The Role of Pd-Pt Bimetallic Catalysts in Ethylene Detection by CMOS-MEMS Gas Sensor Dubbed GMOS
Abstract
1. Introduction and Objectives
- (1)
- Choosing optimal catalyst and operation regimes for the GMOS sensing of ethylene at low and high concentrations.
- (2)
- Comparative analysis of Pt, Pd, and Pd-Pt catalysts for the GMOS sensing of ethylene and ethanol.
2. Experimental
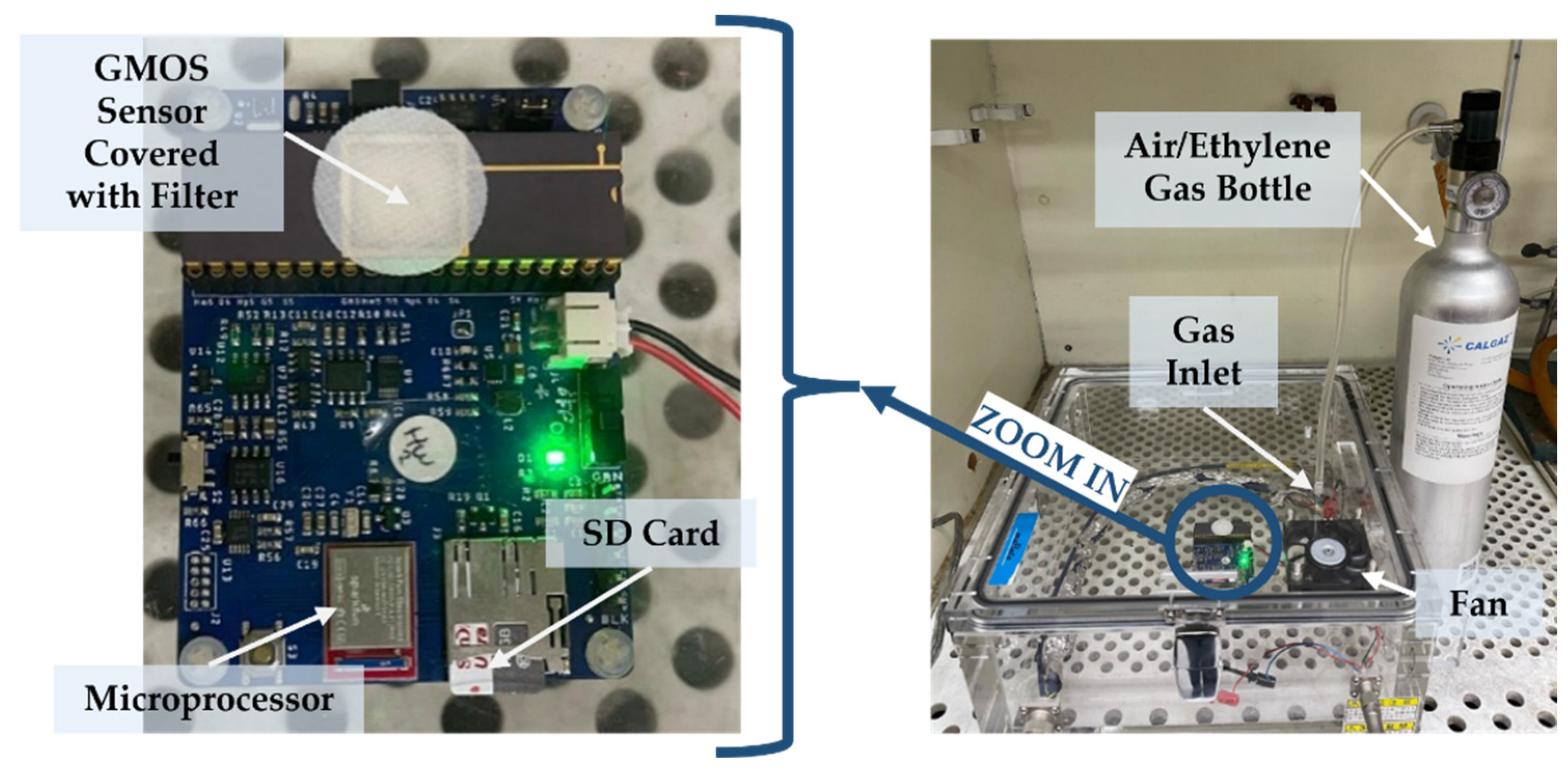
2.1. Measurement of GMOS Response
2.2. Preparation of Catalytic Layers
2.3. Gas Injection Procedure
3. Results and Discussion
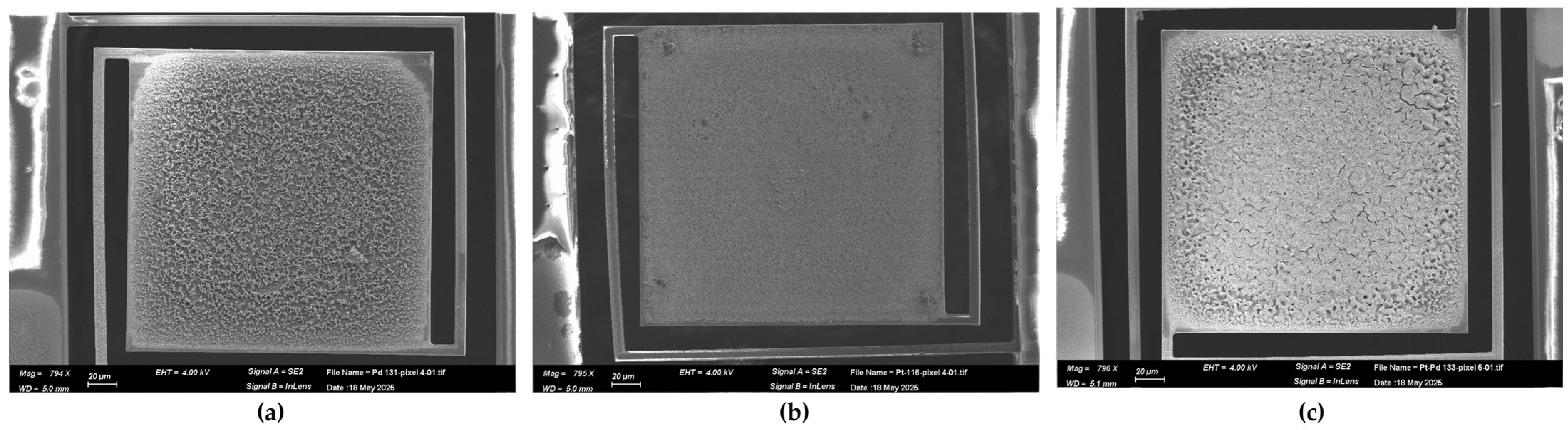
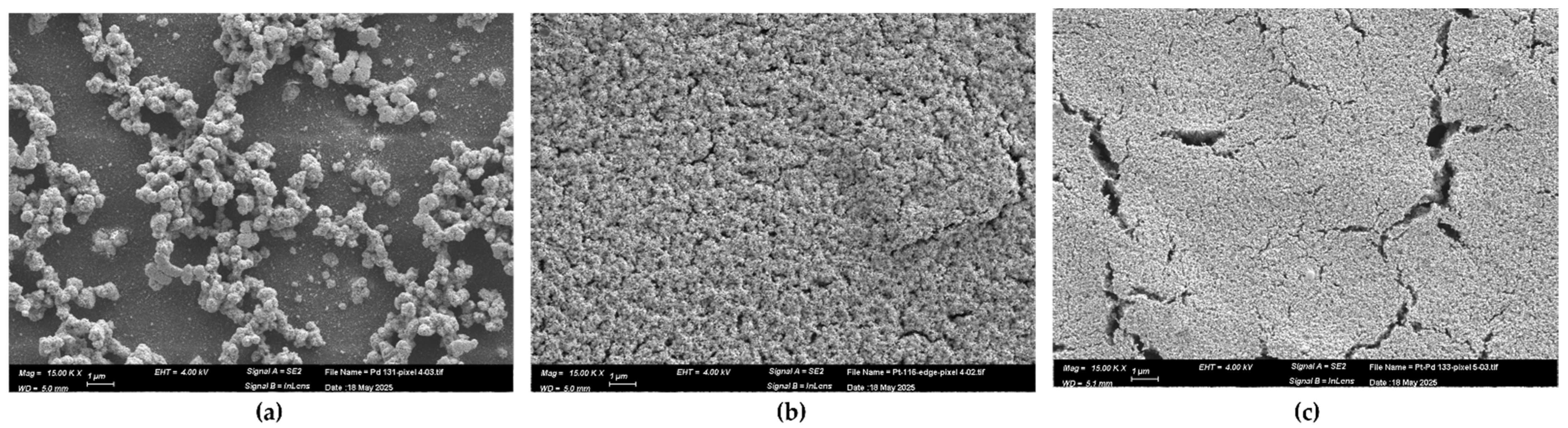
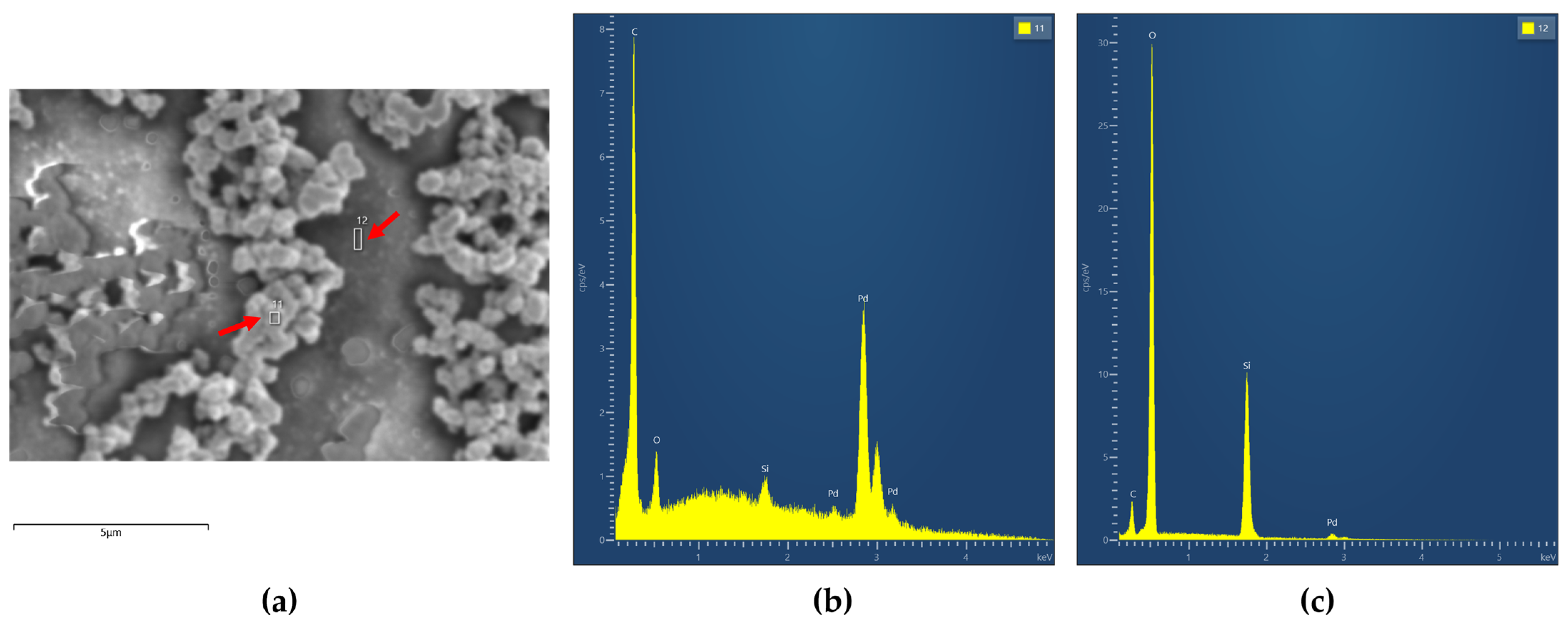
3.1. Morphology and Composition of Catalytic Layers
3.2. Sensing of Ethylene and Ethanol with Different Catalytic Layers
3.2.1. GMOS Response Measurements
3.2.2. Power Savings
3.2.3. Comparison of Sensitivity Measurements
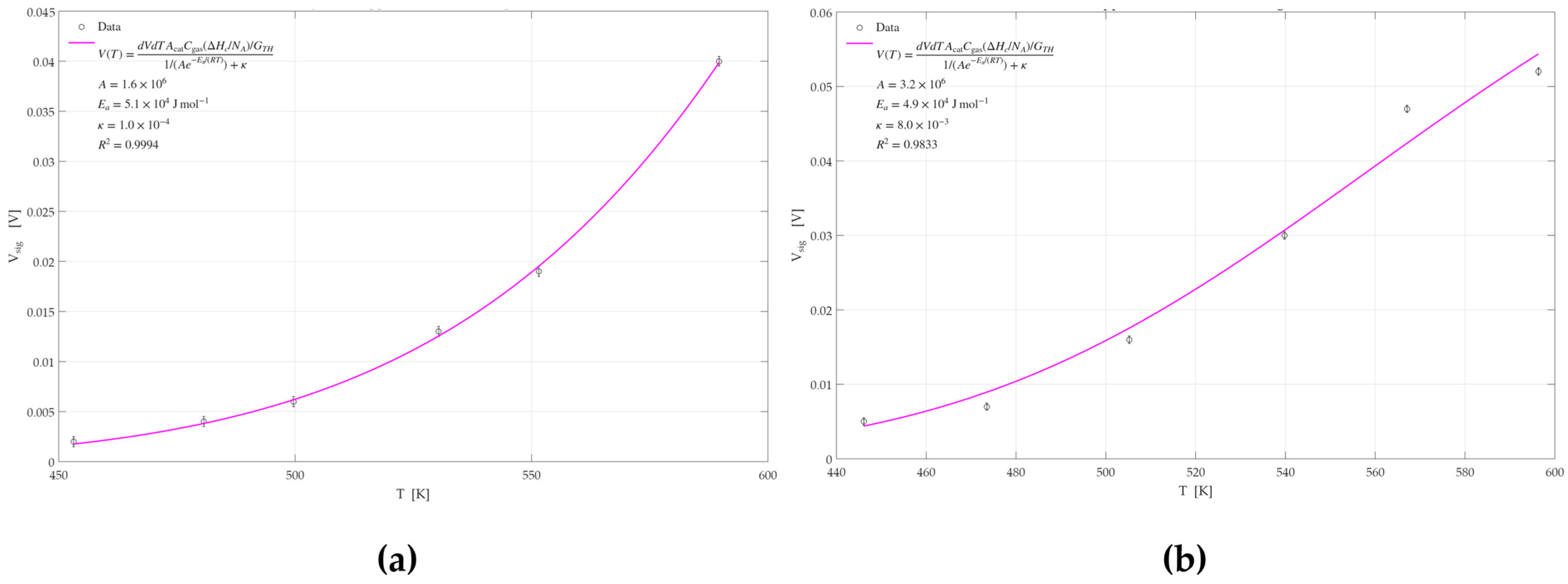
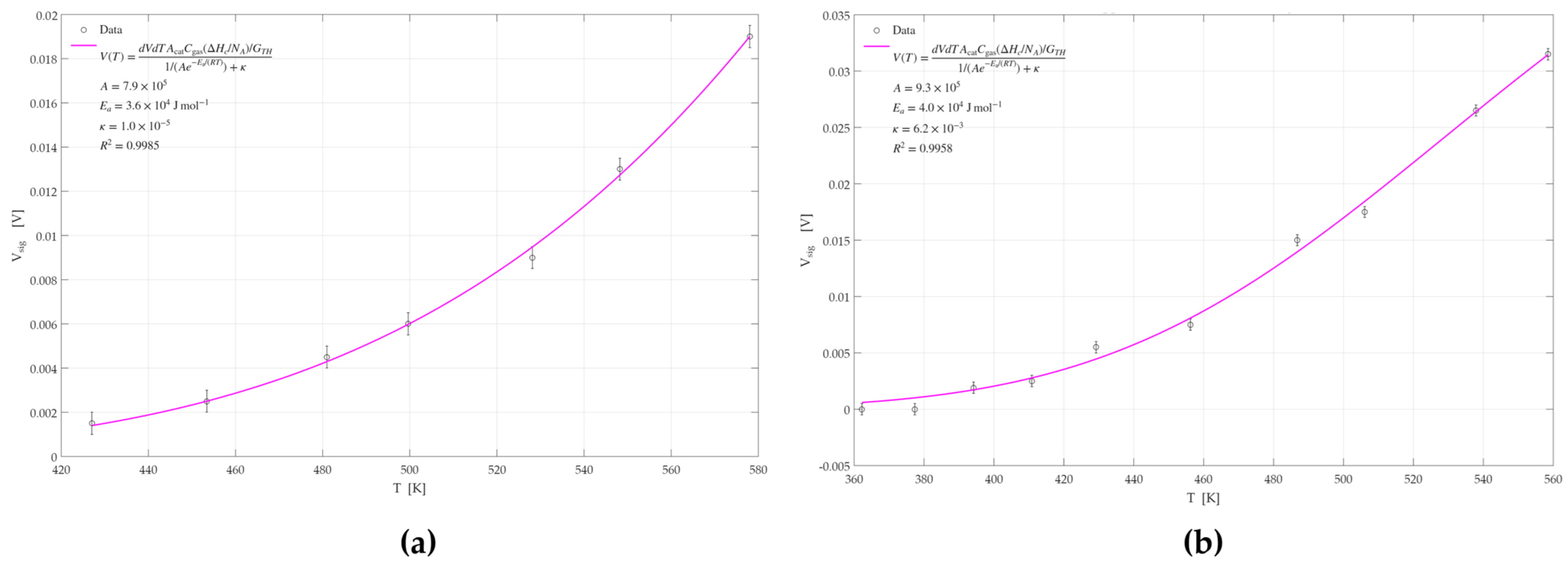
3.2.4. Activation Energy Analysis
3.2.5. Results Discussion
3.3. Sensing of High Ethylene Concentrations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zaidi, N.A.; Tahir, M.W.; Vellekoop, M.J.; Lang, W. A Gas Chromatographic System for the Detection of Ethylene Gas Using Ambient Air as a Carrier Gas. Sensors 2017, 17, 2283. [Google Scholar] [CrossRef] [PubMed]
- Nemirovsky, Y.; Stolyarova, S.; Blank, T.; Bar-Lev, S.; Zviagintsev, A.; Svetlitza, A.; Brouk, I. A new pellistor-like gas sensor based on micro machined CMOS transistor. IEEE Trans. Electron. Devices 2018, 99, 5494–5498. [Google Scholar] [CrossRef]
