Development and Evaluation of Multi-Module Retinal Devices for Artificial Vision Applications
Abstract
1. Introduction
2. Materials and Methods
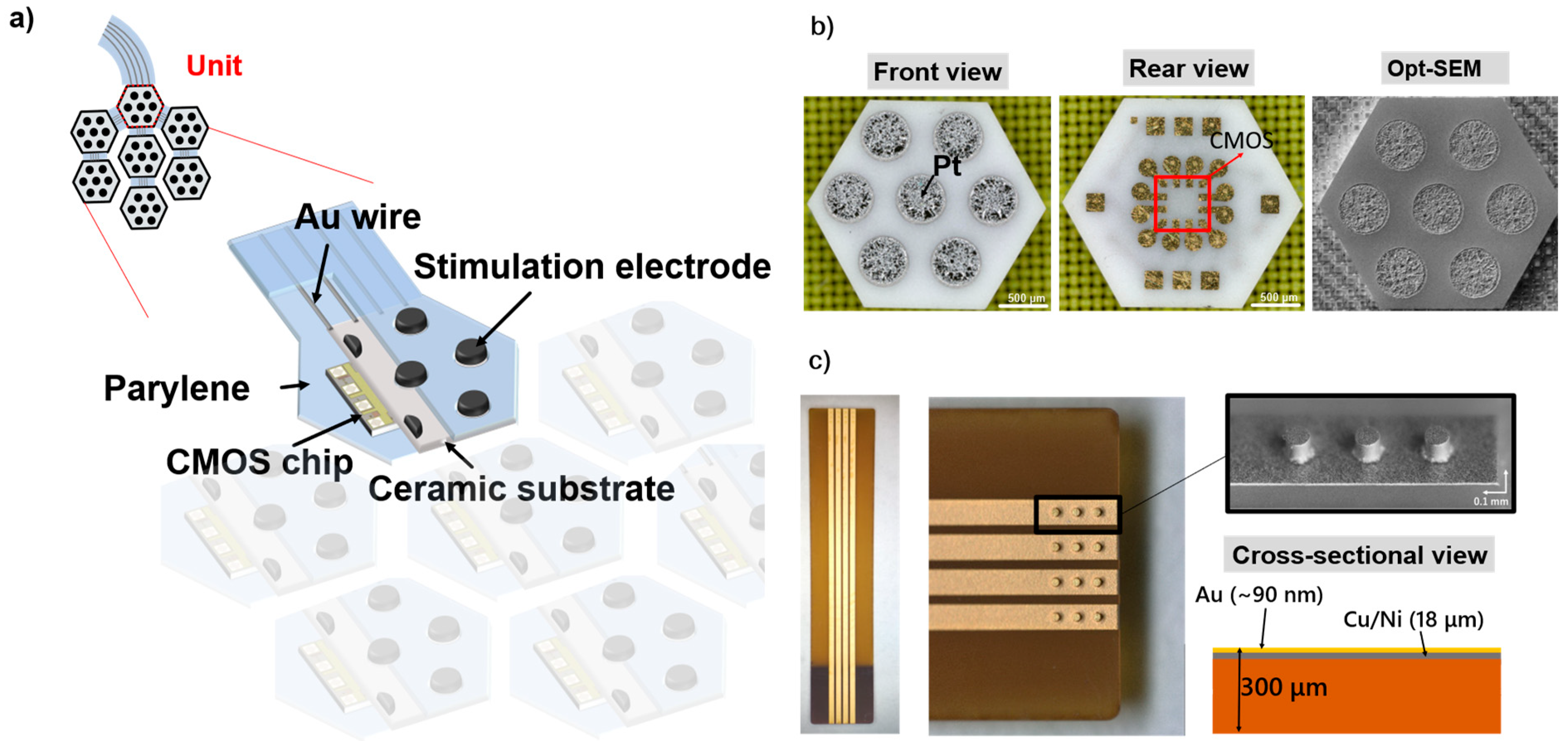
2.1. Structure of the Flexible MMRD
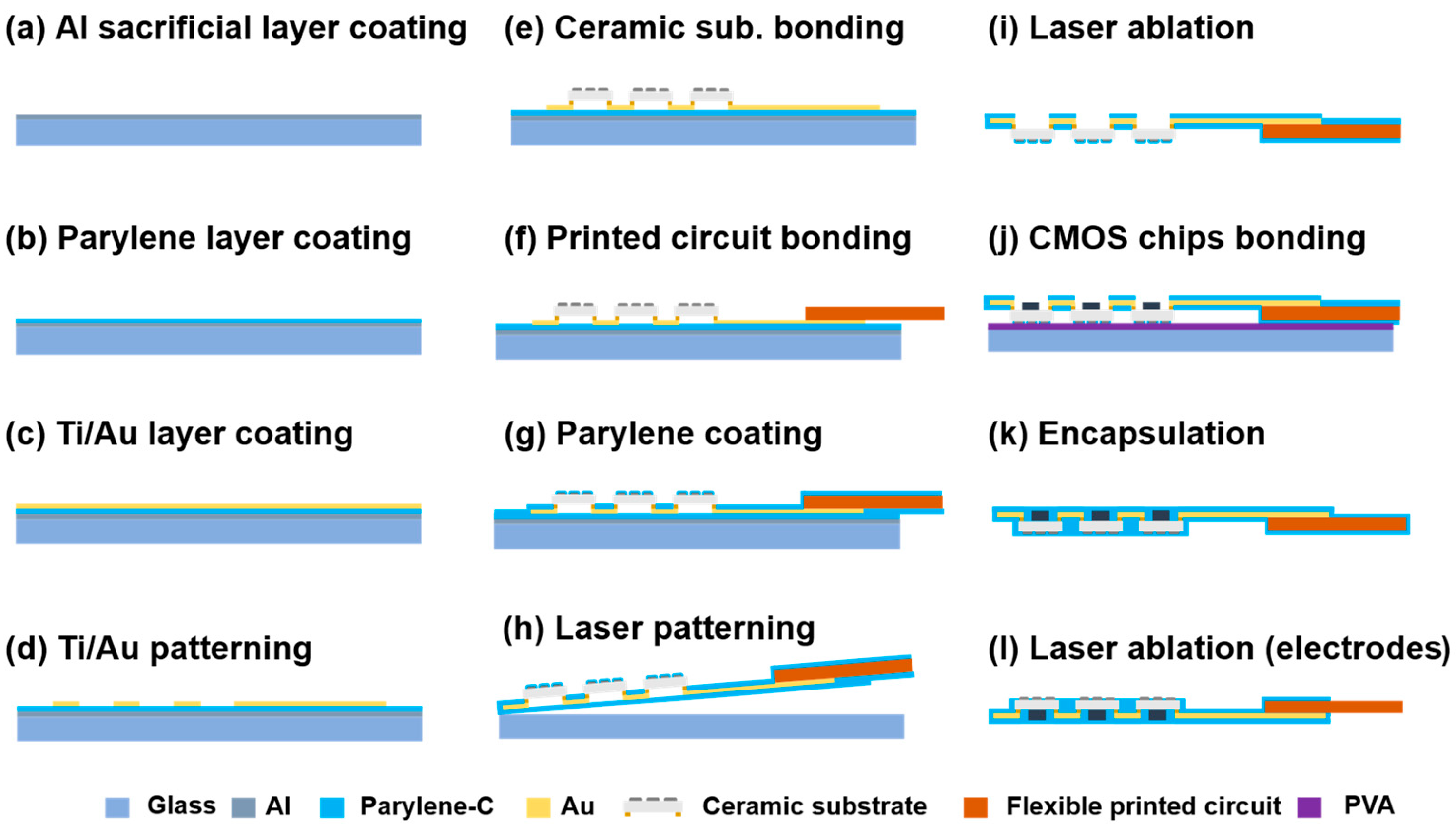
2.2. Fabrication of the Flexible MMRD
2.3. Operating System of the MMRD
2.4. Animal Experiments
2.4.1. Rat Preparation
2.4.2. Rabbit Preparation
2.5. Electrophysiological Recordings
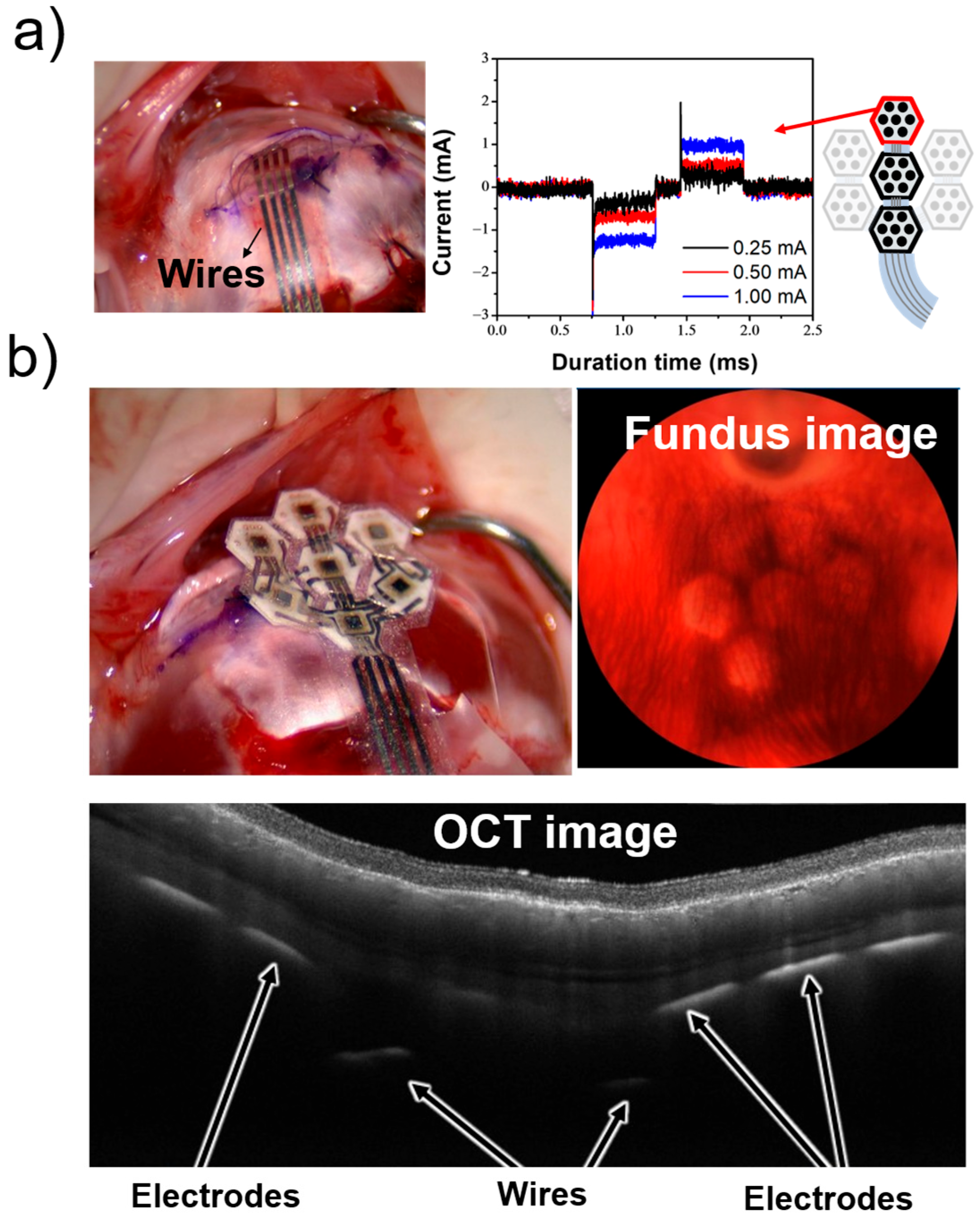
3. Results
3.1. Imaging of the Fabricated Device
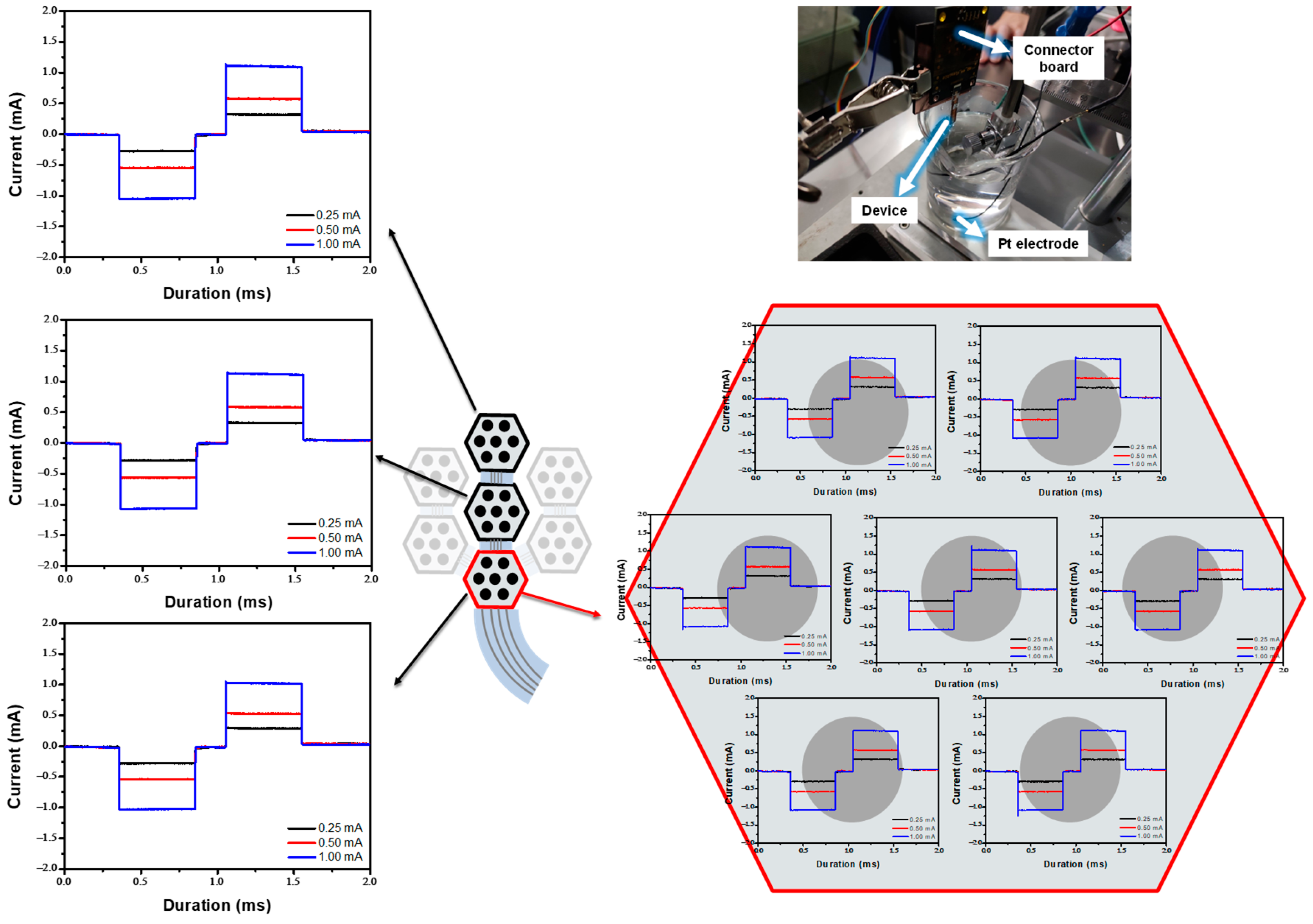
In Vitro Experiment in the PBS
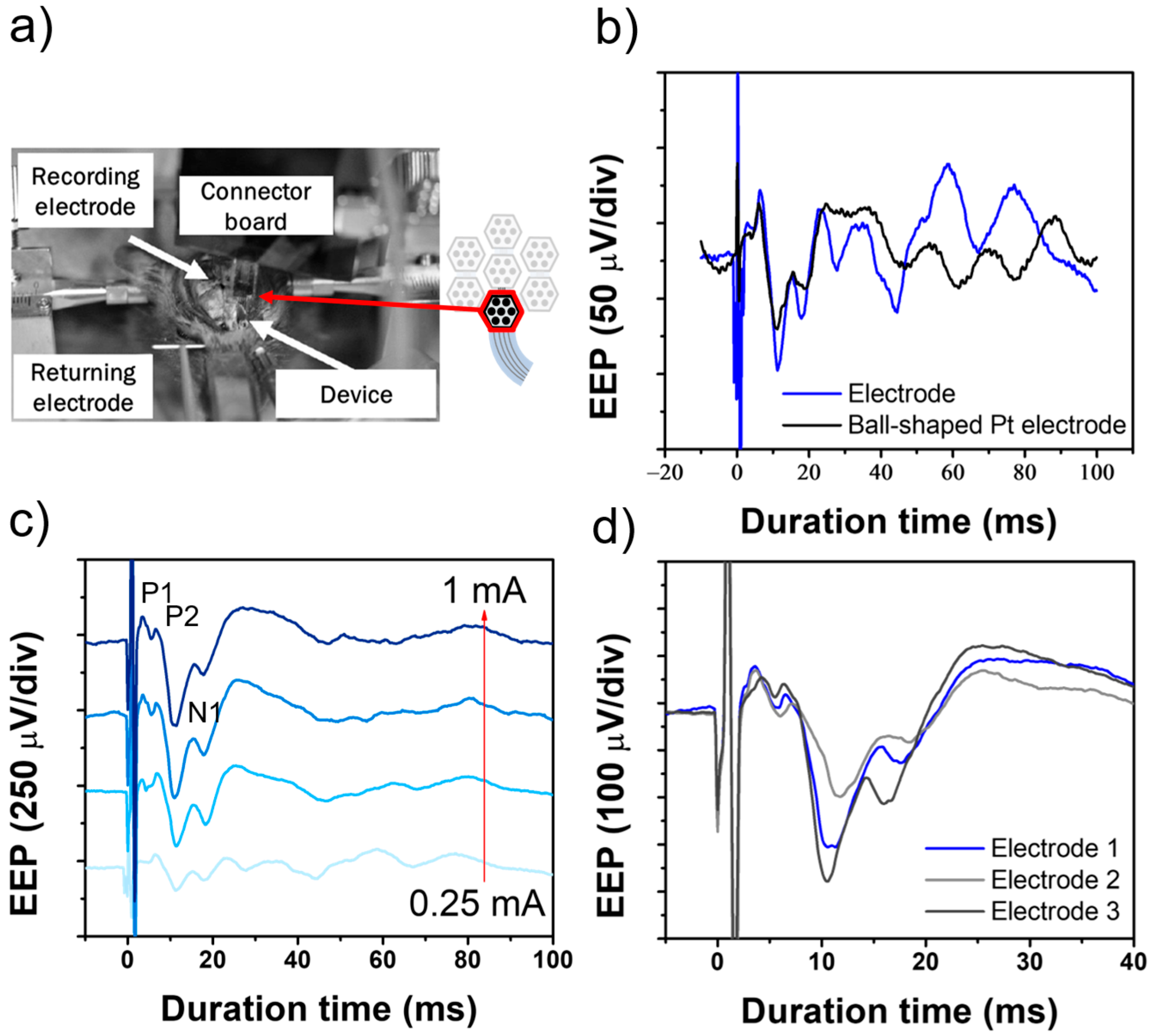
3.2. EEP Signal Recording in the Rat Experiment
3.3. In Vivo Experiment in the Rabbit
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CMOS | Complementary metal-oxide semiconductor |
| STS | Suprachoroidal transretinal stimulation |
| MMRD | Multi-module retinal devices |
| PaC | Parylene-C |
| EEP | Electrically evoked potential |
| OCT | Optical coherence tomography |
| LFT | Local field potential |
References
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.-Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106. [Google Scholar] [CrossRef]
- Friedman, D.S.; O’Colmain, B.J.; Muñoz, B.; Tomany, S.C.; McCarty, C.; de Jong, P.T.; Nemesure, B.; Mitchell, P.; Kempen, J.; Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch. Ophthalmol. 2004, 122, 564. [Google Scholar]
