Roll (R2R) Imprinting and Lamination
2.1.1. Microfluidic Design and Simulation
2.1.2. Roll-to-Roll (R2R) Imprinting
2.1.3. Roll-to-Roll (R2R) Lamination
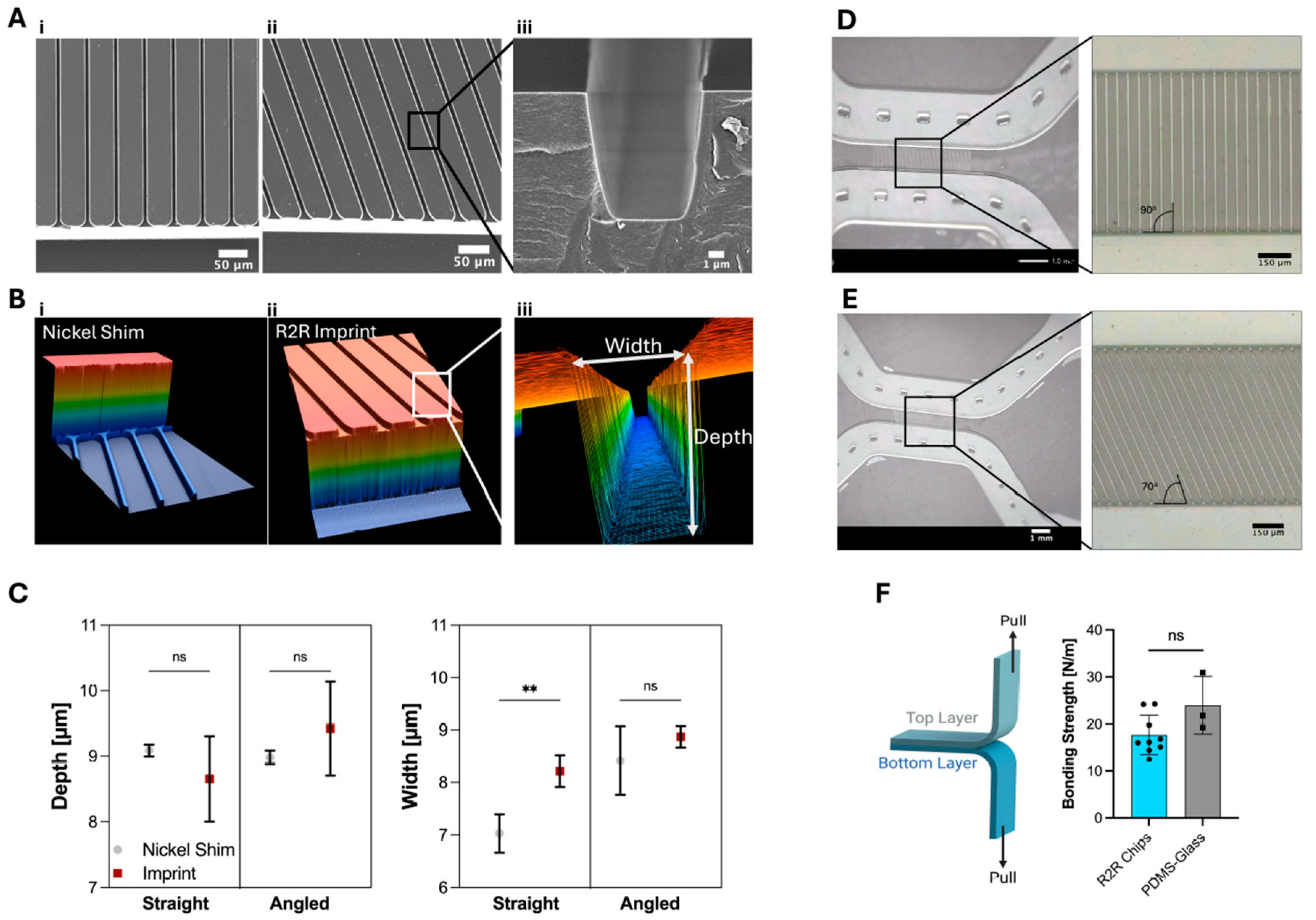
2.1.4. Material Characterization via SEM, Confocal Laser Microscopy, and Tensile Testing
2.2. Cell Culture and Maintenance
