Advancements in Wearable and Implantable BioMEMS Devices: Transforming Healthcare Through Technology
Abstract
1. Introduction
2. Materials for BioMEMS Devices
2.1. Synthetic Polymers
2.1.1. Polydimethylsiloxane (PDMS)
2.1.2. Polyimide
2.1.3. Parylene C
2.1.4. Poly(methyl methacrylate) (PMMA)
2.1.5. Polyurethane (PU)
2.1.6. Polypyrrole (PPy)
2.2. Biodegradable Polymers
2.2.1. Polylactic Acid (PLA)
| Property | Values |
|---|---|
| Specific gravity (g/cm3) | 1–1.5 |
| Surface energy (dynes) | 35–40 |
| Melting temperature (°C) | 150–200 |
| Molecular weight (gms) | 2.7 × 10−19 |
| Mass flow index (g/10 min) | 5–22 |
| Crystallinity (%) | 5–40 |
| Glass transition temperature (°C) | 50–75 |
| Solubility parameters (J/cm) | 21 |
| Mechanical flexibility | Low |
| Thermal conductivity (W/mK) | 0.13–0.22 |
| Electrical conductivity (S/m) | Non-conductive |
| Biocompatibility | Excellent |
2.2.2. Polycaprolactone (PCL)
2.2.3. Polylactic-co-glycolic acid (PLGA)
| Polymer | Modulus (GPa) | Elongation (%) | Mechanical Flexibility | Thermal Conductivity (W/mK) | Electrical Conductivity (S/m) | Biocompatibility | Crystallinity (%) | Degradation Time (Weeks) |
|---|---|---|---|---|---|---|---|---|
| Polyglycolide/ polyglactin | 7 | 15–20 | Low | 0.2–0.4 | Non-conductive | Excellent | 45–55 | 6–12 |
| Poly(L-lactide) | 2.7 | 5–15 | Moderate | 0.2–0.4 | Non-conductive | Excellent | 37 | 12–18 |
| Poly(D, L-lactide) | 2.9 | 3–10 | Moderate | 0.2–0.4 | Non-conductive | Excellent | Amorphous | 11–15 |
| Poly(D, L-lactide-co-glycolide) 85/15 | 2 | 3–10 | Moderate | 0.2–0.4 | Non-conductive | Excellent | Amorphous | 5–6 |
| Poly(D, L-lactide-co-glycolide) 75/25 | 2 | 3–10 | Moderate | 0.2–0.4 | Non-conductive | Excellent | Amorphous | 4–5 |
| Poly(D, L-lactide-co-glycolide) 50/50 | 2 | 3–10 | Moderate | 0.2–0.4 | Non-conductive | Excellent | Amorphous | 1–2 |
2.2.4. Polyhydroxybutyrate (PHB) and Polyhydroxyvalerate (PHV)
2.2.5. Polydioxanone (PDO)
2.3. Natural Polymers
2.3.1. Polysaccharides
2.3.2. Proteins
2.4. Emerging Polymers
2.4.1. Polyphosphazenes (PPZs)
2.4.2. Sundew-Inspired Adhesive Hydrogels
2.4.3. MXenes
2.4.4. Piezoelectric Biomolecular Materials and Transient BioMEMSs
3. Wearable Devices
3.1. Overview of Wearable BioMEMSs
3.2. Types of Wearable BioMEMSs
3.2.1. Wearable Biosensors
3.2.2. Wearable Bioelectronic Devices
3.2.3. Wearable Drug Delivery Devices
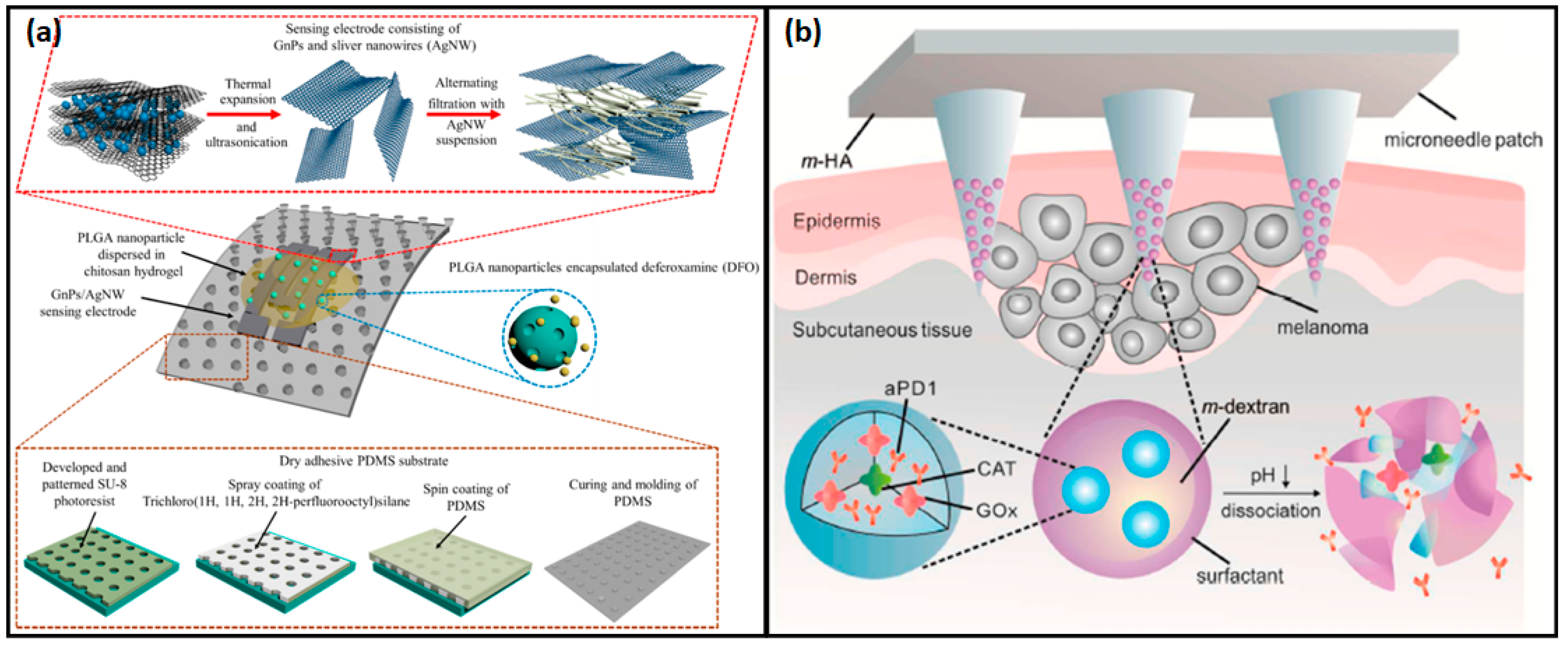
3.2.4. Wearable Motion and Mechanical Sensors
4. Implantable Devices
4.1. Continuous Health Monitoring and Diagnostics
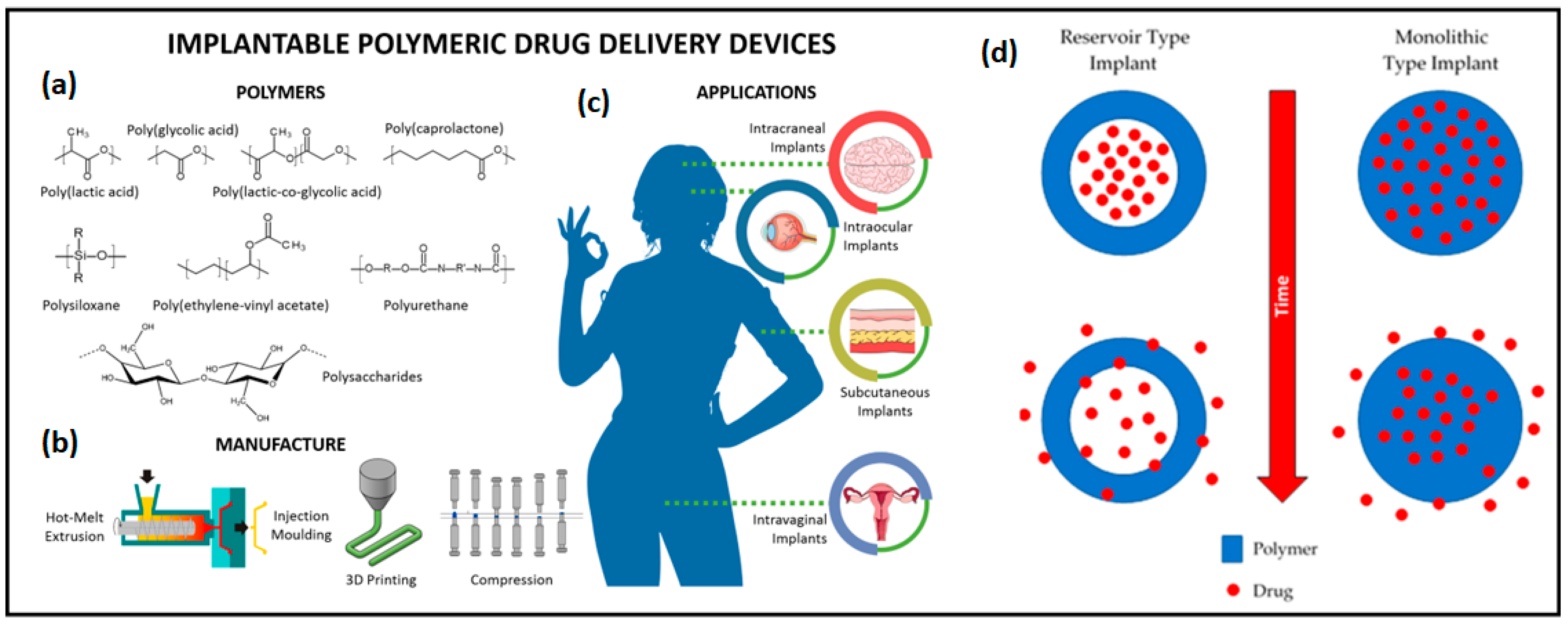
4.2. Implantable Drug Delivery Systems
| Device | Function | Technology | Clinical Application | Manufacturer/Ref. |
|---|---|---|---|---|
| Medtronic Synchromed II | Controlled drug infusion | Programmable pump | Chronic pain, spasticity | Medtronic |
| MicroCHIPS Biotech Implant | Wireless drug delivery | MEMS-based microchip | Osteoporosis, contraceptive implants | MicroCHIPS Biotech |
| Bioresorbable drug-eluting implants | Localized drug release | Biodegradable polymers | Cancer, infections | [457] |
| Nanoporous DDS | Controlled chemotherapy | Nanoporous silicon carriers | Cancer treatment | [458] |
| Reservoir-type subcutaneous implant | Sustained risperidone release | Biodegradable poly(caprolactone) membrane | Schizophrenia treatment | [459] |
| Piroxicam microcapsule-embedded scaffold implant | Sustained NSAID release | PLA/PLGA-based gelatin microcapsules in a scaffold | Arthritis pain management | [460] |
| Artemisinin-loaded PLGA/MSNs Composite nanofibers | Targeted drug delivery | Mesoporous silica nanoparticles (MSNs) and electrospun PLGA nanofibers | Breast cancer treatment | [461] |
| Imidazolium methacrylate–resorcinol dimethacrylate hydrogel implant | Sustained corticosteroid release | 3D-printed photopolymerizable hydrogel with dexamethasone | Inflammatory disease management | [462] |
| Poly(trimethylene carbonate-co-P-dioxanone) implant | Controlled drug release | Biodegradable PTD copolymer with tunable degradation rate | Long-acting drug delivery | [463] |
| 3D-printed EVA28-based subcutaneous implant | Personalized drug delivery | Fused filament fabrication (FFF) of EVA28 with progesterone | Hormonal therapy (progesterone) | [464] |
| Nanochannel delivery system (nDS) implant | Wireless, controlled drug release | Silicon nanofluidic membrane with BLE control | Chronic disease management | [465] |
| 3D-printed hydrogel implant for tenofovir | Prolonged antiviral drug release | Bovine serum albumin hydrogel with methylcellulose reinforcement, semi-solid extrusion 3D printing | Hepatitis B and HIV treatment | [466] |
| Supramolecular hydrogel for glioblastoma therapy | Localized chemotherapy | Peptide-functionalized hyaluronic acid hydrogel with cucurbit [8] uril host–guest interactions | Post-surgical glioblastoma treatment | [467] |
| Implantable scaffold for pancreatic cancer Immunotherapy | Localized immunotherapy | Biodegradable polymer scaffold for sustained drug release | Pancreatic cancer treatment | [468] |
| pH and NIR dual-responsive TiO₂ Nanotube implant | Stimuli-responsive drug release | TiO2 nanotube arrays modified with polydopamine and Fe3+ | Osteoporosis treatment | [469] |
| 3D-printed biodegradable poly(ether ester) implant | Personalized drug release | High-resolution MEAM using poly(ether ester) multiblock copolymers | Chronic disease management | [470] |
| γ-cyclodextrin Hydrogel for josamycin release | Sustained antibiotic release | Crosslinked γ-cyclodextrin hydrogel for prolonged drug delivery | Post-surgical glaucoma treatment | [471] |
| Fluconazole-loaded chitosan nanoparticle composite film | Localized antifungal therapy | Ionic gelation-based chitosan nanoparticles in gelatin–chitosan composite film | Prosthetic joint infection (PJI) | [472] |
| Naringenin-loaded PHBV/PLGA implantable rods | Neuroprotective drug release | Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/PLGA blend via melt- and wet-spinning | Retinal degenerative diseases | [473] |
4.3. Neurostimulation and Bioelectronic Implants
5. Transforming Healthcare
5.1. Chronic Disease Management
5.2. Role of Wearable Technologies in Cardiology
5.2.1. Monitoring Hypertension
5.2.2. Detecting and Monitoring Arrhythmias
5.3. Role of Wearable Technologies in Respiratory Health
5.4. Intraocular Pressure Monitoring in Glaucoma Management
6. Regulatory, Global Standardization, and Societal Determinants
- Complex Approval Processes: The regulatory landscape for BioMEMSs is quite intricate and hence distinguished by lengthy approval processes. Regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) carry out thorough testing to ensure the functionality of such devices [553]. There are strict conditions for implantable BioMEMSs, or any other devices intended for applications such as heart monitoring or other telemetry, where balancing regulatory compliance and innovative ideas is fast becoming a significant challenge. Adequate time and resources must be invested in preclinical and clinical testing, which can lead to delays in product launching and increase costs [554]. Additionally, minimal standardized guidelines for new and upcoming BioMEMS technologies, such as organ-on-chip devices, makes the regulatory pathway more difficult [555].
- Variability in Global Standards: Global variability in regulatory standards is another major bottleneck for BioMEMS developers. Every region has a fixed set of requirements and extensive modifications, or additional testing is needed to fulfil those requirements. This variability in requirements can slow down the global scalability of BioMEMS technologies and increase developmental costs [556]. Coherence of regulatory frameworks, such as the International Medical Device Regulators Forum (IMDRF) initiatives, could make way for faster global acceptance. Joint efforts between regulatory agencies, industry, and academia can expedite better regulatory guidelines for BioMEMSs.
- User-Friendly Design: BioMEMS technologies can be successfully implemented in the long run if these methods are accepted by the patients. Many devices, particularly wearables and implantables, require high levels of comfort, ease of use, and inconspicuousness to ensure acceptance by patients. For example, heavy or intrusive devices may not be ideal for long-term use, and hence they become ineffective in chronic disease management [557]. In order to ensure this long-term usage, BioMEMS designers must focus on user-centered design principles. Flexible and lightweight materials, well-designed user-oriented form factors, and natural interfaces can significantly enhance patient comfort and acceptance. In addition, customizable devices specially made for individual needs could improve patient satisfaction and compliance [558].
- Privacy and Data Security: The amalgamation of BioMEMSs with digital health platforms can result in data privacy and security challenges [559]. Sophisticated devices dedicated to collection and transmission of sensitive health information are vulnerable to cyberattacks, data breaches, and unauthorized access. Secure data encryption coupled with secure communication protocols, and compliance with privacy regulations like HIPAA and the GDPR, will ensure privacy and data security and result in patient satisfaction [29].
- Cultural and Psychological Barriers: Cultural and psychological factors also play a key role in patient acceptance of BioMEMSs. For instance, people from different cultural backgrounds may have certain restrictions about implantable devices due to spiritual or moral beliefs. Likewise, patients with an apprehension of technology or operating procedures may be reluctant to consider BioMEMS-based solutions [560]. Overcoming these barriers necessitates proper planning in education and awareness campaigns to educate patients about the pros and cons of BioMEMSs. Healthcare providers must also be driven by initiative to address patient concerns and facilitate faith and confidence in these technologies [29].
7. Future Directions
- Integration with Advanced Technologies: The integration of BioMEMSs with advanced technologies like AI, ML, and the IoT holds immense promise [537]. AI and ML algorithms can analyze vast amounts of data generated by BioMEMS devices, enabling real-time decision-making and personalized medicine [561]. For instance, wearable BioMEMSs integrated with IoT can provide continuous patient monitoring and predictive analytics, facilitating early intervention for chronic diseases. Furthermore, the incorporation of edge computing into BioMEMS devices can reduce latency and improve energy efficiency. Edge-enabled BioMEMSs could process data locally, ensuring faster response times and enhanced privacy for sensitive health information. Research into secure and energy-efficient edge computing frameworks tailored for BioMEMS applications will likely be a focal point in the coming years [562].
- Development of Multifunctional BioMEMSs: Future BioMEMSs will likely transition from single-function devices to multifunctional systems capable of performing diverse tasks such as sensing, drug delivery, and therapeutic interventions [563]. For example, a BioMEMS device could monitor blood glucose levels and simultaneously deliver insulin, ensuring tighter glucose control for diabetic patients. Advances in microfabrication and materials science will be pivotal in enabling such multifunctionality. Additionally, there is a growing need for BioMEMSs that can integrate with complex biological environments [564]. Hybrid BioMEMSs combining electronic and biological components such as bio-hybrid actuators or bioelectronic interfaces could revolutionize the management of conditions like neurological disorders or organ dysfunction.
- Advances in Materials for BioMEMSs: Material innovation will play a critical role in shaping the next generation of BioMEMSs. Emerging materials like graphene, transition metal dichalcogenides (TMDs), and biocompatible hydrogels offer unique properties, such as high conductivity, flexibility, and biocompatibility. These materials can enhance device performance, longevity, and patient comfort [565]. Research into biodegradable and bioresorbable materials is particularly exciting. Devices made from such materials can safely degrade in the body after their intended function is complete, eliminating the need for surgical removal. This innovation is especially relevant for temporary implants or drug delivery systems [566].
- Personalized and Precision Medicine: The convergence of BioMEMSs and precision medicine presents a transformative opportunity to tailor healthcare interventions to individual patients. BioMEMS devices capable of analyzing genetic, proteomic, or metabolomic data could provide insights into a patient’s unique biological profile, enabling personalized treatment plans [567]. This approach is especially valuable in oncology, where BioMEMS-based platforms can identify biomarkers for specific cancer types and guide targeted therapies. Moreover, microfluidic BioMEMSs can facilitate organ-on-chip technologies, enabling researchers to study disease mechanisms and test potential treatments in a controlled environment [568]. These advancements could significantly accelerate drug discovery and reduce reliance on animal testing.
- Enhancements in Point-of-Care Diagnostics: Point-of-care (POC) diagnostics have already benefited greatly from BioMEMSs, but the future holds potential for even more compact, affordable, and accurate devices [569]. Future POC BioMEMSs could integrate nanoscale sensors and advanced signal processing to detect minute quantities of biomarkers, enabling early diagnosis of diseases such as cancer, infectious diseases, and neurodegenerative conditions [570]. Wearable and implantable BioMEMSs will also play a pivotal role in decentralized healthcare systems. These devices can provide continuous health monitoring, reducing the need for frequent hospital visits and improving patient outcomes. For instance, BioMEMS-enabled wearable sensors could monitor cardiovascular health in real time, alerting patients and physicians to potential risks.
- Miniaturization and Power Efficiency: The ongoing trend of miniaturization in BioMEMSs will continue, driven by advancements in nanotechnology and microfabrication techniques. Smaller devices offer several advantages, including reduced invasiveness, lower material costs, and enhanced portability [303]. However, miniaturization also presents challenges in terms of power consumption and device reliability. Future research will likely focus on energy-efficient power sources for BioMEMSs, such as energy harvesting from the human body. Technologies like piezoelectric nanogenerators and triboelectric energy harvesters could enable self-powered BioMEMSs, extending device longevity and reducing dependency on external power supplies [571].
- Regenerative Medicine and Tissue Engineering: BioMEMSs have significant potential in regenerative medicine and tissue engineering. Microfluidic devices can create 3D tissue constructs by precisely controlling cell deposition and nutrient delivery [572]. These constructs can be used for regenerative therapies, drug testing, or disease modelling. Moreover, BioMEMS-enabled bioprinters could revolutionize organ transplantation by producing patient-specific organs. Research into improving the resolution, speed, and scalability of BioMEMS-based bioprinting technologies will be critical in realizing this vision [573].