- Shlenkevich, D.; Stolyarova, S.; Blank, T.; Brouk, I.; Nemirovsky, Y. A novel miniature and selective combustion on type CMOS gas sensor for gas mixture analysis—Part 1: Emphasis on chemical aspects. Micromachines 2020, 11, 345. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.; Stolyarova, S.; Blank, T.; Bar-Lev, S.; Golan, G.; Nemirovsky, Y. A novel miniature and selective CMOS gas sensor for gas mixture analysis—Part 2: Emphasis on physical aspects. Micromachines 2020, 11, 587. [Google Scholar] [CrossRef] [PubMed]
- Krayden, A.; Shlenkevitch, D.; Blank, T.; Stolyarova, S.; Nemirovsky, Y. Selective Sensing of Mixtures of Gases with CMOS-SOI-MEMS Sensor Dubbed GMOS. Micromachines 2023, 14, 390. [Google Scholar] [CrossRef] [PubMed]
- Avraham, M.; Krayden, A.; Ashkar, H.; Aronin, D.; Stolyarova, S.; Blank, T.; Shlenkevitch, D.; Nemirovsky, Y. A novel miniature and selective CMOS gas sensor for gas mixture analysis—Part 4: The effect of humidity. Micromachines 2024, 15, 264. [Google Scholar] [CrossRef] [PubMed]
- Shlenkevitch, D.; Stolyarova, S.; Blank, T.; Brouk, I.; Levi, Y.; Nemirovsky, Y. Reducing food waste with a tiny CMOS-MEMS gas sensor, dubbed GMOS. In Proceedings of the 7th International Electronic Conference on Sensors and Applications (ECSA-7), Online, 15–30 November 2020. [Google Scholar]
- Dutta, S.; Ray, C.; Sarkar, S.; Roy, A.; Sahoo, R.; Pall, T. Facile synthesis of bimetallic Au–Pt, Pd-Pt, and Au–Pd nanostructures: Enhanced catalytic performance of Pd-Pt analogue towards fuel cell application and electrochemical sensing. Electrochim. Acta 2015, 180, 1075–1084. [Google Scholar] [CrossRef]
- Abbasi, R.; Wu, L.; Wanke, S.E.; Hayes, R.E. Kinetics of methane combustion over Pt and Pt–Pd catalysts. Chem. Eng. Res. Des. 2012, 90, 1930–1942. [Google Scholar] [CrossRef]