2.3. Cell Culture on Neuron-Foil Chips
2.4. Fluorescence Microscopy Imaging on Neuron-Foil Chips
2.5. Quantification and Statistical Analysis
3. Results
3.1. Manufacturing of Neuron-Foil Chips
3.1.1. Characterization of the Roll-to-Roll (R2R) Imprints
3.1.2. Characterization of Roll-to-Roll (R2R) Lamination and Bonding of the Neuron-Foil Chips
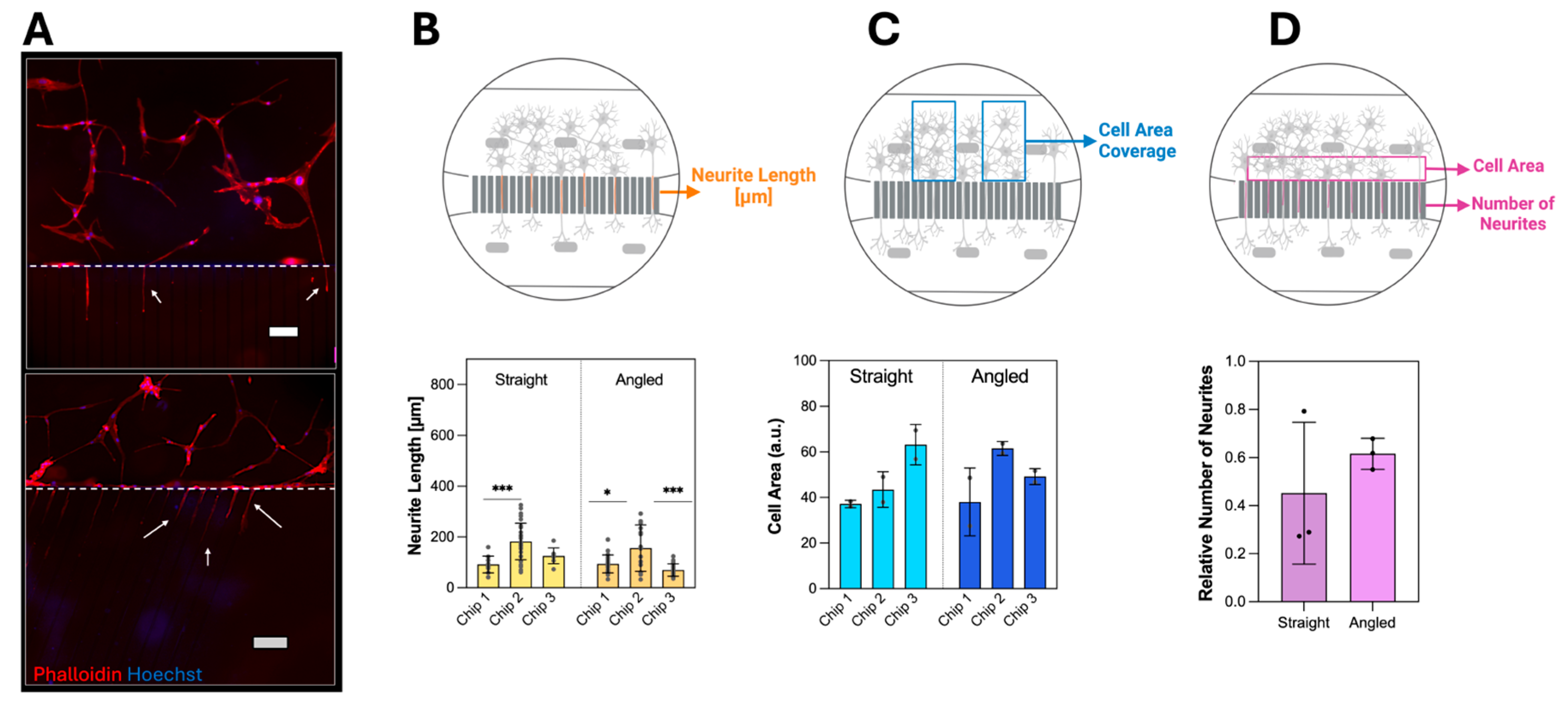
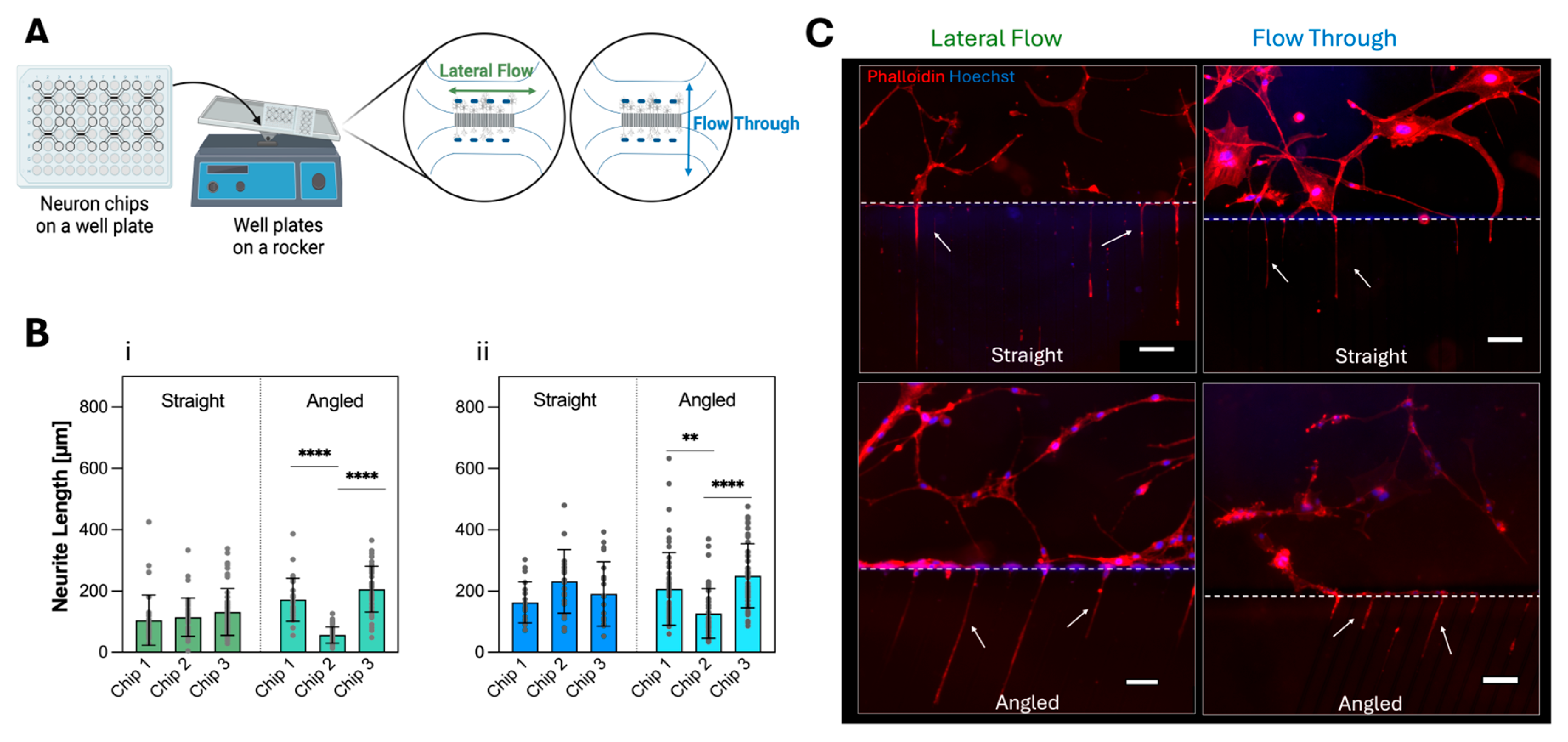
3.2. Neuron Cell Culture in Neuron-Foil Chips
3.2.1. Neurite Outgrowth in Static Culture
3.2.2. Neurite Outgrowth in Dynamic Culture
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). 2024. Available online: www.who.int (accessed on 9 December 2024).
