ZnFe2O4/GQDs Nanoparticles as Peroxidase Mimics for Sensitive and Selective Colorimetric Detection of Glucose in Real Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
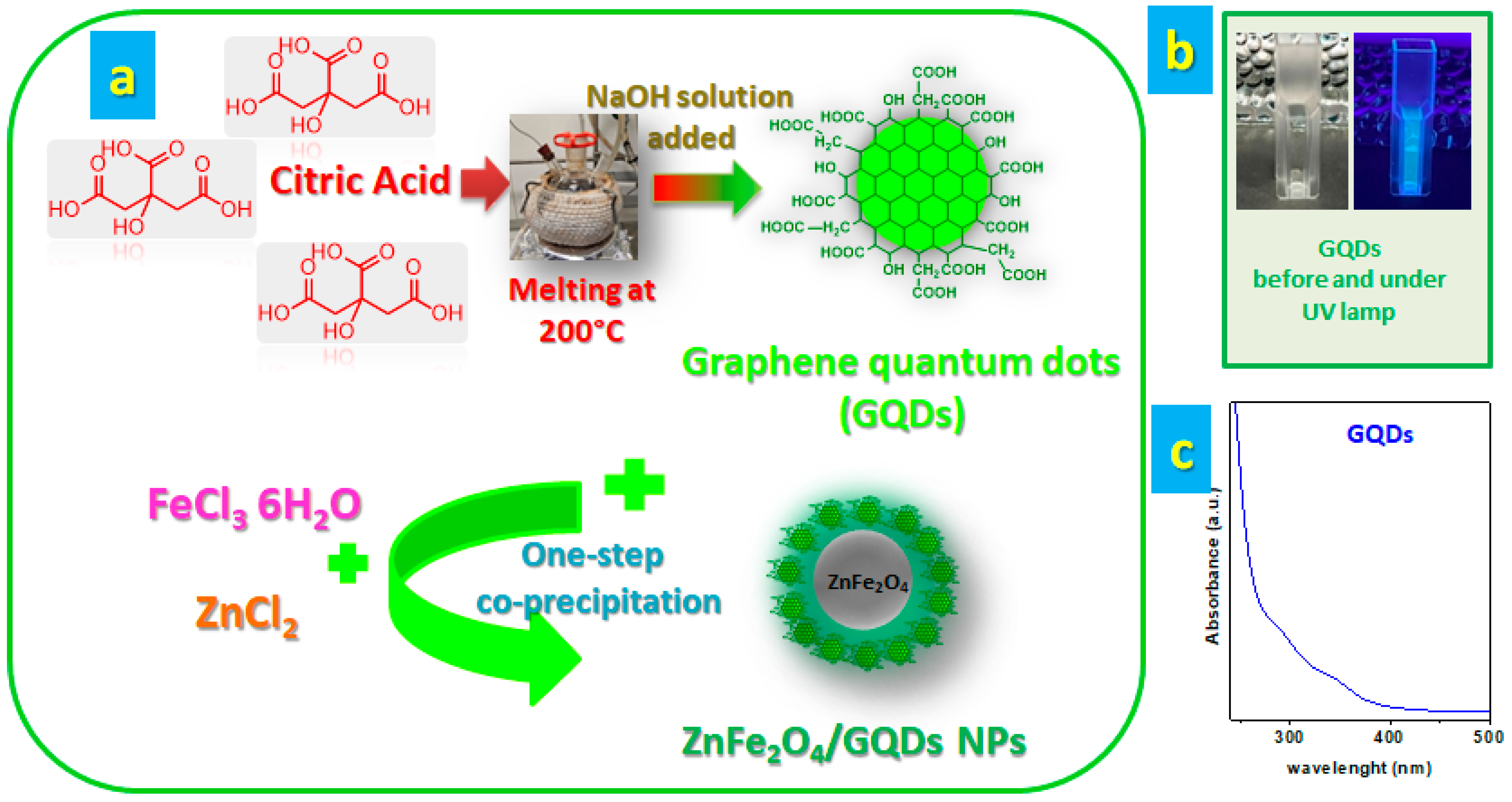
2.2. Synthesis of GQDs
2.3. Synthesis of ZnFe2O4/GQDs NPs
2.4. Characterization Techniques
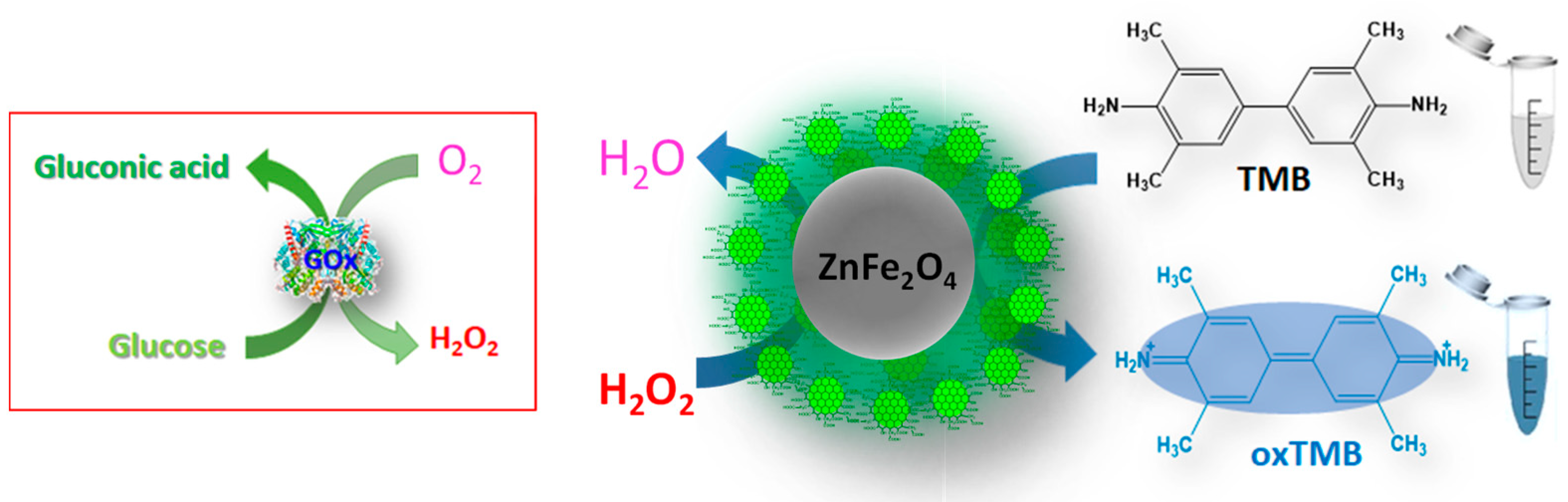
2.5. The Peroxidase-like Catalytic Activity of ZnFe2O4@GQDs NPs
2.6. Steady-State Kinetic Analysis of ZnFe2O4/GQDs NPs
2.7. Active Species Capturing
2.8. H2O2 and Glucose Colorimetric Detection
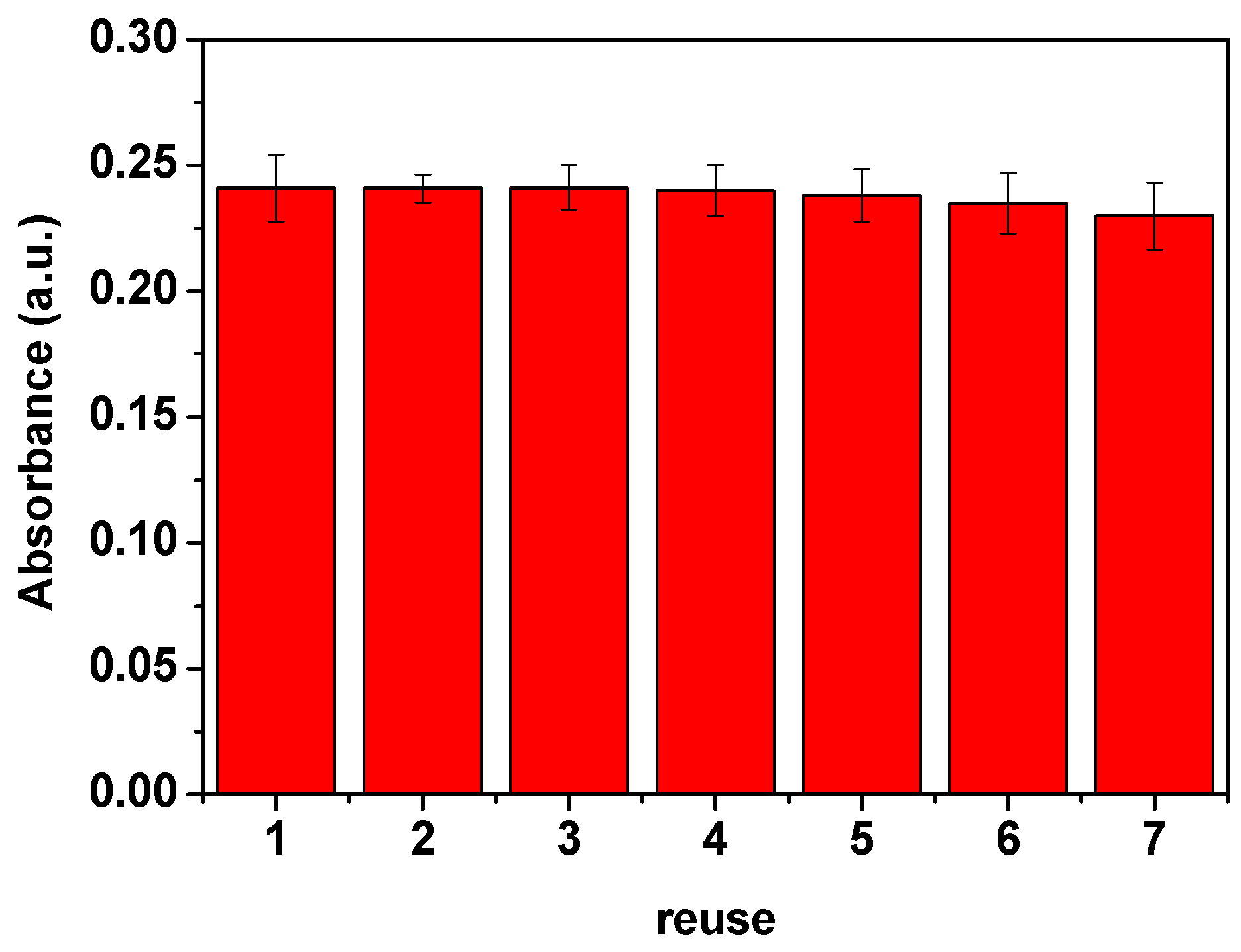
2.9. Reusability Test
3. Result and Discussion
3.1. Preparation of ZnFe2O4/GQDs Nanocomposites
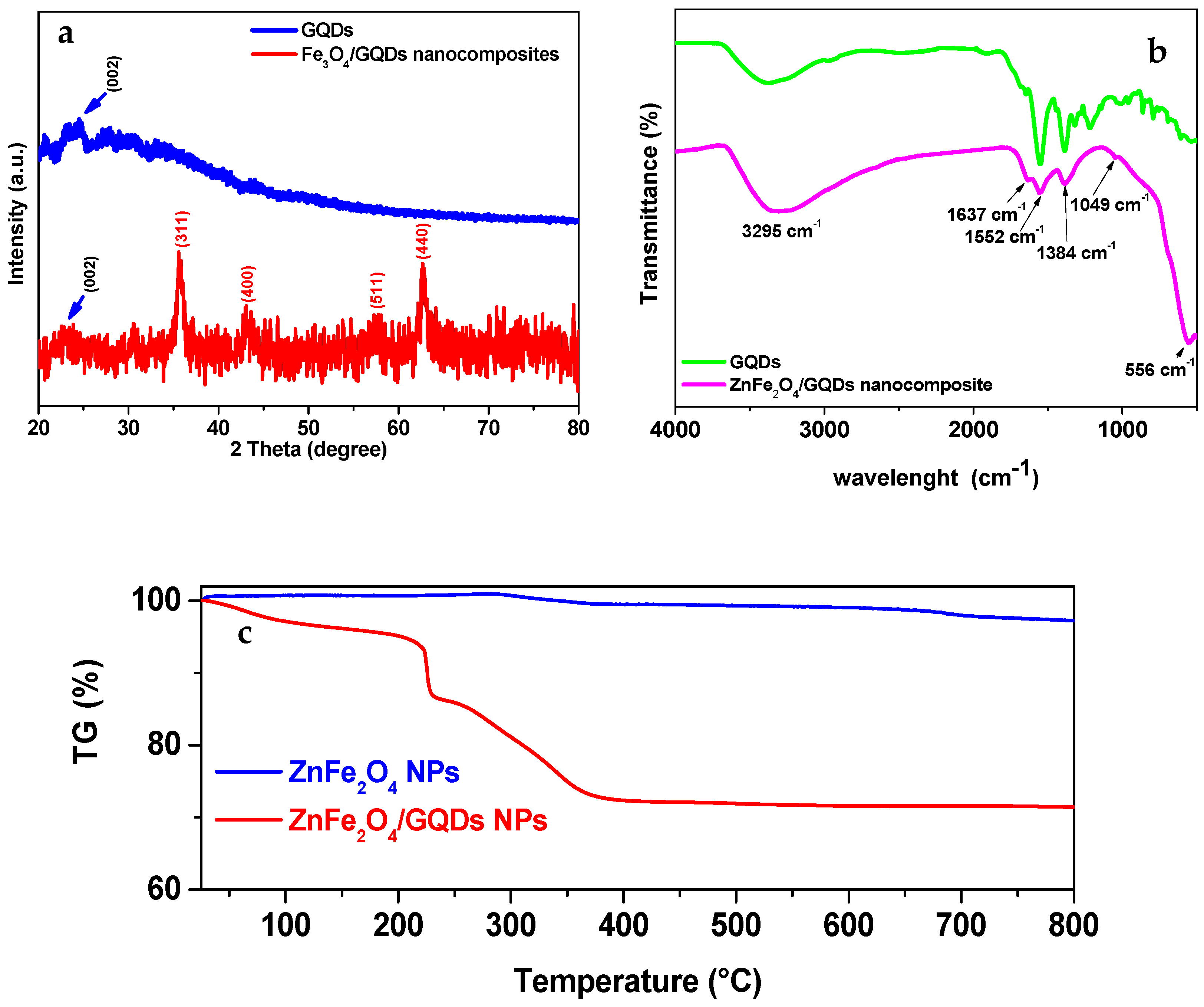
3.2. X-Ray Diffraction Studies
3.3. FT-IR Studies
3.4. Morphological Characterization
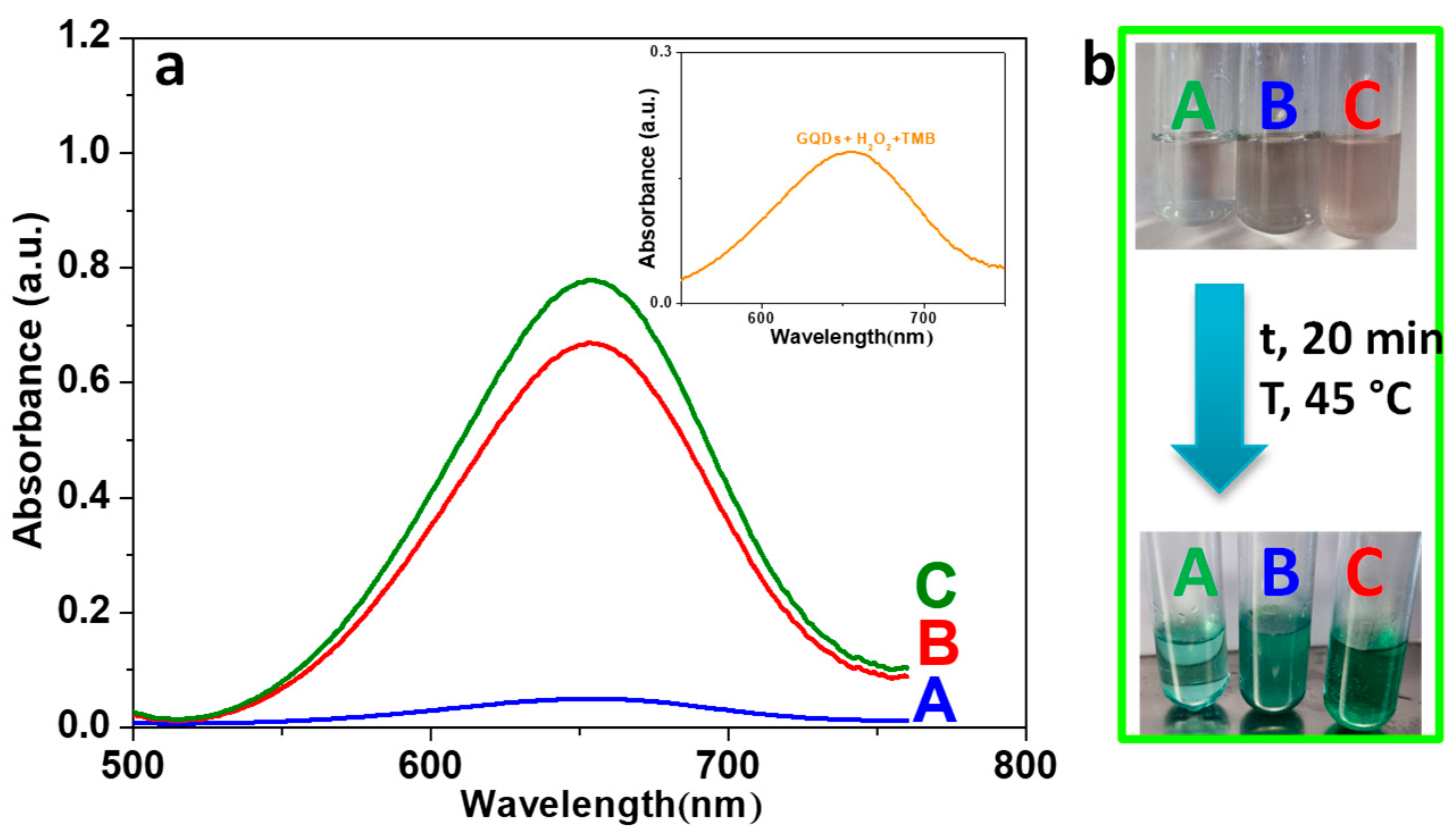
3.5. Peroxidase-like Activity of ZnFe2O4/GQDs Nanoparticles
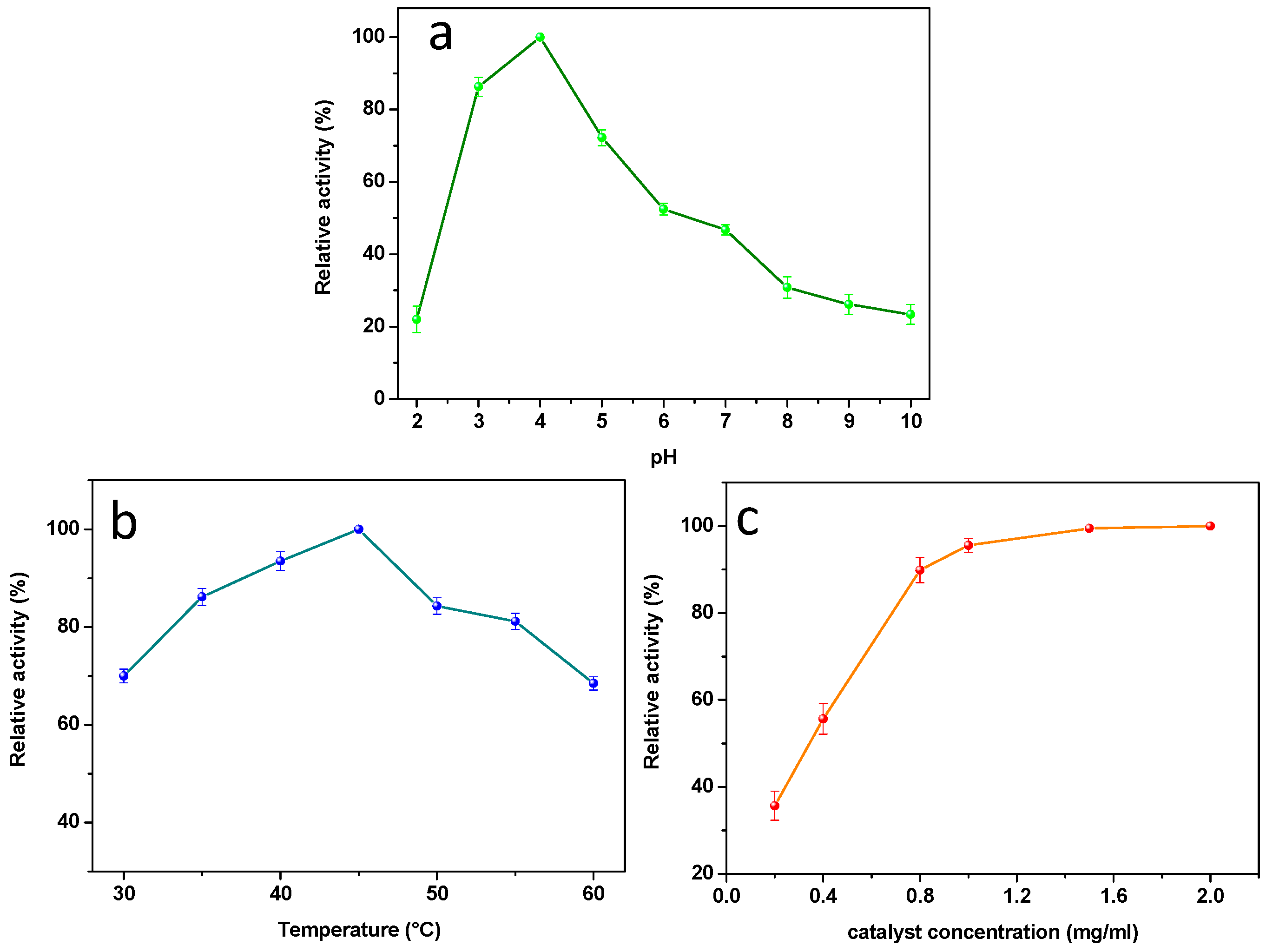
3.6. Experimental Conditions Optimization
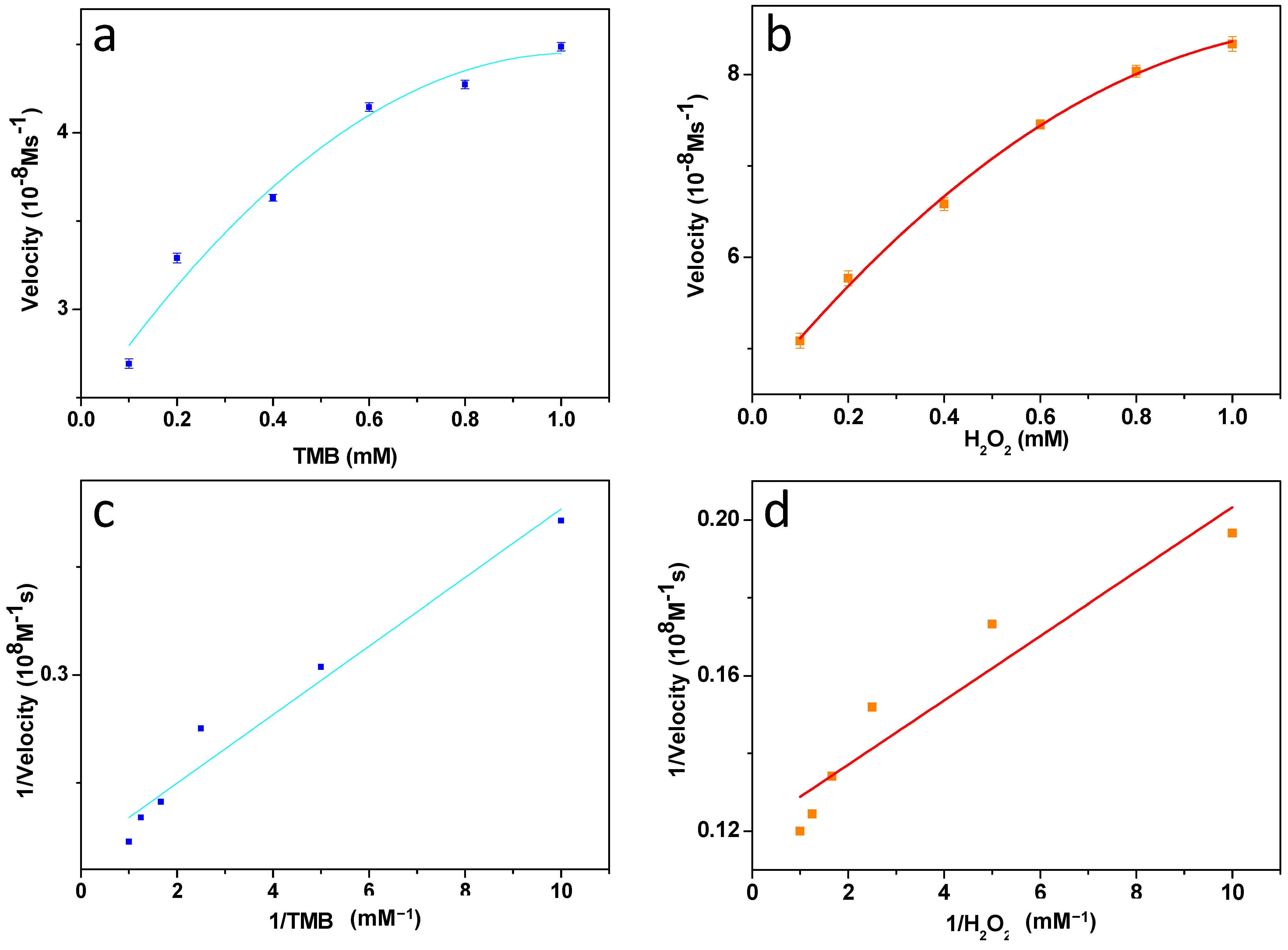
3.7. Steady-State Kinetic Assay of ZnFe2O4/GQDs Nanoparticles
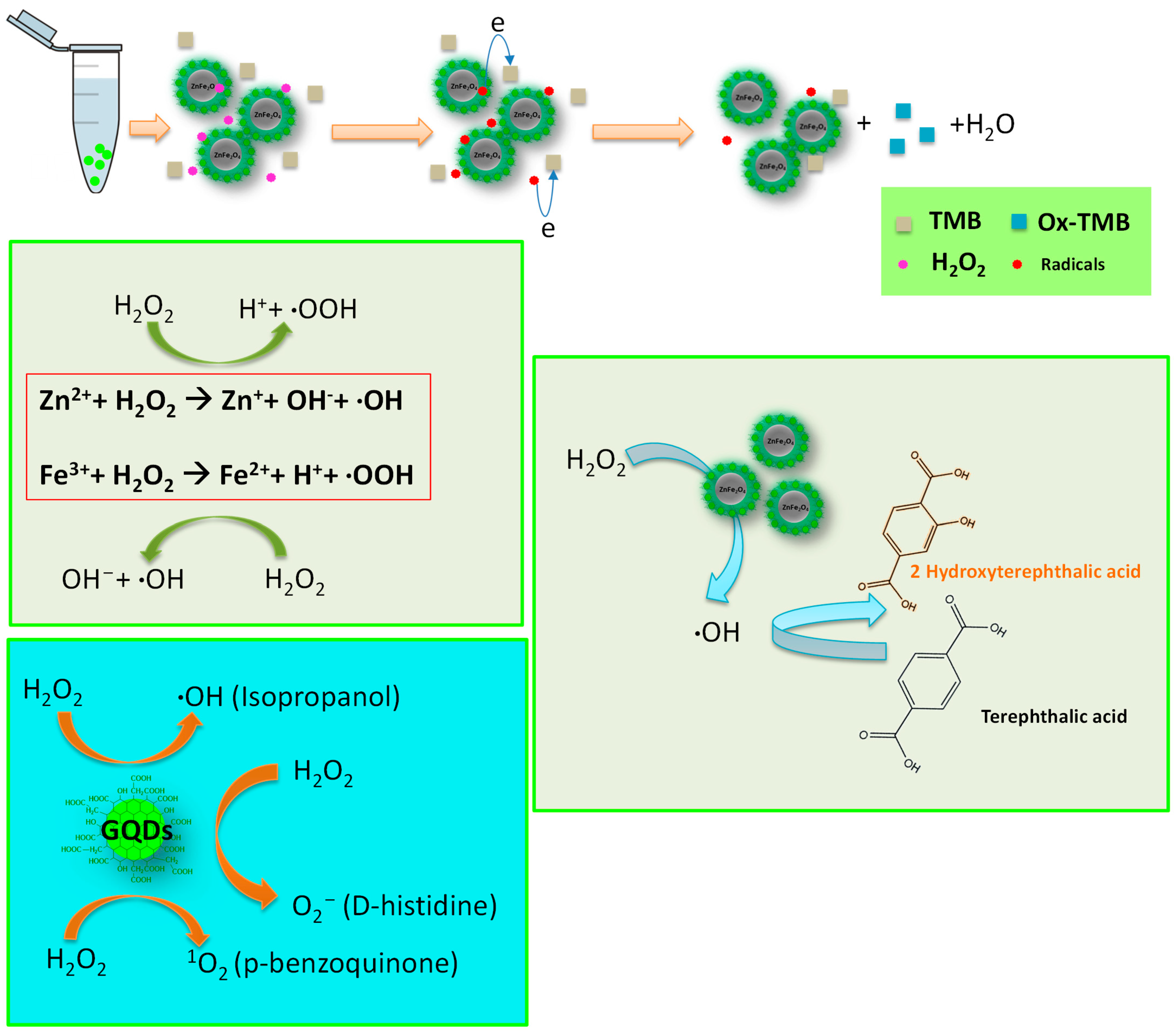
3.8. Catalytic Mechanism
| Catalyst | Substrate | Vmax (×10−8 M/s) | Km (mM) | References |
|---|---|---|---|---|
| Fe3O4@NH2-MIL-101(Fe) | TMB | 10.7 | 0.246 | [77] |
| Fe3O4@NH2-MIL-101(Fe) | H2O2 | 3.65 | 0.105 | [77] |
| HRP | TMB | 10.0 | 0.434 | [19] |
| HRP | H2O2 | 8.71 | 3.70 | [19] |
| Fe3O4 | TMB | 3.44 | 0.0980 | [19] |
| Fe3O4 | H2O2 | 9.78 | 154 | [19] |
| GO-Fe3O4 | TMB | 13.08 | 0.430 | [78] |
| GO-Fe3O4 | H2O2 | 5.31 | 0.710 | [78] |
| CuNPs@C | TMB | 12.1 | 1.65 | [79] |
| CuNPs@C | H2O2 | 5.30 | 1.89 | [79] |
| oS2- Pt74Ag26 | TMB | 7.29 | 25.7 | [86] |
| MoS2- Pt74Ag26 | H2O2 | 3.22 | 0.386 | [80] |
| CDs@Fe3O4 | TMB | 66.67 | 0.17 | [65] |
| CDs@Fe3O4 | H2O2 | 2.21 | 3.16 | [65] |
| FeSe film | TMB | 8.90 | 0.04 | [74] |
| FeSe film | H2O2 | 15.40 | 13.20 | [74] |
| Fe3O4@CeO2 NCs | TMB | 0.64 | 0.15 | [81] |
| Fe3O4@CeO2 NCs | H2O2 | 12.5 | 1.13 | [81] |
| ZnFe2O4 | TMB | 13.31 | 0.85 | [23] |
| ZnFe2O4 | H2O2 | 7.74 | 1.66 | [22] |
| 5-Fe-MSN | TMB | 0.331 | 0.122 | [76] |
| 5-Fe-MSN | H2O2 | 0.3267 | 6.67 | [76] |
| N-GQDs | TMB | 0.38 | 11.90 | [45] |
| N-GQDs | H2O2 | 0.14 | 0.1 | [45] |
| o-GQDs | TMB | 8.389 | 0.1858 | [44] |
| o-GQDs | H2O2 | 7.75 | 0.1363 | [44] |
| ZnFe2O4/GQDs NPs | TMB | 4.58 | 0.072 | This work |
| ZnFe2O4/GQDs NPs | H2O2 | 8.29 | 0.068 | This work |
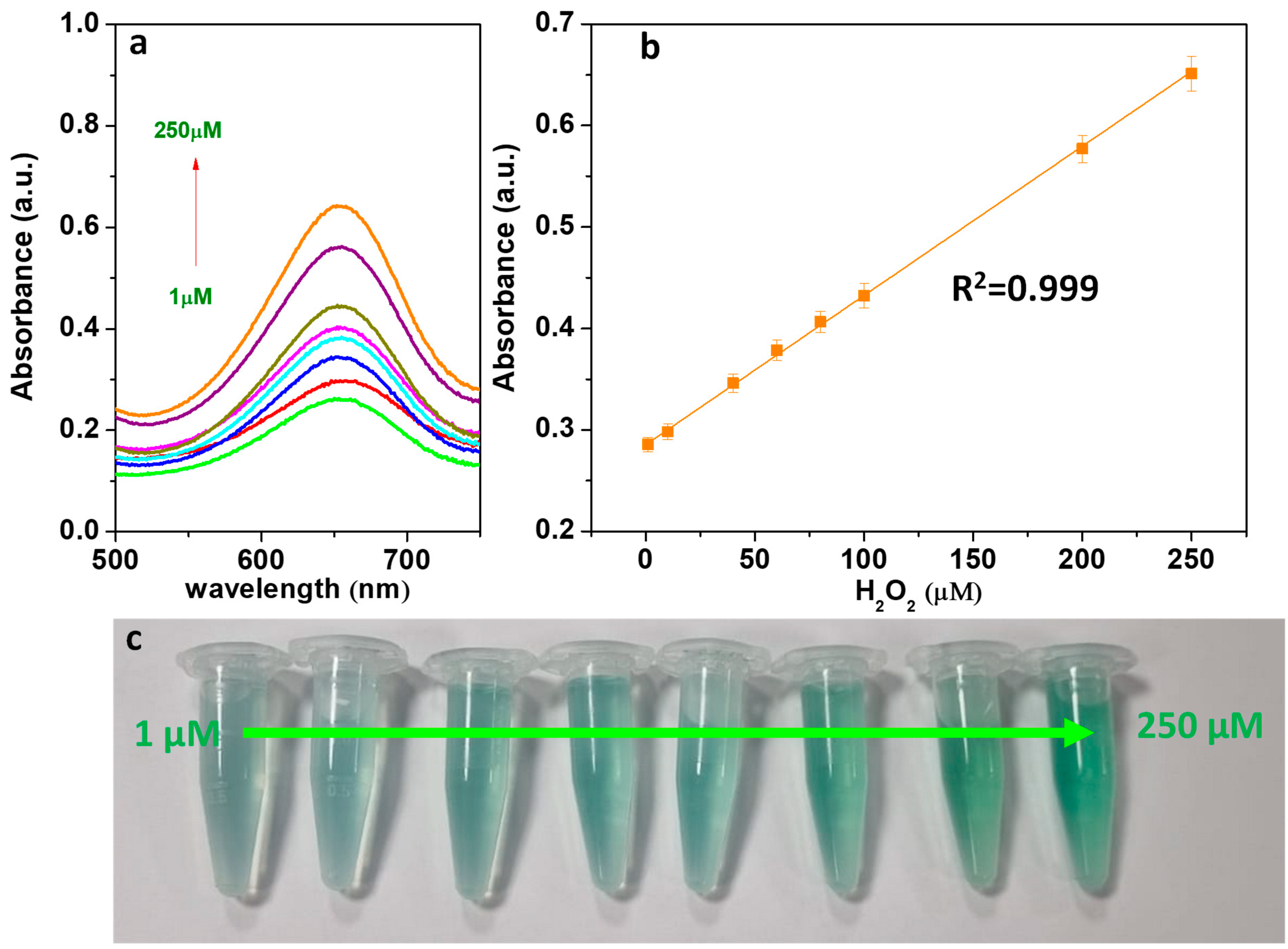
3.9. Detection of H2O2 and Glucose
3.10. Analysis of Glucose in Real Blood Samples
3.11. Reusability of ZnFe2O4@GQDs Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.C.; Lee, A. Recent developments in blood glucose sensors. J. Food Drug Anal. 2015, 23, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Galant, A.; Kaufman, R.C.; Wilson, J.D. Glucose: Detection and analysis. Food Chem. 2015, 188, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Goran, J.M.; Mantilla, S.M.; Stevenson, K.J. Influence of surface adsorption on the interfacial electron transfer of flavin adenine dinucleotide and glucose oxidase at carbon nanotube and Nitrogen-Doped carbon nanotube electrodes. Anal. Chem. 2013, 85, 1571–1581. [Google Scholar] [CrossRef]