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ho, D.; Quake, S.R.; McCabe, E.R.B.; Chng, W.J.; Chow, E.K.; Ding, X.; Gelb, B.D.; Ginsburg, G.S.; Hassenstab, J.; Ho, C.-M.; et al. Enabling Technologies for Personalized and Precision Medicine. Trends Biotechnol. 2020, 38, 497–518. [Google Scholar] [CrossRef] [PubMed]
- Assalve, G.; Lunetti, P.; Di Cagno, A.; De Luca, E.W.; Aldegheri, S.; Zara, V.; Ferramosca, A. Advanced wearable devices for monitoring sweat biochemical markers in athletic performance: A comprehensive review. Biosensors 2024, 14, 574. [Google Scholar] [CrossRef]
- Kassanos, P.; Hourdakis, E. Implantable Passive Sensors for Biomedical Applications. Sensors 2025, 25, 133. [Google Scholar] [CrossRef] [PubMed]
- Erdem, A.; Eksin, E.; Senturk, H.; Yildiz, E.; Maral, M. Recent developments in wearable biosensors for healthcare and biomedical applications. TrAC Trends Anal. Chem. 2024, 171, 117510. [Google Scholar] [CrossRef]
- Vo, D.-K.; Trinh, K.T.L. Advances in Wearable Biosensors for Healthcare: Current Trends, Applications, and Future Perspectives. Biosensors 2024, 14, 560. [Google Scholar] [CrossRef] [PubMed]
- Sanjay; Kumar, V.; Vohra, A. Sensitivity enhancement using triple metal gate work function engineering of junctionless cylindrical gate all around SiNW MOSFET based biosensor for neutral biomolecule species detection for upcoming sub 14 nm technology node. Mater. Sci. Eng. B 2024, 306, 117459. [Google Scholar] [CrossRef]
- Kong, L.; Li, W.; Zhang, T.; Ma, H.; Cao, Y.; Wang, K.; Zhou, Y.; Shamim, A.; Zheng, L.; Wang, X.; et al. Wireless Technologies in Flexible and Wearable Sensing: From Materials Design, System Integration to Applications. Adv. Mater. 2024, 36, 2400333. [Google Scholar] [CrossRef]
- Yogev, D.; Goldberg, T.; Arami, A.; Tejman-Yarden, S.; Winkler, T.E.; Maoz, B.M. Current state of the art and future directions for implantable sensors in medical technology: Clinical needs and engineering challenges. APL Bioeng. 2023, 7, 031506. [Google Scholar] [CrossRef]
- Huanbutta, K.; Puri, V.; Sharma, A.; Singh, I.; Sriamornsak, P.; Sangnim, T. Rise of implantable drugs: A chronicle of breakthroughs in drug delivery systems. Saudi Pharm. J. 2024, 32, 102193. [Google Scholar] [CrossRef]
- Mariello, M.; Proctor, C.M. Wireless Power and Data Transfer Technologies for Flexible Bionic and Bioelectronic Interfaces: Materials and Applications. Adv. Mater. Technol. 2025, 10, 2400797. [Google Scholar] [CrossRef]
- Shuvo, M.M.H.; Titirsha, T.; Amin, N.; Islam, S.K. Energy harvesting in implantable and wearable medical devices for enduring precision healthcare. Energies 2022, 15, 7495. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Q.; Yang, L.; Cui, Y. Materials and biomedical applications of implantable electronic devices. Adv. Mater. Technol. 2023, 8, 2200853. [Google Scholar] [CrossRef]
- Jafleh, E.A.; Alnaqbi, F.A.; Almaeeni, H.A.; Faqeeh, S.; Alzaabi, M.A.; Al Zaman, K.; Alnaqbi, F.; Almaeeni, H.; Alzaabi, M. The role of wearable devices in chronic disease monitoring and patient care: A comprehensive review. Cureus 2024, 16, e68921. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, A.R.; Cheung, J.W.; Lampert, R.; Silva, J.N.A.; Gopinathannair, R.; Sotomonte, J.C.; Tarakji, K.; Fellman, M.; Chrispin, J.; Varma, N.; et al. State of the art of mobile health technologies use in clinical arrhythmia care. Commun. Med. 2024, 4, 218. [Google Scholar] [CrossRef]
- Odeh, V.A.; Chen, Y.; Wang, W.; Ding, X. Recent Advances in the Wearable Devices for Monitoring and Management of Heart Failure. Rev. Cardiovasc. Med. 2024, 25, 386. [Google Scholar] [CrossRef]
- Yin, Z.; Yang, Y.; Hu, C.; Li, J.; Qin, B.; Yang, X. Wearable respiratory sensors for health monitoring. NPG Asia Mater. 2024, 16, 8. [Google Scholar] [CrossRef]
- Wu, K.Y.; Mina, M.; Carbonneau, M.; Marchand, M.; Tran, S.D. Advancements in Wearable and Implantable Intraocular Pressure Biosensors for Ophthalmology: A Comprehensive Review. Micromachines 2023, 14, 1915. [Google Scholar] [CrossRef]
- Pons-Faudoa, F.P.; Ballerini, A.; Sakamoto, J.; Grattoni, A. Advanced implantable drug delivery technologies: Transforming the clinical landscape of therapeutics for chronic diseases. Biomed. Microdevices 2019, 21, 47. [Google Scholar] [CrossRef]
- Perez, F.P.; Walker, B.; Morisaki, J.; Kanakri, H.; Rizkalla, M. Neurostimulation devices to treat Alzheimer’s disease. Explor. Neurosci. 2025, 4, 100674. [Google Scholar] [CrossRef]
- Edwards, C.A.; Kouzani, A.; Lee, K.H.; Ross, E.K. Neurostimulation Devices for the Treatment of Neurologic Disorders. Mayo Clin. Proc. 2017, 92, 1427–1444. [Google Scholar] [CrossRef]
- Junaid, S.B.; Imam, A.A.; Abdulkarim, M.; Surakat, Y.A.; Balogun, A.O.; Kumar, G.; Shuaibu, A.N.; Garba, A.; Sahalu, Y.; Mohammed, A.; et al. Recent Advances in Artificial Intelligence and Wearable Sensors in Healthcare Delivery. Appl. Sci. 2022, 12, 10271. [Google Scholar] [CrossRef]
- Flynn, C.D.; Chang, D. Artificial Intelligence in Point-of-Care Biosensing: Challenges and Opportunities. Diagnostics 2024, 14, 1100. [Google Scholar] [CrossRef]
- Alahi, M.E.E.; Sukkuea, A.; Tina, F.W.; Nag, A.; Kurdthongmee, W.; Suwannarat, K.; Mukhopadhyay, S.C. Integration of IoT-Enabled Technologies and Artificial Intelligence (AI) for Smart City Scenario: Recent Advancements and Future Trends. Sensors 2023, 23, 5206. [Google Scholar] [CrossRef]
- Kang, M.; Yeo, W.-H. Advances in energy harvesting technologies for wearable devices. Micromachines 2024, 15, 884. [Google Scholar] [CrossRef] [PubMed]
- Milivojević, M.; Pajic-Lijaković, I.; Popović, A.; Hasnain, M.S.; Nayak, A.K. Chapter 28-BioMEMS-based biosensors. In Fundamentals of Biosensors in Healthcare; Hasnain, M.S., Nayak, A.K., Aminabhavi, T.M., Eds.; Academic Press: Cambridge, MA, USA, 2025; pp. 661–694. [Google Scholar]
- Xu, J.; Lee, H. Anti-biofouling strategies for long-term continuous use of implantable biosensors. Chemosensors 2020, 8, 66. [Google Scholar] [CrossRef]
- Yao, S.; Yan, H.; Tian, S.; Luo, R.; Zhao, Y.; Wang, J. Anti-fouling coatings for blood-contacting devices. Smart Mater. Med. 2024, 5, 166–180. [Google Scholar] [CrossRef]
- Chavda, V.P.; Gargi, J.; Cláudia, P.-S.A.; Kaushik, A. Biodegradable and removable implants for controlled drug delivery and release application. Expert Opin. Drug Deliv. 2022, 19, 1177–1181. [Google Scholar] [CrossRef]
- Jaime, F.J.; Muñoz, A.; Rodríguez-Gómez, F.; Jerez-Calero, A. Strengthening Privacy and Data Security in Biomedical Microelectromechanical Systems by IoT Communication Security and Protection in Smart Healthcare. Sensors 2023, 23, 8944. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, P.; Vlahu-Gjorgievska, E.; Chow, Y.-W. Security and privacy of technologies in health information systems: A systematic literature review. Computers 2024, 13, 41. [Google Scholar] [CrossRef]
- Brook, M. Silicon in Organic, Organometallic and Polymer Chemistry; John Wiley&Sons. Inc.: New York, NY, USA, 2000. [Google Scholar]
- Selvaganapathy, P.R. 1.04-Polymers. In Comprehensive Microsystems; Gianchandani, Y.B., Tabata, O., Zappe, H., Eds.; Elsevier: Oxford, UK, 2008; pp. 75–105. [Google Scholar]
- Juárez-Moreno, J.; Ávila-Ortega, A.; Oliva, A.; Avilés, F.; Cauich-Rodríguez, J. Effect of wettability and surface roughness on the adhesion properties of collagen on PDMS films treated by capacitively coupled oxygen plasma. Appl. Surf. Sci. 2015, 349, 763–773. [Google Scholar] [CrossRef]
- Chen, D.; Chen, F.; Hu, X.; Zhang, H.; Yin, X.; Zhou, Y. Thermal stability, mechanical and optical properties of novel addition cured PDMS composites with nano-silica sol and MQ silicone resin. Compos. Sci. Technol. 2015, 117, 307–314. [Google Scholar] [CrossRef]
- Johnston, I.D.; McCluskey, D.K.; Tan, C.K.; Tracey, M.C. Mechanical characterization of bulk Sylgard 184 for microfluidics and microengineering. J. Micromech. Microeng. 2014, 24, 035017. [Google Scholar] [CrossRef]
- Fan, X.; Jia, C.; Yang, J.; Li, G.; Mao, H.; Jin, Q.; Zhao, J. A microfluidic chip integrated with a high-density PDMS-based microfiltration membrane for rapid isolation and detection of circulating tumor cells. Biosens. Bioelectron. 2015, 71, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Zhou, W.; Zhao, Y.; Yu, X.; Wang, H.; Chu, P.K. Enhanced cytocompatibility and reduced genotoxicity of polydimethylsiloxane modified by plasma immersion ion implantation. Colloids Surf. B Biointerfaces 2016, 148, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, N.; George, D.; Sen, A. Analytical modeling, simulations and experimental studies of a PZT actuated planar valveless PDMS micropump. Sens. Actuators A Phys. 2015, 225, 81–94. [Google Scholar] [CrossRef]
- Wang, X.; Gu, Y.; Xiong, Z.; Cui, Z.; Zhang, T. Silk-molded flexible, ultrasensitive, and highly stable electronic skin for monitoring human physiological signals. Adv. Mater. (Deerfield Beach Fla.) 2013, 26, 1336–1342. [Google Scholar] [CrossRef]
- Kakati, A.; Das, S. Nano-structured gold strain gauge arrays on PDMS for highly sensitive nems pressure sensor skin. In Proceedings of the 2017 IEEE 12th International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Los Angeles, CA, USA, 9–12 April 2017; pp. 611–614. [Google Scholar]
- Keskin, D.; Mokabbar, T.; Pei, Y.; Van Rijn, P. The relationship between bulk silicone and benzophenone-initiated hydrogel coating properties. Polymers 2018, 10, 534. [Google Scholar] [CrossRef]
- Kim, D.-S.; Jeong, Y.-J.; Lee, B.-K.; Shanmugasundaram, A.; Lee, D.-W. Piezoresistive sensor-integrated PDMS cantilever: A new class of device for measuring the drug-induced changes in the mechanical activity of cardiomyocytes. Sens. Actuators B Chem. 2017, 240, 566–572. [Google Scholar] [CrossRef]
- Kim, K.K.; Hong, S.; Cho, H.M.; Lee, J.; Suh, Y.D.; Ham, J.; Ko, S.H. Highly sensitive and stretchable multidimensional strain sensor with prestrained anisotropic metal nanowire percolation networks. Nano Lett. 2015, 15, 5240–5247. [Google Scholar] [CrossRef]
- Eom, H.; Lee, J.; Pichitpajongkit, A.; Amjadi, M.; Jeong, J.H.; Lee, E.; Lee, J.Y.; Park, I. Ag@ Ni core–shell nanowire network for robust transparent electrodes against oxidation and sulfurization. Small 2014, 10, 4171–4181. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, H.; Yao, G.; Liao, F.; Gao, M.; Huang, Z.; Li, K.; Lin, Y. Highly stretchable, sensitive, and flexible strain sensors based on silver nanoparticles/carbon nanotubes composites. J. Alloys Compd. 2015, 652, 48–54. [Google Scholar] [CrossRef]
- Abhinav, V.; Ranjan, P.; Mahapatra, A.; Belwanshi, V.; Kumar, V. 8-Core-shell oxide nanoparticles and their biomedical applications. In Oxides for Medical Applications; Kumar, P., Kandasamy, G., Singh, J.P., Maurya, P.K., Eds.; Woodhead Publishing: Cambridge, UK, 2023; pp. 197–232. [Google Scholar]
- Amjadi, M.; Kyung, K.U.; Park, I.; Sitti, M. Stretchable, skin-mountable, and wearable strain sensors and their potential applications: A review. Adv. Funct. Mater. 2016, 26, 1678–1698. [Google Scholar] [CrossRef]
- Yang, X.; Shu, J.; Zhou, Z.; Liang, H. Polyimide as a biomedical material: Advantages and applications. Nanoscale Adv. 2024, 6, 4309–4324. [Google Scholar]
- Hiramoto, H. Photosensitive polyimides. MRS Online Proc. Libr. (OPL) 1989, 167, 87. [Google Scholar] [CrossRef]
- Liu, P.; Yao, Z.; Li, L.; Zhou, J. In situ Synthesis and mechanical, thermal properties of polyimide nanocomposite film by addition of functionalized graphene oxide. Polym. Compos. 2016, 37, 907–914. [Google Scholar] [CrossRef]
- Miranda, I.; Souza, A.; Sousa, P.; Ribeiro, J.; Castanheira, E.M.; Lima, R.; Minas, G. Properties and applications of PDMS for biomedical engineering: A review. J. Funct. Biomater. 2021, 13, 2. [Google Scholar] [CrossRef]
- Hasikin, K.; Ibrahim, F.; Soin, N. Modeling of a micro-diaphragm biosensor for human artery pulse wave detection. In Proceedings of the 2008 IEEE International Conference on Semiconductor Electronics, Johor Bahru, Malaysia, 25–27 November 2008; pp. 170–173. [Google Scholar]
- Noh, H.-s.; Moon, K.-s.; Cannon, A.; Hesketh, P.J.; Wong, C. Wafer bonding using microwave heating of parylene intermediate layers. J. Micromechanics Microengineering 2004, 14, 625. [Google Scholar] [CrossRef]
- Redolfi Riva, E.; D’Alessio, A.; Micera, S. Polysaccharide layer-by-layer coating for polyimide-based neural interfaces. Micromachines 2022, 13, 692. [Google Scholar] [CrossRef]
- Fortin, J.B.; Lu, T.-M. Chemical Vapor Deposition Polymerization: The Growth and Properties of Parylene Thin Films; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Sim, W.; Kim, B.; Choi, B.; Park, J.-O. Theoretical and experimental studies on the parylene diaphragms for microdevices. Microsyst. Technol. 2005, 11, 11–15. [Google Scholar] [CrossRef]
- Wang, X.; Hirschberg, A.W.; Xu, H.; Slingsby-Smith, Z.; Lecomte, A.; Scholten, K.; Song, D.; Meng, E. A parylene neural probe array for multi-region deep brain recordings. J. Microelectromechanical Syst. 2020, 29, 499–513. [Google Scholar] [CrossRef]
- Ware, T.; Simon, D.; Arreaga-Salas, D.E.; Reeder, J.; Rennaker, R.; Keefer, E.W.; Voit, W. Fabrication of Responsive, Softening Neural Interfaces. Adv. Funct. Mater. 2012, 22, 3470–3479. [Google Scholar] [CrossRef]
- Gholizadeh, S.; Lincoln, D.M.; Allahyari, Z.; Widom, L.P.; Carter, R.N.; Gaborski, T.R. Optimization of Parylene C and Parylene N thin films for use in cellular co-culture and tissue barrier models. Sci. Rep. 2023, 13, 4262. [Google Scholar] [CrossRef]
- Lecomte, A.; Degache, A.; Descamps, E.; Dahan, L.; Bergaud, C. In vitro and in vivo biostability assessment of chronically-implanted Parylene C neural sensors. Sens. Actuators B Chem. 2017, 251, 1001–1008. [Google Scholar] [CrossRef]
- Kuo, W.-C.; Wu, T.-C.; Wu, C.-F.; Wang, W.-C. Bioperformance analysis of parylene C coating for implanted nickel titanium alloy. Mater. Today Commun. 2021, 27, 102306. [Google Scholar] [CrossRef]
- Uscanga, F.A.R.; Pierce, M.C.; Zahn, J.D. Development of Micromachined Parylene-Based Electroactive Membranes with Embedded Microfluidic Channels For Biological Applications. In Proceedings of the 2022 IEEE 35th International Conference on Micro Electro Mechanical Systems Conference (MEMS), Tokyo, Japan, 9–13 January 2022; pp. 915–918. [Google Scholar]
- Uscanga, F.A.R.; Pierce, M.C.; Zahn, J.D. Fabrication and development of novel micromachined parylene-based electroactive membranes with embedded microfluidic architectures. J. Micromech. Microeng. 2023, 33, 095010. [Google Scholar] [CrossRef]
- Büyükkaya, K. Effects of the fiber diameter on mechanic properties in polymethyl-methacrylate composites reinforced with goose feather fiber. Mater. Sci. Appl. 2017, 8, 811–827. [Google Scholar] [CrossRef]
- Ali, U.; Karim, K.J.B.A.; Buang, N.A. A review of the properties and applications of poly (methyl methacrylate)(PMMA). Polym. Rev. 2015, 55, 678–705. [Google Scholar] [CrossRef]
- Jeffrey, R.; Kear, J.; Kasperczyk, D.; Zhang, X.; Chuprakov, D.; Prioul, R.; Schouten, J. A 2D experimental method with results for hydraulic fractures crossing discontinuities. In Proceedings of the ARMA US Rock Mechanics/Geomechanics Symposium, San Francisco, CA, USA, 28 June–1 July 2015. ARMA–2015-2439. [Google Scholar]
- Lee, H.; Wang, J.; Park, S.-M.; Hong, S.; Kim, N. Analysis of excessive deformation behavior of a PMMA-touch screen panel laminated material in a high temperature condition. Korea-Aust. Rheol. J. 2011, 23, 195–204. [Google Scholar] [CrossRef]
- Tao, S.L.; Lubeley, M.W.; Desai, T.A. Bioadhesive poly (methyl methacrylate) microdevices for controlled drug delivery. J. Control. Release 2003, 88, 215–228. [Google Scholar] [CrossRef]
- Omar, F.; Brousseau, E.; Elkaseer, A.; Kolew, A.; Prokopovich, P.; Dimov, S. Development and experimental validation of an analytical model to predict the demoulding force in hot embossing. J. Micromech. Microeng. 2014, 24, 055007. [Google Scholar] [CrossRef]
- Ishaq, M.; Saeed, K.; Shakirullah, M.; Ahmad, I.; Rehman, T. Effect of coal ash on the morphological, thermal and mechanical properties of poly (Methyl methacrylate). J. Chil. Chem. Soc. 2012, 57, 992–994. [Google Scholar] [CrossRef]
- Ngai, J.H.; Chan, C.C.M.; Ho, C.H.Y.; Ho, J.K.W.; Cheung, S.H.; Yin, H.; So, S.K. A facile and robust approach to prepare fluorinated polymer dielectrics for probing the intrinsic transport behavior of organic semiconductors. Mater. Adv. 2020, 1, 891–898. [Google Scholar] [CrossRef]
- Material Property Database. Available online: https://www.mit.edu/~6.777/matprops/pmma.htm (accessed on 15 March 2025).
- Cui, M.; Chai, Z.; Lu, Y.; Zhu, J.; Chen, J. Developments of polyurethane in biomedical applications: A review. Resour. Chem. Mater. 2023, 2, 262–276. [Google Scholar] [CrossRef]
- Natarajan, N.; Bharathidhasan, S.; Thanigaivelan, R.; Suresh, P. Sisal fiber/glass fiber hybrid nano composite: The tensile and compressive properties. In Proceedings of the 5th International & 26th All India Manufacturing Technology, Design and Research Conference, Assam, India, 12 December 2014; pp. 1–6. [Google Scholar]
- Guoqing, J.; Luchao, Q.; Wenli, V.M.J. Polyurethane reinforced ballasted track: Review, innovation and challenge. Constr. Build. Mater. 2019, 208, 734–748. [Google Scholar]
- Currie, S.; Shariatzadeh, F.J.; Singh, H.; Logsetty, S.; Liu, S. Highly sensitive bacteria-responsive membranes consisting of core–shell polyurethane polyvinylpyrrolidone electrospun nanofibers for in situ detection of bacterial infections. ACS Appl. Mater. Interfaces 2020, 12, 45859–45872. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, W.; Wu, X.-D.; He, X.; Lin, X.; Wang, H.; Li, J.; Jiang, J.; Huang, W. Efficacy of novel nano-hydroxyapatite/polyurethane composite scaffolds with silver phosphate particles in chronic osteomyelitis. J. Mater. Sci. Mater. Med. 2019, 30, 59. [Google Scholar] [CrossRef]
- Deodhar, T.; Nugay, N.; Nugay, T.; Patel, R.; Moggridge, G.; Kennedy, J.P. Synthesis of High-Molecular-Weight and Strength Polyisobutylene-Based Polyurethane and Its Use for the Development of a Synthetic Heart Valve. Macromol. Rapid Commun. 2023, 44, 2200147. [Google Scholar] [CrossRef]
- Hao, L.; Dong, C.; Yu, D. Polypyrrole derivatives: Preparation, properties and application. Polymers 2024, 16, 2233. [Google Scholar] [CrossRef]
- Liang, Y.; Goh, J.C.-H. Polypyrrole-incorporated conducting constructs for tissue engineering applications: A review. Bioelectricity 2020, 2, 101–119. [Google Scholar] [CrossRef]
- Li, X.; Qiu, J.; Liu, X. Antibacterial property and biocompatibility of polypyrrole films treated by oxygen plasma immersion ion implantation. Adv. Mater. Interfaces 2020, 7, 2000057. [Google Scholar] [CrossRef]
- Zare, E.N.; Agarwal, T.; Zarepour, A.; Pinelli, F.; Zarrabi, A.; Rossi, F.; Ashrafizadeh, M.; Maleki, A.; Shahbazi, M.-A.; Maiti, T.K. Electroconductive multi-functional polypyrrole composites for biomedical applications. Appl. Mater. Today 2021, 24, 101117. [Google Scholar] [CrossRef]
- Pang, A.L.; Arsad, A.; Ahmadipour, M. Synthesis and factor affecting on the conductivity of polypyrrole: A short review. Polym. Adv. Technol. 2021, 32, 1428–1454. [Google Scholar] [CrossRef]
- Borges, M.H.; Nagay, B.E.; Costa, R.C.; Souza, J.G.S.; Mathew, M.T.; Barão, V.A. Recent advances of polypyrrole conducting polymer film for biomedical application: Toward a viable platform for cell-microbial interactions. Adv. Colloid Interface Sci. 2023, 314, 102860. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Wang, Y.; Gao, H.; Wei, G.; Huang, Y.; Yu, H.; Gan, Y.; Wang, Y.; Mei, L. Recent progress in drug delivery. Acta Pharm. Sin. B 2019, 9, 1145–1162. [Google Scholar] [CrossRef]
- Hao, L.; Dong, C.; Zhang, L.; Zhu, K.; Yu, D. Polypyrrole nanomaterials: Structure, preparation and application. Polymers 2022, 14, 5139. [Google Scholar] [CrossRef]
- Ariati, R.; Sales, F.; Souza, A.; Lima, R.A.; Ribeiro, J. Polydimethylsiloxane Composites Characterization and Its Applications: A Review. Polymers 2021, 13, 4258. [Google Scholar] [CrossRef] [PubMed]
- Kanyanta, V.; Ivankovic, A. Mechanical characterisation of polyurethane elastomer for biomedical applications. J. Mech. Behav. Biomed. Mater. 2010, 3, 51–62. [Google Scholar] [CrossRef]
- Ogbonna, V.; Popoola, P.; Popoola, O.; Adeosun, S. Enhanced mechanical and electrical properties of ECR-glass reinforced polyimide composites with incorporation of TiO2 for insulation applications. J. Thermoplast. Compos. Mater. 2022, 36, 089270572211422. [Google Scholar] [CrossRef]
- Marhoon, I.I. Mechanical And Physical Properties Of Polyurethane Composites Reinforced With Carbon Black N990 Particles. Int. J. Sci. Technol. Res. 2017, 6, 225–228. [Google Scholar]
- Greer, A.; Vasiev, I.; Della-Rosa, B.; Gadegaard, N. Fluorinated ethylene-propylene: A complementary alternative to PDMS for nanoimprint stamps. Nanotechnology 2016, 27, 155301. [Google Scholar] [CrossRef]
- Gubbels, F. An overview of the chemistry of condensation curing silicone sealants and adhesives. Int. J. Adhes. Adhes. 2024, 132, 103728. [Google Scholar] [CrossRef]
- Stevens, C. Environmental degradation pathways for the breakdown of polydimethylsiloxanes. J. Inorg. Biochem. 1998, 69, 203–207. [Google Scholar] [CrossRef]
- Marois, Y.; Guidoin, R. Biocompatibility of polyurethanes. In Madame Curie Bioscience Database [Internet]; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Kang, H.; Wu, S.; Wang, H.; Liang, Y.; Li, L.; Zhang, Q.; Zhang, L. Sustainable, processable and cytocompatible bioelastomers based on polycaprolactone and biobased polyester elastomer via dynamic vulcanization. Polym. Int. 2023, 72, 832–840. [Google Scholar] [CrossRef]
- Musgrove, H.; Cook, S.; Pompano, R. Parylene-C Coating Protects Resin 3D Printed Devices from Materials Erosion and Cytotoxicity. ChemRxiv 2023, 6, 3079–3083. [Google Scholar] [CrossRef]
- Faruq, O.; Sarkar, K.; Lee, B.-T. Physicochemical property and cytocompatibility of HyA-PEG loaded PMMA based bone cement. Mater. Chem. Phys. 2023, 295, 127142. [Google Scholar] [CrossRef]
- Sabahi, N.; Roohani, I.; Wang, C.H.; Farajzadeh, E.; Li, X. Thermoplastic polyurethane-based shape memory polymers with potential biomedical application: The effect of TPU soft-segment on shape memory effect and cytocompatibility. Polymer 2023, 283, 126189. [Google Scholar] [CrossRef]
- Niwa, O.; Kakuchi, M.; Tamamura, T. Mechanical properties of flexible polypyrrole-based conducting polymer alloy films. Polym. J. 1987, 19, 1293–1301. [Google Scholar] [CrossRef][Green Version]
- POLYPYRROLE. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB7775008.htm (accessed on 15 March 2025).