- GBD 2021 Nervous System Disorders Collaborators. Global, regional, and national burden of disorders affecting the nervous system, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024, 23, 344–381. [Google Scholar] [CrossRef] [PubMed]
- Ristola, M.; Fedele, C.; Hagman, S.; Sukki, L.; Kapucu, F.E.; Mzezewa, R.; Hyvärinen, T.; Kallio, P.; Priimagi, A.; Narkilahti, S. Directional Growth of Human Neuronal Axons in a Microfluidic Device with nanotopography on Azobenzene-Based Material. Adv. Mater. Interfaces 2021, 8, 2100048. [Google Scholar] [CrossRef]
- Babaliari, E.; Ranella, A.; Stratakis, E. Microfluidic Systems for Neural Cell Studies. Bioengineering 2023, 10, 902. [Google Scholar] [CrossRef] [PubMed]
- Babaliari, E.; Kavatzikidou, P.; Mitraki, A.; Papaharilaou, Y.; Ranella, A.; Stratakis, E. Combined effect of shear stress and laser-patterned topography on Schwann cell outgrowth: Synergistic or antagonistic? Biomater. Sci. 2021, 9, 1334–1344. [Google Scholar] [CrossRef]
- De Vitis, E.; La Pesa, V.; Gervaso, F.; Romano, A.; Quattrini, A.; Gigli, G.; Moroni, L.; Polini, A. A microfabricated multi-compartment device for neuron and Schwann cell differentiation. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Taylor, A.M.; Menon, S.; Gupton, S.L. Passive microfluidic chamber for long-term imaging of axon guidance in response to soluble gradients. Lab Chip 2015, 15, 2781–2789. [Google Scholar] [CrossRef]
- Shaik, F.A.; Ihida, S.; Ikeuchi, Y.; Tixier-Mita, A.; Toshiyoshi, H. TFT sensor array for real-time cellular characterization, stimulation, impedance measurement and optical imaging of in-vitro neural cells. Biosens. Bioelectron. 2020, 169, 112546. [Google Scholar] [CrossRef]
- Lee, H.U.; Blasiak, A.; Agrawal, D.R.; Loong, D.T.B.; Thakor, N.V.; All, A.H.; Ho, J.S.; Yang, I.H. Subcellular electrical stimulation of neurons enhances the myelination of axons by oligodendrocytes. PLoS ONE 2017, 12, e0179642. [Google Scholar] [CrossRef]
- Kim, S.; Park, J.; Han, A.; Li, J. Microfluidic systems for axonal growth and regeneration research. Neural Regen. Res. 2014, 9, 1703–1705. [Google Scholar] [CrossRef]
- Bonneau, N.; Potey, A.; Blond, F.; Guerin, C.; Baudouin, C.; Peyrin, J.-M.; Brignole-Baudouin, F.; Réaux-Le Goazigo, A. Assessment of corneal nerve regeneration after axotomy in a compartmentalized microfluidic chip model with automated 3D high resolution live-imaging. Front. Cell. Neurosci. 2024, 18, 1417653. [Google Scholar] [CrossRef]
- Jocher, G.; Mannschatz, S.H.; Offterdinger, M.; Schweigreiter, R. Microfluidics of small-population neurons allows for a precise quantification of the peripheral axonal growth state. Front. Cell. Neurosci. 2018, 12, 166. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Blurton-Jones, M.; Rhee, S.W.; Cribbs, D.H.; Cotman, C.W.; Jeon, N.L. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat. Methods 2005, 2, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Dravid, A.; Raos, B.; Svirskis, D.; O’Carroll, S.J. Optimised techniques for high-throughput screening of differentiated SH-SY5Y cells and application for neurite outgrowth assays. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef]