- Edwards, T.L.; Cottriall, C.L.; Xue, K.; Simunovic, M.P.; Ramsden, J.D.; Zrenner, E.; MacLaren, R.E. Assessment of the electronic retinal implant Alpha AMS in restoring vision to blind patients with end-stage retinitis pigmentosa. Ophthalmology 2018, 125, 432. [Google Scholar] [CrossRef]
- Stingl, K.; Bartz-Schmidt, K.U.; Besch, D.; Chee, C.K.; Cottriall, C.L.; Gekeler, F.; Groppe, M.; Jackson, T.L.; MacLaren, R.E.; Koitschev, A.; et al. Artificial vision with wirelessly powered subretinal electronic implant Alpha-IMS: Implantation and functional outcomes in humans. Vision Res. 2015, 111, 149. [Google Scholar] [CrossRef]
- Luo, Y.H.L.; da Cruz, L. A review and update on the current status of retinal prostheses (bionic eye). Prog. Retin. Eye Res. 2016, 50, 89. [Google Scholar] [CrossRef]
- Dorn, J.D.; Ahuja, A.K.; Caspi, A.; da Cruz, L.; Dagnelie, G.; Sahel, J.A.; Greenberg, R.J.; McMahon, M.; Argus II Study Group. The detection of motion by blind subjects with the epiretinal 60-electrode (Argus II) retinal prosthesis. JAMA Ophthalmol. 2013, 131, 183. [Google Scholar] [CrossRef]
- Fujikado, T.; Morimoto, T.; Kanda, H.; Kusaka, S.; Nakauchi, K.; Ozawa, M.; Matsushita, K.; Sakaguchi, H.; Ikuno, Y.; Kamei, M.; et al. Evaluation of microfabricated retinal stimulating electrodes implanted in rabbit eyes. Graefes Arch. Clin. Exp. Ophthalmol. 2007, 245, 1411. [Google Scholar] [CrossRef]