- Hryniewicz, B.M.; Lima, R.V.; Marchesi, L.F.; Vidotti, M. Impedimetric studies about the degradation of polypyrrole nanotubes during galvanostatic charge and discharge cycles. J. Electroanal. Chem. 2019, 855, 113636. [Google Scholar] [CrossRef]
- Chakraborty, R.; Seesala, V.S.; Manna, J.S.; Saha, P.; Dhara, S. Synthesis, characterization and cytocompatibility assessment of hydroxyapatite-polypyrrole composite coating synthesized through pulsed reverse electrochemical deposition. Mater. Sci. Eng. C 2019, 94, 597–607. [Google Scholar] [CrossRef]
- Ennis, B.; Truong, V.-T. Thermal and electrical stability of polypyrrole at elevated temperatures. Synth. Met. 1993, 59, 387–399. [Google Scholar] [CrossRef]
- Lunn, B.; Unsworth, J.; Booth, N.; Innis, P. Determination of the thermal conductivity of polypyrrole over the temperature range 280–335 K. J. Mater. Sci. 1993, 28, 5092–5098. [Google Scholar] [CrossRef]
- Gideon, O.; Samuel, H.S.; Okino, I.A. Biocompatible materials for next-generation biosensors. Discov. Chem. 2024, 1, 34. [Google Scholar] [CrossRef]
- Sun, J.; Guo, Q.; Dai, W.; Chen, J.L.; Mao, G.; Peng, Y.-K. Conductive Coatings on PDMS, PMMA, and Glass: Comparative Study of Graphene, Graphene Oxide, and Silver Nanoparticle Composites. Electrochem 2024, 5, 25. [Google Scholar] [CrossRef]
- Lima, R.A. The Impact of Polydimethylsiloxane (PDMS) in Engineering: Recent Advances and Applications. Fluids 2025, 10, 41. [Google Scholar] [CrossRef]
- Niranjan, A.; Gupta, P.; Rajoria, M.; Noor, I.M. Polymers as Sensing Layers in MEMS Biosensors for Molecular Diagnosis. Macromol. Symp. 2025, 414, 2400113. [Google Scholar] [CrossRef]
- Jacobsen, S.; Fritz, H.G.; Degée, P.; Dubois, P.; Jérôme, R. Polylactide (PLA)—A new way of production. Polym. Eng. Sci. 1999, 39, 1311–1319. [Google Scholar] [CrossRef]
- Avinc, O.; Khoddami, A. Overview of Poly(lactic acid) (PLA) Fibre. Fibre Chem. 2009, 41, 391–401. [Google Scholar] [CrossRef]
- Basu, P.; Verma, J.; Abhinav, V.; Ratnesh, R.K.; Singla, Y.K.; Kumar, V. Advancements in Lithography Techniques and Emerging Molecular Strategies for Nanostructure Fabrication. Int. J. Mol. Sci. 2025, 26, 3027. [Google Scholar] [CrossRef]
- Corneillie, S.; Smet, M. PLA architectures: The role of branching. Polym. Chem. 2015, 6, 850–867. [Google Scholar] [CrossRef]
- Vert, M.; Schwarch, G.; Coudane, J. Present and Future of PLA Polymers. J. Macromol. Sci. Part A 1995, 32, 787–796. [Google Scholar] [CrossRef]
- Domenek, S.; Ducruet, V. Characteristics and applications of PLA. Biodegradable and Biobased Polymers for Environmental and Biomedical Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; Volume 10, p. 9781119117360. [Google Scholar]
- Tsuji, H. Poly(lactic acid) stereocomplexes: A decade of progress. Adv. Drug Deliv. Rev. 2016, 107, 97–135. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H. Poly(lactide) Stereocomplexes: Formation, Structure, Properties, Degradation, and Applications. Macromol. Biosci. 2005, 5, 569–597. [Google Scholar] [CrossRef] [PubMed]
- Subramaniyan, M.; Karuppan, S.; Radhakrishnan, K.; Rajesh Kumar, R.; Saravana Kumar, K. Investigation of wear properties of 3D-printed PLA components using sandwich structure–A review. Mater. Today Proc. 2022, 66, 1112–1119. [Google Scholar] [CrossRef]
- Ehrmann, G.; Ehrmann, A. Investigation of the Shape-Memory Properties of 3D Printed PLA Structures with Different Infills. Polymers 2021, 13, 164. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.K.; Sanabria-DeLong, N.; Tew, G.N.; Bhatia, S.R. Structural Characterization of PLA−PEO−PLA Solutions and Hydrogels: Crystalline vs. Amorphous PLA Domains. Macromolecules 2008, 41, 1774–1784. [Google Scholar] [CrossRef]
- Ranakoti, L.; Gangil, B.; Mishra, S.K.; Singh, T.; Sharma, S.; Ilyas, R.A.; El-Khatib, S. Critical Review on Polylactic Acid: Properties, Structure, Processing, Biocomposites, and Nanocomposites. Materials 2022, 15, 4312. [Google Scholar] [CrossRef]
- Tripathi, N.; Misra, M.; Mohanty, A.K. Durable Polylactic Acid (PLA)-Based Sustainable Engineered Blends and Biocomposites: Recent Developments, Challenges, and Opportunities. ACS Eng. Au 2021, 1, 7–38. [Google Scholar] [CrossRef]
- Qian, F.; Jia, R.; Cheng, M.; Chaudhary, A.; Melhi, S.; Mekkey, S.D.; Zhu, N.; Wang, C.; Razak, F.; Xu, X.; et al. An overview of polylactic acid (PLA) nanocomposites for sensors. Adv. Compos. Hybrid Mater. 2024, 7, 75. [Google Scholar] [CrossRef]
- Taib, N.-A.A.B.; Rahman, M.R.; Huda, D.; Kuok, K.K.; Hamdan, S.; Bakri, M.K.B.; Julaihi, M.R.M.B.; Khan, A. A review on poly lactic acid (PLA) as a biodegradable polymer. Polym. Bull. 2023, 80, 1179–1213. [Google Scholar] [CrossRef]
- Bikiaris, N.D.; Koumentakou, I.; Samiotaki, C.; Meimaroglou, D.; Varytimidou, D.; Karatza, A.; Kalantzis, Z.; Roussou, M.; Bikiaris, R.D.; Papageorgiou, G.Z. Recent Advances in the Investigation of Poly(lactic acid) (PLA) Nanocomposites: Incorporation of Various Nanofillers and their Properties and Applications. Polymers 2023, 15, 1196. [Google Scholar] [CrossRef]
- Li, X.; Lin, Y.; Liu, M.; Meng, L.; Li, C. A review of research and application of polylactic acid composites. J. Appl. Polym. Sci. 2023, 140, e53477. [Google Scholar] [CrossRef]
- Trivedi, A.K.; Gupta, M.K.; Singh, H. PLA based biocomposites for sustainable products: A review. Adv. Ind. Eng. Polym. Res. 2023, 6, 382–395. [Google Scholar] [CrossRef]
- Hussain, M.; Khan, S.M.; Shafiq, M.; Abbas, N. A review on PLA-based biodegradable materials for biomedical applications. Giant 2024, 18, 100261. [Google Scholar] [CrossRef]
- Pérez-Davila, S.; González-Rodríguez, L.; Lama, R.; López-Álvarez, M.; Oliveira, A.L.; Serra, J.; Novoa, B.; Figueras, A.; González, P. 3D-Printed PLA Medical Devices: Physicochemical Changes and Biological Response after Sterilisation Treatments. Polymers 2022, 14, 4117. [Google Scholar] [CrossRef]
- Bao, Z.; Liu, Z.; Sun, B. Fabrication of the Flexible, Biocompatible, and Permeable Graphene/PLA Strain Sensor for Wearable Devices. Fibers Polym. 2024, 25, 1613–1621. [Google Scholar] [CrossRef]
- Tasangtong, B.; Sirichan, K.; Hasoon, C.; Na Nongkhai, P.; Rodthongkum, N.; Sameenoi, Y. Fabrication of biocompatible and biodegradable cloth-based sweat sensors using polylactic acid (PLA) via stencil transparent film-printing. Sens. Actuators B Chem. 2024, 408, 135513. [Google Scholar] [CrossRef]
- Ma, X.; Hu, Q.; Dai, Y.; He, P.; Zhang, X. Disposable sensors based on biodegradable polylactic acid piezoelectret films and their application in wearable electronics. Sens. Actuators A Phys. 2022, 346, 113834. [Google Scholar] [CrossRef]
- Ramezani Dana, H.; Ebrahimi, F. Synthesis, properties, and applications of polylactic acid-based polymers. Polym. Eng. Sci. 2023, 63, 22–43. [Google Scholar] [CrossRef]
- Tirado-Garcia, I.; Garcia-Gonzalez, D.; Garzon-Hernandez, S.; Rusinek, A.; Robles, G.; Martinez-Tarifa, J.M.; Arias, A. Conductive 3D printed PLA composites: On the interplay of mechanical, electrical and thermal behaviours. Compos. Struct. 2021, 265, 113744. [Google Scholar] [CrossRef]
- Houshyar, S.; Rifai, A.; Zizhou, R.; Dekiwadia, C.; Booth, M.A.; John, S.; Fox, K.; Truong, V.K. Liquid metal polymer composite: Flexible, conductive, biocompatible, and antimicrobial scaffold. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 1131–1139. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Yusoh, K. A review on the recent research of polycaprolactone (PCL). Adv. Mater. Res. 2016, 1134, 249–255. [Google Scholar] [CrossRef]
- Abedalwafa, M.; Wang, F.; Wang, L.; Li, C. Biodegradable poly-epsilon-caprolactone (PCL) for tissue engineering applications: A review. Rev. Adv. Mater. Sci. 2013, 34, 123–140. [Google Scholar]
- Demir, P.; Akman, F. Molecular structure, spectroscopic characterization, HOMO and LUMO analysis of PU and PCL grafted onto PEMA-co-PHEMA with DFT quantum chemical calculations. J. Mol. Struct. 2017, 1134, 404–415. [Google Scholar] [CrossRef]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef]
- Choi, E.-J.; Kim, C.-H.; Park, J.-K. Structure–property relationship in PCL/starch blend compatibilized with starch-g-PCL copolymer. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 2430–2438. [Google Scholar] [CrossRef]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef]
- Fortelny, I.; Ujcic, A.; Fambri, L.; Slouf, M. Phase Structure, Compatibility, and Toughness of PLA/PCL Blends: A Review. Front. Mater. 2019, 6, 206. [Google Scholar] [CrossRef]
- Fernández-Tena, A.; Pérez-Camargo, R.A.; Coulembier, O.; Sangroniz, L.; Aranburu, N.; Guerrica-Echevarria, G.; Liu, G.; Wang, D.; Cavallo, D.; Müller, A.J. Effect of Molecular Weight on the Crystallization and Melt Memory of Poly(ε-caprolactone) (PCL). Macromolecules 2023, 56, 4602–4620. [Google Scholar] [CrossRef]
- Siddiqui, N.; Asawa, S.; Birru, B.; Baadhe, R.; Rao, S. PCL-Based Composite Scaffold Matrices for Tissue Engineering Applications. Mol. Biotechnol. 2018, 60, 506–532. [Google Scholar] [CrossRef]
- Archer, E.; Torretti, M.; Madbouly, S. Biodegradable polycaprolactone (PCL) based polymer and composites. Phys. Sci. Rev. 2023, 8, 4391–4414. [Google Scholar] [CrossRef]
- Tamayo-Belda, M.; Pulido-Reyes, G.; González-Pleiter, M.; Martín-Betancor, K.; Leganés, F.; Rosal, R.; Fernández-Piñas, F. Identification and toxicity towards aquatic primary producers of the smallest fractions released from hydrolytic degradation of polycaprolactone microplastics. Chemosphere 2022, 303, 134966. [Google Scholar] [CrossRef]
- Richert, A.; Dąbrowska, G.B. Enzymatic degradation and biofilm formation during biodegradation of polylactide and polycaprolactone polymers in various environments. Int. J. Biol. Macromol. 2021, 176, 226–232. [Google Scholar] [CrossRef]
- Hegyesi, N.; Balogh-Weiser, D.; Pukánszky, B. Covalent immobilization of an enzyme on a layered silicate to catalyze the self-degradation of PCL. Polym. Degrad. Stab. 2024, 229, 111003. [Google Scholar] [CrossRef]
- Muñoz-González, A.M.; Clavijo-Grimaldo, D.; Leal-Marin, S.; Glasmacher, B. Optimizing Electroconductive PPy-PCL Scaffolds for Enhanced Tissue Engineering Performance. J. Biomed. Mater. Res. Part B Appl. Biomater. 2024, 112, e35511. [Google Scholar] [CrossRef] [PubMed]
- Shabankhah, M.; Moghaddaszadeh, A.; Najmoddin, N. 3D printed conductive PCL/GO scaffold immobilized with gelatin/CuO accelerates H9C2 cells attachment and proliferation. Prog. Org. Coat. 2024, 186, 108013. [Google Scholar] [CrossRef]
- Choi, S.B.; Meena, J.S.; Joo, J.; Kim, J.-W. Autonomous self-healing wearable flexible heaters enabled by innovative MXene/polycaprolactone composite fibrous networks and silver nanowires. Adv. Compos. Hybrid Mater. 2023, 6, 227. [Google Scholar] [CrossRef]
- Yan, X.; Wang, S.; Nie, G.; Gao, Y.; Li, L.; Zhang, T.; Long, Y.-Z.; Han, W. The iontronic sensor based on biodegradable polycaprolactone for interfacial capacitive pressure sensing. J. Mater. Sci. Mater. Electron. 2024, 35, 1093. [Google Scholar] [CrossRef]
- Giuliani, C.; De Stefano, I.; Mancuso, M.; Fiaschini, N.; Hein, L.A.; Mirabile Gattia, D.; Scatena, E.; Zenobi, E.; Del Gaudio, C.; Galante, F.; et al. Advanced Electrospun Composites Based on Polycaprolactone Fibers Loaded with Micronized Tungsten Powders for Radiation Shielding. Polymers 2024, 16, 2590. [Google Scholar] [CrossRef]
- Delgado-Rivera, R.; García-Rodríguez, W.; López, L.; Cunci, L.; Resto, P.J.; Domenech, M. PCL/PEO Polymer Membrane Prevents Biofouling in Wearable Detection Sensors. Membranes 2023, 13, 728. [Google Scholar] [CrossRef]
- Dedeloudi, A.; Farzeen, F.; Lesutan, V.-N.; Irwin, R.; Wylie, M.P.; Andersen, S.; Eastwood, M.P.; Lamprou, D.A. Biopolymeric 3D printed scaffolds as a versatile tissue engineering treatment for congenital diaphragmatic hernia. Int. J. Pharm. 2025, 672, 125313. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Oyunbaatar, N.-E.; Jeong, Y.-J.; Park, J.; Kim, S.-H.; Kwon, K.; Lee, H.; Won, Y.; Kim, D.-S.; Lee, D.-W. Enhancing flexibility of smart bioresorbable vascular scaffolds through 3D printing using polycaprolactone and polylactic acid. Sens. Actuators B Chem. 2025, 422, 136667. [Google Scholar] [CrossRef]
- Schmitt, P.R.; Dwyer, K.D.; Coulombe, K.L.K. Current Applications of Polycaprolactone as a Scaffold Material for Heart Regeneration. ACS Appl. Bio Mater. 2022, 5, 2461–2480. [Google Scholar] [CrossRef]
- Chountoulesi, M.; Selianitis, D.; Pispas, S.; Pippa, N. Recent Advances on PEO-PCL Block and Graft Copolymers as Nanocarriers for Drug Delivery Applications. Materials 2023, 16, 2298. [Google Scholar] [CrossRef] [PubMed]
- Soares, Í.; Faria, J.; Marques, A.; Ribeiro, I.A.C.; Baleizão, C.; Bettencourt, A.; Ferreira, I.M.M.; Baptista, A.C. Drug Delivery from PCL/Chitosan Multilayer Coatings for Metallic Implants. ACS Omega 2022, 7, 23096–23106. [Google Scholar] [CrossRef]
- Ganbaatar, S.E.; Kim, H.-K.; Kang, N.-U.; Kim, E.C.; U, H.J.; Cho, Y.-S.; Park, H.-H. Calcium Phosphate (CaP) Composite Nanostructures on Polycaprolactone (PCL): Synergistic Effects on Antibacterial Activity and Osteoblast Behavior. Polymers 2025, 17, 200. [Google Scholar] [CrossRef]
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A unique polymer for drug delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef]
- Singh, S.; Singha, P. Effect of Modifications in Poly (Lactide-co-Glycolide) (PLGA) on Drug Release and Degradation Characteristics: A Mini Review. Curr. Drug Deliv. 2021, 18, 1378–1390. [Google Scholar] [CrossRef]
- Bazgir, M.; Zhang, W.; Zhang, X.; Elies, J.; Saeinasab, M.; Coates, P.; Youseffi, M.; Sefat, F. Degradation and Characterisation of Electrospun Polycaprolactone (PCL) and Poly(lactic-co-glycolic acid) (PLGA) Scaffolds for Vascular Tissue Engineering. Materials 2021, 14, 4773. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Chen, L.; Ci, T.; Yu, L.; Ding, J. Effects of Molecular Weight and Its Distribution of PEG Block on Micellization and Thermogellability of PLGA–PEG–PLGA Copolymer Aqueous Solutions. Macromolecules 2015, 48, 3662–3671. [Google Scholar] [CrossRef]
- Zhang, K.; Tang, X.; Zhang, J.; Lu, W.; Lin, X.; Zhang, Y.; Tian, B.; Yang, H.; He, H. PEG–PLGA copolymers: Their structure and structure-influenced drug delivery applications. J. Control. Release 2014, 183, 77–86. [Google Scholar] [CrossRef]
- Ganesan, M.; Paranthaman, S. Molecular structure, interactions, and antimicrobial properties of curcumin-PLGA Complexes—A DFT study. J. Mol. Model. 2021, 27, 329. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.; Handler, R.A.; Blaisten-Barojas, E. Structure, energetics and thermodynamics of PLGA condensed phases from Molecular Dynamics. Polymer 2020, 206, 122903. [Google Scholar] [CrossRef]
- Ko, K.-W.; Choi, B.; Kang, E.Y.; Shin, S.-W.; Baek, S.-W.; Han, D.K. The antagonistic effect of magnesium hydroxide particles on vascular endothelial activation induced by acidic PLGA degradation products. Biomater. Sci. 2021, 9, 892–907. [Google Scholar] [CrossRef]
- Gaur, M.; Maurya, S.; Akhtar, M.S.; Yadav, A.B. Synthesis and Evaluation of BSA-Loaded PLGA–Chitosan Composite Nanoparticles for the Protein-Based Drug Delivery System. ACS Omega 2023, 8, 18751–18759. [Google Scholar] [CrossRef]
- Chen, Z.; Fu, Y.; Zhang, X.; Weng, Y. Research progress in degradation behavior and applications of PLGA. China Plast. 2024, 38, 92–99. [Google Scholar] [CrossRef]
- Walker, J.; Albert, J.; Liang, D.; Sun, J.; Schutzman, R.; Kumar, R.; White, C.; Beck-Broichsitter, M.; Schwendeman, S.P. In vitro degradation and erosion behavior of commercial PLGAs used for controlled drug delivery. Drug Deliv. Transl. Res. 2023, 13, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Narmani, A.; Jahedi, R.; Bakhshian-Dehkordi, E.; Ganji, S.; Nemati, M.; Ghahramani-Asl, R.; Moloudi, K.; Hosseini, S.M.; Bagheri, H.; Kesharwani, P.; et al. Biomedical applications of PLGA nanoparticles in nanomedicine: Advances in drug delivery systems and cancer therapy. Expert Opin. Drug Deliv. 2023, 20, 937–954. [Google Scholar] [CrossRef]
- Joiner, J.B.; Prasher, A.; Young, I.C.; Kim, J.; Shrivastava, R.; Maturavongsadit, P.; Benhabbour, S.R. Effects of Drug Physicochemical Properties on In-Situ Forming Implant Polymer Degradation and Drug Release Kinetics. Pharmaceutics 2022, 14, 1188. [Google Scholar] [CrossRef]
- Yang, F.; Stahnke, R.; Lawal, K.; Mahnen, C.; Duffy, P.; Xu, S.; Durig, T. Development of poly (lactic-co-glycolic acid) (PLGA) based implants using hot melt extrusion (HME) for sustained release of drugs: The impacts of PLGA’s material characteristics. Int. J. Pharm. 2024, 663, 124556. [Google Scholar] [CrossRef] [PubMed]
- Khadka, B.; Lee, B.; Kim, K.-T. Drug Delivery Systems for Personal Healthcare by Smart Wearable Patch System. Biomolecules 2023, 13, 929. [Google Scholar] [CrossRef] [PubMed]
- Kar, A.; Ahamad, N.; Dewani, M.; Awasthi, L.; Patil, R.; Banerjee, R. Wearable and implantable devices for drug delivery: Applications and challenges. Biomaterials 2022, 283, 121435. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Xu, Y.; Gao, Z.; Yuan, T.; Liu, Q.; Yang, R.; Zhang, B.; Peng, L. Recent Advances in Intelligent Wearable Medical Devices Integrating Biosensing and Drug Delivery. Adv. Mater. 2022, 34, 2108491. [Google Scholar] [CrossRef]
- Giri, B.R.; Jakka, D.; Sandoval, M.A.; Kulkarni, V.R.; Bao, Q. Advancements in Ocular Therapy: A Review of Emerging Drug Delivery Approaches and Pharmaceutical Technologies. Pharmaceutics 2024, 16, 1325. [Google Scholar] [CrossRef]
- Sciurti, E.; Primavera, R.; Di Mascolo, D.; Rizzo, A.; Balena, A.; Padmanabhan, S.K.; Rizzi, F.; Decuzzi, P.; De Vittorio, M. Ultrasound-induced deformation of PLGA-microPlates for on-command drug release. Microelectron. Eng. 2020, 229, 111360. [Google Scholar] [CrossRef]
- Seo, I.; Hassan, R.U.; Ryu, B.; Koh, W.-G.; Ryu, W. Electrohydrodynamic Printing of Biodegradable PLGA Micro-Patterns on 3D Polymer Structures. Adv. Mater. Technol. 2024, 9, 2400230. [Google Scholar] [CrossRef]