- Chung, B.G.; Flanagan, L.A.; Schwartz, P.H.; Rhee, S.W.; Jeon, N.L.; Lee, A.P.; Monuki, E.S. Human neural stem cell growth and differentiation in a gradient-generating microfluidic device. Lab Chip 2005, 5, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Lotlikar, M.S.; Tarantino, M.B.; Jorfi, M.; Kovacs, D.M.; Tanzi, R.E.; Bhattacharyya, R. Microfluidic separation of axonal and somal compartments of neural progenitor cells differentiated in a 3D matrix. STAR Protoc. 2022, 3, 101028. [Google Scholar] [CrossRef]
- Ristola, M.; Sukki, L.; Azevedo, M.M.; Seixas, A.I.; Relvas, J.B.; Narkilahti, S.; Kallio, P. A compartmentalized neuron-oligodendrocyte co-culture device for myelin research: Design, fabrication and functionality testing. J. Micromech. Microeng. 2019, 29, 065009. [Google Scholar] [CrossRef]
- Vulić, K.; Amos, G.; Ruff, T.; Kasm, R.; Ihle, S.J.; Küchler, J.; Vörös, J.; Weaver, S. Impact of microchannel width on axons for brain-on-chip applications. Lab Chip 2024, 24, 5155–5166. [Google Scholar] [CrossRef]
- Lee, C.; Robinson, M.; Willerth, S.M. Direct Reprogramming of Glioblastoma Cells into Neurons Using Small Molecules. ACS Chem. Neurosci. 2018, 9, 3175–3185. [Google Scholar] [CrossRef]
- Liu, H.; Xia, J.; Wang, T.; Li, W.; Song, Y.; Tan, G. Differentiation of human glioblastoma U87 cells into cholinergic neuron. Neurosci. Lett. 2019, 704, 1–7. [Google Scholar] [CrossRef]
- Campenot, R.B. Local control of neurite development by nerve growth factor (chemotaxis/culture methods/retrograde transport/sympathetic ganglia). Proc. Natl. Acad. Sci. USA 1977, 74, 4516–4519. [Google Scholar] [CrossRef]
- Morbioli, G.G.; Speller, N.C.; Stockton, A.M. A practical guide to rapid-prototyping of PDMS-based microfluidic devices: A tutorial. Anal. Chim. Acta 2020, 1135, 150–174. [Google Scholar] [CrossRef] [PubMed]
- Borók, A.; Laboda, K.; Bonyár, A. PDMS bonding technologies for microfluidic applications: A review. Biosensors 2021, 11, 292. [Google Scholar] [CrossRef] [PubMed]
- Miranda, I.; Castanheira, E.M.S.; Lima, R.; Minas, G.; Souza, A.; Ribeiro, J.; Sousa, P. Properties and applications of PDMS for biomedical engineering: A review. J. Funct. Biomater. 2021, 13, 2. [Google Scholar] [CrossRef]
- Raj M, K.; Chakraborty, S. PDMS microfluidics: A mini review. J. Appl. Polym. Sci. 2020, 137, 48958. [Google Scholar] [CrossRef]
- Moutaux, E.; Charlot, B.; Genoux, A.; Saudou, F.; Cazorla, M. An integrated microfluidic/microelectrode array for the study of activity-dependent intracellular dynamics in neuronal networks. Lab Chip 2018, 18, 3425–3435. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Sun, H.; Li, W.; Hu, C.; Ren, K. Recent progresses in microfabricating perfluorinated polymers (Teflons) and the associated new applications in microfluidics. Microphysiol. Syst. 2018, 1, 1. [Google Scholar] [CrossRef]
- Shakeri, A.; Khan, S.; Didar, T.F. Conventional and emerging strategies for the fabrication and functionalization of PDMS-based microfluidic devices. Lab Chip 2021, 21, 3053–3075. [Google Scholar] [CrossRef]