- Li, H.; Liu, C.; Wang, D.; Zhang, C. Chemiluminescence cloth-based glucose test sensors (CCGTSs): A new class of chemiluminescence glucose sensors. Biosens. Bioelectron. 2017, 91, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Weng, J. Enhanced peroxidase-like activity of MoS2/graphene oxide hybrid with light irradiation for glucose detection. Biosens. Bioelectron. 2017, 89, 652–658. [Google Scholar] [CrossRef]
- Lin, T.; Zhong, L.; Guo, L.; Fu, F.; Chen, G. Seeing diabetes: Visual detection of glucose based on the intrinsic peroxidase-like activity of MoS2 nanosheets. Nanoscale 2014, 6, 11856–11862. [Google Scholar] [CrossRef]
- Jiang, D.; Chu, Z.; Peng, J.; Luo, J.; Mao, Y.; Yang, P.; Jin, W. One-step synthesis of three-dimensional Co(OH)2/rGO nano-flowers as enzyme-mimic sensors for glucose detection. Electrochim. Acta 2018, 270, 147–155. [Google Scholar] [CrossRef]
- Xie, W.Q.; Gong, Y.X.; Yu, K.X. Rapid quantitative detection of glucose content in glucose injection by reaction headspace gas chromatography. J. Chromatogr. A 2017, 1520, 143–146. [Google Scholar] [CrossRef]
- Dehghan, G.; Shaghaghi, M.; Alizadeh, P. A novel ultrasensitive and non-enzymatic “turn-on-off” fluorescence nanosensor for direct determination of glucose in the serum: As an alternative approach to the other optical and electrochemical methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 214, 459–468. [Google Scholar] [CrossRef]
- Darabdhara, G.; Bordoloi, J.; Manna, P.; Das, M.R. Biocompatible bimetallic Au-Ni doped graphitic carbon nitride sheets: A novel peroxidase-mimicking artificial enzyme for rapid and highly sensitive colorimetric detection of glucose. Sens. Actuators B Chem. 2019, 285, 277–290. [Google Scholar] [CrossRef]
- Liu, T.; Wu, L.; Zai, Y.; Zhang, Y.; Su, E.; Gu, N. Paper-based colorimetric glucose sensor using Prussian blue nanoparticles as mimic peroxidase. Biosens. Bioelectron. 2023, 219, 114787. [Google Scholar] [CrossRef]
- Cheng, X.; Huang, L.; Yang, X.; Elzatahry, A.A.; Alghamdi, A.; Deng, Y. Rational design of a stable peroxidase mimic for colorimetric detection of H2O2 and glucose: A synergistic CeO2/Zeolite Y nanocomposite. J. Colloid Interface Sci. 2019, 535, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Yu, J.; Lv, F.; Yan, L.; Zheng, L.R.; Gu, Z.; Zhao, Y. Functionalized Nano-MoS2 with Peroxidase Catalytic and Near-Infrared Photothermal Activities for Safe and Synergetic Wound Antibacterial Applications. ACS Nano. 2016, 10, 11000–11011. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060. [Google Scholar] [CrossRef]
- Jangi, A.R.H.; Jangi, M.R.H.; Jangi, S.R.H. Detection mechanism and classification of design principles of peroxidase mimic based colorimetric sensors: A brief overview. Chin. J. Chem. Eng. 2020, 28, 1492–1503. [Google Scholar] [CrossRef]
- Cirillo, C.; Iuliano, M.; Navarrete Astorga, E.; Sarno, M. Structural tuning of MgFe2O4/PVP@ZIF-67@Ag nanozymes for colorimetric detection of sulfide ions. Microchem. J. 2025, 212, 113381. [Google Scholar] [CrossRef]