- Ciocîlteu, M.V.; Mocanu, A.G.; Biță, A.; Manda, C.V.; Nicolicescu, C.; Rău, G.; Belu, I.; Pîrvu, A.S.; Balasoiu, M.; Nănescu, V.; et al. Development of Hybrid Implantable Local Release Systems Based on PLGA Nanoparticles with Applications in Bone Diseases. Polymers 2024, 16, 3064. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, H.; Luo, Y.; Chen, Y.; Wang, M.; Wu, C.; Hu, P. Recent Applications of PLGA in Drug Delivery Systems. Polymers 2024, 16, 2606. [Google Scholar] [CrossRef]
- Sah, R.K.; Kumar, B.S. Development of PLGA-SPC3 functionalized gefitinib mesoporous silica nano-scaffolds for breast cancer targeting: Biodistribution and cytotoxicity analysis. Pharm. Dev. Technol. 2025, 30, 160–176. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An Overview of Poly(lactic-co-glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]
- Rocha, C.V.; Gonçalves, V.; da Silva, M.C.; Bañobre-López, M.; Gallo, J. PLGA-Based Composites for Various Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 2034. [Google Scholar] [CrossRef]
- Yang, X.; Zhong, Y.; Zhang, L.; Liu, Y.; Zhuo, F.; Wang, J.; Ge, L.; Zhang, L.; Zeng, X.; Tan, W.; et al. Conductive/Insulating Bioinks with Multitechnology Compatibility and Adjustable Performance. ACS Biomater. Sci. Eng. 2024, 10, 5352–5361. [Google Scholar] [CrossRef]
- Ozcicek, I.; Aysit, N.; Balcikanli, Z.; Ayturk, N.U.; Aydeger, A.; Baydas, G.; Aydin, M.S.; Altintas, E.; Erim, U.C. Development of BDNF/NGF/IKVAV Peptide Modified and Gold Nanoparticle Conductive PCL/PLGA Nerve Guidance Conduit for Regeneration of the Rat Spinal Cord Injury. Macromol. Biosci. 2024, 24, 2300453. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef]
- Kulkarni, S.O.; Kanekar, P.P.; Nilegaonkar, S.S.; Sarnaik, S.S.; Jog, J.P. Production and characterization of a biodegradable poly (hydroxybutyrate-co-hydroxyvalerate) (PHB-co-PHV) copolymer by moderately haloalkalitolerant Halomonas campisalis MCM B-1027 isolated from Lonar Lake, India. Bioresour. Technol. 2010, 101, 9765–9771. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; Wang, M. Production and evaluation of biodegradable composites based on PHB–PHV copolymer. Biomaterials 2002, 23, 2631–2639. [Google Scholar] [CrossRef] [PubMed]
- Volova, T.; Shishatskaya, E.; Sevastianov, V.; Efremov, S.; Mogilnaya, O. Results of biomedical investigations of PHB and PHB/PHV fibers. Biochem. Eng. J. 2003, 16, 125–133. [Google Scholar] [CrossRef]
- Barham, P.J.; Barker, P.; Organ, S.J. Physical properties of poly(hydroxybutyrate) and copolymers of hydroxybutyrate and hydroxyvalerate. FEMS Microbiol. Lett. 1992, 103, 289–298. [Google Scholar] [CrossRef]
- Sato, H.; Ando, Y.; Dybal, J.; Iwata, T.; Noda, I.; Ozaki, Y. Crystal Structures, Thermal Behaviors, and C−H···O═C Hydrogen Bondings of Poly(3-hydroxyvalerate) and Poly(3-hydroxybutyrate) Studied by Infrared Spectroscopy and X-ray Diffraction. Macromolecules 2008, 41, 4305–4312. [Google Scholar] [CrossRef]
- Marlina, D.; Sato, H.; Hoshina, H.; Ozaki, Y. Intermolecular interactions of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (P(HB-co-HV)) with PHB-type crystal structure and PHV-type crystal structure studied by low-frequency Raman and terahertz spectroscopy. Polymer 2018, 135, 331–337. [Google Scholar] [CrossRef]
- Langford, A.; Chan, C.M.; Pratt, S.; Garvey, C.J.; Laycock, B. The morphology of crystallisation of PHBV/PHBV copolymer blends. Eur. Polym. J. 2019, 112, 104–119. [Google Scholar] [CrossRef]
- Policastro, G.; Panico, A.; Fabbricino, M. Improving biological production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) co-polymer: A critical review. Rev. Environ. Sci. Bio/Technol. 2021, 20, 479–513. [Google Scholar] [CrossRef]
- Urbelienė, N.; Gasparavičiūtė, R.; Vaitekūnas, J.; Meškienė, R.; Valantinaitė, U.; Kruopis, P.; Gudiukaitė, R.; Meškys, R. A screening method for polyester films-degrading microorganisms and enzymes. J. Hazard. Mater. 2025, 487, 137177. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Koelling, K.; Vodovotz, Y. Assessment of PHB with varying hydroxyvalerate content for potential packaging applications. Eur. Polym. J. 2011, 47, 179–186. [Google Scholar] [CrossRef]
- Yuan, Q.; Chen, S.; Chen, Y.; Zhang, X.; Lou, Y.; Li, X.; Liang, Q.; Zhang, Y.; Sun, Y. Evaluating AGS efficiency in PHA synthesis and extraction integrated with nutrient removal: The impact of COD concentrations. Chemosphere 2024, 368, 143708. [Google Scholar] [CrossRef]
- Reay, M.K.; Graf, M.; Greenfield, L.M.; Bargiela, R.; Onyije, C.; Lloyd, C.E.; Bull, I.D.; Evershed, R.P.; Golyshin, P.N.; Chadwick, D.R. Microbial degradation of bioplastic (PHBV) is limited by nutrient availability at high microplastic loadings. Environ. Sci. Adv. 2025, 4, 133–146. [Google Scholar] [CrossRef]
- Gao, L.; Drozdov, A.D. Exploring the performance of bio-based PLA/PHB blends: A comprehensive analysis. Polym. Renew. Resour. 2024, 15, 358–374. [Google Scholar] [CrossRef]
- Zytner, P.; Pal, A.K.; Mohanty, A.K.; Misra, M. Performance evaluation of biodegradable polymer PHBV and PBAT blends with adjustable melt flow behaviour, heat deflection temperature, and morphological transition. Can. J. Chem. Eng. 2024, 102, 2805–2817. [Google Scholar] [CrossRef]
- Paloyan, A.; Tadevosyan, M.; Ghevondyan, D.; Khoyetsyan, L.; Karapetyan, M.; Margaryan, A.; Antranikian, G.; Panosyan, H. Biodegradation of polyhydroxyalkanoates: Current state and future prospects. Front. Microbiol. 2025, 16, 1542468. [Google Scholar] [CrossRef]
- El-Hadi, A.; Schnabel, R.; Straube, E.; Müller, G.; Henning, S. Correlation between degree of crystallinity, morphology, glass temperature, mechanical properties and biodegradation of poly (3-hydroxyalkanoate) PHAs and their blends. Polym. Test. 2002, 21, 665–674. [Google Scholar] [CrossRef]
- Norazlina, H. Bioinspired Materials: Polyhydroxyalkanoates-based Graphene Nanocomposites. Malays. J. Appl. Sci. 2024, 9, 105–114. [Google Scholar] [CrossRef]
- Goonoo, N.; Bhaw-Luximon, A.; Passanha, P.; Esteves, S.; Schönherr, H.; Jhurry, D. Biomineralization potential and cellular response of PHB and PHBV blends with natural anionic polysaccharides. Mater. Sci. Eng. C 2017, 76, 13–24. [Google Scholar] [CrossRef]
- Alam, F.; Ashfaq Ahmed, M.; Jalal, A.H.; Siddiquee, I.; Adury, R.Z.; Hossain, G.M.M.; Pala, N. Recent Progress and Challenges of Implantable Biodegradable Biosensors. Micromachines 2024, 15, 475. [Google Scholar] [CrossRef]
- Rakkan, T.; Zhang, S.; Lehner, S.; Hufenus, R.; Sangkharak, K.; Ren, Q. Bio-based modification of polyhydroxyalkanoates (PHA) towards increased antimicrobial activities and reduced cytotoxicity. Int. J. Biol. Macromol. 2024, 275, 133132. [Google Scholar] [CrossRef]
- Sun, S.; Tan, Y.; Cheng, Q.; Cai, Y.; Zheng, J.; Wang, W.; Xu, L.; Li, G.; Wang, D.; Zhang, L.; et al. Thermoplastic PHB-Reinforced Chitosan Piezoelectric Films for Biodegradable Pressure Sensors. ACS Appl. Bio Mater. 2024, 7, 6823–6831. [Google Scholar] [CrossRef]
- Mohapatra, S.; Mohanty, D.; Sharma, S.; Dikshit, S.; Kohli, I.; Samantaray, D.; Kumar, R.; Kathpalia, M. Biomedical application of polymeric biomaterial: Polyhydroxybutyrate. In Bioresource Utilization and Management; Apple Academic Press: Cambridge, MA, USA, 2021; pp. 111–124. [Google Scholar]
- Khan, A.; Joshi, R.; Sharma, M.K.; Huang, C.-J.; Yu, J.-H.; Wang, Y.-L.; Lin, Z.-H. The potential of organic piezoelectric materials for next-generation implantable biomedical devices. Nano Trends 2024, 6, 100032. [Google Scholar] [CrossRef]
- Cesarino, I.; Moroz, I.; Carbonera, A.F.; Martins, G.d.O.; Fernandes, L.G.L.; Leao, A.L. Chapter 6-PHA/PHB/PHBV-based composites: Development and biomedical applications. In Bioresorbable Polymers and Their COMPOSITES; Verma, D., Okhawilai, M., Goh, K.L., Ramakrishna, S., Pasbakhsh, P., Sharma, M., Eds.; Woodhead Publishing: Cambridge, UK, 2024; pp. 107–123. [Google Scholar]
- Engler, L.G.; Giustina, M.D.; Giovanela, M.; Roesch-Ely, M.; Gately, N.; Major, I.; Crespo, J.S.; Devine, D.M. Exploring the Synergy of Metallic Antimicrobial Agents in Ternary Blends of PHB/PLA/PCL. J. Biomed. Mater. Res. Part A 2025, 113, e37857. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.A.; Lach, A.A.; Morris, H.L.; Carr, A.J.; Mouthuy, P.-A. Polydioxanone implants: A systematic review on safety and performance in patients. J. Biomater. Appl. 2020, 34, 902–916. [Google Scholar] [CrossRef]
- Miele, D.; Nomicisio, C.; Musitelli, G.; Boselli, C.; Cornaglia, A.I.; Sànchez-Espejo, R.; Vigani, B.; Viseras, C.; Rossi, S.; Sandri, G. Design and development of polydioxanone scaffolds for skin tissue engineering manufactured via green process. Int. J. Pharm. 2023, 634, 122669. [Google Scholar] [CrossRef]
- Goonoo, N.; Jeetah, R.; Bhaw-Luximon, A.; Jhurry, D. Polydioxanone-based bio-materials for tissue engineering and drug/gene delivery applications. Eur. J. Pharm. Biopharm. 2015, 97, 371–391. [Google Scholar] [CrossRef] [PubMed]
- Saska, S.; Pilatti, L.; Silva, E.S.d.S.; Nagasawa, M.A.; Câmara, D.; Lizier, N.; Finger, E.; Dyszkiewicz Konwińska, M.; Kempisty, B.; Tunchel, S. Polydioxanone-based membranes for bone regeneration. Polymers 2021, 13, 1685. [Google Scholar] [CrossRef]
- Kumar, P.; Kandasamy, G.; Singh, J.P.; Maurya, P.K. Oxides for Medical Applications; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Xie, Y.; Ning, J. Application of polydioxanone sutures in the Nuss procedure. Thorac. Cardiovasc. Surg. 2022, 70, 077–082. [Google Scholar] [CrossRef] [PubMed]
- Molea, G.; Schonauer, F.; Bifulco, G.; D’angelo, D. Comparative study on biocompatibility and absorption times of three absorbable monofilament suture materials (Polydioxanone, Poliglecaprone 25, Glycomer 631). Br. J. Plast. Surg. 2000, 53, 137–141. [Google Scholar] [CrossRef]
- Harding, S.E.; Tombs, M.P.; Adams, G.G.; Paulsen, B.S.; Inngjerdingen, K.T.; Barsett, H. An Introduction to Polysaccharide Biotechnology; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Maji, B. 1-Introduction to natural polysaccharides. In Functional Polysaccharides for Biomedical Applications; Maiti, S., Jana, S., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 1–31. [Google Scholar]
- Aspinall, G.O. The Polysaccharides; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Saji, S.; Hebden, A.; Goswami, P.; Du, C. A Brief Review on the Development of Alginate Extraction Process and Its Sustainability. Sustainability 2022, 14, 5181. [Google Scholar] [CrossRef]
- Hecht, H.; Srebnik, S. Structural Characterization of Sodium Alginate and Calcium Alginate. Biomacromolecules 2016, 17, 2160–2167. [Google Scholar] [CrossRef]
- Kabir, I.I.; Sorrell, C.C.; Mofarah, S.S.; Yang, W.; Yuen, A.C.Y.; Nazir, M.T.; Yeoh, G.H. Alginate/Polymer-Based Materials for Fire Retardancy: Synthesis, Structure, Properties, and Applications. Polym. Rev. 2021, 61, 357–414. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Sudhakar, M.; Nallasamy, V.D.; Dharani, G.; Buschmann, A.H. Applications of seaweed biopolymers and its composites in dental applications. J. Appl. Biol. Biotechnol. 2024, 12, 62–68. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Z.; Wang, H.; Song, Z.; Yu, D.; Li, G.; Liu, X.; Liu, W. Progress in Research on Metal Ion Crosslinking Alginate-Based Gels. Gels 2024, 11, 16. [Google Scholar] [CrossRef]
- Argüelles-Monal, W.M.; Lizardi-Mendoza, J.; Fernández-Quiroz, D.; Recillas-Mota, M.T.; Montiel-Herrera, M. Chitosan Derivatives: Introducing New Functionalities with a Controlled Molecular Architecture for Innovative Materials. Polymers 2018, 10, 342. [Google Scholar] [CrossRef]
- Roberts, G.A.F. Structure of Chitin and Chitosan. In Chitin Chemistry; Roberts, G.A.F., Ed.; Macmillan Education UK: London, UK, 1992; pp. 1–53. [Google Scholar]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Román-Doval, R.; Torres-Arellanes, S.P.; Tenorio-Barajas, A.Y.; Gómez-Sánchez, A.; Valencia-Lazcano, A.A. Chitosan: Properties and Its Application in Agriculture in Context of Molecular Weight. Polymers 2023, 15, 2867. [Google Scholar] [CrossRef]
- Domard, A.; Domard, M. Chitosan: Structure-properties relationship and biomedical applications. Polym. Biomater. 2002, 9, 187–212. [Google Scholar]
- Kou, S.; Peters, L.; Mucalo, M. Chitosan: A review of molecular structure, bioactivities and interactions with the human body and micro-organisms. Carbohydr. Polym. 2022, 282, 119132. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, Y.; Huang, S.; Guo, B. Chitosan-based self-healing hydrogel dressing for wound healing. Adv. Colloid Interface Sci. 2024, 332, 103267. [Google Scholar] [CrossRef]
- Naghib, S.M.; Ahmadi, B.; Mikaeeli Kangarshahi, B.; Mozafari, M.R. Chitosan-based smart stimuli-responsive nanoparticles for gene delivery and gene therapy: Recent progresses on cancer therapy. Int. J. Biol. Macromol. 2024, 278, 134542. [Google Scholar] [CrossRef] [PubMed]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef]
- Ward, P.D.; Thibeault, S.L.; Gray, S.D. Hyaluronic Acid: Its Role in Voice. J. Voice 2002, 16, 303–309. [Google Scholar] [CrossRef]
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic Acid: Redefining Its Role. Cells 2020, 9, 1743. [Google Scholar] [CrossRef]
- Atkins, E.D.T.; Sheehan, J.K. Structure for Hyaluronic Acid. Nat. New Biol. 1972, 235, 253–254. [Google Scholar] [CrossRef] [PubMed]
- Ström, A.; Larsson, A.; Okay, O. Preparation and physical properties of hyaluronic acid-based cryogels. J. Appl. Polym. Sci. 2015, 132, 42194. [Google Scholar] [CrossRef]
- Sudha, P.N.; Rose, M.H. Chapter Nine-Beneficial Effects of Hyaluronic Acid. In Advances in Food and Nutrition Research; Kim, S.-K., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 72, pp. 137–176. [Google Scholar]
- Salih, A.R.C.; Farooqi, H.M.U.; Amin, H.; Karn, P.R.; Meghani, N.; Nagendran, S. Hyaluronic acid: Comprehensive review of a multifunctional biopolymer. Future J. Pharm. Sci. 2024, 10, 63. [Google Scholar] [CrossRef]
- Chang, W.; Chen, L.; Chen, K. The bioengineering application of hyaluronic acid in tissue regeneration and repair. Int. J. Biol. Macromol. 2024, 270, 132454. [Google Scholar] [CrossRef]
- Lavanya, D.; Kulkarni, P.; Dixit, M.; Raavi, P.K.; Krishna, L.N.V. Sources of cellulose and their applications—A review. Int. J. Drug Formul. Res. 2011, 2, 19–38. [Google Scholar]
- Kobayashi, S.; Sakamoto, J.; Kimura, S. In vitro synthesis of cellulose and related polysaccharides. Prog. Polym. Sci. 2001, 26, 1525–1560. [Google Scholar] [CrossRef]
- Heinze, T. Cellulose: Structure and Properties. In Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials; Rojas, O.J., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–52. [Google Scholar]
- Liu, J.; Lv, S.; Mu, Y.; Tong, J.; Liu, L.; He, T.; Zeng, Q.; Wei, D. Applied research and recent advances in the development of flexible sensing hydrogels from cellulose: A review. Int. J. Biol. Macromol. 2024, 281, 136100. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Ding, L.; Bai, X.; Cao, Y.; Rahmaninia, M.; Li, B.; Li, B. Cellulose-based suture: State of art, challenge, and future outlook. J. Bioresour. Bioprod. 2024. [Google Scholar] [CrossRef]
- Branden, C.I.; Tooze, J. Introduction to Protein Structure; Garland Science: New York, NY, USA, 2012. [Google Scholar]
- Lesk, A. Introduction to Protein Science: Architecture, Function, and Genomics. Oxford University Press: Oxford, MS, USA, 2010. [Google Scholar]
- Kessel, A.; Ben-Tal, N. Introduction to Proteins: Structure, Function, and Motion; Chapman and Hall/CRC: Boca Raton, FL, USA, 2018. [Google Scholar]
- DeGrado, W.F. Introduction: Protein Design. Chem. Rev. 2001, 101, 3025–3026. [Google Scholar] [CrossRef][Green Version]
- Fratzl, P. Collagen: Structure and mechanics, an introduction. In Collagen: Structure and Mechanics; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–13. [Google Scholar]
- Shenoy, M.; Abdul, N.S.; Qamar, Z.; Al Bahri, B.M.; Al Ghalayini, K.Z.K.; Kakti, A.; Murad, M.; Fadul, M.; Alamri, M.A.M.; Alqarni, M.A. Collagen structure, synthesis, and its applications: A systematic review. Cureus 2022, 14, e24856. [Google Scholar] [CrossRef]
- Zhao, C.; Xiao, Y.; Ling, S.; Pei, Y.; Ren, J. Structure of collagen. In Fibrous Proteins: Design, Synthesis, and Assembly; Springer: Berlin/Heidelberg, Germany, 2021; pp. 17–25. [Google Scholar]
- Kolliopoulos, V.; Harley, B.A.C. Mineralized collagen scaffolds for regenerative engineering applications. Curr. Opin. Biotechnol. 2024, 86, 103080. [Google Scholar] [CrossRef] [PubMed]
- Dille, M.J.; Haug, I.J.; Draget, K.I. Chapter 34-Gelatin and collagen. In Handbook of Hydrocolloids, 3rd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Cambridge, UK, 2021; pp. 1073–1097. [Google Scholar]
- Mikhailov, O.V. Gelatin as It Is: History and Modernity. Int. J. Mol. Sci. 2023, 24, 3583. [Google Scholar] [CrossRef] [PubMed]
- Rather, J.A.; Akhter, N.; Ashraf, Q.S.; Mir, S.A.; Makroo, H.A.; Majid, D.; Barba, F.J.; Khaneghah, A.M.; Dar, B.N. A comprehensive review on gelatin: Understanding impact of the sources, extraction methods, and modifications on potential packaging applications. Food Packag. Shelf Life 2022, 34, 100945. [Google Scholar] [CrossRef]
- Alipal, J.; Mohd Pu’ad, N.A.S.; Lee, T.C.; Nayan, N.H.M.; Sahari, N.; Basri, H.; Idris, M.I.; Abdullah, H.Z. A review of gelatin: Properties, sources, process, applications, and commercialisation. Mater. Today Proc. 2021, 42, 240–250. [Google Scholar] [CrossRef]
- Lukin, I.; Erezuma, I.; Maeso, L.; Zarate, J.; Desimone, M.F.; Al-Tel, T.H.; Dolatshahi-Pirouz, A.; Orive, G. Progress in Gelatin as Biomaterial for Tissue Engineering. Pharmaceutics 2022, 14, 1177. [Google Scholar] [CrossRef]
- Koh, L.-D.; Cheng, Y.; Teng, C.-P.; Khin, Y.-W.; Loh, X.-J.; Tee, S.-Y.; Low, M.; Ye, E.; Yu, H.-D.; Zhang, Y.-W.; et al. Structures, mechanical properties and applications of silk fibroin materials. Prog. Polym. Sci. 2015, 46, 86–110. [Google Scholar] [CrossRef]