- Ferrando, R.; Jellinek, J.; Johnston, R.L. Nanoalloys: From theory to applications of alloy clusters and nanoparticles. Chem. Rev. 2008, 108, 845–910. [Google Scholar] [CrossRef] [PubMed]
- Carbonea, L.; Cozzoli, P.D. Colloidal heterostructured nanocrystals: Synthesis and growth mechanism. Nano Today 2010, 5, 449. [Google Scholar] [CrossRef]
- Wilson, A.R.; Sun, K.; Chi, M.; White, R.M.; LeBeau, J.M.; Lamb, H.H.; Wiley, B.J. From core–shell to alloys: The preparation and characterization of solution-synthesized AuPd nanoparticle catalysts. J. Phys. Chem. C 2013, 117, 17557. [Google Scholar] [CrossRef]
- Maroun, F.; Ozanam, F.; Magnussen, O.M.; Behm, R.J. The role of atomic ensembles in the reactivity of bimetallic electrocatalysts. Science 2001, 293, 1811. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.; Pilcher, G. Thermochemistry of Organic and Organometallic Compounds; Academic Press: New York, NY, USA, 1970. [Google Scholar]
- Schwartz, A.; Holbrook, L.L.; Wise, H. Catalytic oxidation studies with platinum and palladium. J. Catal. 1971, 21, 199–207. [Google Scholar] [CrossRef]
- Fraunhofer Institute. Available online: https://www.dresden.fraunhofer.de/en.html (accessed on 23 April 2025).
- Heraeus Group. Available online: https://www.heraeus-group.com/en/heraeus-businesses/heraeus-precious-metals/ (accessed on 23 April 2025).
- Calgaz. Cylinders—Calgaz. Available online: https://www.calgaz.com/products/cylinders (accessed on 26 May 2025).
- Liu, S. An Overview of Chemical Reaction Analysis. In Bioprocess Engineering, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017; Chapter 3.12, Eq. (3.118). [Google Scholar]
- Biolab. Biolab–Smart Solutions for Biology. Available online: https://www.biolab-biology.com/ (accessed on 27 May 2025).