- Ayton, L.N.; Blamey, P.J.; Guymer, R.H.; Luu, C.D.; Nayagam, D.A.; Sinclair, N.C.; Shivdasani, M.N.; Yeoh, J.; McCombe, M.F.; Briggs, R.J.; et al. First-in-human trial of a novel suprachoroidal retinal prosthesis. PLoS ONE 2014, 9, e115239. [Google Scholar] [CrossRef]
- Fujikado, T.; Kamei, M.; Sakaguchi, H.; Kanda, H.; Morimoto, T.; Ikuno, Y.; Nishida, K.; Kishima, H.; Maruo, T.; Konoma, K.; et al. Testing of semichronic implantation of suprachoroidal-transretinal stimulation prosthesis in patients with advanced retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 2011, 52, 4726. [Google Scholar] [CrossRef]
- Ho, E.; Lei, X.; Flores, T.; Lorach, H.; Huang, T.; Galambos, L.; Kamins, T.; Harris, J.; Mathieson, K.; Palanker, D. Photovoltaic retinal prosthesis for restoring sight to the blind. J. Neural Eng. 2019, 16, 066027. [Google Scholar]
- Stieglitz, T. Development of neural interfaces: Critical review and perspectives. J. Neural Eng. 2009, 6, 065005. [Google Scholar]
- Tong, W.; Stamp, M.; Apollo, N.V.; Ganesan, K.; Meffin, H.; Prawer, S.; Garrett, D.J.; Ibbotson, M.R. Optimizing mechanical stimulation of retinal neurons with carbon fiber microelectrodes. J. Neural Eng. 2019, 17, 016018. [Google Scholar]
- Bareket, L.; Barriga-Rivera, A.; Zapf, M.P.; Lovell, N.H.; Suaning, G.J. Progress towards a high-density retinal implant: A review of the Alpha AMS, Argus II, and PRIMA Systems. J. Neural Eng. 2017, 14, 045002. [Google Scholar]