- Sarno, M.; Galvagno, S.; Scudieri, C.; Borriello, C.; Cirillo, C. Dopamine sensor in real sample based on thermal plasma silicon carbide nanopowders. J. Phys. Chem. Solids 2019, 131, 213–222. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, X.; He, S.; Huang, Y. CoFe2O4 magnetic nanoparticles as a peroxidase mimic mediated chemiluminescence for hydrogen peroxide and glucose. Chem. Commun. 2011, 47, 10785. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Song, H.; Zhu, L.; Li, Y.; Lou, Z.; Xiao, M.; Ye, Z. Preparation of ZnFe2O4 nanostructures and highly efficient visible-light-driven hydrogen generation with the assistance of nanoheterostructures. J. Mater. Chem. A Mater. Energy Sustain. 2015, 3, 8353–8360. [Google Scholar] [CrossRef]
- Šutka, A.; Zavickis, J.; Mežinskis, G.; Jakovļevs, D.; Barloti, J. Ethanol monitoring by ZnFe2O4 thin film obtained by spray pyrolysis. Sens. Actuators B Chem. 2013, 176, 330–334. [Google Scholar] [CrossRef]
- Wan, J.; Jiang, X.; Li, H.; Chen, K. Facile synthesis of zinc ferrite nanoparticles as non-lanthanide T1 MRI contrast agents. J. Mater. Chem. 2012, 22, 13500. [Google Scholar] [CrossRef]
- Su, L.; Feng, J.; Zhou, X.; Ren, C.; Li, H.; Chen, X. Colorimetric detection of urine glucose based ZNFE2O4 magnetic nanoparticles. Anal. Chem. 2012, 84, 5753–5758. [Google Scholar] [CrossRef] [PubMed]
- Mokoloko, L.L.; Forbes, R.P.; Coville, N.J. The behavior of carbon dots in catalytic reactions. Catalysts 2023, 13, 1201. [Google Scholar] [CrossRef]
- Baker, S.N.; Baker, G.A. Luminescent Carbon NanoDots: Emergent Nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef]
- Sharma, A.; Das, J. Small molecules derived carbon dots: Synthesis and applications in sensing, catalysis, imaging, and biomedicine. J. Nanobiotechnol. 2019, 17, 92. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F. The role of carbon materials in heterogeneous catalysis. Carbon 1998, 36, 159–175. [Google Scholar] [CrossRef]
- Berseth, P.A.; Harter, A.G.; Zidan, R.; Blomqvist, A.; Araújo, C.M.; Scheicher, R.H.; Ahuja, R.; Jena, P. Carbon nanomaterials as catalysts for hydrogen uptake and release in NAALH4. Nano Lett. 2009, 9, 1501–1505. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, C.; Zheng, X.; Gao, L.; Cui, Z.; Yang, H.; Guo, C.; Chi, Y.; Li, C.M. One-step and high yield simultaneous preparation of single- and multi-layer graphene quantum dots from CX-72 carbon black. J. Mater. Chem. 2012, 22, 8764. [Google Scholar] [CrossRef]
- Ahmadian-Fard-Fini, S.; Ghanbari, D.; Amiri, O.; Salavati-Niasari, M. Electro-spinning of cellulose acetate nanofibers/Fe/carbon dot as photoluminescence sensor for mercury (II) and lead (II) ions. Carbohydr. Polym. 2020, 229, 115428. [Google Scholar] [CrossRef]
- Prakash, S.H.; Roopan, S.M. A comprehensive review on recent developments in the graphene quantum dot framework for organic transformations. J. Organomet. Chem. 2023, 997, 122790. [Google Scholar] [CrossRef]
- Shen, J.; Zhu, Y.; Yang, X.; Zong, J.; Zhang, J.; Li, C. One-pot hydrothermal synthesis of graphenequantum dots surface-passivated by polyethylene glycol and their photoelectric conversion under near-infrared light. New J. Chem. 2012, 36, 97–101. [Google Scholar] [CrossRef]