- Gasso, S.; Carrier, J.; Radu, D.; Lai, C.-Y. Novel Gas Sensing Approach: ReS2/Ti3C2Tx Heterostructures for NH3 Detection in Humid Environments. ACS Sens. 2024, 9, 4788–4802. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Dong, F.; Han, W.; Tang, Z.; Ding, Y. Recent advances and future challenges in the catalytic combustion of light hydrocarbon VOCs. J. Mater. Chem. A 2024, 12, 7470–7507. [Google Scholar] [CrossRef]
- Li, L.; Zhang, N.; Wu, R.; Song, L.; Zhang, G.; He, H. Comparative study of moisture-treated Pd@CeO2/Al2O3 and Pd/CeO2/Al2O3 catalysts for automobile exhaust emission reactions: Effect of core-shell interface. ACS Appl. Mater. Interfaces 2020, 12, 10350–10358. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.X.; Yang, B.; Wang, H.; Anderson, S.K.; Law, C.K. Kinetics of catalytic oxidation of ethylene over palladium oxide. Proc. Combust. Inst. 2015, 35, 2233–2240. [Google Scholar] [CrossRef]
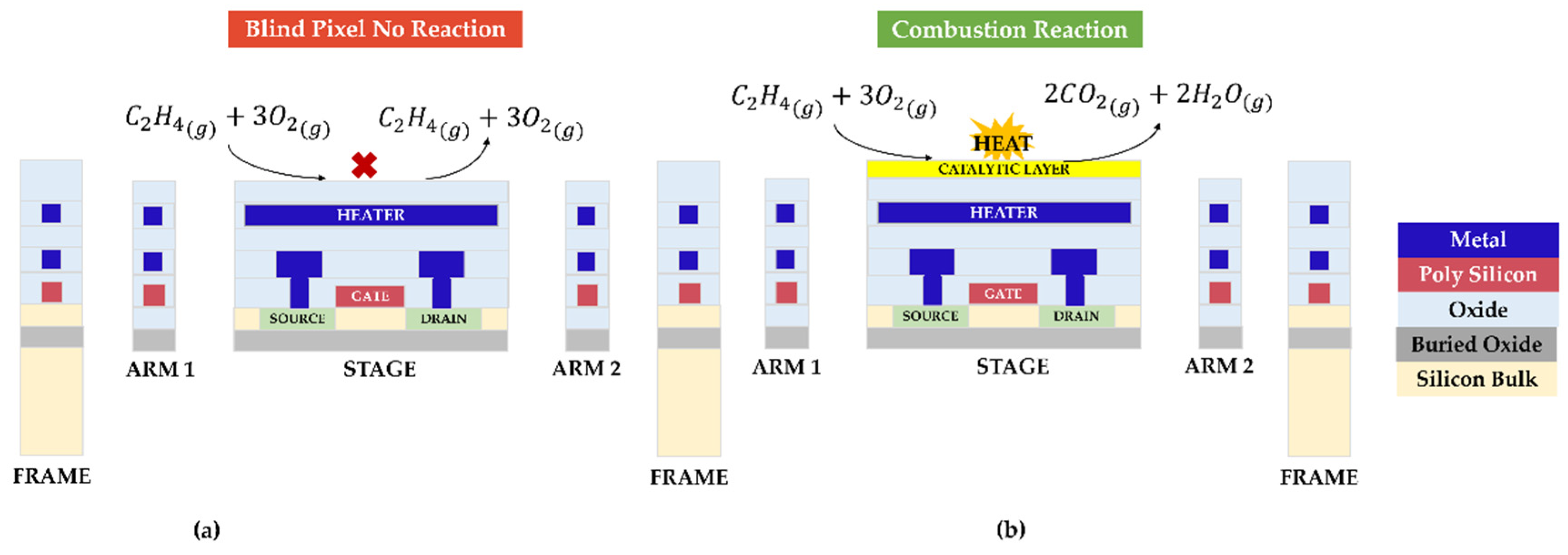
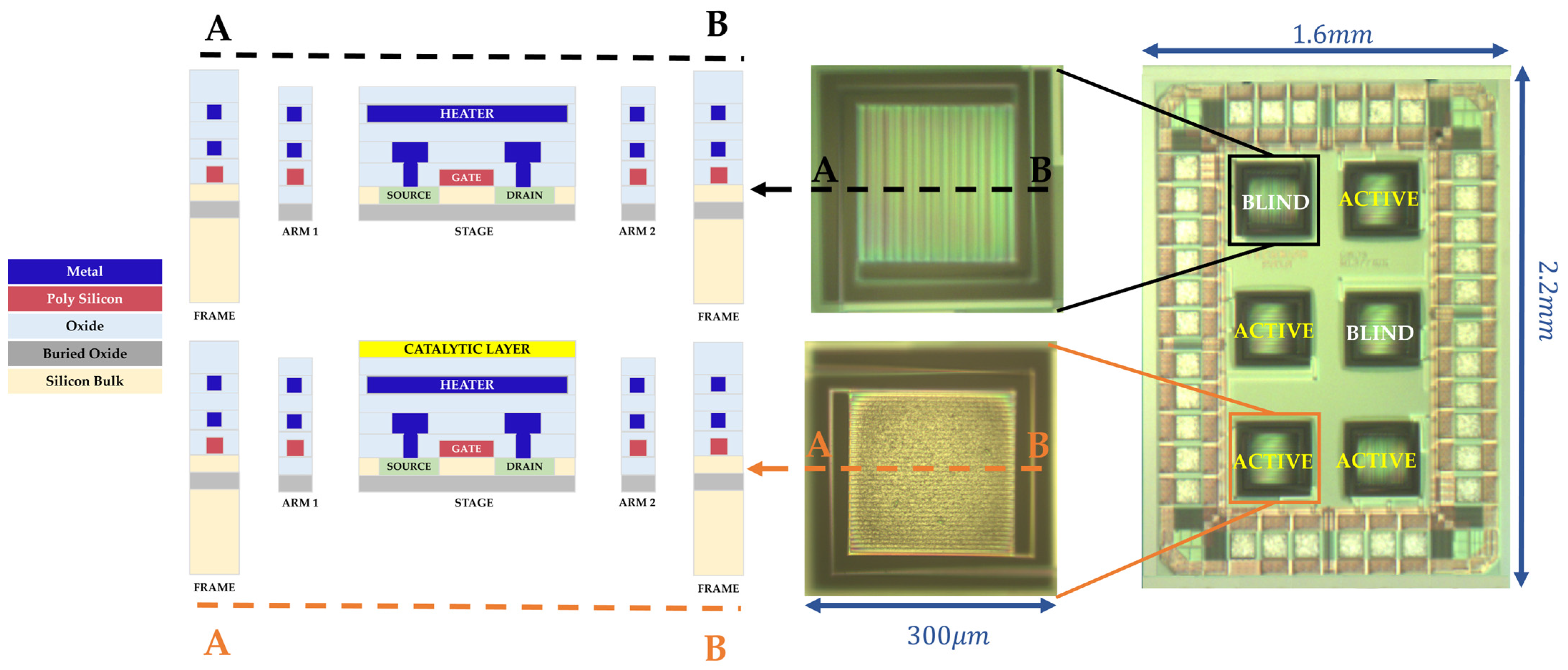
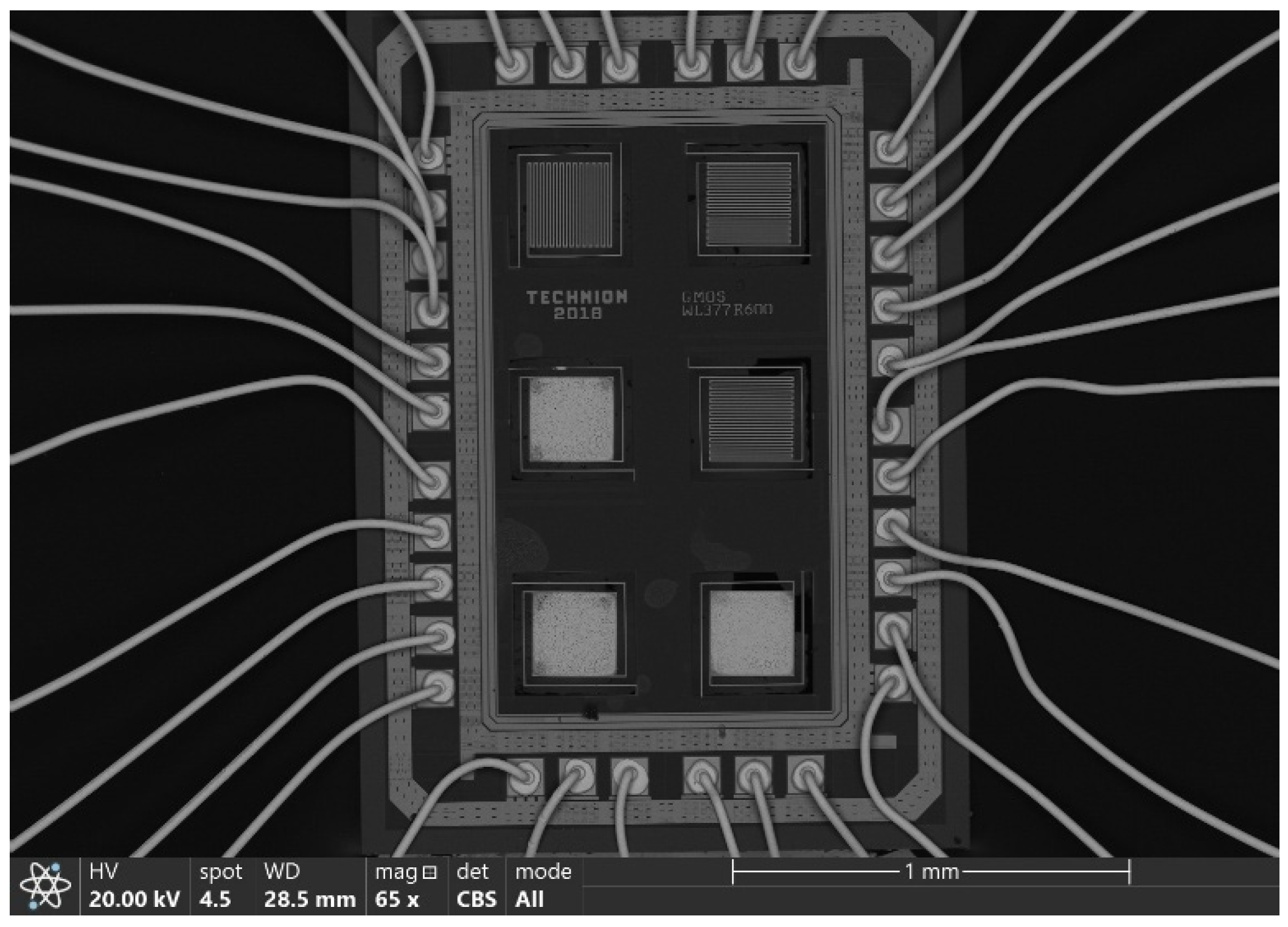
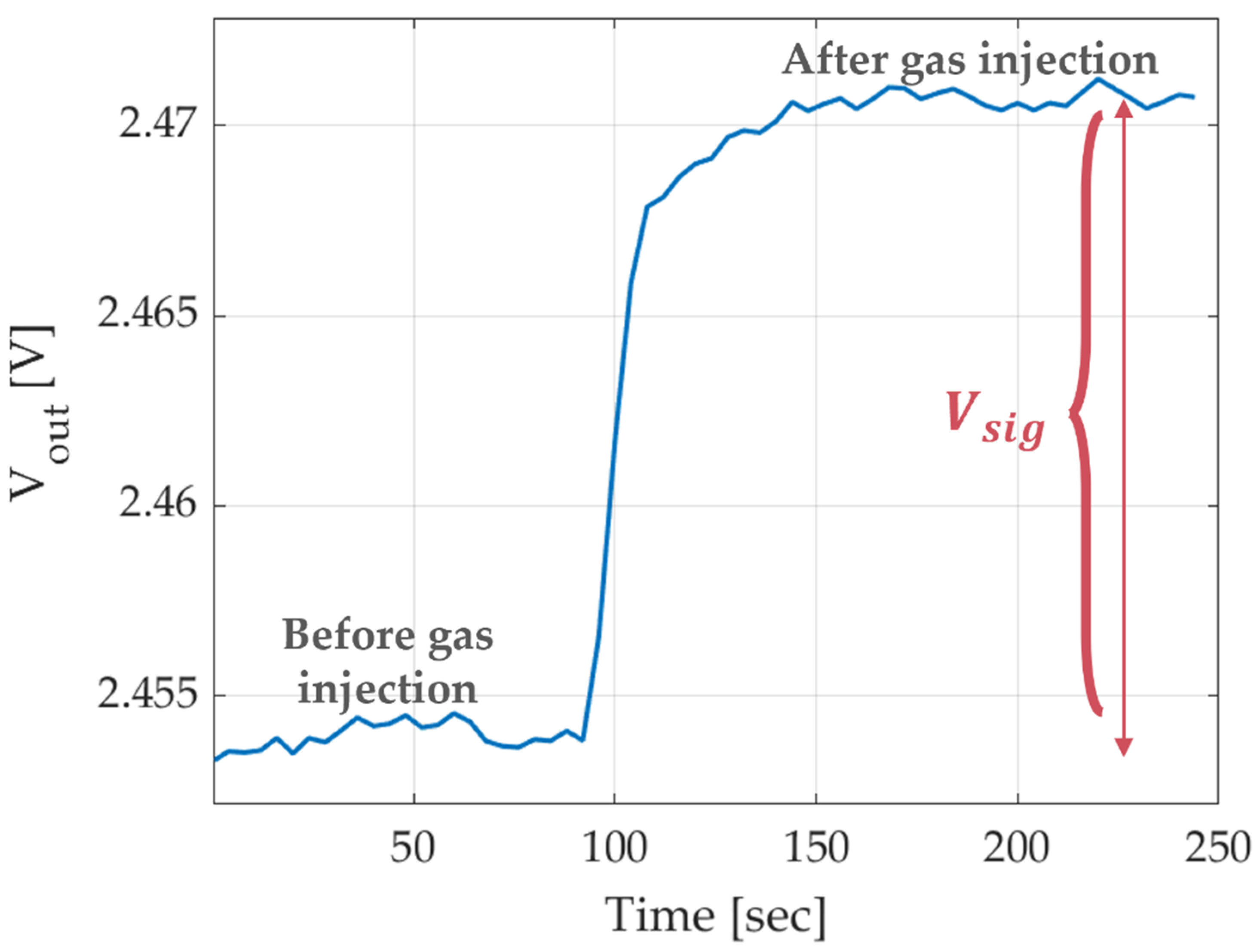

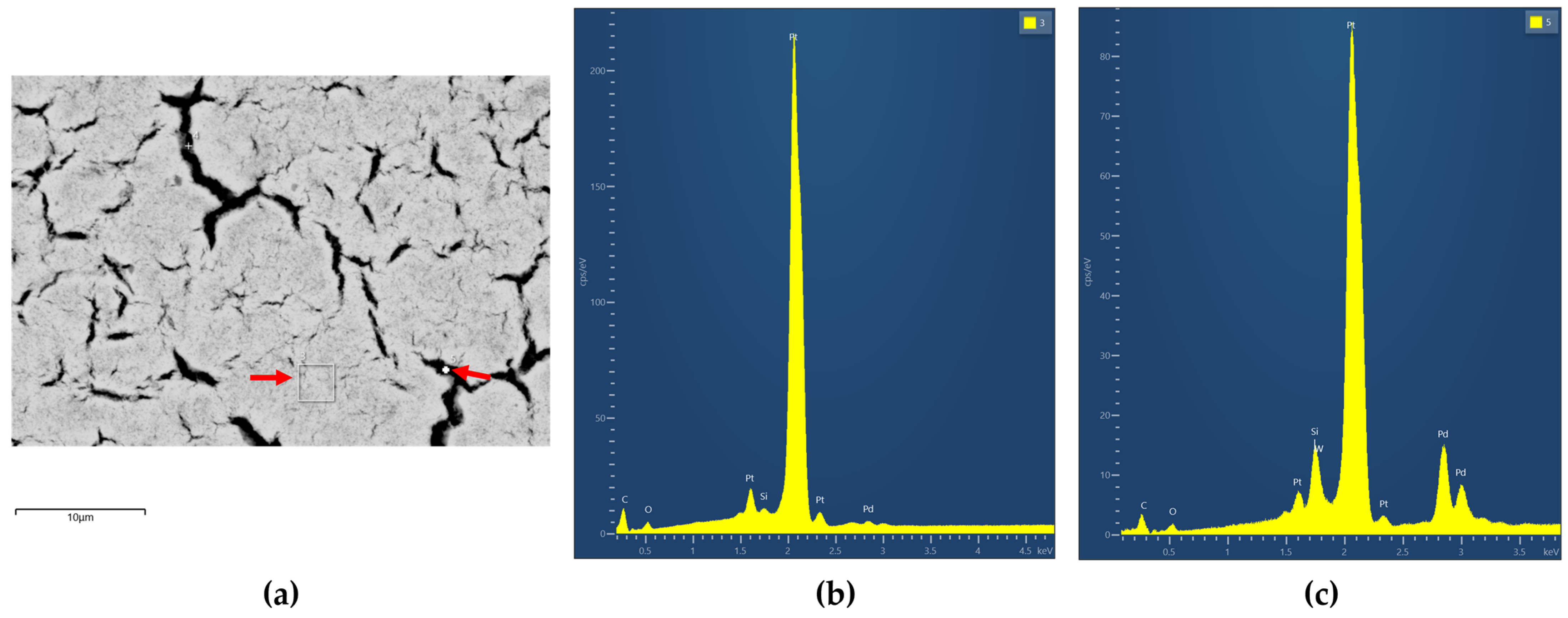
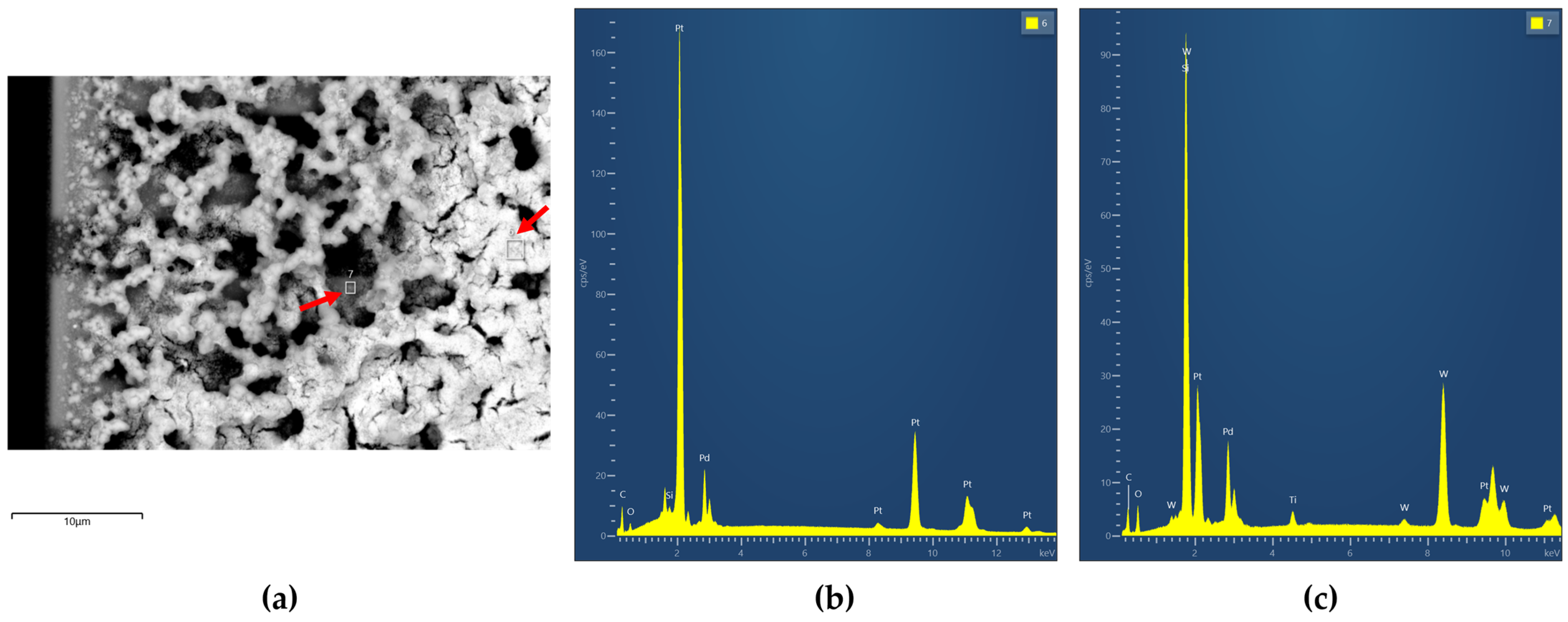
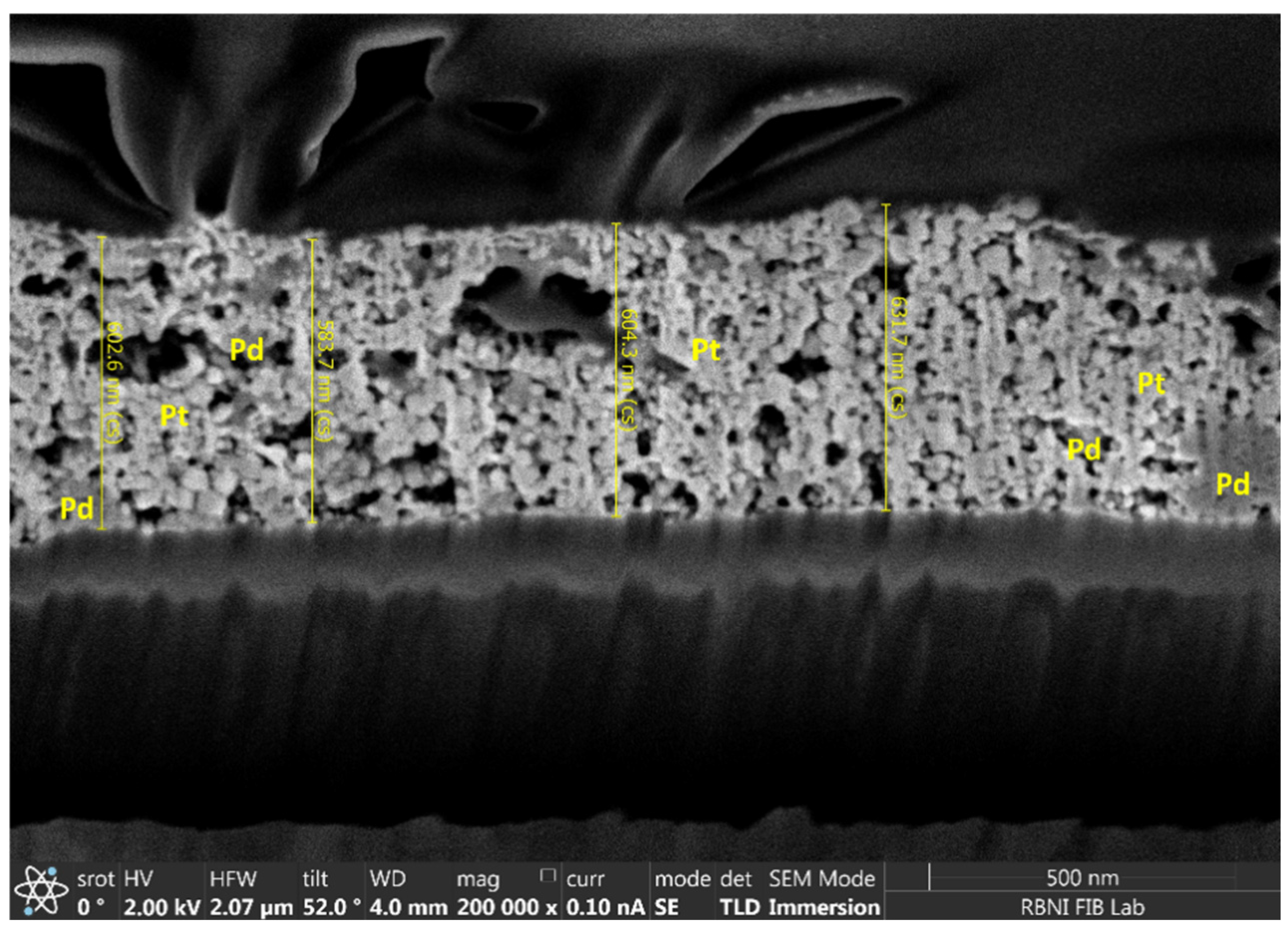
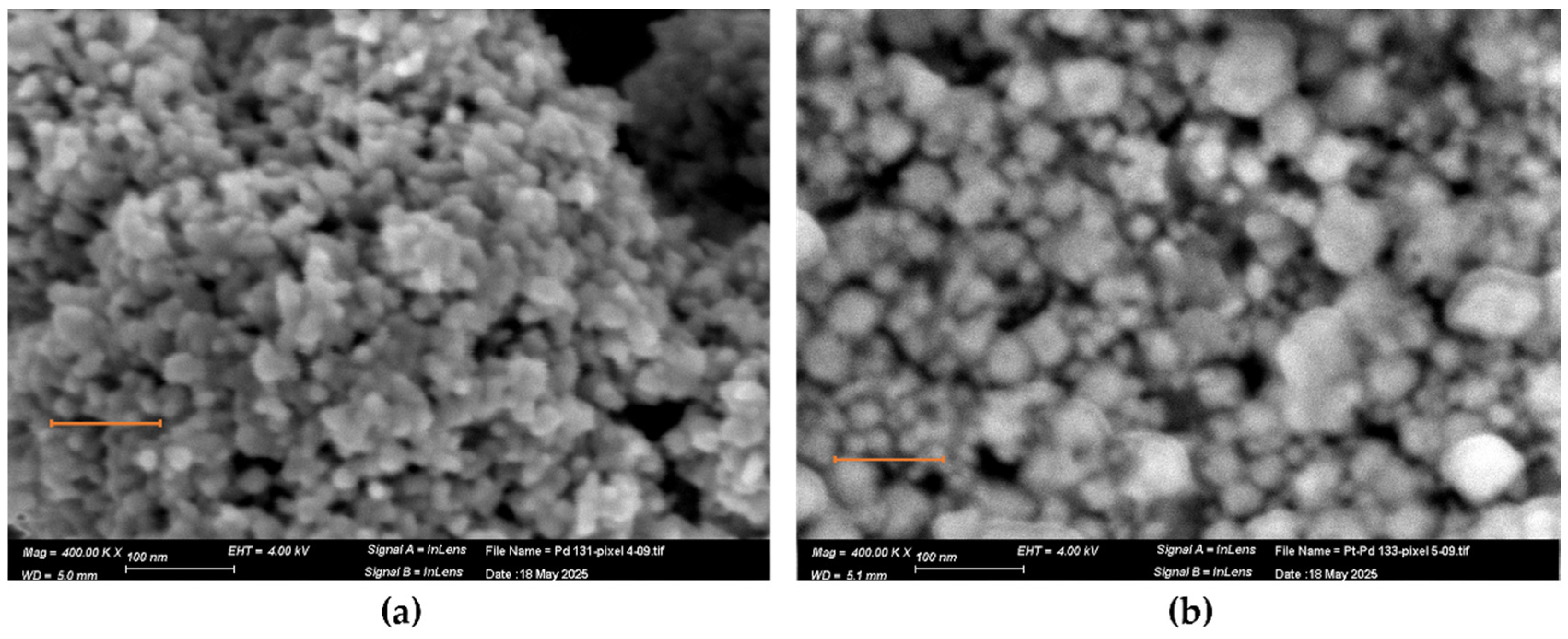
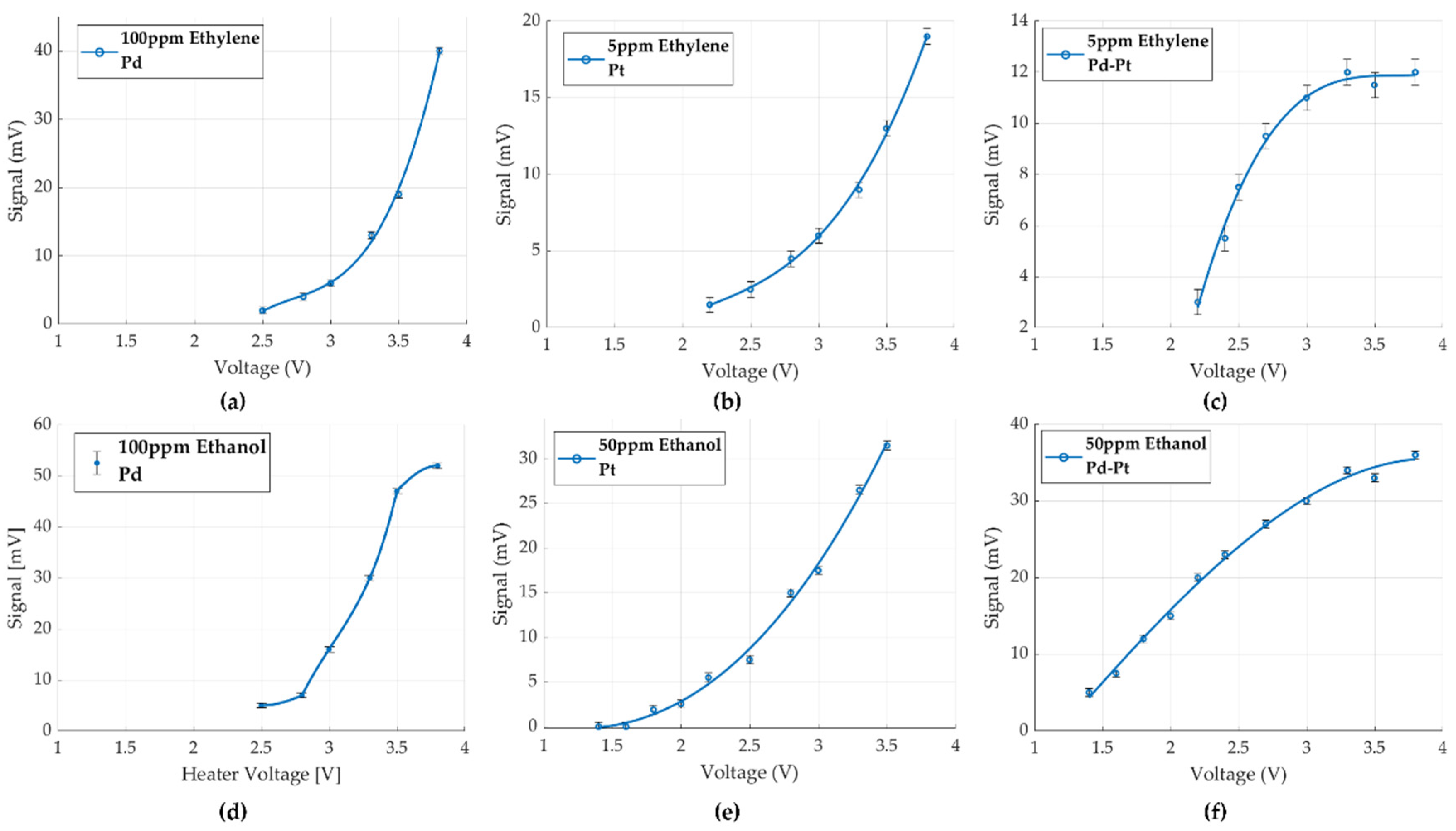
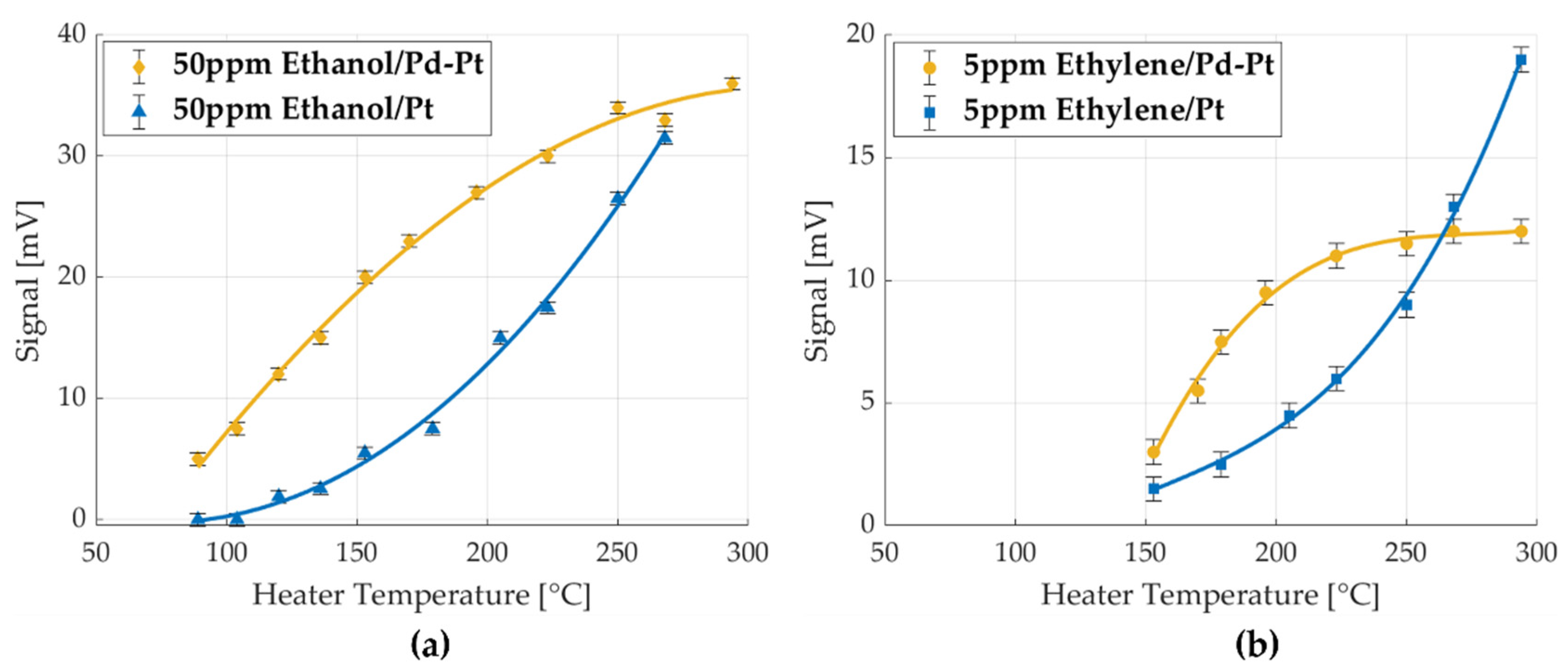