- Ayton, L.N.; Barnes, N.; Dagnelie, G.; Fujikado, T.; Goetz, G.; Hornig, R.; Jones, B.W.; Muqit, M.M.K.; Rathbun, D.L.; Stingl, K.; et al. An update on retinal prostheses. Clin. Neurophysiol. 2020, 6, 1383. [Google Scholar] [CrossRef]
- Bloch, E.; Luo, Y.; da Cruz, L. Advances in retinal prosthesis systems. Ther. Adv. Ophthalmol. 2019, 11, 2515841418817501. [Google Scholar] [CrossRef]
- Lorach, H.; Goetz, G.; Smith, R.; Lei, X.; Mandel, Y.; Kamins, T.; Mathieson, K.; Huie, P.; Harris, J.; Sher, A.; et al. Photovoltaic restoration of sight with high visual acuity. Nat. Med. 2015, 21, 476. [Google Scholar] [CrossRef]
- Chenais, N.A.L.; Airaghi Leccardi, M.J.I.; Ghezzi, D. Photovoltaic retinal prosthesis restores high-resolution responses to single-pixel stimulation in blind retinas. Commun. Mater. 2021, 2, 28. [Google Scholar] [CrossRef]
- Schaffrath, K.; Lohmann, T.; Seifert, J.; Ingensiep, C.; Raffelberg, P.; Waschkowski, F.; Viga, R.; Kokozinski, R.; Mokwa, W.; Johnen, S.; et al. New epiretinal implant with integrated sensor chips for optical capturing shows a good biocompatibility profile in vitro and in vivo. Biomed. Eng. Online 2021, 20, 102. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Sung, W.-J.; Kuo, P.-H.; Tzeng, C.-K.; Chiao, C.-C.; Tsai, Y.-C. The design of CMOS self-powered 256-pixel implantable chip with on-chip photovoltaic cells and active pixel sensors for subretinal prostheses. In Proceedings of the 2015 IEEE Biomed. Circuits Syst. Conf. (BioCAS), Atlanta, GA, USA, 22–24 October 2015; Volume 1. [Google Scholar]