- Dwivedi, N.; Kumar, S.; Malik, H.K.; Govind Rauthan, C.M.S.; Panwar, O.S. Correlation of sp3 and sp2 fraction of carbon with electrical, optical and nano-mechanical properties of argon-diluted diamond-like carbon films. Appl. Surf. Sci. 2011, 257, 6804–6810. [Google Scholar] [CrossRef]
- Zhu, S.; Bai, X.; Wang, T.; Shi, Q.; Zhu, J.; Wang, B. One-step synthesis of fluorescent graphene quantum dots as an effective fluorescence probe for vanillin detection. RSC Adv. 2021, 11, 9121–9129. [Google Scholar] [CrossRef]
- Liu, W.; Li, M.; Jiang, G.; Li, G.; Zhu, J.; Xiao, M.; Zhu, Y.; Gao, R.; Yu, A.; Feng, M.; et al. Graphene Quantum Dots-Based Advanced Electrode Materials: Design, synthesis and their applications in electrochemical energy storage and electrocatalysis. Adv. Energy Mater. 2020, 10, 2001275. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Chen, N.; Qu, L. Graphene quantum dots: An emerging material for energy-related applications and beyond. Energy Environ. Sci. 2012, 5, 8869. [Google Scholar] [CrossRef]
- Bak, S.; Kim, D.; Lee, H. Graphene quantum dots and their possible energy applications: A review. Curr. Appl. Phys. 2016, 16, 1192–1201. [Google Scholar] [CrossRef]
- Tetsuka, H. 2D/0D graphene hybrids for visible-blind flexible UV photodetectors. Sci. Rep. 2017, 7, 5544. [Google Scholar] [CrossRef]
- Tajik, S.; Dourandish, Z.; Zhang, K.; Beitollahi, H.; Van Le, Q.; Jang, H.W.; Shokouhimehr, M. Carbon and graphene quantum dots: A review on syntheses, characterization, biological and sensing applications for neurotransmitter determination. RSC Adv. 2020, 10, 15406–15429. [Google Scholar] [CrossRef]
- Younis, M.R.; He, G.; Lin, J.; Huang, P. Recent advances on graphene quantum dots for bioimaging applications. Front. Chem. 2020, 8, 424. [Google Scholar] [CrossRef]
- Xu, A.; He, P.; Ye, C.; Liu, Z.; Gu, B.; Gao, B.; Li, Y.; Dong, H.; Chen, D.; Wang, G.; et al. Polarizing Graphene Quantum Dots toward Long-Acting Intracellular Reactive Oxygen Species Evaluation and Tumor Detection. ACS Appl. Mater. Interfaces 2020, 12, 10781–10790. [Google Scholar] [CrossRef]
- Tong, X.; Wei, Q.; Zhan, X.; Zhang, G.; Sun, S. The new Graphene Family Materials: Synthesis and applications in oxygen reduction reaction. Catalysts 2016, 7, 1. [Google Scholar] [CrossRef]
- Wang, H.; Liu, C.; Liu, Z.; Ren, J.; Qu, X. Specific oxygenated groups enriched graphene quantum dots as highly efficient enzyme mimics. Small 2018, 14, 1703710. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Song, X.; Chen, Y.; Rong, M.; Zhao, T.; Wang, Y.; Jiang, Y.; Chen, X. Intrinsic peroxidase-like catalytic activity of nitrogen-doped graphene quantum dots and their application in the colorimetric detection of H2O2 and glucose. Anal. Chim. Acta 2015, 869, 89–95. [Google Scholar] [CrossRef]
- Ouyang, B.; Zhong, Q.; Ouyang, P.; Yuan, Y.; Wu, X.; Yang, S. Graphene quantum dots enhance the biological nitrogen fixation by up-regulation of cellular metabolism and electron transport. Chem. Eng. J. 2024, 487, 150694. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, C.; Zhou, X.; Wu, X.; Yang, Y.; Wu, H.; Guo, S.; Zhang, J. Graphene quantum dots/gold electrode and its application in living cell H2O2 detection. Nanoscale 2013, 5, 1816. [Google Scholar] [CrossRef]