| Heater Voltage [V] | 1.4 | 1.6 | 1.8 | 2 | 2.2 | 2.4 | 2.5 | 2.7 | 2.8 | 3 | 3.3 | 3.5 | 3.8 |
| Temperature [°C] | 89 | 104 | 120 | 136 | 153 | 170 | 179 | 196 | 205 | 223 | 250 | 268 | 294 |
| Ethylene Sensitivity [mV/ppm] | |||
|---|---|---|---|
| Catalyst | Conc. [ppm] | Vheater = 3.5 V | Vheater = 3.8 V |
| Pd | 100 | 0.2 ± 0.01 | 0.4 ± 0.01 |
| Pt | 5 | 2.6 ± 0.08 | 3.8 ± 0.08 |
| Pd-Pt | 5 | 2.4 ± 0.09 | 2.4 ± 0.09 |
| Ethanol Sensitivity [mV/ppm] | |||
| Catalyst | Conc. [ppm] | Vheater = 3.5 V | Vheater = 3.8 V |
| Pd | 100 | 0.48 ± 0.01 | 0.52 ± 0.01 |
| Pt | 50 | 0.52 ± 0.01 | 0.62 ± 0.01 |
| Pd-Pt | 50 | 0.7 ± 0.02 | 0.72 ± 0.02 |
| Ethylene | Ethanol | ||||
|---|---|---|---|---|---|
