A New Means to Generate Liposomes by Rehydrating Engineered Lipid Nanoconstructs
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Supplies
2.2. Preparation of Glass Supports
2.3. Contact Angle Measurements
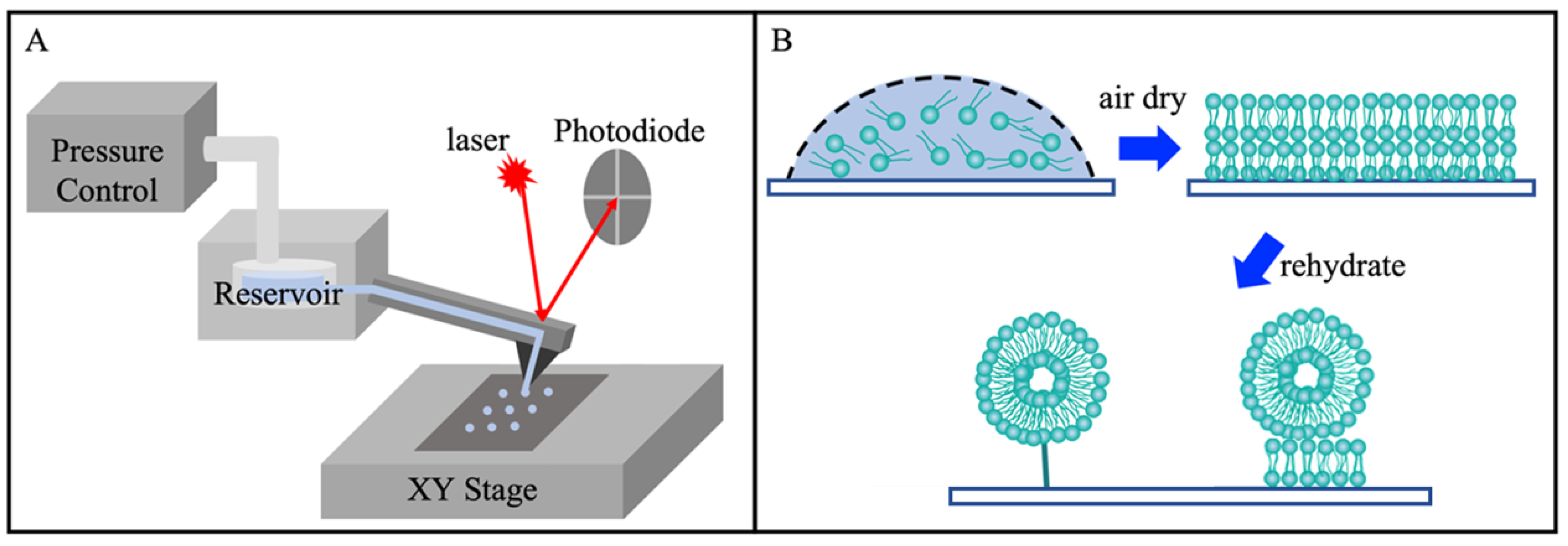
2.4. Lipid Printing and Rehydration
2.5. Atomic Force Microscopy Imaging
2.6. Laser Scanning Confocal Microscopy Imaging
3. Results and Discussion
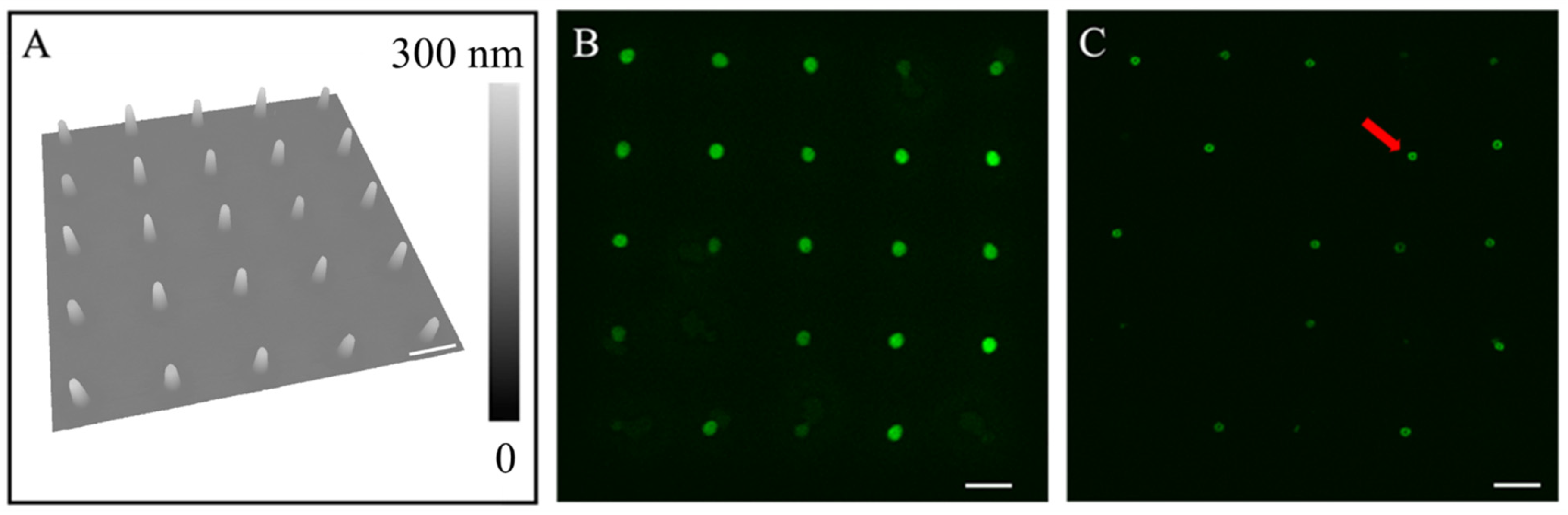
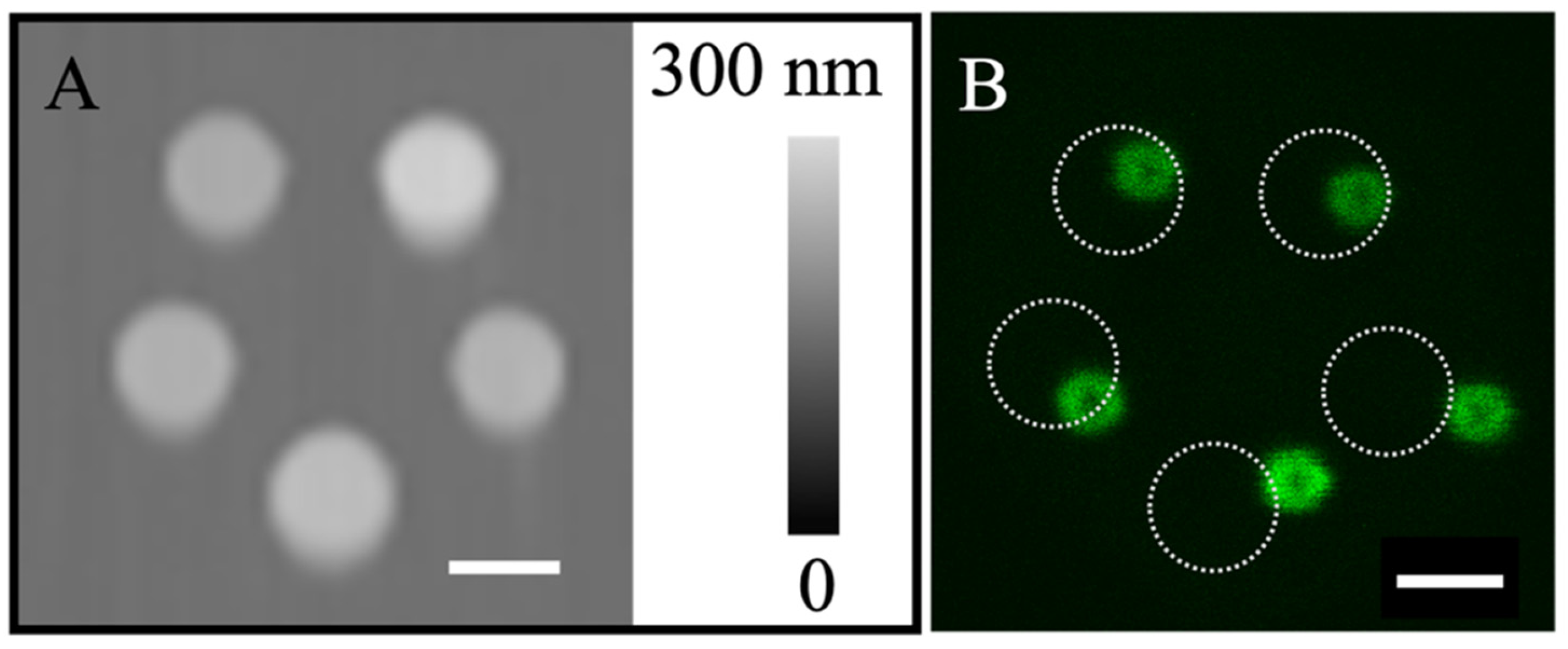
3.1. Rehydration of Lipid Nanostructures Leads to Formation of Liposomes
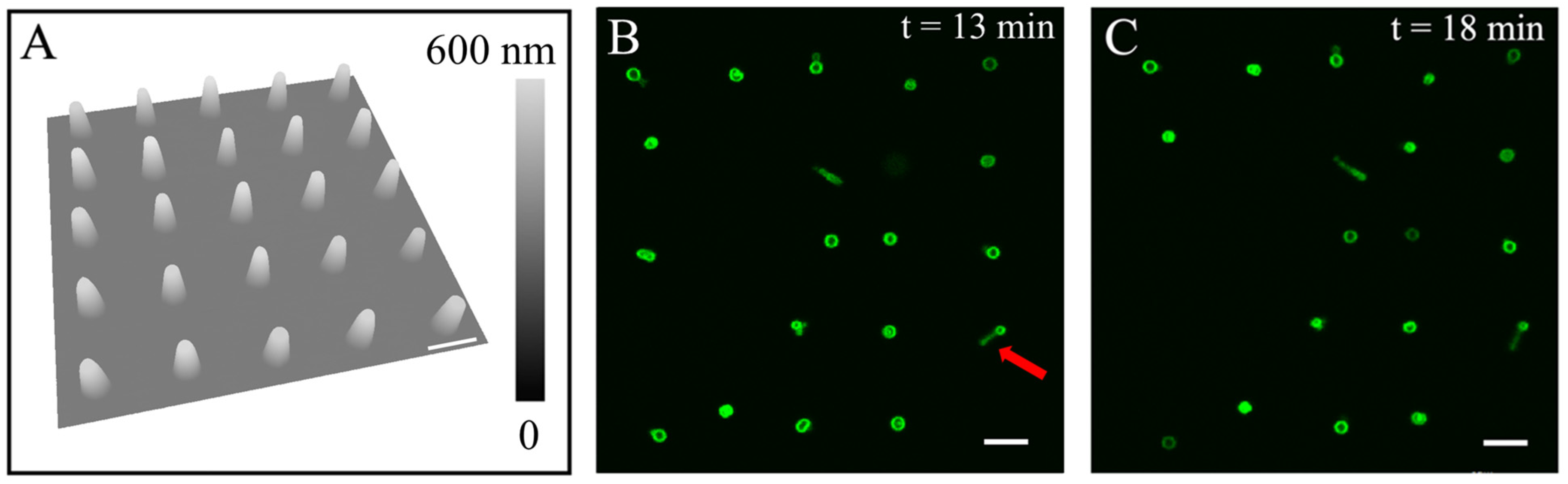
3.2. Liposomes Formed via Rehydrating Lipid Constructs Exhibit High Stability
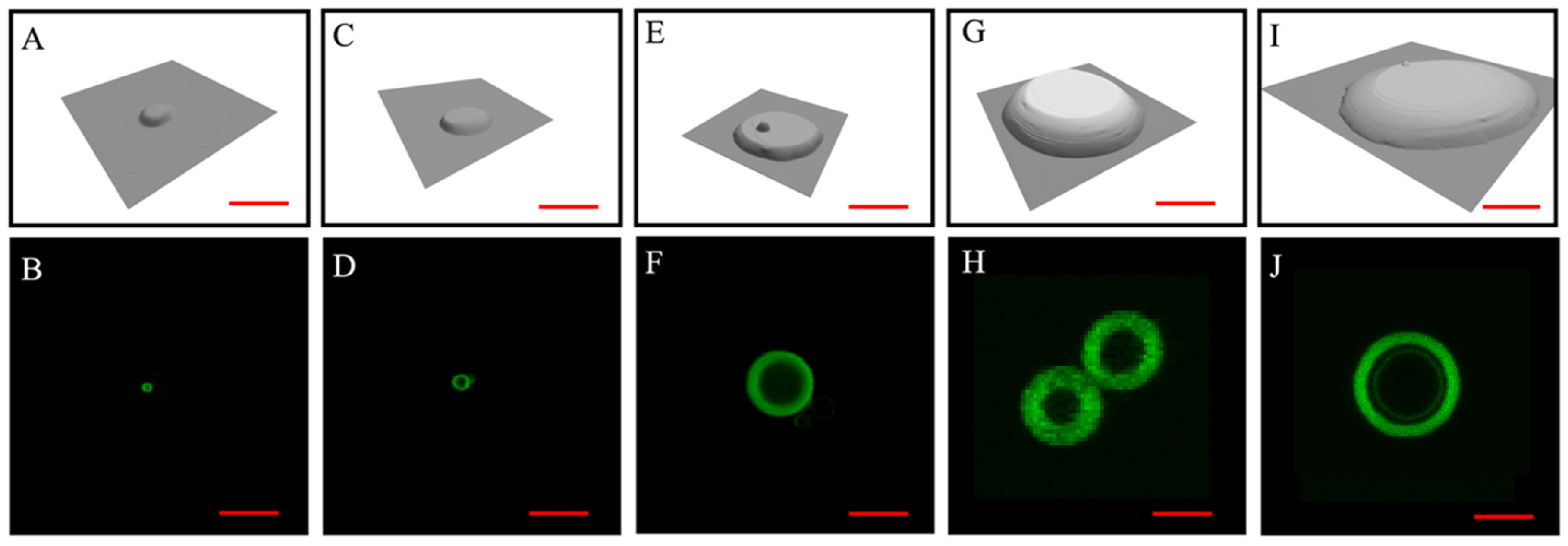
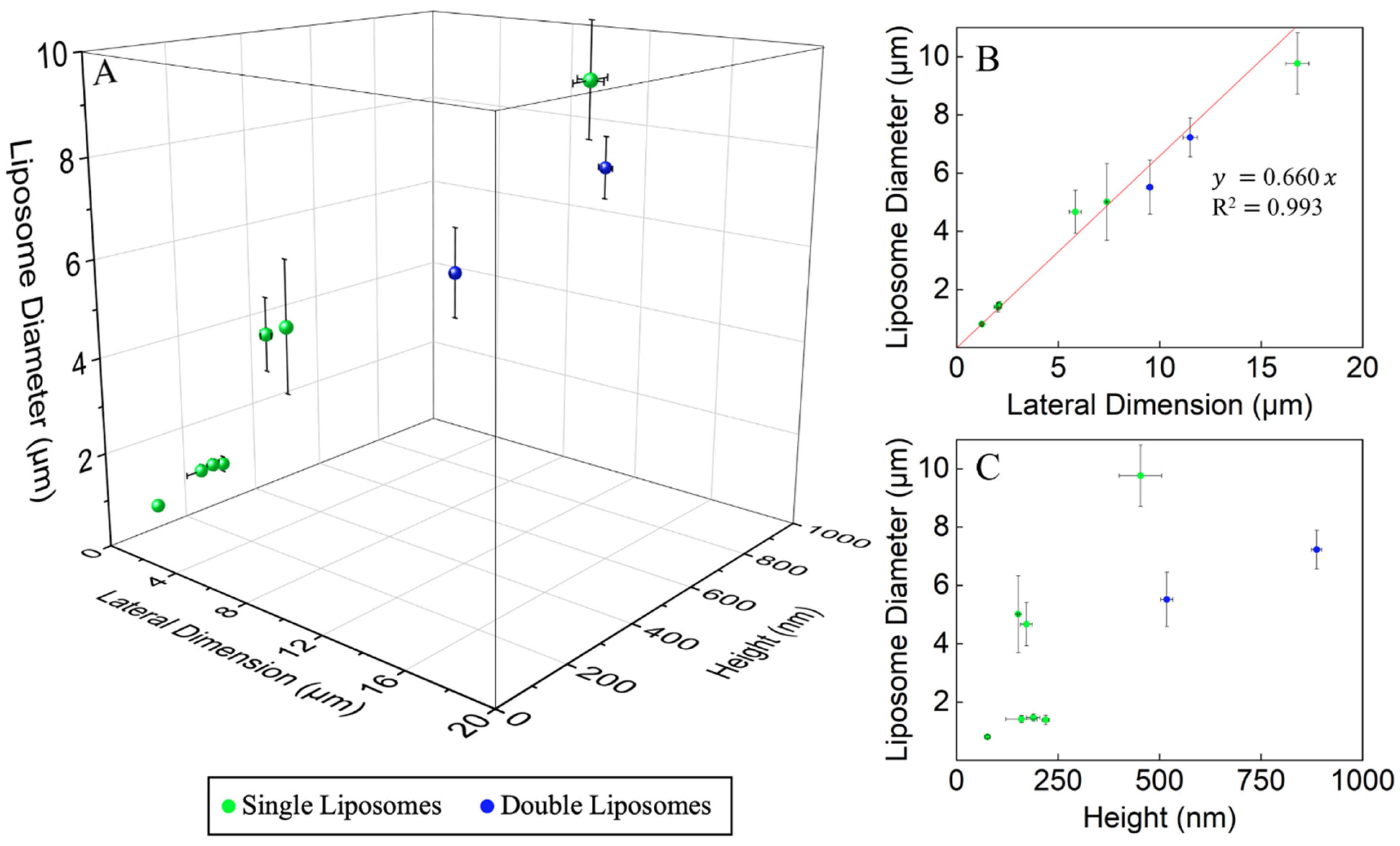
3.3. The Dimension of the Lipid Constructs Dictates the Liposomal Size
3.4. Potential to Produce Hierarchical Structures of Liposomes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rovira-Bru, M.; Thompson, D.H.; Szleifer, I. Size and structure of spontaneously forming liposomes in lipid/PEG-lipid mixtures. Biophys. J. 2002, 83, 2419–2439. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.J.; Semple, S.C.; Klimuk, S.K.; Ansell, S.; Maurer, N.; Cullis, P.R. Characterization of the drug retention and pharmacokinetic properties of liposomal nanoparticles containing dihydrosphingomyelin. Biochim. Biophys. Acta 2007, 1768, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Hofheinz, R.-D.; Gnad-Vogt, S.U.; Beyer, U.; Hochhaus, A. Liposomal encapsulated anti-cancer drugs. Anti-Cancer Drugs 2005, 16, 691–707. [Google Scholar] [CrossRef]