- Allen, P.J.; Kolic, M.; Baglin, E.K.; Titchener, S.A.; Kvansakul, J.; Nayagam, D.A.X.; Yeoh, J.; Briggs, R.J.; Villalobos, J.; Williams, C.E.; et al. Second-Generation (44-Channel) Suprachoroidal Retinal Prosthesis: Surgical Stability and Safety During a 2-Year Clinical Trial. Clin. Exp. Ophthalmol. 2025; early view. [Google Scholar] [CrossRef]
- Petoe, M.A.; Abbott, C.J.; Titchener, S.A.; Kolic, M.; Kentler, W.G.; Nayagam, D.A.X.; Baglin, E.K.; Kvansakul, J.; Barnes, N.; Walker, J.G.; et al. A Second-Generation (44-Channel) Suprachoroidal Retinal Prosthesis: A Single-Arm Clinical Trial of Feasibility. Ophthalmol. Sci. 2025, 1, 100525. [Google Scholar] [CrossRef]
- Petoe, M.A.; Titchener, S.A.; Kolic, M.; Kentler, W.G.; Abbott, C.J.; Nayagam, D.A.X.; Baglin, E.K.; Kvansakul, J.; Barnes, N.; Walker, J.G.; et al. A Second-Generation (44-Channel) Suprachoroidal Retinal Prosthesis: Interim Clinical Trial Results. Transl. Vis. Sci. Technol. 2021, 10, 12. [Google Scholar] [CrossRef]
- Terasawa, Y.; Tashiro, H.; Nakano, Y.; Ohta, J. Safety and efficacy of semichronic suprachoroidal transretinal stimulation with femtosecond laser-induced porosity and smooth-surface electrodes. Sens. Mater. 2018, 30, 235. [Google Scholar] [CrossRef]