- Zheng, H.; Zuo, B. Functional silk fibroin hydrogels: Preparation, properties and applications. J. Mater. Chem. B 2021, 9, 1238–1258. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, D.; Zhang, Y.; Li, M.; Chai, R. Silk fibroin hydrogels for biomedical applications. Smart Med. 2022, 1, e20220011. [Google Scholar] [CrossRef]
- Lujerdean, C.; Baci, G.-M.; Cucu, A.-A.; Dezmirean, D.S. The Contribution of Silk Fibroin in Biomedical Engineering. Insects 2022, 13, 286. [Google Scholar] [CrossRef]
- Weisel, J.W.; Litvinov, R.I. Fibrin Formation, Structure and Properties. In Fibrous Proteins: Structures and Mechanisms; Parry, D.A.D., Squire, J.M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 405–456. [Google Scholar]
- Pieters, M.; Wolberg, A.S. Fibrinogen and fibrin: An illustrated review. Res. Pract. Thromb. Haemost. 2019, 3, 161–172. [Google Scholar] [CrossRef]
- Murano, G. The molecular structure of fibrinogen. Semin. Thromb. Hemost. 2024, 50, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Neerman-Arbez, M.; Casini, A. Fifty years of fibrinogen structure and function. Semin. Thromb. Hemost. 2024, 50, 148–150. [Google Scholar] [CrossRef]
- Sierra-Sánchez, Á.; Sanabria-de la Torre, R.; Ubago-Rodríguez, A.; Quiñones-Vico, M.I.; Montero-Vílchez, T.; Sánchez-Díaz, M.; Arias-Santiago, S. Blood Plasma, Fibrinogen or Fibrin Biomaterial for the Manufacturing of Skin Tissue-Engineered Products and Other Dermatological Treatments: A Systematic Review. J. Funct. Biomater. 2025, 16, 79. [Google Scholar] [CrossRef]
- Wu, X.; Yu, X.; Chen, C.; Chen, C.; Wang, Y.; Su, D.; Zhu, L. Fibrinogen and tumors. Front. Oncol. 2024, 14, 1393599. [Google Scholar] [CrossRef]
- Jin, G.-W.; Rejinold, N.S.; Choy, J.-H. Polyphosphazene-based biomaterials for biomedical applications. Int. J. Mol. Sci. 2022, 23, 15993. [Google Scholar] [CrossRef] [PubMed]
- Ogueri, K.S.; Ogueri, K.S.; Ude, C.C.; Allcock, H.R.; Laurencin, C.T. Biomedical applications of polyphosphazenes. Med. Devices Sens. 2020, 3, e10113. [Google Scholar] [CrossRef] [PubMed]
- Ilayaperumal, P.; Chelladurai, P.; Vairan, K.; Anilkumar, P.; Balagurusamy, B. Polyphosphazenes—A promising candidate for Drug Delivery, Bioimaging, and tissue Engineering: A review. Macromol. Mater. Eng. 2023, 308, 2200553. [Google Scholar] [CrossRef]
- Teasdale, I.; Brüggemann, O. Polyphosphazenes: Multifunctional, biodegradable vehicles for drug and gene delivery. Polymers 2013, 5, 161–187. [Google Scholar] [CrossRef]
- Ogueri, K.S.; Ogueri, K.S.; Allcock, H.R.; Laurencin, C.T. Polyphosphazene polymers: The next generation of biomaterials for regenerative engineering and therapeutic drug delivery. J. Vac. Sci. Technol. B 2020, 38, 030801. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Sun, L.; Agrawal, R.; Zhang, M. Sundew adhesive: A naturally occurring hydrogel. J. R. Soc. Interface 2015, 12, 20150226. [Google Scholar] [CrossRef]
- Choi, J.H.; Son, D.; Shin, M. Sundew-Inspired Adhesive Hydrogel Threads through Reversible Complexation of Polyphenol and Boronic Acid. Appl. Sci. 2021, 11, 8591. [Google Scholar] [CrossRef]
- Gong, Z.; Zhang, Y.; Yu, X. Review on bio-inspired materials with nanotechnology applications in medical devices. J. Phys. Conf. Ser. 2021, 1948, 012227. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, X.; Ma, X.; Wang, W.; Yan, F.; Zhao, X.; Chu, X.; Xu, W.; Sun, C. A review of the properties and applications of bioadhesive hydrogels. Polym. Chem. 2021, 12, 3721–3739. [Google Scholar] [CrossRef]
- Sun, L.; Huang, Y.; Bian, Z.; Petrosino, J.; Fan, Z.; Wang, Y.; Park, K.H.; Yue, T.; Schmidt, M.; Galster, S. Sundew-inspired adhesive hydrogels combined with adipose-derived stem cells for wound healing. ACS Appl. Mater. Interfaces 2016, 8, 2423–2434. [Google Scholar] [CrossRef] [PubMed]
- Zamhuri, A.; Lim, G.P.; Ma, N.L.; Tee, K.S.; Soon, C.F. MXene in the lens of biomedical engineering: Synthesis, applications and future outlook. Biomed. Eng. Online 2021, 20, 33. [Google Scholar] [CrossRef]
- Huang, J.; Li, Z.; Mao, Y.; Li, Z. Progress and biomedical applications of MXenes. Nano Sel. 2021, 2, 1480–1508. [Google Scholar] [CrossRef]
- Seidi, F.; Arabi Shamsabadi, A.; Dadashi Firouzjaei, M.; Elliott, M.; Saeb, M.R.; Huang, Y.; Li, C.; Xiao, H.; Anasori, B. MXenes antibacterial properties and applications: A review and perspective. Small 2023, 19, 2206716. [Google Scholar] [CrossRef]
- Huang, H.; Dong, C.; Feng, W.; Wang, Y.; Huang, B.; Chen, Y. Biomedical engineering of two-dimensional MXenes. Adv. Drug Deliv. Rev. 2022, 184, 114178. [Google Scholar] [CrossRef]
- Li, H.; Fan, R.; Zou, B.; Yan, J.; Shi, Q.; Guo, G. Roles of MXenes in biomedical applications: Recent developments and prospects. J. Nanobiotechnology 2023, 21, 73. [Google Scholar] [CrossRef]
- Kim, D.; Han, S.A.; Kim, J.H.; Lee, J.-H.; Kim, S.-W.; Lee, S.-W. Biomolecular Piezoelectric Materials: From Amino Acids to Living Tissues. Adv. Mater. 2020, 32, 1906989. [Google Scholar] [CrossRef]
- Panda, S. Biomolecular Piezoelectric Materials for Biosensors. Prabha Mater. Sci. Lett. 2022, 1, 37–49. [Google Scholar] [CrossRef]
- Guerin, S.; Tofail, S.A.M.; Thompson, D. Organic piezoelectric materials: Milestones and potential. NPG Asia Mater. 2019, 11, 10. [Google Scholar] [CrossRef]
- Menon, K.; Joy, R.A.; Sood, N.; Mittal, R.K. The Applications of BioMEMS in Diagnosis, Cell Biology, and Therapy: A Review. BioNanoScience 2013, 3, 356–366. [Google Scholar] [CrossRef]
- Guerin, S. Predictive Design of Biomolecular Crystal Piezoelectricity. Ph.D. Thesis, University of Limerick, Limerick, Ireland, 2023. [Google Scholar]
- Sharoyko, V.V.; Shemchuk, O.S.; Meshcheriakov, A.A.; Andoskin, P.A.; Ageev, S.V.; Petrov, A.V.; Rumyantsev, A.M.; Sambuk, E.V.; Murin, I.V.; Maystrenko, D.N.; et al. Synthesis, characterization and biocompatibility of glycine modified graphene oxide. Fuller. Nanotub. Carbon Nanostruct. 2024, 32, 288–299. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Zhou, S.; Chen, Z.; Xu, J.; Cui, N.; Yuan, M.; Li, B.; Gu, L. A high-performance, biocompatible, and fully biodegradable piezo-triboelectric hybrid nanogenerator based on PVA/Glycine/PVA heterostructured piezoelectric film. Nano Energy 2024, 122, 109310. [Google Scholar] [CrossRef]
- Kholkin, A.; Alikin, D.; Shur, V.; Dishon, S.; Ehre, D.; Lubomirsky, I. Local Piezoelectric Properties of Doped Biomolecular Crystals. Materials 2021, 14, 4922. [Google Scholar] [CrossRef]
- Bai, Y.; Meng, H.; Li, Z.; Wang, Z.L. Degradable piezoelectric biomaterials for medical applications. Med Mat 2024, 1, 40–49. [Google Scholar] [CrossRef]
- Lin, Q.; Zhang, Y.; Chen, L.; Zhang, H.; An, C.; Li, C.; Wang, Q.; Song, J.; He, W.; Wang, H. Glycine/alginate-based piezoelectric film consisting of a single, monolithic β-glycine spherulite towards flexible and biodegradable force sensor. Regen. Biomater. 2024, 11, rbae047. [Google Scholar] [CrossRef]
- Silva, L.R.; Sulzbach, J.F.; Nunes, J.P.P.; Almeida, T.V.e.S.L.d. An Open-Source BioMEMS Platform for Generalized Early Cancer Detection. In Proceedings of the 2024 8th International Symposium on Instrumentation Systems, Circuits and Transducers (INSCIT), Joao Pessoa, Brazil, 2–6 September 2024; pp. 1–6. [Google Scholar]
- Javor, J. Biomems for Cardiac Tissue Monitoring and Maturation. Ph.D. Thesis, Boston University, Boston, MA, USA, 2021. [Google Scholar]
- Mousavi, M.; Alzgool, M.; Davaji, B.; Towfighian, S. Event-driven MEMS vibration sensor: Integration of triboelectric nanogenerator and low-frequency switch. Mech. Syst. Signal Process. 2023, 187, 109921. [Google Scholar] [CrossRef]
- Chircov, C.; Grumezescu, A.M. Microelectromechanical Systems (MEMS) for Biomedical Applications. Micromachines 2022, 13, 164. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Tang, Y.-Y.; Gu, Z.-X.; Wang, P.; Chen, X.-G.; Lv, H.-P.; Li, P.-F.; Jiang, Q.; Gu, N.; Ren, S.; et al. Biodegradable ferroelectric molecular crystal with large piezoelectric response. Science 2024, 383, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, J.; Long, Y.; Zhang, Z.; Wang, L.; Sui, J.; Dong, Y.; Wang, Y.; Taylor, R.; Ni, D.; et al. Wafer-scale heterostructured piezoelectric bio-organic thin films. Science 2021, 373, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, X.; Peng, Z.; Yan, X.; Liu, S.; Hong, Y.; Shan, Y.; Xu, X.; Jin, L.; Liu, B.; et al. Active self-assembly of piezoelectric biomolecular films via synergistic nanoconfinement and in-situ poling. Nat. Commun. 2023, 14, 4094. [Google Scholar] [CrossRef]
- Le, T.T.; Curry, E.J.; Vinikoor, T.; Das, R.; Liu, Y.; Sheets, D.; Tran, K.T.M.; Hawxhurst, C.J.; Stevens, J.F.; Hancock, J.N.; et al. Piezoelectric Nanofiber Membrane for Reusable, Stable, and Highly Functional Face Mask Filter with Long-Term Biodegradability. Adv. Funct. Mater. 2022, 32, 2113040. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Z.; Peng, Z.; Yang, X.; Li, X.; Shan, Y.; Liu, B.; Xu, X.; Gao, Y.; Yang, Z. Self-charging and long-term face masks leveraging low-cost, biodegradable and sustainable piezoelectric nanofiber membrane. Nano Mater. Sci. 2025, 7, 113–122. [Google Scholar] [CrossRef]
- Sharma, A.; Badea, M.; Tiwari, S.; Marty, J.L. Wearable Biosensors: An Alternative and Practical Approach in Healthcare and Disease Monitoring. Molecules 2021, 26, 748. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Upadhyay, A.; Sayson, D.; Nguyen, B.H.; Tran, S.D. Advances in Medical Wearable Biosensors: Design, Fabrication and Materials Strategies in Healthcare Monitoring. Molecules 2022, 27, 165. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.-F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Smith, A.A.; Li, R.; Tse, Z.T.H. Reshaping healthcare with wearable biosensors. Sci. Rep. 2023, 13, 4998. [Google Scholar] [CrossRef]
- Research, G.V. Wearable Medical Devices Market Trends. Available online: https://www.grandviewresearch.com/industry-analysis/wearable-medical-devices-market (accessed on 18 March 2025).
- Johnston, L.; Wang, G.; Hu, K.; Qian, C.; Liu, G. Advances in Biosensors for Continuous Glucose Monitoring Towards Wearables. Front. Bioeng. Biotechnol. 2021, 9, 733810. [Google Scholar] [CrossRef]
- Huang, X.; Yao, C.; Huang, S.; Zheng, S.; Liu, Z.; Liu, J.; Wang, J.; Chen, H.-j.; Xie, X. Technological Advances of Wearable Device for Continuous Monitoring of In Vivo Glucose. ACS Sens. 2024, 9, 1065–1088. [Google Scholar] [CrossRef]
- Mathew, M.; Radhakrishnan, S.; Vaidyanathan, A.; Chakraborty, B.; Rout, C.S. Flexible and wearable electrochemical biosensors based on two-dimensional materials: Recent developments. Anal. Bioanal. Chem. 2021, 413, 727–762. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Cui, Z.; Du, Y.; Xu, Q.; Deng, Q.; Zhu, N. Multifunctional Prussian blue/graphene ink for flexible biosensors and supercapacitors. Electrochim. Acta 2021, 387, 138496. [Google Scholar] [CrossRef]
- Arduini, F. Nanomaterials and Cross-Cutting Technologies for Fostering Smart Electrochemical Biosensors in the Detection of Chemical Warfare Agents. Appl. Sci. 2021, 11, 720. [Google Scholar] [CrossRef]
- Pérez, D.; Orozco, J. Wearable electrochemical biosensors to measure biomarkers with complex blood-to-sweat partition such as proteins and hormones. Microchim. Acta 2022, 189, 127. [Google Scholar] [CrossRef] [PubMed]
- Parrilla, M.; Detamornrat, U.; Domínguez-Robles, J.; Donnelly, R.F.; De Wael, K. Wearable hollow microneedle sensing patches for the transdermal electrochemical monitoring of glucose. Talanta 2022, 249, 123695. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Li, G.; Yan, D.; Liu, C.; Xu, T.; Zhang, X. Ultra-Small Wearable Flexible Biosensor for Continuous Sweat Analysis. ACS Sens. 2022, 7, 3102–3107. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Fan, Z.; Sun, Y.; Sun, Y.; Yang, Y.; Zhang, Y.; Ma, J.; Wang, Z.; Zhu, Z. A Dual-Function Wearable Electrochemical Sensor for Uric Acid and Glucose Sensing in Sweat. Biosensors 2023, 13, 105. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, D.; Cai, Z.; Du, B.; Zou, M.; Tang, S.; Li, B.; Xiong, C.; Ji, P.; Zhang, L.; et al. A Wearable Breath Sensor Based on Fiber-Tip Microcantilever. Biosensors 2022, 12, 168. [Google Scholar] [CrossRef]
- Yin, B.; Wan, X.; Qian, C.; Sohan, A.S.M.M.F.; Zhou, T.; Yue, W. Enzyme Method-Based Microfluidic Chip for the Rapid Detection of Copper Ions. Micromachines 2021, 12, 1380. [Google Scholar] [CrossRef]
- Saeidi, M.; Chenani, H.; Orouji, M.; Adel Rastkhiz, M.; Bolghanabadi, N.; Vakili, S.; Mohamadnia, Z.; Hatamie, A.; Simchi, A. Electrochemical Wearable Biosensors and Bioelectronic Devices Based on Hydrogels: Mechanical Properties and Electrochemical Behavior. Biosensors 2023, 13, 823. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Peng, S.; He, S.; Tang, S.-Y.; Goda, K.; Wang, C.H.; Li, M. Wearable Electrochemical Biosensors for Advanced Healthcare Monitoring. Adv. Sci. 2025, 12, 2411433. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, J.; Ji, G.; Wang, H.; Zhu, B.; Chen, C.; Zhou, H.; Wang, Y.; Gao, Z. Universal Flexible Wearable Biosensors for Noninvasive Health Monitoring. ACS Appl. Mater. Interfaces 2025, 17, 20741–20755. [Google Scholar] [CrossRef]
- Yoon, J.; Kwon, N.; Lee, Y.; Kim, S.; Lee, T.; Choi, J.-W. Nanotechnology-Based Wearable Electrochemical Biosensor for Disease Diagnosis. ACS Sens. 2025, 10, 1675–1689. [Google Scholar] [CrossRef]
- Hua, J.; Li, J.; Jiang, Y.; Xie, S.; Shi, Y.; Pan, L. Skin-Attachable Sensors for Biomedical Applications. Biomed. Mater. Devices 2023, 1, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, H.; Zheng, Y.; Zhai, Y.; Liu, X.; Liu, C.; Mi, L.; Guo, Z.; Shen, C. Highly Compressible and Robust Polyimide/Carbon Nanotube Composite Aerogel for High-Performance Wearable Pressure Sensor. ACS Appl. Mater. Interfaces 2019, 11, 42594–42606. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, X.; Wang, W.; Yu, D. Flexible cellulose/polyvinyl alcohol/PEDOT:PSS electrodes for ECG monitoring. Cellulose 2021, 28, 4913–4926. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, T.; Liu, Z.; Wang, J.; Xu, J.; Chen, K.; Yu, L. Flexible and Robust 3D a-SiGe Radial Junction Near-Infrared Photodetectors for Rapid Sphygmic Signal Monitoring. Adv. Funct. Mater. 2022, 32, 2107040. [Google Scholar] [CrossRef]
- Tang, L.; Chang, S.J.; Chen, C.-J.; Liu, J.-T. Non-Invasive Blood Glucose Monitoring Technology: A Review. Sensors 2020, 20, 6925. [Google Scholar] [CrossRef]
- Chen, Y.; He, Z.; Wu, Y.; Bai, X.; Li, Y.; Yang, W.; Liu, Y.; Li, R.-W. A Wearable Molecularly Imprinted Electrochemical Sensor for Cortisol Stable Monitoring in Sweat. Biosensors 2025, 15, 194. [Google Scholar] [CrossRef]
- Singh, N.K.; Chung, S.; Chang, A.-Y.; Wang, J.; Hall, D.A. A non-invasive wearable stress patch for real-time cortisol monitoring using a pseudoknot-assisted aptamer. Biosens. Bioelectron. 2023, 227, 115097. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.-I.; Chen, J.; Wang, W.; Kim, J.-Y.; Kwon, M.-K.; Moradnia, M.; Pouladi, S.; Ryou, J.-H. Skin-Attached Arrayed Piezoelectric Sensors for Continuous and Safe Monitoring of Oculomotor Movements. Adv. Healthc. Mater. 2024, 13, 2303581. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lyu, Y.; Liu, D.; Shi, X.; Ji, Z.; Liu, D.; Jia, X.; Wang, X. Skin-Mountable Thermo-responsive Structured Hydrogel for Optical and Adhesion Coupled Functional Sensing. Small 2025, 21, 2411808. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Shi, B.; Li, Y.; Wang, Y.; Shuai, K.; Li, Y.; Ming, G.; Song, T.; Pei, W.; et al. Freestanding Transparent Organic–Inorganic Mesh E-Tattoo for Breathable Bioelectrical Membranes with Enhanced Capillary-Driven Adhesion. ACS Appl. Mater. Interfaces 2025, 17, 22337–22351. [Google Scholar] [CrossRef]
- Parrilla, M.; Cuartero, M.; Padrell Sánchez, S.; Rajabi, M.; Roxhed, N.; Niklaus, F.; Crespo, G.A. Wearable All-Solid-State Potentiometric Microneedle Patch for Intradermal Potassium Detection. Anal. Chem. 2019, 91, 1578–1586. [Google Scholar] [CrossRef]
- Abhinav, V.; Naik, T.R. Low-Cost, Point-of-Care Potassium Ion Sensing Electrode in EGFET Configuration for Ultra-High Sensitivity. IEEE Access 2024, 12, 121837–121845. [Google Scholar] [CrossRef]
- Piro, B.; Mattana, G.; Noël, V. Recent Advances in Skin Chemical Sensors. Sensors 2019, 19, 4376. [Google Scholar] [CrossRef]
- Khonina, S.N.; Kazanskiy, N.L. Trends and Advances in Wearable Plasmonic Sensors Utilizing Surface-Enhanced Raman Spectroscopy (SERS): A Comprehensive Review. Sensors 2025, 25, 1367. [Google Scholar] [CrossRef]
- Haque Chowdhury, M.A.; Tasnim, N.; Hossain, M.; Habib, A. Flexible, stretchable, and single-molecule-sensitive SERS-active sensor for wearable biosensing applications. RSC Adv. 2023, 13, 20787–20798. [Google Scholar] [CrossRef]
- Liu, L.; Martinez Pancorbo, P.; Xiao, T.-H.; Noguchi, S.; Marumi, M.; Segawa, H.; Karhadkar, S.; Gala de Pablo, J.; Hiramatsu, K.; Kitahama, Y.; et al. Highly Scalable, Wearable Surface-Enhanced Raman Spectroscopy. Adv. Opt. Mater. 2022, 10, 2200054. [Google Scholar] [CrossRef]
- Cheng, Y.; Feng, S.; Ning, Q.; Li, T.; Xu, H.; Sun, Q.; Cui, D.; Wang, K. Dual-signal readout paper-based wearable biosensor with a 3D origami structure for multiplexed analyte detection in sweat. Microsyst. Nanoeng. 2023, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; Kang, D.; Xue, Y.; Lee, S.; Pielak, R.M.; Kim, J.; Hwang, T.; Min, S.; Banks, A.; Bastien, P.; et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med. 2016, 8, 366ra165. [Google Scholar] [CrossRef] [PubMed]
- Ardalan, S.; Hosseinifard, M.; Vosough, M.; Golmohammadi, H. Towards smart personalized perspiration analysis: An IoT-integrated cellulose-based microfluidic wearable patch for smartphone fluorimetric multi-sensing of sweat biomarkers. Biosens. Bioelectron. 2020, 168, 112450. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Guo, K.; Shen, X.; Ning, H.; Liang, H.; Zhong, J.; Xu, W.; Tang, B.; Yao, R.; Peng, J. Physical Simulation Model of WO3 Electrochromic Films Based on Continuous Electron-Transfer Kinetics and Experimental Verification. ACS Appl. Mater. Interfaces 2021, 13, 4768–4776. [Google Scholar] [CrossRef]