- Wisdom, K. Lung tumor microphysiological system with 3D endothelium to evaluate modulators of T-cell migration_suppl. ALTEX 2023, 40, 649–664. [Google Scholar] [CrossRef]
- Linares, G.R.; Li, Y.; Chang, W.-H.; Rubin-Sigler, J.; Mendonca, S.; Hong, S.; Eoh, Y.; Guo, W.; Huang, Y.-H.; Chang, J.; et al. SYF2 suppression mitigates neurodegeneration in models of diverse forms of ALS. Cell Stem Cell 2023, 30, 171–187.e14. [Google Scholar] [CrossRef]
- Lohse, M.; Thesen, M.W.; Haase, A.; Smolka, M.; Iceta, N.B.; Ayerdi Izquierdo, A.; Ramos, I.; Salado, C.; Schleunitz, A. Novel concept of micro patterned micro titer plates fabricated via uv-nil for automated neuronal cell assay read-out. Nanomaterials 2021, 11, 902. [Google Scholar] [CrossRef]
- Metwally, K.; Robert, L.; Queste, S.; Bernard, G.M.; Chantal, K.M. Roll manufacturing of flexible microfluidic devices in thin PMMA and COC foils by embossing and lamination. Microsyst. Technol. 2012, 18, 199–207. [Google Scholar] [CrossRef]
- Azizgolshani, H.; Coppeta, J.R.; Vedula, E.M.; Marr, E.E.; Cain, B.P.; Luu, R.J.; Lech, M.P.; Kann, S.H.; Mulhern, T.J.; Tandon, V.; et al. High-throughput organ-on-chip platform with integrated programmable fluid flow and real-time sensing for complex tissue models in drug development workflows. Lab Chip 2021, 21, 1454–1474. [Google Scholar] [CrossRef]
- Liu, M.; Wu, A.; Liu, J.; Huang, H.-W.; Shi, Q.; Wang, H. Arched Microfluidic Channel for the Promotion of Axonal Growth Performance. iScience 2024, 27, 110885. [Google Scholar] [CrossRef]
- Toren, P.; Smolka, M.; Haase, A.; Palfinger, U.; Nees, D.; Ruttloff, S.; Kuna, L.; Schaude, C.; Jauk, S.; Rumpler, M.; et al. High-throughput roll-to-roll production of polymer biochips for multiplexed DNA detection in point-of-care diagnostics. Lab Chip 2020, 20, 4106–4117. [Google Scholar] [CrossRef]
- Hoang, T.; Truong, H.; Han, J.; Lee, S.; Lee, J.; Parajuli, S.; Lee, J.; Cho, G. Room temperature roll-to-roll additive manufacturing of polydimethylsiloxane-based centrifugal microfluidic device for on-site isolation of ribonucleic acid from whole blood. Mater. Today Bio 2023, 23, 100838. [Google Scholar] [CrossRef]
- Smolka, M.; Ruttloff, S.; Nees, D.; Prietl, C.; Satzinger, V.; Lamprecht, B.; Hütter, P.; Hesse, J.; Kokkinis, G.; Kriechhammer, G.; et al. High Throughput Roll-to-Roll Production of Microfluidic Chips. Proceedings 2018, 2, 1054. [Google Scholar] [CrossRef]
- Liedert, C.; Rannaste, L.; Kokkonen, A.; Huttunen, O.-H.; Liedert, R.; Hiltunen, J.; Hakalahti, L. Roll-to-Roll Manufacturing of Integrated Immunodetection Sensors. ACS Sens. 2020, 5, 2010–2017. [Google Scholar] [CrossRef]
- Rannaste, L.; Huttunen, O.H.; Hiitola-Keinanen, J.; Hiltunen, J.; Liedert, C.; Hakalahti, L. Roll-to-Roll Fabricated Self-Filling Polydimethylsiloxane Diagnostic Platforms for Multiplexed Pathogen Nucleic Acid Detection. In Proceedings of the BioCAS 2022—IEEE Biomedical Circuits and Systems Conference: Intelligent Biomedical Systems for a Better Future, Proceedings, Taipei, Taiwan, 13–15 October 2022; pp. 115–119. [Google Scholar] [CrossRef]