- Dutta, A.K.; Maji, S.K.; Biswas, P.; Adhikary, B. New peroxidase-substrate 3,5-di-tert-butylcatechol for colorimetric determination of blood glucose in presence of Prussian Blue-modified iron oxide nanoparticles. Sens. Actuators B Chem. 2013, 177, 676–683. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Z.-G.; Cole, I.; Li, Q. Structural evolution of graphene quantum dots during thermal decomposition of citric acid and the corresponding photoluminescence. Carbon 2015, 82, 304–313. [Google Scholar] [CrossRef]
- Arvand, M.; Hemmati, S. Magnetic nanoparticles embedded with graphene quantum dots and multiwalled carbon nanotubes as a sensing platform for electrochemical detection of progesterone. Sens. Actuators B Chem. 2017, 238, 346–356. [Google Scholar] [CrossRef]
- Li, J.; Qiu, X.; Lin, Y.; Liu, X.; Gao, R.; Wang, A. A study of modified Fe3O4 nanoparticles for the synthesis of ionic ferrofluids. Appl. Surf. Sci. 2010, 256, 6977–6981. [Google Scholar] [CrossRef]
- Zhang, X.; Gong, S.W.; Zhang, Y.; Yang, T.; Wang, C.; Gu, N. Prussian blue modified iron oxide magnetic nanoparticles and their high peroxidase-like activity. J. Mater. Chem. 2010, 20, 5110. [Google Scholar] [CrossRef]
- Wang, F.; Liao, L.; Liu, X.; Zhang, J.; Wu, F. Porphyrin-based porous organic frameworks as efficient peroxidase mimics for selective detection of hydrogen peroxide and glucose. Inorg. Chem. Commun. 2023, 155, 111011. [Google Scholar] [CrossRef]
- Mu, J.; Wang, Y.; Zhao, M. Intrinsic peroxidase-like activity and catalase-like activity of Co3O4 nanoparticles. Chem. Commun. 2012, 48, 2540. [Google Scholar] [CrossRef] [PubMed]
- Kansara, V.; Shukla, R.; Flora, S.J.S.; Bahadur, P.; Tiwari, S. Graphene quantum dots: Synthesis, optical properties and navigational applications against cancer. Mater. Today Commun. 2022, 31, 103359. [Google Scholar] [CrossRef]
- Zhang, M.; Bai, L.; Shang, W.; Xie, W.; Ma, H.; Fu, Y.; Fang, D.; Sun, H.; Fan, L.; Han, M.; et al. Facile synthesis of water-soluble, highly fluorescent graphene quantum dots as a robust biological label for stem cells. J. Mater. Chem. 2012, 22, 7461. [Google Scholar] [CrossRef]
- Vinosha, P.A.; Mely, L.A.; Jeronsia, J.E.; Krishnan, S.; Das, S.J. Synthesis and properties of spinel ZnFe2O4 nanoparticles by facile co-precipitation route. Optik 2017, 134, 99–108. [Google Scholar] [CrossRef]
- Pooresmaeil, M.; Namazi, H. pH-sensitive ternary Fe3O4/GQDs@G hybrid microspheres; Synthesis, characterization, and drug delivery application. J. Alloys Compd. 2020, 846, 156419. [Google Scholar] [CrossRef]
- Alvand, M.; Shemirani, F. A Fe3O4@SiO2@graphene quantum dot core-shell structured nanomaterial as a fluorescent probe and for magnetic removal of mercury(II) ion. Microchim. Acta 2017, 184, 1621–1629. [Google Scholar] [CrossRef]
- Arvand, M.; Abbasnejad, S.; Ghodsi, N. Graphene quantum dots decorated with Fe3O4 nanoparticles/functionalized multiwalled carbon nanotubes as a new sensing platform for electrochemical determination of l-DOPA in agricultural products. Anal. Methods 2016, 8, 5861–5868. [Google Scholar] [CrossRef]