- Omri, A.; Suntres, Z.E.; Shek, P.N. Enhanced activity of liposomal polymyxin B against Pseudomonas aeruginosa in a rat model of lung infection. Biochem. Pharmacol. 2002, 64, 1407–1413. [Google Scholar] [CrossRef]
- Chandrawati, R.; Caruso, F. Biomimetic Liposome- and Polymersome-Based Multicompartmentalized Assemblies. Langmuir 2012, 28, 13798–13807. [Google Scholar] [CrossRef]
- Herianto, S.; Subramani, B.; Chen, B.-R.; Chen, C.-S. Recent advances in liposome development for studying protein-lipid interactions. Crit. Rev. Biotechnol. 2022, 44, 1–14. [Google Scholar] [CrossRef]
- Ahmad, A.; Ahsan, H. Lipid-based formulations in cosmeceuticals and biopharmaceuticals. Biomed. Dermatol. 2020, 4, 12. [Google Scholar] [CrossRef]
- Shukla, S.; Haldorai, Y.; Hwang, S.K.; Bajpai, V.K.; Huh, Y.S.; Han, Y.-K. Current Demands for Food-Approved Liposome Nanoparticles in Food and Safety Sector. Front. Microbiol. 2017, 8, 2398. [Google Scholar] [CrossRef]
- Subramani, T.; Ganapathyswamy, H. An overview of liposomal nano-encapsulation techniques and its applications in food and nutraceutical. J. Food Sci. Technol. 2020, 57, 3545–3555. [Google Scholar] [CrossRef]
- Emami, S.; Azadmard-Damirchi, S.; Peighambardoust, S.H.; Valizadeh, H.; Hesari, J. Liposomes as carrier vehicles for functional compounds in food sector. J. Exp. Nanosci. 2016, 11, 737–759. [Google Scholar] [CrossRef]
- Huang, F.; Xue, H.; Fu, Y.; Ouyang, Y.; Chen, D.; Xia, F.; Willner, I. Three Compartment Liposome Fusion: Functional Protocells for Biocatalytic Cascades and Operation of Dynamic DNA Machineries. Adv. Funct. Mater. 2023, 33, 2302814. [Google Scholar] [CrossRef]