| Catalyst | Ea [KJ/mol] | R2 | Catalyst | Ea [KJ/mol] | R2 |
| Pd | 50.8 ± 0.5 | 0.9994 | Pd | 48.9 ± 0.5 | 0.9833 |
| Pt | 35.5 ± 0.4 | 0.9985 | Pt | 39.9 ± 0.2 | 0.9958 |
| Pd-Pt | 29.7 ± 1.1 | 0.8805 | Pd-Pt | 31.8 ± 1.1 | 0.9951 |
| Vheater [V] | Vsig Obtained for 100 ppm Ethylene [mV] | Vsig Obtained for 1000 ppm Ethylene [mV] |
|---|---|---|
| 3.0 | 180 | 130 |
| 3.5 | 235 | 2360 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashkar, H.; Stolyarova, S.; Blank, T.; Nemirovsky, Y. The Role of Pd-Pt Bimetallic Catalysts in Ethylene Detection by CMOS-MEMS Gas Sensor Dubbed GMOS. Micromachines 2025, 16, 672. https://doi.org/10.3390/mi16060672
Ashkar H, Stolyarova S, Blank T, Nemirovsky Y. The Role of Pd-Pt Bimetallic Catalysts in Ethylene Detection by CMOS-MEMS Gas Sensor Dubbed GMOS. Micromachines. 2025; 16(6):672. https://doi.org/10.3390/mi16060672
Chicago/Turabian StyleAshkar, Hanin, Sara Stolyarova, Tanya Blank, and Yael Nemirovsky. 2025. "The Role of Pd-Pt Bimetallic Catalysts in Ethylene Detection by CMOS-MEMS Gas Sensor Dubbed GMOS" Micromachines 16, no. 6: 672. https://doi.org/10.3390/mi16060672
APA StyleAshkar, H., Stolyarova, S., Blank, T., & Nemirovsky, Y. (2025). The Role of Pd-Pt Bimetallic Catalysts in Ethylene Detection by CMOS-MEMS Gas Sensor Dubbed GMOS. Micromachines, 16(6), 672. https://doi.org/10.3390/mi16060672