- Zhang, L.; Hai, X.; Xia, C.; Chen, X.; Wang, J. Growth of CuO nanoneedles on graphene quantum dots as peroxidase mimics for sensitive colorimetric detection of hydrogen peroxide and glucose. Sens. Actuators B Chem. 2017, 248, 374–384. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Y.; Han, T.; Wu, H.; Guo, S.; Zhang, J. Composite of graphene quantum dots and Fe3O4nanoparticles: Peroxidase activity and application in phenolic compound removal. RSC Adv. 2014, 4, 3299–3305. [Google Scholar] [CrossRef]
- Chen, Q.; Ma, X.; Xie, L.; Chen, W.; Xu, Z.; Song, E.; Zhu, X.; Song, Y. Iron-based nanoparticles for MR imaging-guided ferroptosis in combination with photodynamic therapy to enhance cancer treatment. Nanoscale 2021, 13, 4855–4870. [Google Scholar] [CrossRef]
- Chen, S.; Hai, X.; Chen, X.; Wang, J. In situ growth of silver nanoparticles on graphene quantum dots for ultrasensitive colorimetric detection of H2O2 and glucose. Anal. Chem. 2014, 86, 6689–6694. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.; Shepherd, H.; Boggavarapu, K.; Paudyal, J. Colorimetric detection of dopamine based on peroxidase-like activity of Β-CD functionalized AUNPs. Molecules 2025, 30, 423. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Zhao, B.; Luo, X.; Wu, F. Glucose-sensitive colorimetric sensor based on peroxidase mimics activity of carbon dots-functionalized Fe3O4 nanocomposites. Diam. Relat. Mater. 2023, 136, 109914. [Google Scholar] [CrossRef]
- Al-Bagmi, M.S.; Khan, M.S.; Ismael, M.A.; Al-Senaidy, A.M.; Bacha, A.B.; Husain, F.M.; Alamery, S.F. An efficient methodology for the purification of date palm peroxidase: Stability comparison with horseradish peroxidase (HRP). Saudi J. Biol. Sci. 2018, 26, 301–307. [Google Scholar] [CrossRef]
- Yin, X.; Liu, P.; Xu, X.; Pan, J.; Li, X.; Niu, X. Breaking the pH limitation of peroxidase-like CoFe2O4 nanozyme via vitriolization for one-step glucose detection at physiological pH. Sens. Actuators B Chem. 2021, 328, 129033. [Google Scholar] [CrossRef]
- Niu, X.; Xu, X.; Li, X.; Pan, J.; Qiu, F.; Zhao, H.; Lan, M. Surface charge engineering of nanosized CuS via acidic amino acid modification enables high peroxidase-mimicking activity at neutral pH for one-pot detection of glucose. Chem. Commun. 2018, 54, 13443–13446. [Google Scholar] [CrossRef]
- He, Y.; Li, X.; Xu, X.; Pan, J.; Niu, X. A cobalt-based polyoxometalate nanozyme with high peroxidase-mimicking activity at neutral pH for one-pot colorimetric analysis of glucose. J. Mater. Chem. B 2018, 6, 5750–5755. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, B.; Wang, M.; Pan, J.; Xu, L.; Hu, P.; Niu, X. Amorphous Fe-Containing Phosphotungstates Featuring Efficient Peroxidase-like Activity at Neutral pH: Toward Portable Swabs for Pesticide Detection with Tandem Catalytic Amplification. Anal. Chem. 2023, 95, 4776–4785. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, H.; Wang, X. Bivalent metal ions tethered fluorescent gold nanoparticles as a reusable peroxidase mimic nanozyme. J. Anal. Test. 2019, 3, 269–276. [Google Scholar] [CrossRef]
- Herrasti, Z.; Martínez, F.R.; Baldrich, E. Detection of uric acid at reversibly nanostructured thin-film microelectrodes. Sens. Actuators B Chem. 2016, 234, 667–673. [Google Scholar] [CrossRef]