- Matsuo, M.; Ohyama, S.; Sakurai, K.; Toyota, T.; Suzuki, K.; Sugawara, T. A sustainable self-reproducing liposome consisting of a synthetic phospholipid. Chem. Phys. Lipids 2019, 222, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shew, R.L.; Deamer, D.W. A novel method for encapsulation of macromolecules in liposomes. Biochim. Biophys. Acta BBA Biomembr. 1985, 816, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Visht, S.; Awasthi, R.; Rai, R.; Srivastav, P. Development of Dehydration-Rehydration Liposomal System Using Film Hydration Technique Followed by Sonication. Curr. Drug Deliv. 2014, 11, 763–770. [Google Scholar] [CrossRef]
- Szoka, F.; Papahadjopoulos, D. Comparative Properties and Methods of Preparation of Lipid Vesicles (Liposomes). Annu. Rev. Biophys. 1980, 9, 467–508. [Google Scholar] [CrossRef]
- Faizi, H.A.; Tsui, A.; Dimova, R.; Vlahovska, P.M. Bending Rigidity, Capacitance, and Shear Viscosity of Giant Vesicle Membranes Prepared by Spontaneous Swelling, Electroformation, Gel-Assisted, and Phase Transfer Methods: A Comparative Study. Langmuir 2022, 38, 10548–10557. [Google Scholar] [CrossRef]
- Genç, R.; Ortiz, M.; O’Sullivan, C.K. Curvature-Tuned Preparation of Nanoliposomes. Langmuir 2009, 25, 12604–12613. [Google Scholar] [CrossRef]
- Gutman, M. The pH Jump: Probing of Macromolecules and Solutions by a Laser-Induced, Ultrashort Proton Pulse—Theory and Applications in Biochemistry. In Methods of Biochemical Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1984; pp. 1–103. [Google Scholar]
- Hauser, H.; Gains, N. Spontaneous vesiculation of phospholipids: A simple and quick method of forming unilamellar vesicles. Proc. Natl. Acad. Sci. USA 1982, 79, 1683–1687. [Google Scholar] [CrossRef]
- Szoka, F.; Papahadjopoulos, D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc. Natl. Acad. Sci. USA 1978, 75, 4194–4198. [Google Scholar] [CrossRef]