- Kim, K.; Kim, J.; Kim, B.; Ko, S. Fabrication of Microfluidic Structure Based Biosensor Using Roll-to-Roll Gravure Printing. Int. J. Precis. Eng. Manuf.-Green Technol. 2018, 5, 369–374. [Google Scholar] [CrossRef]
- Maize, K.; Mi, Y.; Cakmak, M.; Shakouri, A. Real-Time Metrology for Roll-To-Roll and Advanced Inline Manufacturing: A Review. Adv. Mater. Technol. 2023, 8, 2200173. [Google Scholar] [CrossRef]
- Deshmukh, S.S.; Goswami, A. Current innovations in roller embossing—A comprehensive review. Microsyst. Technol. 2022, 28, 1077–1114. [Google Scholar] [CrossRef]
- Taylor, A.M.; Dieterich, D.C.; Ito, H.T.; Kim, S.A.; Schuman, E.M. Microfluidic Local Perfusion Chambers for the Visualization and Manipulation of Synapses. Neuron 2010, 66, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Götz, J.; Rueda, A.A.; Ruttloff, S.; Kuna, L.; Belegratis, M.; Palfinger, U.; Nees, D.; Hartmann, P.; Stadlober, B. Finite Element Simulations of Filling and Demolding in Roll-To-Roll UV Nanoimprinting of Micro-and Nanopatterns. ACS Appl. Nano Mater. 2022, 5, 3434–3449. [Google Scholar] [CrossRef]
- Leitgeb, M.; Nees, D.; Ruttloff, S.; Palfinger, U.; Götz, J.; Liska, R.; Belegratis, M.R.; Stadlober, B. Multilength Scale Patterning of Functional Layers by Roll-to-Roll Ultraviolet-Light-Assisted Nanoimprint Lithography. ACS Nano 2016, 10, 4926–4941. [Google Scholar] [CrossRef]
- Oláh, M.; Farkas, E.; Székács, I.; Horvath, R.; Székács, A. Cytotoxic effects of Roundup Classic and its components on NE-4C and MC3T3-E1 cell lines determined by biochemical and flow cytometric assays. Toxicol. Rep. 2022, 9, 914–926. [Google Scholar] [CrossRef]
- Meijering, E. Neuron tracing in perspective. Cytom. Part A 2010, 77, 693–704. [Google Scholar] [CrossRef]
- Meijering, E.; Jacob, M.; Sarria, J.-C.F.; Steiner, P.; Hirling, H.; Unser, M. Design and Validation of a Tool for Neurite Tracing and Analysis in Fluorescence Microscopy Images. Cytom. Part A J. Int. Soc. Anal. Cytol. 2004, 58, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Spijkers, X.M.; Pasteuning-Vuhman, S.; Dorleijn, J.C.; Vulto, P.; Wevers, N.R.; Pasterkamp, R.J. A directional 3D neurite outgrowth model for studying motor axon biology and disease. Sci. Rep. 2021, 11, 2080. [Google Scholar] [CrossRef]
- Moeck, A.; Bianchi, S.R.; Helsby, D. Shrinkage of UV Oligomers and Monomers. Available online: https://www.radtech.org/2014proceedings/papers/technical-conference/Formulation/Moeck%20-%20Shrinkage%20of%20UV%20Oligomers%20and%20Monomers.pdf (accessed on 10 February 2025).
- Park, J.W.; Shim, G.S.; Back, J.H.; Kim, H.J.; Shin, S.; Hwang, T.S. Characteristic shrinkage evaluation of photocurable materials. Polym. Test. 2016, 56, 344–353. [Google Scholar] [CrossRef]
- Heathcote, R.D.; Sargent, P.B. Growth and morphogenesis of an autonomic ganglion. I. Matching neurons with target. J. Neurosci. 1987, 7, 2493–2501. [Google Scholar] [PubMed] [PubMed Central]
- UNE-EN-ISO 10993-5; Biological Evaluation of Medical Devices Part 5: Tests for In Vitro Cytotoxicity. Edition 3, DIN: Geneva, Switzerland, 2009.