- Abhinav, V.; Patkar, R.; Vinchurkar, M.; Naik, T.R.; Baghini, M.S. Enhancing Potentiometric Response Using Modified Ion Sensitive Transistor. ECS Meet. Abstr. 2019, MA2019-01, 2022. [Google Scholar] [CrossRef]
- Desai, S.R.; Bhoraniya, R.B.; Koladiya, M.; Bhopekar, V.V.; Patel, C.R.; Mori, T.; Modha, S.G. Solvatochromism and halochromism in nitro lophine derivatives: Photophysical study, computational calculations and applications as pH sensing material. J. Photochem. Photobiol. A Chem. 2024, 455, 115751. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Lasalde-Ramírez, J.A.; Mahato, K.; Wang, J.; Gao, W. Wearable chemical sensors for biomarker discovery in the omics era. Nat. Rev. Chem. 2022, 6, 899–915. [Google Scholar] [CrossRef]
- Sekine, Y.; Kim, S.B.; Zhang, Y.; Bandodkar, A.J.; Xu, S.; Choi, J.; Irie, M.; Ray, T.R.; Kohli, P.; Kozai, N.; et al. A fluorometric skin-interfaced microfluidic device and smartphone imaging module for in situ quantitative analysis of sweat chemistry. Lab A Chip 2018, 18, 2178–2186. [Google Scholar] [CrossRef]
- Polat, E.O.; Mercier, G.; Nikitskiy, I.; Puma, E.; Galan, T.; Gupta, S.; Montagut, M.; Piqueras, J.J.; Bouwens, M.; Durduran, T.; et al. Flexible graphene photodetectors for wearable fitness monitoring. Sci. Adv. 2019, 5, eaaw7846. [Google Scholar] [CrossRef]
- Yu, X.; Xing, Y.; Zhang, Y.; Zhang, P.; He, Y.; Ghamsari, F.; Ramasubramanian, M.K.; Wang, Y.; Ai, H.; Oberholzer, J. Smartphone-microfluidic fluorescence imaging system for studying islet physiology. Front. Endocrinol. 2022, 13, 1039912. [Google Scholar] [CrossRef]
- Roda, A.; Arduini, F.; Mirasoli, M.; Zangheri, M.; Fabiani, L.; Colozza, N.; Marchegiani, E.; Simoni, P.; Moscone, D. A challenge in biosensors: Is it better to measure a photon or an electron for ultrasensitive detection? Biosens. Bioelectron. 2020, 155, 112093. [Google Scholar] [CrossRef] [PubMed]
- Leal-Junior, A.; Avellar, L.; Frizera, A.; Marques, C. Smart textiles for multimodal wearable sensing using highly stretchable multiplexed optical fiber system. Sci. Rep. 2020, 10, 13867. [Google Scholar] [CrossRef]
- Padash, M.; Enz, C.; Carrara, S. Microfluidics by Additive Manufacturing for Wearable Biosensors: A Review. Sensors 2020, 20, 4236. [Google Scholar] [CrossRef] [PubMed]
- Boobphahom, S.; Nguyet Ly, M.; Soum, V.; Pyun, N.; Kwon, O.-S.; Rodthongkum, N.; Shin, K. Recent Advances in Microfluidic Paper-Based Analytical Devices toward High-Throughput Screening. Molecules 2020, 25, 2970. [Google Scholar] [CrossRef]
- Pour, S.R.S.; Calabria, D.; Emamiamin, A.; Lazzarini, E.; Pace, A.; Guardigli, M.; Zangheri, M.; Mirasoli, M. Microfluidic-Based Non-Invasive Wearable Biosensors for Real-Time Monitoring of Sweat Biomarkers. Biosensors 2024, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Yang, T.; Zhao, X.; Cai, Z.; Chen, G.; Yao, M.; Chen, K.; Bick, M.; Wang, J.; Li, S.; et al. A wireless energy transmission enabled wearable active acetone biosensor for non-invasive prediabetes diagnosis. Nano Energy 2020, 74, 104941. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Yang, J.; Zhou, J.; Zhang, Y.; Zhang, H.; Li, Y. A Fully Integrated Flexible Tunable Chemical Sensor Based on Gold-Modified Indium Selenide Nanosheets. ACS Sens. 2022, 7, 1183–1193. [Google Scholar] [CrossRef]
- Kumar, A.; Kim, J.H.; Chang, D.W. Flexible and Ultra Low Weight Energy Harvesters Based on 2D Phosphorene or Black phosphorus (BP): Current and Futuristic Prospects. ChemSusChem 2024, 17, e202301718. [Google Scholar] [CrossRef]
- Ma, C.; Ma, M.-G.; Si, C.; Ji, X.-X.; Wan, P. Flexible MXene-Based Composites for Wearable Devices. Adv. Funct. Mater. 2021, 31, 2009524. [Google Scholar] [CrossRef]
- Sun, Y.; He, W.; Jiang, C.; Li, J.; Liu, J.; Liu, M. Wearable Biodevices Based on Two-Dimensional Materials: From Flexible Sensors to Smart Integrated Systems. Nano-Micro Lett. 2025, 17, 109. [Google Scholar] [CrossRef]
- Barki, H.; Chung, W.-Y. Mental Stress Detection Using a Wearable In-Ear Plethysmography. Biosensors 2023, 13, 397. [Google Scholar] [CrossRef] [PubMed]
- Florea, A.D.; Arghir, S.-N.; Chira, A.I.; Sollars-Castellanos, A.-M.; Sipele-Siale, O.; Calvo-Córdoba, A. Advancements in Monitoring Physical Fatigue in Aviation: A Comprehensive Analysis of State-of-the-Art ECG Sensor Technologies; SciTePress: Setúbal, Portugal, 2024. [Google Scholar]
- Kargarandehkordi, A.; Li, S.; Lin, K.; Phillips, K.T.; Benzo, R.M.; Washington, P. Fusing Wearable Biosensors with Artificial Intelligence for Mental Health Monitoring: A Systematic Review. Biosensors 2025, 15, 202. [Google Scholar] [CrossRef]
- Betti, S.; Lova, R.M.; Rovini, E.; Acerbi, G.; Santarelli, L.; Cabiati, M.; Ry, S.D.; Cavallo, F. Evaluation of an Integrated System of Wearable Physiological Sensors for Stress Monitoring in Working Environments by Using Biological Markers. IEEE Trans. Biomed. Eng. 2018, 65, 1748–1758. [Google Scholar] [CrossRef] [PubMed]
- Anusha, A.S.; Sukumaran, P.; Sarveswaran, V.; Shyam, A.; Akl, T.J.; Preejith, S.P.; Sivaprakasam, M. Electrodermal Activity Based Pre-surgery Stress Detection Using a Wrist Wearable. IEEE J. Biomed. Health Inform. 2020, 24, 92–100. [Google Scholar] [CrossRef]
- Aristizabal, S.; Byun, K.; Wood, N.; Mullan, A.F.; Porter, P.M.; Campanella, C.; Jamrozik, A.; Nenadic, I.Z.; Bauer, B.A. The Feasibility of Wearable and Self-Report Stress Detection Measures in a Semi-Controlled Lab Environment. IEEE Access 2021, 9, 102053–102068. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, X.; Liu, Y.; Fan, L.; Gan, J.; Liu, W.; Zhao, Y.; Sun, L. Polydopamine Decorated Microneedles with Fe-MSC-Derived Nanovesicles Encapsulation for Wound Healing. Adv. Sci. 2022, 9, 2103317. [Google Scholar] [CrossRef]
- Jang, M.-H.; Lei, Y.; Conners, R.T.; Wang, G. Self-powered triboelectric wearable biosensor using Scotch tape. J. Mater. Chem. B 2023, 11, 10640–10650. [Google Scholar] [CrossRef]
- Wang, M.; Ye, C.; Yang, Y.; Mukasa, D.; Wang, C.; Xu, C.; Min, J.; Solomon, S.A.; Tu, J.; Shen, G.; et al. Printable molecule-selective core–shell nanoparticles for wearable and implantable sensing. Nat. Mater. 2025, 24, 589–598. [Google Scholar] [CrossRef]
- Torrente-Rodríguez, R.M.; Tu, J.; Yang, Y.; Min, J.; Wang, M.; Song, Y.; Yu, Y.; Xu, C.; Ye, C.; IsHak, W.W.; et al. Investigation of Cortisol Dynamics in Human Sweat Using a Graphene-Based Wireless mHealth System. Matter 2020, 2, 921–937. [Google Scholar] [CrossRef]
- Martinek, R.; Ladrova, M.; Sidikova, M.; Jaros, R.; Behbehani, K.; Kahankova, R.; Kawala-Sterniuk, A. Advanced Bioelectrical Signal Processing Methods: Past, Present and Future Approach—Part I: Cardiac Signals. Sensors 2021, 21, 5186. [Google Scholar] [CrossRef]
- Tian, Z.; Zhao, Z.; Yan, F. Organic electrochemical transistor in wearable bioelectronics: Profiles, applications, and integration. Wearable Electron. 2024, 1, 1–25. [Google Scholar] [CrossRef]
- Ershad, F.; Patel, S.; Yu, C. Wearable bioelectronics fabricated in situ on skins. npj Flex. Electron. 2023, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.M.; Noghanian, S.; Khan, Z.U.; Alzahrani, S.; Alharbi, S.; Alhartomi, M.; Alsulami, R. Wearable and Flexible Sensor Devices: Recent Advances in Designs, Fabrication Methods, and Applications. Sensors 2025, 25, 1377. [Google Scholar] [CrossRef]
- Alberto, J.; Leal, C.; Fernandes, C.; Lopes, P.A.; Paisana, H.; de Almeida, A.T.; Tavakoli, M. Fully Untethered Battery-free Biomonitoring Electronic Tattoo with Wireless Energy Harvesting. Sci. Rep. 2020, 10, 5539. [Google Scholar] [CrossRef]
- Rachim, V.P.; Lee, J.; Kim, Y.-C.; Oh, J.; Jeong, U.; Park, S.-M. A Scalable Laser-Centric Fabrication of an Epidermal Cardiopulmonary Patch. Adv. Mater. Technol. 2022, 7, 2200242. [Google Scholar] [CrossRef]
- Chung, H.U.; Kim, B.H.; Lee, J.Y.; Lee, J.; Xie, Z.; Ibler, E.M.; Lee, K.; Banks, A.; Jeong, J.Y.; Kim, J.; et al. Binodal, wireless epidermal electronic systems with in-sensor analytics for neonatal intensive care. Science 2019, 363, eaau0780. [Google Scholar] [CrossRef]
- Bin Heyat, M.B.; Akhtar, F.; Abbas, S.J.; Al-Sarem, M.; Alqarafi, A.; Stalin, A.; Abbasi, R.; Muaad, A.Y.; Lai, D.; Wu, K. Wearable Flexible Electronics Based Cardiac Electrode for Researcher Mental Stress Detection System Using Machine Learning Models on Single Lead Electrocardiogram Signal. Biosensors 2022, 12, 427. [Google Scholar] [CrossRef]
- Alimbayeva, Z.; Alimbayev, C.; Ozhikenov, K.; Bayanbay, N.; Ozhikenova, A. Wearable ECG Device and Machine Learning for Heart Monitoring. Sensors 2024, 24, 4201. [Google Scholar] [CrossRef] [PubMed]
- Pazuelo, J.; Juez, J.Y.; Moumane, H.; Pyrzowski, J.; Mayor, L.; Segura-Quijano, F.E.; Valderrama, M.; Le Van Quyen, M. Evaluating the Electroencephalographic Signal Quality of an In-Ear Wearable Device. Sensors 2024, 24, 3973. [Google Scholar] [CrossRef]
- Takagi, T.; Tomita, N.; Sato, S.; Yamamoto, M.; Takamatsu, S.; Itoh, T. Wearable EMG Measurement Device Using Polyurethane Foam for Motion Artifact Suppression. Sensors 2024, 24, 2985. [Google Scholar] [CrossRef]
- Casson, A.J. Wearable EEG and beyond. Biomed. Eng. Lett. 2019, 9, 53–71. [Google Scholar] [CrossRef]
- Xu, Y.; De la Paz, E.; Paul, A.; Mahato, K.; Sempionatto, J.R.; Tostado, N.; Lee, M.; Hota, G.; Lin, M.; Uppal, A.; et al. In-ear integrated sensor array for the continuous monitoring of brain activity and of lactate in sweat. Nat. Biomed. Eng. 2023, 7, 1307–1320. [Google Scholar] [CrossRef]
- Carvalho, F.M.; Lopes, P.; Carneiro, M.; Serra, A.; Coelho, J.; de Almeida, A.T.; Tavakoli, M. Nondrying, Sticky Hydrogels for the Next Generation of High-Resolution Conformable Bioelectronics. ACS Appl. Electron. Mater. 2020, 2, 3390–3401. [Google Scholar] [CrossRef]
- Kim, M.K.; Kantarcigil, C.; Kim, B.; Baruah, R.K.; Maity, S.; Park, Y.; Kim, K.; Lee, S.; Malandraki, J.B.; Avlani, S.; et al. Flexible submental sensor patch with remote monitoring controls for management of oropharyngeal swallowing disorders. Sci. Adv. 2019, 5, eaay3210. [Google Scholar] [CrossRef]
- Rdest, M.; Janas, D. Carbon Nanotube Wearable Sensors for Health Diagnostics. Sensors 2021, 21, 5847. [Google Scholar] [CrossRef]
- Kim, T.Y.; Hong, S.H.; Jeong, S.H.; Bae, H.; Cheong, S.; Choi, H.; Hahn, S.K. Multifunctional Intelligent Wearable Devices Using Logical Circuits of Monolithic Gold Nanowires. Adv. Mater. 2023, 35, 2303401. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhang, F.; Yan, Z.; Chen, P.-Y. Wearable bioelectronics based on emerging nanomaterials for telehealth applications. Device 2025, 3, 100676. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Tian, G.; Chen, J.; Liu, Y.; Fatima, E.; Qiu, J.; Malek, N.A.N.N.; Qi, D. Neural electrodes for brain-computer interface system: From rigid to soft. BMEMat 2025, e12130. [Google Scholar] [CrossRef]
- Zhang, W.; Su, Z.; Zhang, X.; Wang, W.; Li, Z. Recent progress on PEDOT-based wearable bioelectronics. VIEW 2022, 3, 20220030. [Google Scholar] [CrossRef]
- Linh, V.T.N.; Han, S.; Koh, E.; Kim, S.; Jung, H.S.; Koo, J. Advances in wearable electronics for monitoring human organs: Bridging external and internal health assessments. Biomaterials 2025, 314, 122865. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Goldstein, C. Clinical applications of artificial intelligence in sleep medicine: A sleep clinician’s perspective. Sleep Breath. 2023, 27, 39–55. [Google Scholar] [CrossRef]
- Antonijevic, M.; Zivkovic, M.; Arsic, S.; Jevremovic, A. Using AI-Based Classification Techniques to Process EEG Data Collected during the Visual Short-Term Memory Assessment. J. Sens. 2020, 2020, 8767865. [Google Scholar] [CrossRef]
- Brunmair, J.; Gotsmy, M.; Niederstaetter, L.; Neuditschko, B.; Bileck, A.; Slany, A.; Feuerstein, M.L.; Langbauer, C.; Janker, L.; Zanghellini, J.; et al. Finger sweat analysis enables short interval metabolic biomonitoring in humans. Nat. Commun. 2021, 12, 5993. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Dong, W.; Li, Y.; Li, F.; Geng, J.; Zhu, M.; Chen, T.; Zhang, H.; Sun, L.; Lee, C. An epidermal sEMG tattoo-like patch as a new human–machine interface for patients with loss of voice. Microsyst. Nanoeng. 2020, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Bu, N.; Hu, X. Recent trends in electronic skin for transdermal drug delivery. Intell. Pharm. 2023, 1, 183–191. [Google Scholar] [CrossRef]
- Sen, O.; Poddar, P.; Sarkar, P.; Das, S.; Manna, S. Current advancements in microneedle technology for therapeutic and biomedical applications. Sens. Int. 2025, 6, 100325. [Google Scholar] [CrossRef]
- Feng, M.; Li, Y.; Sun, Y.; Liu, T.; Ergashovich, Y.; Jiang, G. Integration of metformin-loaded MIL-100(Fe) into hydrogel microneedles for prolonged regulation of blood glucose levels. Biomed. Phys. Eng. Express 2024, 10, 045004. [Google Scholar] [CrossRef]
- Luo, X.; Yu, Q.; Yang, L.; Cui, Y. Wearable, Sensing-Controlled, Ultrasound-Based Microneedle Smart System for Diabetes Management. ACS Sens. 2023, 8, 1710–1722. [Google Scholar] [CrossRef]
- Ranjan Yadav, P.; Iqbal Nasiri, M.; Vora, L.K.; Larrañeta, E.; Donnelly, R.F.; Pattanayek, S.K.; Bhusan Das, D. Super-swelling hydrogel-forming microneedle based transdermal drug delivery: Mathematical modelling, simulation and experimental validation. Int. J. Pharm. 2022, 622, 121835. [Google Scholar] [CrossRef]
- Xiao, M.; Zifeng, W.; Yanru, A.; Yingqi, D.; Xinghao, W.; and Zhu, Z. Fabrication and mechanical modelling of dissolvable PVA/PVP composite microneedles with biocompatibility for efficient transdermal delivery of ibuprofen. J. Biomater. Sci. Polym. Ed. 2024, 35, 1439–1454. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Ye, R.; Zheng, Y.; Li, X.; Chen, Y.; Xie, X.; Jiang, L. Smartphone-powered iontophoresis-microneedle array patch for controlled transdermal delivery. Microsyst. Nanoeng. 2020, 6, 112. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Q.; Ye, L.; Yang, L.; Cui, Y. A wearable, minimally-invasive, fully electrochemically-controlled feedback minisystem for diabetes management. Lab A Chip 2023, 23, 421–436. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Liu, T.; Kopecki, Z.; Cowin, A.; Lee, I.; Pai, J.-H.; Lowe, S.E.; Zhong, Y.L. A Multifunctional Wearable Device with a Graphene/Silver Nanowire Nanocomposite for Highly Sensitive Strain Sensing and Drug Delivery. C 2019, 5, 17. [Google Scholar] [CrossRef]
- Cicha, I.; Priefer, R.; Severino, P.; Souto, E.B.; Jain, S. Biosensor-Integrated Drug Delivery Systems as New Materials for Biomedical Applications. Biomolecules 2022, 12, 1198. [Google Scholar] [CrossRef]
- Amano, T.; Fujii, N.; Quan, Y.-S.; Kenny, G.P.; Kondo, N.; Yamashita, H.; Inoue, Y. In vivo assessments of microneedle arrays and iontophoresis of pilocarpine in human palmar sweating. J. Control. Release 2023, 358, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ye, R.; Gong, X.; Yang, J.; Liu, B.; Xu, Y.; Nie, G.; Xie, X.; Jiang, L. Iontophoresis-driven microneedle patch for the active transdermal delivery of vaccine macromolecules. Microsyst. Nanoeng. 2023, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.; Heath, B. Iontophoresis and electroporation-assisted microneedles: Advancements and therapeutic potentials in transdermal drug delivery. In Drug Delivery and Translational Research; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar] [CrossRef]
- Wang, G.; Moriyama, N.; Tottori, S.; Nishizawa, M. Recent advances in iontophoresis-assisted microneedle devices for transdermal biosensing and drug delivery. Mater. Today Bio 2025, 31, 101504. [Google Scholar] [CrossRef]
- Raikar, A.S.; Kumar, P.; Raikar, G.S.; Somnache, S.N. Advances and Challenges in IoT-Based Smart Drug Delivery Systems: A Comprehensive Review. Appl. Syst. Innov. 2023, 6, 62. [Google Scholar] [CrossRef]
- Chan, K.S.; Chan, Y.M.; Tan, A.H.M.; Liang, S.; Cho, Y.T.; Hong, Q.; Yong, E.; Chong, L.R.C.; Zhang, L.; Tan, G.W.L.; et al. Clinical validation of an artificial intelligence-enabled wound imaging mobile application in diabetic foot ulcers. Int. Wound J. 2022, 19, 114–124. [Google Scholar] [CrossRef]
- Preethichandra, D.M.G.; Piyathilaka, L.; Izhar, U.; Samarasinghe, R.; Silva, L.C.D. Wireless Body Area Networks and Their Applications—A Review. IEEE Access 2023, 11, 9202–9220. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled drug delivery systems: Current status and future directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Lu, Y. Physical stimuli-responsive polymeric patches for healthcare. Bioact. Mater. 2025, 43, 342–375. [Google Scholar] [CrossRef]
- Fazio, R.D.; Spongano, L.; Visconti, P.; Velazquez, R.; Soto, C.D.V. Neural Network-based Wearable Devices for Limb Rehabilitation by Inertial Signal Classification. In Proceedings of the 2024 International Symposium on Electronics and Telecommunications (ISETC), Timisoara, Romania, 7–8 September 2024; pp. 1–4. [Google Scholar]
- Wang, X.; Yu, H.; Kold, S.; Rahbek, O.; Bai, S. Wearable sensors for activity monitoring and motion control: A review. Biomim. Intell. Robot. 2023, 3, 100089. [Google Scholar] [CrossRef]
- Alzahrani, A.; Ullah, A. Advanced biomechanical analytics: Wearable technologies for precision health monitoring in sports performance. Digit. Health 2024, 10, 20552076241256745. [Google Scholar] [CrossRef]
- Hui, Z.; Qi, W.; Zhang, Y.; Wang, M.; Zhang, J.; Li, D.; Zhu, D. Efficacy of a Soft Robotic Exoskeleton to Improve Lower Limb Motor Function in Children with Spastic Cerebral Palsy: A Single-Blinded Randomized Controlled Trial. Brain Sci. 2024, 14, 425. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, S.M.; Silva, A.F.D.; Santos, C.P. Smart Vest for Real-Time Postural Biofeedback and Ergonomic Risk Assessment. IEEE Access 2020, 8, 107583–107592. [Google Scholar] [CrossRef]