- Göpfrich, K.; Haller, B.; Staufer, O.; Dreher, Y.; Mersdorf, U.; Platzman, I.; Spatz, J.P. One-Pot Assembly of Complex Giant Unilamellar Vesicle-Based Synthetic Cells. ACS Synth. Biol. 2019, 8, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.-Q.; Qi, X.-R. Preparation of Drug Liposomes by Reverse-Phase Evaporation. In Liposome-Based Drug Delivery Systems; Lu, W.-L., Qi, X.-R., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–10. [Google Scholar]
- Stano, P.; Bufali, S.; Pisano, C.; Bucci, F.; Barbarino, M.; Santaniello, M.; Carminati, P.; Luisi, P.L. Novel camptothecin analogue (gimatecan)-containing liposomes prepared by the ethanol injection method. J. Liposome Res. 2004, 14, 87–109. [Google Scholar] [CrossRef] [PubMed]
- Maherani, B.; Arab-Tehrany, E.R.; Mozafari, M.; Gaiani, C.; Linder, M. Liposomes: A Review of Manufacturing Techniques and Targeting Strategies. Curr. Nanosci. 2011, 7, 436–452. [Google Scholar] [CrossRef]
- Has, C.; Sunthar, P. A comprehensive review on recent preparation techniques of liposomes. J. Liposome Res. 2020, 30, 336–365. [Google Scholar] [CrossRef]
- Jaafar-Maalej, C.; Charcosset, C.; Fessi, H. A new method for liposome preparation using a membrane contactor. J. Liposome Res. 2011, 21, 213–220. [Google Scholar] [CrossRef]
- Schubert, R. Liposome preparation by detergent removal. Methods Enzymol. 2003, 367, 46–70. [Google Scholar]
- Schwendener, R.A.; Asanger, M.; Weder, H.G. n-alkyl-glucosides as detergents for the preparation of highly homogeneous bilayer liposomes of variable sizes (60–240 nm φ) applying defined rates of detergent removal by dialysis. Biochem. Biophys. Res. Commun. 1981, 100, 1055–1062. [Google Scholar] [CrossRef]