- Kim, T.H.; Lee, J.M.; Ahrberg, C.D.; Chung, B.G. Development of the Microfluidic Device to Regulate Shear Stress Gradients. Biochip J. 2018, 12, 294–303. [Google Scholar] [CrossRef]
- Li, L.; Ren, L.; Liu, W.; Wang, J.-C.; Wang, Y.; Tu, Q.; Xu, J.; Liu, R.; Zhang, Y.; Yuan, M.-S.; et al. Spatiotemporally controlled and multifactor involved assay of neuronal compartment regeneration after chemical injury in an integrated microfluidics. Anal. Chem. 2012, 84, 6444–6453. [Google Scholar] [CrossRef] [PubMed]
- Wevers, N.R.; Nair, A.L.; Fowke, T.M.; Pontier, M.; Kasi, D.G.; Spijkers, X.M.; Hallard, C.; Rabussier, G.; van Vught, R.; Vulto, P.; et al. Modeling ischemic stroke in a triculture neurovascular unit on-a-chip. Fluids Barriers CNS 2021, 18, 59. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.Q.; Wang, P.; Yan, J.W.; Ba, L.N.; Shi, P.L.; Wu, H.M.; Guan, X.Y.; Cao, Y.G.; Sun, H.L.; Mao, X.Y. Shear Stress Rescued the Neuronal Impairment Induced by Global Cerebral Ischemia Reperfusion via Activating PECAM-1-eNOS-NO Pathway. Front. Cell Dev. Biol. 2021, 8, 631286. [Google Scholar] [CrossRef] [PubMed]
- Gladkov, A.; Pigareva, Y.; Kutyina, D.; Kolpakov, V.; Bukatin, A.; Mukhina, I.; Kazantsev, V.; Pimashkin, A. Design of Cultured Neuron Networks in vitro with Predefined Connectivity Using Asymmetric Microfluidic Channels. Sci. Rep. 2017, 7, 15625. [Google Scholar] [CrossRef]
- Takada, N.; Hagiwara, S.; Abe, N.; Yamazaki, R.; Tsuneishi, K.; Yasuda, K. Open-End Control of Neurite Outgrowth Lengths with Steep Bending Confinement Microchannel Patterns for Miswiring-Free Neuronal Network Formation. Micromachines 2024, 15, 1374. [Google Scholar] [CrossRef]
- Francisco, H.; Yellen, B.B.; Halverson, D.S.; Friedman, G.; Gallo, G. Regulation of Axon Guidance and Extension by 3-Dimensional Constraints. Biomaterials 2007, 28, 3398–3407. [Google Scholar] [CrossRef]
- Girardi, G.; Zumpano, D.; Raybould, H.; Seker, E. Microfluidic compartmentalization of rat vagal afferent neurons to model gut-brain axis. Bioelectron. Med. 2024, 10, 3. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atak, N.; Smolka, M.; Haase, A.; Lorenz, A.; Schobesberger, S.; Ruttloff, S.; Wolf, C.; Ayerdi-Izquierdo, A.; Ertl, P.; Briz Iceta, N.; et al. Roll-to-Roll (R2R) High-Throughput Manufacturing of Foil-Based Microfluidic Chips for Neurite Outgrowth Studies. Micromachines 2025, 16, 713. https://doi.org/10.3390/mi16060713
Atak N, Smolka M, Haase A, Lorenz A, Schobesberger S, Ruttloff S, Wolf C, Ayerdi-Izquierdo A, Ertl P, Briz Iceta N, et al. Roll-to-Roll (R2R) High-Throughput Manufacturing of Foil-Based Microfluidic Chips for Neurite Outgrowth Studies. Micromachines. 2025; 16(6):713. https://doi.org/10.3390/mi16060713
Chicago/Turabian StyleAtak, Nihan, Martin Smolka, Anja Haase, Alexandra Lorenz, Silvia Schobesberger, Stephan Ruttloff, Christian Wolf, Ana Ayerdi-Izquierdo, Peter Ertl, Nerea Briz Iceta, and et al. 2025. "Roll-to-Roll (R2R) High-Throughput Manufacturing of Foil-Based Microfluidic Chips for Neurite Outgrowth Studies" Micromachines 16, no. 6: 713. https://doi.org/10.3390/mi16060713
APA StyleAtak, N., Smolka, M., Haase, A., Lorenz, A., Schobesberger, S., Ruttloff, S., Wolf, C., Ayerdi-Izquierdo, A., Ertl, P., Briz Iceta, N., Hesse, J., & Frauenlob, M. (2025). Roll-to-Roll (R2R) High-Throughput Manufacturing of Foil-Based Microfluidic Chips for Neurite Outgrowth Studies. Micromachines, 16(6), 713. https://doi.org/10.3390/mi16060713