- Noda, T.; Nakano, Y.; Terasawa, Y.; Haruta, M.; Sasagawa, K.; Tokuda, T.; Ohta, J. Performance improvement and in vivo demonstration of a sophisticated retinal stimulator using smart electrodes with built-in CMOS microchips. Jpn. J. Appl. Phys. 2018, 57, 1002B3. [Google Scholar] [CrossRef]
- Tso, K.C.; Fukunari, Y.; Hagita, M.; Haruta, M.; Takehara, H.; Tashiro, H.; Sasagawa, K.; Wu, P.W.; Ohta, J. Honeycomb-type retinal device using chemically derived iridium oxide biointerfaces. AIP Adv. 2021, 11, 095305. [Google Scholar] [CrossRef]
- Pan, K.; Hagiwara, T.; Tso, K.C.; Siwadamrongpong, R.; Akbar, L.; Nakano, Y.; Kono, T.; Terasawa, Y.; Haruta, M.; Takehara, H.; et al. A flexible retinal device with CMOS smart electrodes fabricated on parylene C thin-film and bioceramic substrate. Jpn. J. Appl. Phys. 2023, 62, SC1022. [Google Scholar] [CrossRef]
- Nakano, Y.; Terasawa, Y.; Kanda, H.; Ohta, J.; Sawai, H.; Fujikado, T. Effects of an asymmetric electrical pulse on retinal excitement for retinal prostheses. Sens. Mater. 2018, 30, 315. [Google Scholar] [CrossRef]
- Ortigoza-Diaz, J.; Scholten, K.; Larson, C.; Cobo, A.; Hudson, T.; Yoo, J.; Baldwin, A.; Weltman Hirschberg, A.; Meng, E. Techniques and Considerations in the Microfabrication of Parylene C Microelectromechanical Systems. Micromachines 2018, 9, 422. [Google Scholar] [CrossRef]