- Milsmann, M.H.W.; Schwendener, R.A.; Weder, H.-G. The preparation of large single bilayer liposomes by a fast and controlled dialysis. Biochim. Biophys. Acta BBA Biomembr. 1978, 512, 147–155. [Google Scholar] [CrossRef]
- Jiskoot, W.; Teerlink, T.; Beuvery, E.C.; Crommelin, D.J.A. Preparation of liposomes via detergent removal from mixed micelles by dilution. Pharm. Weekbl. 1986, 8, 259–265. [Google Scholar] [CrossRef]
- Yu, B.; Lee, R.J.; Lee, L.J. Chapter 7—Microfluidic Methods for Production of Liposomes. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2009; Volume 465, pp. 129–141. [Google Scholar]
- Carugo, D.; Bottaro, E.; Owen, J.; Stride, E.; Nastruzzi, C. Liposome production by microfluidics: Potential and limiting factors. Sci. Rep. 2016, 6, 25876. [Google Scholar] [CrossRef]
- Gkionis, L.; Aojula, H.; Harris, L.K.; Tirella, A. Microfluidic-assisted fabrication of phosphatidylcholine-based liposomes for controlled drug delivery of chemotherapeutics. Int. J. Pharm. 2021, 604, 120711. [Google Scholar] [CrossRef] [PubMed]
- Patil, Y.P.; Jadhav, S. Novel methods for liposome preparation. Chem. Phys. Lipids 2014, 177, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.; Caspi, Y.; Meijering, A.E.C.; Dekker, C. Octanol-assisted liposome assembly on chip. Nat. Commun. 2016, 7, 10447. [Google Scholar] [CrossRef] [PubMed]
- Osaki, T.; Kamiya, K.; Kawano, R.; Kuribayashi-Shigetomi, K.; Takeuchi, S. Controlled Self-Assembly of Vesicles by Electrospray Deposition. Small Struct. 2024, 5, 2300543. [Google Scholar] [CrossRef]