- Kavitha, S.; Kala, S.M.J.; Christus, A.a.B.; Ravikumar, A. Colorimetric determination of cysteine and copper based on the peroxidase-like activity of Prussian blue nanocubes. RSC Adv. 2021, 11, 37162–37170. [Google Scholar] [CrossRef]
- Dutta, A.K.; Maji, S.K.; Mondal, A.; Karmakar, B.; Biswas, P.; Adhikary, B. Iron selenide thin film: Peroxidase-like behavior, glucose detection and amperometric sensing of hydrogen peroxide. Sens. Actuators B Chem. 2012, 173, 724–731. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, D.; Li, J.; Guo, S.; Wang, E.; Dong, S. Hemin−Graphene Hybrid Nanosheets with Intrinsic Peroxidase-like Activity for Label-free Colorimetric Detection of Single-Nucleotide Polymorphism. ACS Nano 2011, 5, 1282–1290. [Google Scholar] [CrossRef]
- Kumari, S.; Dhar, B.B.; Panda, C.; Meena, A.; Gupta, S.S. Fe-TAML encapsulated inside mesoporous silica nanoparticles as peroxidase MiMic: Femtomolar protein detection. ACS Appl. Mater. Interfaces 2014, 6, 13866–13873. [Google Scholar] [CrossRef]
- Yao, X.; Si, H.; Sun, D.; Hou, X. Magnetic Fe3O4@NH2-MIL-101(Fe) nanocomposites with peroxidase-like activity for colorimetric detection of glucose. Microchem. J. 2020, 156, 104929. [Google Scholar] [CrossRef]
- Dong, Y.-L.; Zhang, H.-G.; Rahman, Z.U.; Su, L.; Chen, X.-J.; Hu, J.; Chen, X.-G. Graphene oxide–Fe3O4 magnetic nanocomposites with peroxidase-like activity for colorimetric detection of glucose. Nanoscale 2012, 4, 3969. [Google Scholar] [CrossRef]
- Tan, H.; Ma, C.; Gao, L.; Li, Q.; Song, Y.; Xu, F.; Wang, T.; Wang, L. Metal–Organic Framework-Derived Copper Nanoparticle@Carbon nanocomposites as peroxidase mimics for colorimetric sensing of ascorbic acid. Chem. Eur. J. 2014, 20, 16377–16383. [Google Scholar] [CrossRef]
- Cai, S.; Han, Q.; Qi, C.; Lian, Z.; Jia, X.; Yang, R.; Wang, C. Pt74Ag26 nanoparticle-decorated ultrathin MoS2 nanosheets as novel peroxidase mimics for highly selective colorimetric detection of H2O2 and glucose. Nanoscale 2016, 8, 3685–3693. [Google Scholar] [CrossRef]
- Huang, F.; Wang, J.; Chen, W.; Wan, Y.; Wang, X.; Cai, N.; Liu, J.; Yu, F. Synergistic peroxidase-like activity of CeO2-coated hollow Fe3O4 nanocomposites as an enzymatic mimic for low detection limit of glucose. J. Taiwan Inst. Chem. Eng. 2018, 83, 40–49. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, F.; Song, Z.; Webster Na, S.; Luo, H.; Gao, Y. Synthesis and formation mechanism of VO2(A) nanoplates with intrinsic peroxidase-like activity. RSC Adv. 2015, 5, 61371–61379. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Z.; Zhou, Q.; Xu, C.; Hou, J. Nanoporous platinum-copper flowers for non-enzymatic sensitive detection of hydrogen peroxide and glucose at near-neutral pH values. Microchim. Acta 2019, 186, 631. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, X.; Zhao, C.; Qu, K.; Ren, J.; Qu, X. Label-Free colorimetric detection of single nucleotide polymorphism by using Single-Walled carbon nanotube intrinsic Peroxidase-Like activity. Chem. Eur. J. 2010, 16, 3617–3621. [Google Scholar] [CrossRef]