- Nanbakhsh, K.; Van Gompel, M.; Ritasalo, R.; Gollhardt, A.; Horváth, D.; Tóth, K.; Meszéna, D.; Ulbert, I.; Serdijn, W.; Giagka, V. An In Vivo Biostability Evaluation of ALD and Parylene-ALD Multilayers as Micro-Packaging Solutions for Small Single-Chip Implants. Small 2025, 21, 2410141. [Google Scholar] [CrossRef]
- Wong, Y.T.; Chen, S.C.; Seo, J.M.; Morley, J.W.; Lovell, N.H.; Suaning, G.J. Focal activation of the feline retina via a suprachoroidal electrode array. Vision Res. 2009, 49, 825. [Google Scholar] [CrossRef]
- Villalobos, J.; Fallon, J.B.; Nayagam, D.A.X.; Shivdasani, M.N.; Luu, C.D.; Allen, P.J.; Shepherd, R.K.; Williams, C.E. Cortical activation following chronic passive implantation of a wide-field suprachoroidal retinal prosthesis. J. Neural Eng. 2014, 11, 046017. [Google Scholar] [CrossRef]
- Liang, T.; Zhao, L.; Sui, X.; Zhou, C.; Ren, Q.; Qi, Y. Threshold suprachoroidal-transretinal stimulation current required by different-size electrodes in rabbit eyes. Ophthal. Res. 2011, 45, 113. [Google Scholar] [CrossRef]
- Xie, H.; Wang, Y.; Ye, Z.; Fang, S.; Xu, Z.; Wu, T.; Chan, L.L.H. Monitoring cortical response and electrode-retina impedance under epiretinal stimulation in rats. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 1178. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Sui, X.; Liu, W.; Lu, Y.; Cao, P.; Ma, Z.; Chen, Y.; Chai, X.; Li, L. Spatial characteristics of evoked potentials elicited by a MEMS microelectrode array for suprachoroidal-transretinal stimulation in a rabbit. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 1515. [Google Scholar] [CrossRef]