- Huang, Y.; Karsai, A.; Sambre, P.D.; Su, W.-C.; Faller, R.; Parikh, A.N.; Liu, G.-y. Production of Lipid Constructs by Design via Three-Dimensional Nanoprinting. Micromachines 2023, 14, 372. [Google Scholar] [CrossRef]
- Zhang, J.; Piunova, V.A.; Liu, Y.; Tek, A.; Yang, Q.; Frommer, J.; Liu, G.-y.; Sly, J. Controlled Molecular Assembly via Dynamic Confinement of Solvent. J. Phys. Chem. Lett. 2018, 9, 6232–6237. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, H.; Harris, B.; Zheng, Y.; Celik, U.; Na, L.; Faller, R.; Chen, X.; Haudenschild, D.R.; Liu, G.-y. New Means to Control Molecular Assembly. J. Phys. Chem. C 2020, 124, 6405–6412. [Google Scholar] [CrossRef]
- Yuan, W.; van Ooij, W.J. Characterization of Organofunctional Silane Films on Zinc Substrates. J. Colloid Interface Sci. 1997, 185, 197–209. [Google Scholar] [CrossRef]
- Abbott, N.L.; Gorman, C.B.; Whitesides, G.M. Active Control of Wetting Using Applied Electrical Potentials and Self- Assembled Monolayers. Langmuir 1995, 11, 16–18. [Google Scholar] [CrossRef]
- Bain, C.D.; Whitesides, G.M. A study by contact angle of the acid-base behavior of monolayers containing .omega.-mercaptocarboxylic acids adsorbed on gold: An example of reactive spreading. Langmuir 1989, 5, 1370–1378. [Google Scholar] [CrossRef]
- Grüter, R.R.; Vörös, J.; Zambelli, T. FluidFM as a lithography tool in liquid: Spatially controlled deposition of fluorescent nanoparticles. Nanoscale 2013, 5, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Meister, A.; Gabi, M.; Behr, P.; Studer, P.; Vörös, J.; Niedermann, P.; Bitterli, J.; Polesel-Maris, J.; Liley, M.; Heinzelmann, H.; et al. FluidFM: Combining Atomic Force Microscopy and Nanofluidics in a Universal Liquid Delivery System for Single Cell Applications and Beyond. Nano Lett. 2009, 9, 2501–2507. [Google Scholar] [CrossRef] [PubMed]