- Shaheen, H.M.; Ayesh, A.; Sayeh, F.; Fawaghra, N. The Impact of Ergonomic Technology on the Prevalence of Work-Related Low Back Pain Among Physical Therapists in the South West Bank. Ahliya J. Allied Med. Technol. Sci. 2024, 1, 30–35. [Google Scholar] [CrossRef]
- Figueira, V.; Silva, S.; Costa, I.; Campos, B.; Salgado, J.; Pinho, L.; Freitas, M.; Carvalho, P.; Marques, J.; Pinho, F. Wearables for Monitoring and Postural Feedback in the Work Context: A Scoping Review. Sensors 2024, 24, 1341. [Google Scholar] [CrossRef]
- Seçkin, A.Ç.; Ateş, B.; Seçkin, M. Review on Wearable Technology in Sports: Concepts, Challenges and Opportunities. Appl. Sci. 2023, 13, 10399. [Google Scholar] [CrossRef]
- Ashry, S.; Ogawa, T.; Gomaa, W. CHARM-Deep: Continuous Human Activity Recognition Model Based on Deep Neural Network Using IMU Sensors of Smartwatch. IEEE Sens. J. 2020, 20, 8757–8770. [Google Scholar] [CrossRef]
- Kim, H.-C.; Hwang, H.-S.; Lee, K.-H.; Kim, M.-H. Vest-type System on Machine Learning-based Algorithm to Detect and Predict Falls. PNF Mov. 2024, 22, 43–54. [Google Scholar] [CrossRef]
- Rebelo, A.; Martinho, D.V.; Valente-dos-Santos, J.; Coelho-e-Silva, M.J.; Teixeira, D.S. From data to action: A scoping review of wearable technologies and biomechanical assessments informing injury prevention strategies in sport. BMC Sports Sci. Med. Rehabil. 2023, 15, 169. [Google Scholar] [CrossRef] [PubMed]
- Mangi, M.A.; Elahi, H.; Ali, A.; Jabbar, H.; Aqeel, A.B.; Farrukh, A.; Bibi, S.; Altabey, W.A.; Kouritem, S.A.; Noori, M. Applications of piezoelectric-based sensors, actuators, and energy harvesters. Sens. Actuators Rep. 2025, 9, 100302. [Google Scholar] [CrossRef]
- Ju, M.; Dou, Z.; Li, J.-W.; Qiu, X.; Shen, B.; Zhang, D.; Yao, F.-Z.; Gong, W.; Wang, K. Piezoelectric Materials and Sensors for Structural Health Monitoring: Fundamental Aspects, Current Status, and Future Perspectives. Sensors 2023, 23, 543. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Pu, Y.; Fan, J.; Yan, Y.; Liu, W.; Luo, K.; Wang, Y.; Zhao, G.; Chen, T.; Puiu, P.D.; et al. Wearable Sensors, Data Processing, and Artificial Intelligence in Pregnancy Monitoring: A Review. Sensors 2024, 24, 6426. [Google Scholar] [CrossRef]
- Huang, X.; Xue, Y.; Ren, S.; Wang, F. Sensor-Based Wearable Systems for Monitoring Human Motion and Posture: A Review. Sensors 2023, 23, 9047. [Google Scholar] [CrossRef]
- Mu, G.; Zhang, Y.; Yan, Z.; Yu, Q.; Wang, Q. Recent advancements in wearable sensors: Integration with machine learning for human–machine interaction. RSC Adv. 2025, 15, 7844–7854. [Google Scholar] [CrossRef]
- Lu, T.; Ji, S.; Jin, W.; Yang, Q.; Luo, Q.; Ren, T.-L. Biocompatible and Long-Term Monitoring Strategies of Wearable, Ingestible and Implantable Biosensors: Reform the Next Generation Healthcare. Sensors 2023, 23, 2991. [Google Scholar] [CrossRef]
- Li, P.; Lee, G.-H.; Kim, S.Y.; Kwon, S.Y.; Kim, H.-R.; Park, S. From Diagnosis to Treatment: Recent Advances in Patient-Friendly Biosensors and Implantable Devices. ACS Nano 2021, 15, 1960–2004. [Google Scholar] [CrossRef]
- Wu, J.; Liu, H.; Chen, W.; Ma, B.; Ju, H. Device integration of electrochemical biosensors. Nat. Rev. Bioeng. 2023, 1, 346–360. [Google Scholar] [CrossRef]
- Dimov, I.B.; Moser, M.; Malliaras, G.G.; McCulloch, I. Semiconducting Polymers for Neural Applications. Chem. Rev. 2022, 122, 4356–4396. [Google Scholar] [CrossRef]
- Kim, H.; Rigo, B.; Wong, G.; Lee, Y.J.; Yeo, W.-H. Advances in Wireless, Batteryless, Implantable Electronics for Real-Time, Continuous Physiological Monitoring. Nano-Micro Lett. 2023, 16, 52. [Google Scholar] [CrossRef]
- Sang, M.; Cho, M.; Lim, S.; Min, I.S.; Han, Y.; Lee, C.; Shin, J.; Yoon, K.; Yeo, W.-H.; Lee, T.; et al. Fluorescent-based biodegradable microneedle sensor array for tether-free continuous glucose monitoring with smartphone application. Sci. Adv. 2023, 9, eadh1765. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.; Meehan, J.; Ward, C.; Langdon, S.P.; Kunkler, I.H.; Murray, A.; Argyle, D. Implantable biosensors and their contribution to the future of precision medicine. Vet. J. 2018, 239, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Hamade, F.; Bubniene, U.; Engblom, J.; Ramanavicius, A.; Ramanaviciene, A.; Ruzgas, T. In-vitro model for assessing glucose diffusion through skin. Biosens. Bioelectron. 2018, 110, 175–179. [Google Scholar] [CrossRef]
- Tolu-Akinnawo, O.; Akhtar, N.; Zalavadia, N.; Guglin, M.; Zalavadiya, N. CardioMEMS Heart Failure System: An Up-to-Date Review. Cureus 2025, 17, e77816. [Google Scholar] [CrossRef] [PubMed]
- Farzan, F. 10-Brain-computer interface. In Handbook of Neural Engineering; Willerth, S., Ed.; Academic Press: Cambridge, MA, USA, 2025; pp. 351–388. [Google Scholar]
- Ong, J.S.; Wong, S.N.; Arulsamy, A.; Watterson, J.L.; Shaikh, M.F. Medical Technology: A Systematic Review on Medical Devices Utilized for Epilepsy Prediction and Management. Curr. Neuropharmacol. 2022, 20, 950–964. [Google Scholar] [CrossRef]
- Yu, M.; Peng, Y.; Wang, X.; Ran, F. Emerging Design Strategies Toward Developing Next-Generation Implantable Batteries and Supercapacitors. Adv. Funct. Mater. 2023, 33, 2301877. [Google Scholar] [CrossRef]
- Janićijević, Ž.; Huang, T.; Bojórquez, D.I.S.; Tonmoy, T.H.; Pané, S.; Makarov, D.; Baraban, L. Design and Development of Transient Sensing Devices for Healthcare Applications. Adv. Sci. 2024, 11, 2307232. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Z.; Wang, H.; Jin, B.; Meng, C.; Chen, X.; Li, R.; Wang, H.; Xin, M.; Zhao, Z.; et al. Self-Healing, Reconfigurable, Thermal-Switching, Transformative Electronics for Health Monitoring. Adv. Mater. 2023, 35, 2207742. [Google Scholar] [CrossRef]
- Nelson, B.D.; Karipott, S.S.; Wang, Y.; Ong, K.G. Wireless Technologies for Implantable Devices. Sensors 2020, 20, 4604. [Google Scholar] [CrossRef]
- Magill, E.; Demartis, S.; Gavini, E.; Permana, A.D.; Thakur, R.R.S.; Adrianto, M.F.; Waite, D.; Glover, K.; Picco, C.J.; Korelidou, A.; et al. Solid implantable devices for sustained drug delivery. Adv. Drug Deliv. Rev. 2023, 199, 114950. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.A.; Domínguez-Robles, J.; Donnelly, R.F.; Larrañeta, E. Implantable Polymeric Drug Delivery Devices: Classification, Manufacture, Materials, and Clinical Applications. Polymers 2018, 10, 1379. [Google Scholar] [CrossRef] [PubMed]
- Blackshear, P.; Rohde, T.; Prosl, F.; Buchwald, H. The implantable infusion pump: A new concept in drug delivery. Med. Prog. Through Technol. 1979, 6, 149–161. [Google Scholar]
- Greatbatch, W.; Holmes, C.F. History of implantable devices. IEEE Eng. Med. Biol. Mag. 1991, 10, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Losic, D.; Simovic, S. Self-ordered nanopore and nanotube platforms for drug delivery applications. Expert Opin. Drug Deliv. 2009, 6, 1363–1381. [Google Scholar] [CrossRef]
- Ehrick, J.D.; Luckett, M.R.; Khatwani, S.; Wei, Y.; Deo, S.K.; Bachas, L.G.; Daunert, S. Glucose Responsive Hydrogel Networks Based on Protein Recognition. Macromol. Biosci. 2009, 9, 864–868. [Google Scholar] [CrossRef]
- Talebian, S.; Foroughi, J.; Wade, S.J.; Vine, K.L.; Dolatshahi-Pirouz, A.; Mehrali, M.; Conde, J.; Wallace, G.G. Biopolymers for Antitumor Implantable Drug Delivery Systems: Recent Advances and Future Outlook. Adv. Mater. 2018, 30, 1706665. [Google Scholar] [CrossRef]
- Stoian, A.B.; Demetrescu, I.; Ionita, D. Nanotubes and nano pores with chitosan construct on TiZr serving as drug reservoir. Colloids Surf. B Biointerfaces 2020, 185, 110535. [Google Scholar] [CrossRef]
- Foulady-Dehaghi, R.; Sohrabnezhad, S.; Hadavi, M. A new biocompatible COF-MCM nanoporous hybrid DDS for pH-controlled delivery of curcumin. Sci. Rep. 2024, 14, 32077. [Google Scholar] [CrossRef]
- Li, L.; Permana, A.D.; Domínguez-Robles, J.; Amir, M.N.; Habibie, H.; Anjani, Q.K.; Zhao, L.; Moreno-Castellanos, N.; Donnelly, R.F.; Larrañeta, E. Reservoir-Type Subcutaneous Implantable Devices Containing Porous Rate Controlling Membranes for Sustained Delivery of Risperidone. Adv. Healthc. Mater. 2025, 2403689. [Google Scholar] [CrossRef]
- Kumar, S.; Mohan, M. Empowering Arthritis Patients: Optimized Drug Delivery through Piroxicam Microcapsule-Embedded Scaffold Implants via Box-Behnken Experimental Design. Pharm. Nanotechnol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Eslami Vaghar, M.; Dadashpour, M.; Derakhshan, E.; Vahedian, V.; Shahrtash, S.A.; Firouzi Amandi, A.; Eslami, M.; Hasannia, M.; Mehrabi, Z.; Roshangar, L. Artemisinin-loaded mesoporous silica nanoparticles/electrospun poly(lactic-co-glycolic acid) composite nanofibers for enhanced anticancer efficiency in breast cancer cells. Cancer Nanotechnol. 2024, 15, 58. [Google Scholar] [CrossRef]
- Bezerra, J.M.S.; Lima, M.J.S.; Santos, D.K.D.N.; Maciel, L.G.; Alves-Jr, S.; dos Anjos, J.V. Exploring the potential of imidazolium methacrylate crosslinking with resorcinol dimethacrylate for drug delivery systems. J. Appl. Polym. Sci. 2025, 142, e56425. [Google Scholar] [CrossRef]
- Zang, T.; Zhang, J.; Liu, Y.; Chen, S.; Han, S.; Guo, J.; Hu, J.; Hou, Z.; Yang, L.; Cui, H. Enhancing the degradation properties of poly (trimethylene carbonate) by simple and effective copolymerization of trimethylene carbonate with p-dioxanone. Polym. Degrad. Stab. 2025, 231, 111086. [Google Scholar] [CrossRef]
- Brandl, B.; Eder, S.; Hirtler, A.; Khinast, G.; Haley, J.; Schneider, C.; Theissl, S.; Bramboeck, A.; Treffer, D.; Heupl, S.; et al. An alternative filament fabrication method as the basis for 3D-printing personalized implants from elastic ethylene vinyl acetate copolymer. Sci. Rep. 2024, 14, 22773. [Google Scholar] [CrossRef]
- Bono, F.D.; Trani, N.D.; Demarchi, D.; Grattoni, A.; Ros, P.M. System Integration of an Implantable Drug Delivery Device for Long-Term In-Vivo Experiments. In Proceedings of the 2024 IEEE SENSORS, Kobe, Japan, 20–23 October 2024; pp. 1–4. [Google Scholar]
- Sharan, M.K.V.S.; Choudhury, D.; Mohapatra, P.; Banerjee, S. 3D printed subcutaneous implant for prolonged delivery of tenofovir with desired release capability, biocompatibility, and viability. J. Mol. Struct. 2025, 1319, 139559. [Google Scholar] [CrossRef]
- Cavanagh, R.J.; Baquain, S.; Alexander, C.; Scherman, O.A.; Rahman, R. Supramolecular hydrogels enable co-delivery of chemotherapeutics with synergistic efficacy against patient-derived glioblastoma cells and spheroids. RSC Pharm. 2024, 1, 742–754. [Google Scholar] [CrossRef]
- Minaei, E.; Ranson, M.; Aghmesheh, M.; Sluyter, R.; Vine, K.L. Enhancing pancreatic cancer immunotherapy: Leveraging localized delivery strategies through the use of implantable devices and scaffolds. J. Control. Release 2024, 373, 145–160. [Google Scholar] [CrossRef]
- Liu, N.; Tu, S.; Zhang, S.; Chen, D.; Xiao, X.; Zhang, T.; Zheng, H. A pH and near-infrared light dual-responsive drug delivery system based on TiO2 nanotube arrays modified with polydopamine and Fe3+. J. Drug Deliv. Sci. Technol. 2024, 99, 106003. [Google Scholar] [CrossRef]
- Brandl, B.; Eder, S.; Palanisamy, A.; Heupl, S.; Terzic, I.; Katschnig, M.; Nguyen, T.; Senck, S.; Roblegg, E.; Spoerk, M. Toward high-resolution 3D-printing of pharmaceutical implants–A holistic analysis of relevant material properties and process parameters. Int. J. Pharm. 2024, 660, 124356. [Google Scholar] [CrossRef]
- Huling, J.; Stefan, O.; Helge, L.; Anna, S.K.; Thomas, S.; Jana, M.; Oliver, S.; Steffen, M.; Nasrullah, U.; Anita, P.; et al. γ-Cyclodextrin hydrogel for the sustained release of josamycin for potential ocular application. Drug Deliv. 2024, 31, 2361168. [Google Scholar] [CrossRef]
- Kale, R.; Deshkar, S.S.; Deshmane, P.D.; Whagmare, B. Development of Nanoparticle-Based Biodegradable Polymer Composite Film for Antifungal Drug Delivery in Prosthetic Joint Infection. Indian J. Pharm. Educ. Res. 2024, 58, s468–s483. [Google Scholar] [CrossRef]
- Pecorini, G.; Votta, A.; Tiralongo, G.; Volpi, D.; Ferraro, E.; Puppi, D. Naringenin-loaded poly(3-hydroxybutyrate-co-3-hydroxyvalerate)-based devices have an anti-inflammatory activity on microglia. J. Drug Deliv. Sci. Technol. 2024, 98, 105895. [Google Scholar] [CrossRef]
- Tng, D.J.H.; Hu, R.; Song, P.; Roy, I.; Yong, K.-T. Approaches and Challenges of Engineering Implantable Microelectromechanical Systems (MEMS) Drug Delivery Systems for in Vitro and in Vivo Applications. Micromachines 2012, 3, 615–631. [Google Scholar] [CrossRef]
- Meng, E.; Hoang, T. Micro- and nano-fabricated Implantable drug-delivery Systems. Ther. Deliv. 2012, 3, 1457–1467. [Google Scholar] [CrossRef]
- Uguz, I.; Proctor, C.M.; Curto, V.F.; Pappa, A.-M.; Donahue, M.J.; Ferro, M.; Owens, R.M.; Khodagholy, D.; Inal, S.; Malliaras, G.G. A Microfluidic Ion Pump for In Vivo Drug Delivery. Adv. Mater. 2017, 29, 1701217. [Google Scholar] [CrossRef] [PubMed]
- Arany, P.; Papp, I.; Zichar, M.; Csontos, M.; Elek, J.; Regdon, G.; Budai, I.; Béres, M.; Gesztelyi, R.; Fehér, P.; et al. In Vitro Tests of FDM 3D-Printed Diclofenac Sodium-Containing Implants. Molecules 2020, 25, 5889. [Google Scholar] [CrossRef]
- Quarterman, J.C.; Geary, S.M.; Salem, A.K. Evolution of drug-eluting biomedical implants for sustained drug delivery. Eur. J. Pharm. Biopharm. 2021, 159, 21–35. [Google Scholar] [CrossRef]
- Picco, C.J.; Domínguez-Robles, J.; Utomo, E.; Paredes, A.J.; Volpe-Zanutto, F.; Malinova, D.; Donnelly, R.F.; Larrañeta, E. 3D-printed implantable devices with biodegradable rate-controlling membrane for sustained delivery of hydrophobic drugs. Drug Deliv. 2022, 29, 1038–1048. [Google Scholar] [CrossRef]
- Korelidou, A.; Domínguez-Robles, J.; Magill, E.R.; Eleftheriadou, M.; Cornelius, V.A.; Donnelly, R.F.; Margariti, A.; Larrañeta, E. 3D-printed reservoir-type implants containing poly(lactic acid)/poly(caprolactone) porous membranes for sustained drug delivery. Biomater. Adv. 2022, 139, 213024. [Google Scholar] [CrossRef]
- Al-Litani, K.; Ali, T.; Robles Martinez, P.; Buanz, A. 3D printed implantable drug delivery devices for women’s health: Formulation challenges and regulatory perspective. Adv. Drug Deliv. Rev. 2023, 198, 114859. [Google Scholar] [CrossRef] [PubMed]
- Fayzullin, A.; Bakulina, A.; Mikaelyan, K.; Shekhter, A.; Guller, A. Implantable Drug Delivery Systems and Foreign Body Reaction: Traversing the Current Clinical Landscape. Bioengineering 2021, 8, 205. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, J.; Lim, J.; Nolan, J.K.; Lee, H.; Lee, C.H. Wearable Glucose Monitoring and Implantable Drug Delivery Systems for Diabetes Management. Adv. Healthc. Mater. 2021, 10, 2100194. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, M.; Al Fatease, A.; Alany, R.G.; Abdelkader, H. Recent Advances of Ocular Drug Delivery Systems: Prominence of Ocular Implants for Chronic Eye Diseases. Pharmaceutics 2023, 15, 1746. [Google Scholar] [CrossRef]
- Farra, R.; Sheppard, N.F.; McCabe, L.; Neer, R.M.; Anderson, J.M.; Santini, J.T.; Cima, M.J.; Langer, R. First-in-Human Testing of a Wirelessly Controlled Drug Delivery Microchip. Sci. Transl. Med. 2012, 4, 122ra121. [Google Scholar] [CrossRef] [PubMed]
- Lerman, I.; Bu, Y.; Singh, R.; Silverman, H.A.; Bhardwaj, A.; Mann, A.J.; Widge, A.; Palin, J.; Puleo, C.; Lim, H. Next generation bioelectronic medicine: Making the case for non-invasive closed-loop autonomic neuromodulation. Bioelectron. Med. 2025, 11, 1. [Google Scholar] [CrossRef]
- Lee, S.K.; Jeakins, G.S.; Tukiainen, A.; Hewage, E.; Armitage, O.E. Next-Generation Bioelectric Medicine: Harnessing the Therapeutic Potential of Neural Implants. Bioelectricity 2020, 2, 321–327. [Google Scholar] [CrossRef]
- Zhao, H. Optogenetic Implants. In Handbook of Biochips: Integrated Circuits and Systems for Biology and Medicine; Sawan, M., Ed.; Springer: New York, NY, USA, 2022; pp. 179–206. [Google Scholar]
- Beisteiner, R.; Hallett, M.; Lozano, A.M. Ultrasound Neuromodulation as a New Brain Therapy. Adv. Sci. 2023, 10, 2205634. [Google Scholar] [CrossRef]
- Carnicer-Lombarte, A.; Malliaras, G.G.; Barone, D.G. The Future of Biohybrid Regenerative Bioelectronics. Adv. Mater. 2025, 37, 2408308. [Google Scholar] [CrossRef]
- Dinis, H.; Mendes, P.M. A comprehensive review of powering methods used in state-of-the-art miniaturized implantable electronic devices. Biosens. Bioelectron. 2021, 172, 112781. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Avti, P. Implantable Biofuel Cells for Biomedical Applications. In Biofuel Cells; Wiley: Hoboken, NJ, USA, 2021; pp. 69–95. [Google Scholar]
- Park, Y.; Jung, J.; Chang, M. Research Progress on Conducting Polymer-Based Biomedical Applications. Appl. Sci. 2019, 9, 1070. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Conducting Polymers in the Design of Biosensors and Biofuel Cells. Polymers 2021, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.; Robinson, J.T. Wireless Power Delivery Techniques for Miniature Implantable Bioelectronics. Adv. Healthc. Mater. 2021, 10, 2100664. [Google Scholar] [CrossRef]
- Wright, J.P.; Mughrabi, I.T.; Wong, J.; Mathew, J.; Jayaprakash, N.; Crosfield, C.; Chang, E.H.; Chavan, S.S.; Tracey, K.J.; Pavlov, V.A.; et al. A fully implantable wireless bidirectional neuromodulation system for mice. Biosens. Bioelectron. 2022, 200, 113886. [Google Scholar] [CrossRef]
- Magisetty, R.; Park, S.-M. New Era of Electroceuticals: Clinically Driven Smart Implantable Electronic Devices Moving towards Precision Therapy. Micromachines 2022, 13, 161. [Google Scholar] [CrossRef] [PubMed]
- Pak, A.; Nanbakhsh, K.; Hölck, O.; Ritasalo, R.; Sousa, M.; Van Gompel, M.; Pahl, B.; Wilson, J.; Kallmayer, C.; Giagka, V. Thin Film Encapsulation for LCP-Based Flexible Bioelectronic Implants: Comparison of Different Coating Materials Using Test Methodologies for Life-Time Estimation. Micromachines 2022, 13, 544. [Google Scholar] [CrossRef]