- Kim, J.-H.; Heller, D.A.; Jin, H.; Barone, P.W.; Song, C.; Zhang, J.; Trudel, L.J.; Wogan, G.N.; Tannenbaum, S.R.; Strano, M.S. The rational design of nitric oxide selectivity in single-walled carbon nanotube near-infrared fluorescence sensors for biological detection. Nat. Chem. 2009, 1, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Heller, D.A.; Jin, H.; Martinez, B.M.; Patel, D.; Miller, B.M.; Yeung, T.-K.; Jena, P.V.; Höbartner, C.; Ha, T.; Silverman, S.K.; et al. Multimodal optical sensing and analyte specificity using single-walled carbon nanotubes. Nature Nanotechnol. 2009, 4, 114–120. [Google Scholar] [CrossRef]
- Singh, E.; Kaur, M.; Sharma, S. Structural tuning of CTAB@MgFe2O4 nanocomposite as peroxidase mimic for H2O2 and glucose sensing. Mater. Chem. Phys. 2021, 271, 124851. [Google Scholar] [CrossRef]
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef]
- Hu, T.; Xu, K.; Qiu, S.; Han, Y.; Chen, J.; Xu, J.; Chen, K.; Sun, Z.; Yi, H.; Ni, Z. Colorimetric detection of urine glucose using a C/CdTe QDs–GOx aerogel based on a microfluidic assay sensor. J. Mater. Chem. B 2020, 8, 7160–7165. [Google Scholar] [CrossRef]
- Tian, L.; Zhao, B.; Zhang, J.; Luo, X.; Wu, F. Magnetic covalent organic framework nanospheres with enhanced peroxidase-like activity for colorimetric detection of H2O2 and glucose. Colloid Surf. A Physicochem. Eng. Asp. 2023, 666, 131309. [Google Scholar] [CrossRef]
- Flockhart, M.; Larsen, F.J. Continuous glucose monitoring in Endurance Athletes: Interpretation and relevance of measurements for improving performance and health. Sports Med. 2024, 54, 247–255. [Google Scholar] [CrossRef]

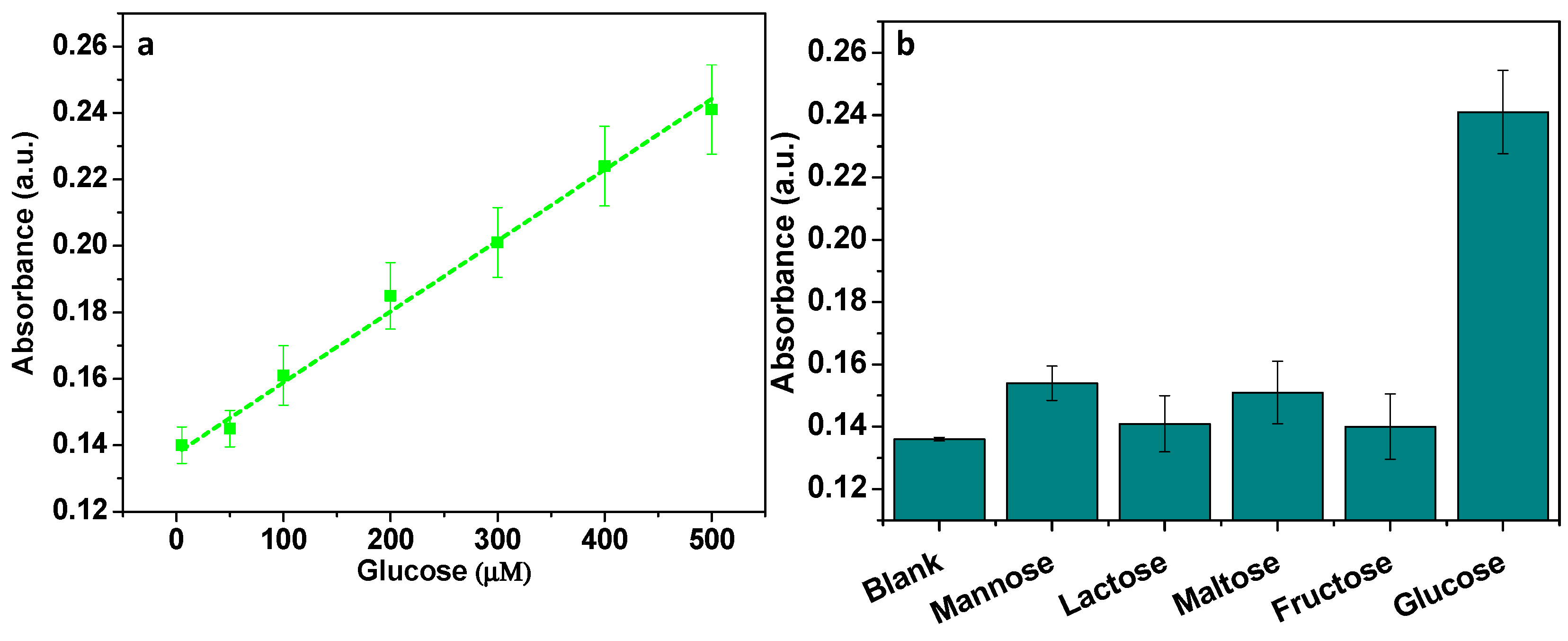
| State-of-the-Art Catalysts | Detection Limit (μM) | Linear Range (μM) | References |
|---|---|---|---|
| CTAB@MgFe2O4 | 5 | 10–1000 | [87] |
| PTB-GOx | 22.2 | 75–7500 | [88] |
| Fe3O4@COFs | 6.0 | 50–900 | [90] |
| C/CdTe | 223 | 0–13,000 | [89] |
| Co3O4 | 5 | 10–1000 | [53] |
| ZnFe2O4/GQDs NPs | 7.0 | 5–500 | This work |
| Sample | Current Colorimetric Method (mM ± SD, n = 3) | Standard Pathological Laboratory Method (mM) |
|---|---|---|
| Serum 1 | 4.65 ± 0.15 | 4.72 |
| Serum 2 | 4.57 ± 0.21 | 4.61 |
| Serum 3 | 5.83 ± 0.08 | 5.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cirillo, C.; Iuliano, M.; Sarno, M. ZnFe2O4/GQDs Nanoparticles as Peroxidase Mimics for Sensitive and Selective Colorimetric Detection of Glucose in Real Samples. Micromachines 2025, 16, 520. https://doi.org/10.3390/mi16050520
Cirillo C, Iuliano M, Sarno M. ZnFe2O4/GQDs Nanoparticles as Peroxidase Mimics for Sensitive and Selective Colorimetric Detection of Glucose in Real Samples. Micromachines. 2025; 16(5):520. https://doi.org/10.3390/mi16050520
Chicago/Turabian StyleCirillo, Claudia, Mariagrazia Iuliano, and Maria Sarno. 2025. "ZnFe2O4/GQDs Nanoparticles as Peroxidase Mimics for Sensitive and Selective Colorimetric Detection of Glucose in Real Samples" Micromachines 16, no. 5: 520. https://doi.org/10.3390/mi16050520
APA StyleCirillo, C., Iuliano, M., & Sarno, M. (2025). ZnFe2O4/GQDs Nanoparticles as Peroxidase Mimics for Sensitive and Selective Colorimetric Detection of Glucose in Real Samples. Micromachines, 16(5), 520. https://doi.org/10.3390/mi16050520