- Berganza, E.; Hirtz, M. Direct-Write Patterning of Biomimetic Lipid Membranes In Situ with FluidFM. ACS Appl. Mater. Interfaces 2021, 13, 50774–50784. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.N.; Wang, S.; Ventrici de Souza, J.; Kuhl, T.L.; Liu, G.-y. New Algorithm to Enable Construction and Display of 3D Structures from Scanning Probe Microscopy Images Acquired Layer-by-Layer. J. Phys. Chem. A 2018, 122, 5756–5763. [Google Scholar] [CrossRef]
- Kang, W.; McNaughton, R.L.; Yavari, F.; Minary-Jolandan, M.; Safi, A.; Espinosa, H.D. Microfluidic Parallel Patterning and Cellular Delivery of Molecules with a Nanofountain Probe. SLAS Technol. 2014, 19, 100–109. [Google Scholar] [CrossRef]
- Ventrici de Souza, J.; Liu, Y.; Wang, S.; Dörig, P.; Kuhl, T.L.; Frommer, J.; Liu, G.-y. Three-Dimensional Nanoprinting via Direct Delivery. J. Phys. Chem. B 2018, 122, 956–962. [Google Scholar] [CrossRef]
- Moga, A.; Yandrapalli, N.; Dimova, R.; Robinson, T. Optimization of the Inverted Emulsion Method for High-Yield Production of Biomimetic Giant Unilamellar Vesicles. ChemBioChem 2019, 20, 2674–2682. [Google Scholar] [CrossRef]
- Tran, V.; Karsai, A.; Fong, M.C.; Cai, W.; Yik, J.H.N.; Klineberg, E.; Haudenschild, D.R.; Liu, G.-y. Label-Free and Direct Visualization of Multivalent Binding of Bone Morphogenetic Protein-2 with Cartilage Oligomeric Matrix Protein. J. Phys. Chem. B 2019, 123, 39–46. [Google Scholar] [CrossRef]
- Li, G.; Kim, J.; Huang, Z.; St. Clair, J.R.; Brown, D.A.; London, E. Efficient replacement of plasma membrane outer leaflet phospholipids and sphingolipids in cells with exogenous lipids. Proc. Natl. Acad. Sci. USA 2016, 113, 14025–14030. [Google Scholar] [CrossRef]