- Chowdhury, V.; Morley, J.W.; Coroneo, M.T. Stimulation of the retina with a multielectrode extraocular visual prosthesis. ANZ J. Surg. 2005, 75, 697. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tso, K.-C.; Sunaga, Y.; Nakanishi, Y.; Terasawa, Y.; Haruta, M.; Sasagawa, K.; Ohta, J. Development and Evaluation of Multi-Module Retinal Devices for Artificial Vision Applications. Micromachines 2025, 16, 580. https://doi.org/10.3390/mi16050580
Tso K-C, Sunaga Y, Nakanishi Y, Terasawa Y, Haruta M, Sasagawa K, Ohta J. Development and Evaluation of Multi-Module Retinal Devices for Artificial Vision Applications. Micromachines. 2025; 16(5):580. https://doi.org/10.3390/mi16050580
Chicago/Turabian StyleTso, Kuang-Chih, Yoshinori Sunaga, Yuki Nakanishi, Yasuo Terasawa, Makito Haruta, Kiyotaka Sasagawa, and Jun Ohta. 2025. "Development and Evaluation of Multi-Module Retinal Devices for Artificial Vision Applications" Micromachines 16, no. 5: 580. https://doi.org/10.3390/mi16050580
APA StyleTso, K.-C., Sunaga, Y., Nakanishi, Y., Terasawa, Y., Haruta, M., Sasagawa, K., & Ohta, J. (2025). Development and Evaluation of Multi-Module Retinal Devices for Artificial Vision Applications. Micromachines, 16(5), 580. https://doi.org/10.3390/mi16050580