- Ho, J.C.S.; Su, W.-C.; Chun Wang, X.; Parikh, A.N.; Liedberg, B. Nonequilibrium Self-Organization of Lipids into Hierarchically Ordered and Compositionally Graded Cylindrical Smectics. Langmuir 2022, 38, 1045–1056. [Google Scholar] [CrossRef]
- Sugihara, K.; Chami, M.; Derényi, I.; Vörös, J.; Zambelli, T. Directed Self-Assembly of Lipid Nanotubes from Inverted Hexagonal Structures. ACS Nano 2012, 6, 6626–6632. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, K.; Rustom, A.; Spatz, J.P. Freely drawn single lipid nanotube patterns. Soft Matter 2015, 11, 2029–2035. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Xu, Z.; Celik, U.; Carnahan, C.F.; Faller, R.; Parikh, A.N.; Liu, G.-y. A New Means to Generate Liposomes by Rehydrating Engineered Lipid Nanoconstructs. Micromachines 2025, 16, 138. https://doi.org/10.3390/mi16020138
Huang Y, Xu Z, Celik U, Carnahan CF, Faller R, Parikh AN, Liu G-y. A New Means to Generate Liposomes by Rehydrating Engineered Lipid Nanoconstructs. Micromachines. 2025; 16(2):138. https://doi.org/10.3390/mi16020138
Chicago/Turabian StyleHuang, Yuqi, Ziqian Xu, Umit Celik, Christopher F. Carnahan, Roland Faller, Atul N. Parikh, and Gang-yu Liu. 2025. "A New Means to Generate Liposomes by Rehydrating Engineered Lipid Nanoconstructs" Micromachines 16, no. 2: 138. https://doi.org/10.3390/mi16020138
APA StyleHuang, Y., Xu, Z., Celik, U., Carnahan, C. F., Faller, R., Parikh, A. N., & Liu, G.-y. (2025). A New Means to Generate Liposomes by Rehydrating Engineered Lipid Nanoconstructs. Micromachines, 16(2), 138. https://doi.org/10.3390/mi16020138