- Chen, J.C.; Kan, P.; Yu, Z.; Alrashdan, F.; Garcia, R.; Singer, A.; Lai, C.S.E.; Avants, B.; Crosby, S.; Li, Z.; et al. A wireless millimetric magnetoelectric implant for the endovascular stimulation of peripheral nerves. Nat. Biomed. Eng. 2022, 6, 706–716. [Google Scholar] [CrossRef]
- Boys, A.J.; Carnicer-Lombarte, A.; Güemes-Gonzalez, A.; van Niekerk, D.C.; Hilton, S.; Barone, D.G.; Proctor, C.M.; Owens, R.M.; Malliaras, G.G. 3D Bioelectronics with a Remodellable Matrix for Long-Term Tissue Integration and Recording. Adv. Mater. 2023, 35, 2207847. [Google Scholar] [CrossRef]
- Valle, G.; Aiello, G.; Ciotti, F.; Cvancara, P.; Martinovic, T.; Kravic, T.; Navarro, X.; Stieglitz, T.; Bumbasirevic, M.; Raspopovic, S. Multifaceted understanding of human nerve implants to design optimized electrodes for bioelectronics. Biomaterials 2022, 291, 121874. [Google Scholar] [CrossRef]
- Luo, T.; Zheng, L.; Chen, D.; Zhang, C.; Liu, S.; Jiang, C.; Xie, Y.; Du, D.; Zhou, W. Implantable microfluidics: Methods and applications. Analyst 2023, 148, 4637–4654. [Google Scholar] [CrossRef] [PubMed]
- Shade, J.K.; Prakosa, A.; Popescu, D.M.; Yu, R.; Okada, D.R.; Chrispin, J.; Trayanova, N.A. Predicting risk of sudden cardiac death in patients with cardiac sarcoidosis using multimodality imaging and personalized heart modeling in a multivariable classifier. Sci. Adv. 2021, 7, eabi8020. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zhu, R.; Peng, I.; Xu, Z.; Jiang, Y. Wearable and implantable biosensors: Mechanisms and applications in closed-loop therapeutic systems. J. Mater. Chem. B 2024, 12, 8577–8604. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Jekal, J.; Liu, J.; Kim, J.; Park, J.-U.; Lee, T.; Jang, K.-I. Bioelectronic Implantable Devices for Physiological Signal Recording and Closed-Loop Neuromodulation. Adv. Funct. Mater. 2024, 34, 2403562. [Google Scholar] [CrossRef]
- Villa, J.; Cury, J.; Kessler, L.; Tan, X.; Richter, C.-P. Enhancing biocompatibility of the brain-machine interface: A review. Bioact. Mater. 2024, 42, 531–549. [Google Scholar] [CrossRef]
- Canali, S.; Schiaffonati, V.; Aliverti, A. Challenges and recommendations for wearable devices in digital health: Data quality, interoperability, health equity, fairness. PLOS Digit. Health 2022, 1, e0000104. [Google Scholar] [CrossRef]
- Nethan, S.; Sinha, D.; Mehrotra, R. Non Communicable Disease Risk Factors and their Trends in India. Asian Pac. J. Cancer Prev. 2017, 18, 2005–2010. [Google Scholar] [CrossRef]
- Gagnon, M.-P.; Ouellet, S.; Attisso, E.; Supper, W.; Amil, S.; Rhéaume, C.; Paquette, J.-S.; Chabot, C.; Laferrière, M.-C.; Sasseville, M. Wearable Devices for Supporting Chronic Disease Self-Management: Scoping Review. Interact. J. Med. Res. 2024, 13, e55925. [Google Scholar] [CrossRef]
- Phillips, S.M.; Cadmus-Bertram, L.; Rosenberg, D.; Buman, M.P.; Lynch, B.M. Wearable Technology and Physical Activity in Chronic Disease: Opportunities and Challenges. Am. J. Prev. Med. 2018, 54, 144–150. [Google Scholar] [CrossRef]
- Mattison, G.; Canfell, O.; Forrester, D.; Dobbins, C.; Smith, D.; Töyräs, J.; Sullivan, C. The Influence of Wearables on Health Care Outcomes in Chronic Disease: Systematic Review. J. Med. Internet. Res. 2022, 24, e36690. [Google Scholar] [CrossRef]
- Darwish, B.A.; Rehman, S.U.; Sadek, I.; Salem, N.M.; Kareem, G.; Mahmoud, L.N. From lab to real-life: A three-stage validation of wearable technology for stress monitoring. MethodsX 2025, 14, 103205. [Google Scholar] [CrossRef]
- Bulut, M.G.; Unal, S.; Hammad, M.; Pławiak, P. Deep CNN-based detection of cardiac rhythm disorders using PPG signals from wearable devices. PLoS ONE 2025, 20, e0314154. [Google Scholar] [CrossRef] [PubMed]
- Damery, S.; Jones, J.; Harrison, A.; Hinde, S.; Jolly, K. Technology-enabled hybrid cardiac rehabilitation: Qualitative study of healthcare professional and patient perspectives at three cardiac rehabilitation centres in England. PLoS ONE 2025, 20, e0319619. [Google Scholar] [CrossRef] [PubMed]
- Hakizimana, A.; Pooja, D.; and Gaillard, E.A. Current technological advancement in asthma care. Expert Rev. Respir. Med. 2024, 18, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Baghersalimi, S.; Amirshahi, A.; Forooghifar, F.; Teijeiro, T.; Aminifar, A.; Atienza, D. M2SKD: Multi-to-Single Knowledge Distillation of Real-Time Epileptic Seizure Detection for Low-Power Wearable Systems. ACM Trans. Intell. Syst. Technol. 2024, 15, 102. [Google Scholar] [CrossRef]
- Goltz, F.; Dirkx, M.F.; Schoffelen, J.M.; Okun, M.S.; Hu, W.; Hess, C.W.; Nonnekes, J.; Bloem, B.R.; Helmich, R.C. A prospective controlled study of a wearable rhythmic vibrotactile device for tremor in Parkinson’s disease. Clin. Neurophysiol. 2025, 172, 51–60. [Google Scholar] [CrossRef]
- Yang, Q.; Yu, M.; Zhang, H.; Li, N.; Du, J.; Xu, L.; Xu, J. Triboelectric nanogenerator based on well-dispersed and oxide-free liquid metal-doped conductive hydrogel as self-powered wearable sensor for respiratory and thyroid cartilage signal monitoring. Nano Energy 2025, 134, 110530. [Google Scholar] [CrossRef]
- Cromack, S.C.; Walter, J.R. Consumer wearables and personal devices for tracking the fertile window. Am. J. Obstet. Gynecol. 2024, 231, 516–523. [Google Scholar] [CrossRef]
- Esmaeilian, A.; Yazdian, F.; Saberi, A.; Hosseini, A.; Rahdar, A.; Fathi-karkan, S.; Pandey, S. Innovations in Self-Powered glucose Biosensors: From energy harvesting to Continuous, Non-Invasive monitoring. Inorg. Chem. Commun. 2025, 174, 114076. [Google Scholar] [CrossRef]
- Onasanya, A.; Elshakankiri, M. Smart integrated IoT healthcare system for cancer care. Wireless Netw 2021, 27, 4297–4312. [Google Scholar] [CrossRef]
- Zhao, Y.; Choudhury, T. Evaluate Closed-Loop, Mindless Intervention in-the-Wild: A Micro-Randomized Trial on Offset Heart Rate Biofeedback. In Proceedings of the Companion of the 2024 on ACM International Joint Conference on Pervasive and Ubiquitous Computing, Melbourne, VIC, Australia, 5–9 October 2024; pp. 307–312. [Google Scholar]
- Pullano, S.A.; Oliva, G.; Lagana, F.; Fiorillo, A.S.; Prattico, D.; Presta, P.; Carullo, N.; Musolino, M.; Andreucci, M.; Bolignano, D.; et al. Development of a Wearable Device for Arteriovenous Fistula Monitoring. In Proceedings of the 2024 IEEE Sensors Applications Symposium (SAS), Naples, Italy, 23–25 July 2024; pp. 1–4. [Google Scholar]
- Han, S.; Yamamoto, S.; Jung, C.-Y.; Jin, D.Y.; Lee, T.; Kim, J.-S. Wearable sensors for monitoring chronic kidney disease. Commun. Mater. 2024, 5, 153. [Google Scholar] [CrossRef]
- Khayat, R.; Kim, J.; Lee, E.; Chaudhari, A. 1131: A Novel Sensor to Track Transient Blood Pressure Changes During Sleep. Sleep 2024, 47, A485. [Google Scholar] [CrossRef]
- Lee, E.K.; Wang, S.; Yip, B.H.; Yu, E.Y.; Leung, S.Y.; Han, J.; Choi, Y.K.; Chow, K.F.; Chung, W.H.; Yan, B.P.; et al. Home blood pressure during night-time sleep–A feasible treatment target for patients with hypertension: A proof-of-concept randomised controlled trial. J. Pharm. Policy Pract. 2025, 18, 2463435. [Google Scholar] [CrossRef] [PubMed]
- Breen, C.; Hendriks, J. Advancing cardiovascular health: Photoplethysmography as a tool for electrocardiogram signal acquisition. Eur. J. Cardiovasc. Nurs. 2025, 24, 314–315. [Google Scholar] [CrossRef]
- Kwon, S.; Hong, J.; Choi, E.-K.; Lee, B.; Baik, C.; Lee, E.; Jeong, E.-R.; Koo, B.-K.; Oh, S.; Yi, Y. Detection of Atrial Fibrillation Using a Ring-Type Wearable Device (CardioTracker) and Deep Learning Analysis of Photoplethysmography Signals: Prospective Observational Proof-of-Concept Study. J. Med. Internet Res. 2020, 22, e16443. [Google Scholar] [CrossRef] [PubMed]
- Kwun, J.-S.; Lee, J.H.; Park, B.E.; Park, J.S.; Kim, H.J.; Kim, S.-H.; Jeon, K.-H.; Cho, H.-w.; Kang, S.-H.; Lee, W.; et al. Diagnostic Value of a Wearable Continuous Electrocardiogram Monitoring Device (AT-Patch) for New-Onset Atrial Fibrillation in High-Risk Patients: Prospective Cohort Study. J. Med. Internet Res. 2023, 25, e45760. [Google Scholar] [CrossRef]
- Bogár, B.; Pető, D.; Sipos, D.; Füredi, G.; Keszthelyi, A.; Betlehem, J.; Pandur, A.A. Detection of Arrhythmias Using Smartwatches—A Systematic Literature Review. Healthcare 2024, 12, 892. [Google Scholar] [CrossRef]
- Bhaltadak, V.; Ghewade, B.; Yelne, S. A comprehensive review on advancements in wearable technologies: Revolutionizing cardiovascular medicine. Cureus 2024, 16, e61312. [Google Scholar] [CrossRef]
- Spatz, E.S.; Ginsburg, G.S.; Rumsfeld, J.S.; Turakhia, M.P. Wearable Digital Health Technologies for Monitoring in Cardiovascular Medicine. New Engl. J. Med. 2024, 390, 346–356. [Google Scholar] [CrossRef]
- Park, J.; Park, S.; Ahn, S.; Cho, Y.; Park, J.J.; Shin, H. Wearable Strain Sensor Using Conductive Yarn Sewed on Clothing for Human Respiratory Monitoring. IEEE Sens. J. 2020, 20, 12628–12636. [Google Scholar] [CrossRef]
- Whitlock, J.; Sill, J.; Jain, S. A-spiro: Towards continuous respiration monitoring. Smart Health 2020, 15, 100105. [Google Scholar] [CrossRef]
- Villar, R.; Beltrame, T.; Hughson, R.L. Validation of the Hexoskin wearable vest during lying, sitting, standing, and walking activities. Appl. Physiol. Nutr. Metab. 2015, 40, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- de Sena, S.; Häggman, M.; Ranta, J.; Roienko, O.; Ilén, E.; Acosta, N.; Salama, J.; Kirjavainen, T.; Stevenson, N.; Airaksinen, M.; et al. NAPping PAnts (NAPPA): An open wearable solution for monitoring Infant’s sleeping rhythms, respiration and posture. Heliyon 2024, 10, e33295. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, J.; Wang, S.; Zhang, Y. A review of IoT applications in healthcare. Neurocomputing 2024, 565, 127017. [Google Scholar] [CrossRef]
- Kim, D.; Lee, J.; Park, M.K.; Ko, S.H. Recent developments in wearable breath sensors for healthcare monitoring. Commun. Mater. 2024, 5, 41. [Google Scholar] [CrossRef]
- Chihara, E. Assessment of True Intraocular Pressure: The Gap Between Theory and Practical Data. Surv. Ophthalmol. 2008, 53, 203–218. [Google Scholar] [CrossRef]
- Liu, Z.; Pei, W.; Wang, G.; Chen, H. Three-Dimensional Graphene as Sensing Element for Intraocular Pressure Monitoring. In Proceedings of the 2019 9th International IEEE/EMBS Conference on Neural Engineering (NER), San Francisco, CA, USA, 20–23 March 2019; pp. 803–806. [Google Scholar]
- Nasreldin, M.; Delattre, R.; Ramuz, M.; Lahuec, C.; Djenizian, T.; de Bougrenet de la Tocnaye, J.-L. Flexible Micro-Battery for Powering Smart Contact Lens. Sensors 2019, 19, 2062. [Google Scholar] [CrossRef]
- Maeng, B.; Chang, H.-k.; Park, J. Photonic crystal-based smart contact lens for continuous intraocular pressure monitoring. Lab A Chip 2020, 20, 1740–1750. [Google Scholar] [CrossRef]
- Chen, P.J.; Saati, S.; Varma, R.; Humayun, M.S.; Tai, Y.C. Wireless Intraocular Pressure Sensing Using Microfabricated Minimally Invasive Flexible-Coiled LC Sensor Implant. J. Microelectromech. Syst. 2010, 19, 721–734. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Fernandes, J.; Kim, J.J.; Croft, M.A.; Liu, H.; Kaufman, P.L.; Jiang, H. Two-Dimensional Plasmonic Grating for Intraocular Pressure Sensing. IEEE Sens. Lett. 2019, 3, 3501904. [Google Scholar] [CrossRef]
- Medeiros, F.A.; Weinreb, R.N. Evaluation of the Influence of Corneal Biomechanical Properties on Intraocular Pressure Measurements Using the Ocular Response Analyzer. J. Glaucoma 2006, 15, 364–370. [Google Scholar] [CrossRef]
- Kida, T.; Liu, J.H.K.; Weinreb, R.N. Effect of 24-Hour Corneal Biomechanical Changes on Intraocular Pressure Measurement. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4422–4426. [Google Scholar] [CrossRef] [PubMed]
- Varel, Ç.; Shih, Y.-C.; Otis, B.P.; Shen, T.S.; Böhringer, K.F. A wireless intraocular pressure monitoring device with a solder-filled microchannel antenna. J. Micromech. Microeng. 2014, 24, 045012. [Google Scholar] [CrossRef]
- Shin, M.-K.; Ji, Y.W.; Moon, C.-E.; Lee, H.; Kang, B.; Jinn, W.-S.; Ki, J.; Mun, B.; Kim, M.-H.; Lee, H.K.; et al. Matrix metalloproteinase 9-activatable peptide-conjugated hydrogel-based fluorogenic intraocular-lens sensor. Biosens. Bioelectron. 2020, 162, 112254. [Google Scholar] [CrossRef] [PubMed]
- Nasr, F.H.; Khoee, S.; Dehghan, M.M.; Chaleshtori, S.S.; Shafiee, A. Preparation and Evaluation of Contact Lenses Embedded with Polycaprolactone-Based Nanoparticles for Ocular Drug Delivery. Biomacromolecules 2016, 17, 485–495. [Google Scholar] [CrossRef]
- Lee, S.-H.; Shin, K.-S.; Kim, J.-W.; Kang, J.-Y.; Kim, J.-K. Stimulus-Responsive Contact Lens for IOP Measurement or Temperature-Triggered Drug Release. Transl. Vis. Sci. Technol. 2020, 9, 1. [Google Scholar] [CrossRef]
- Tsironi, S.; Almaliotis, D.; Ntonti, P.; Sidiropoulos, G.; Theodoridou, E.; Theofrastou, E.; Karachrysafi, S.; Psimenidou, E.; Sarafi, A.; Kapourani, V.; et al. Clinical Outcomes of the Implementation of IOP Monitoring, in and out of Office Time, to 1500 Patients—A Cohort Study. Vision 2022, 6, 69. [Google Scholar] [CrossRef]
- Shean, R.; Yu, N.; Guntipally, S.; Nguyen, V.; He, X.; Duan, S.; Gokoffski, K.; Zhu, Y.; Xu, B. Advances and Challenges in Wearable Glaucoma Diagnostics and Therapeutics. Bioengineering 2024, 11, 138. [Google Scholar] [CrossRef]
- Gherghescu, I.; Delgado-Charro, M.B. The Biosimilar Landscape: An Overview of Regulatory Approvals by the EMA and FDA. Pharmaceutics 2021, 13, 48. [Google Scholar] [CrossRef]
- Guerra Bretaña, R.; Flórez, A. Impact of regulations on innovation in the field of medical devices. Res. Biomed. Eng. 2018, 34, 356–367. [Google Scholar] [CrossRef]
- Gurman, P.; Rabinovitz-Harison, O.; Hunter, T.B. Regulatory challenges in biomaterials: Focus on medical devices. Biomater. Sci. Integr. Clin. Eng. Approach 2012, 223, 48. [Google Scholar]
- Amaral, C.; Paiva, M.; Rodrigues, A.R.; Veiga, F.; Bell, V. Global Regulatory Challenges for Medical Devices: Impact on Innovation and Market Access. Appl. Sci. 2024, 14, 9304. [Google Scholar] [CrossRef]
- Yin, Z.; Yan, J.; Fang, S.; Wang, D.; Han, D. User acceptance of wearable intelligent medical devices through a modified unified theory of acceptance and use of technology. Ann. Transl. Med. 2022, 10, 629. [Google Scholar] [CrossRef] [PubMed]
- Ledel Solem, I.K.; Varsi, C.; Eide, H.; Kristjansdottir, O.B.; Børøsund, E.; Schreurs, K.M.G.; Waxenberg, L.B.; Weiss, K.E.; Morrison, E.J.; Haaland-Øverby, M.; et al. A User-Centered Approach to an Evidence-Based Electronic Health Pain Management Intervention for People With Chronic Pain: Design and Development of EPIO. J. Med. Internet Res. 2020, 22, e15889. [Google Scholar] [CrossRef] [PubMed]
- Turkstani, H.A.; Almutawah, F.N.; AlZamel, N.A.; Zaid, M.; Alshammari, A.A.A.; Algharbi, M.T.; Alsuayri, A.M.; Badie, M.; Gong, J.S.A.; Alnemer10, A.F. Privacy and Confidentiality in Healthcare: Best Practices for Protecting Patient Information. J. Healthc. Sci. 2025, 5. [Google Scholar] [CrossRef]
- Hasan, M.; Tabassum, T.; Tabassum, T.; Tanbir, M.; Kibria, M.; Chowduary, M.; Nambiar, R. Navigating Cultural Diversity in the Selection of Cardiovascular Device Treatments: A Comprehensive Review. Cureus 2023, 15, e38934. [Google Scholar] [CrossRef] [PubMed]
- Shajari, S.; Kuruvinashetti, K.; Komeili, A.; Sundararaj, U. The Emergence of AI-Based Wearable Sensors for Digital Health Technology: A Review. Sensors 2023, 23, 9498. [Google Scholar] [CrossRef]
- Anzanpour, A.; Amiri, D.; Azimi, I.; Levorato, M.; Dutt, N.; Liljeberg, P.; Rahmani, A.M. Edge-Assisted Control for Healthcare Internet of Things: A Case Study on PPG-Based Early Warning Score. ACM Trans. Internet Things 2020, 2, 1–21. [Google Scholar] [CrossRef]
- Cobo, A.; Sheybani, R.; Meng, E. MEMS: Enabled Drug Delivery Systems. Adv. Healthc. Mater. 2015, 4, 969–982. [Google Scholar] [CrossRef]
- Huang, S.-J.; Lin, M.-T.; Chiang, C.-C.; Arun Dwivedi, K.; Abbas, A. Recent Advancements in Biological Microelectromechanical Systems (BioMEMS) and Biomimetic Coatings. Coatings 2022, 12, 1800. [Google Scholar] [CrossRef]
- Luo, X.; Tan, H.; Wen, W. Recent Advances in Wearable Healthcare Devices: From Material to Application. Bioengineering 2024, 11, 358. [Google Scholar] [CrossRef] [PubMed]
- Kandi, R. Recent Advances on the Development of Additive Manufactured Biodegradable Implants. In Additive Manufacturing for Biomedical Applications: Recent Trends and Challenges; Dixit, A., Kumar, A., Pathak, D.K., Eds.; Springer Nature: Singapore, 2024; pp. 161–173. [Google Scholar]
- Molla, G.; Bitew, M. Revolutionizing Personalized Medicine: Synergy with Multi-Omics Data Generation, Main Hurdles, and Future Perspectives. Biomedicines 2024, 12, 2750. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hui, J.; Yang, P.; Mao, H. Microfluidic Organ-on-a-Chip System for Disease Modeling and Drug Development. Biosensors 2022, 12, 370. [Google Scholar] [CrossRef] [PubMed]
- Asci Erkocyigit, B.; Ozufuklar, O.; Yardim, A.; Guler Celik, E.; Timur, S. Biomarker Detection in Early Diagnosis of Cancer: Recent Achievements in Point-of-Care Devices Based on Paper Microfluidics. Biosensors 2023, 13, 387. [Google Scholar] [CrossRef]
- Wasilewski, T.; Kamysz, W.; Gębicki, J. AI-Assisted Detection of Biomarkers by Sensors and Biosensors for Early Diagnosis and Monitoring. Biosensors 2024, 14, 356. [Google Scholar] [CrossRef]
- Kiran Kolluri, S.S.; Ananiah Durai, S. Wearable micro-electro-mechanical systems pressure sensors in health care: Advancements and trends—A review. IET Wirel. Sens. Syst. 2024, 14, 233–247. [Google Scholar] [CrossRef]
- Borenstein, J.T.; Vunjak-Novakovic, G. Engineering Tissue with BioMEMS. IEEE Pulse 2011, 2, 28–34. [Google Scholar] [CrossRef]
- Liu, S.; Yu, J.-M.; Gan, Y.-C.; Qiu, X.-Z.; Gao, Z.-C.; Wang, H.; Chen, S.-X.; Xiong, Y.; Liu, G.-H.; Lin, S.-E.; et al. Biomimetic natural biomaterials for tissue engineering and regenerative medicine: New biosynthesis methods, recent advances, and emerging applications. Mil. Med. Res. 2023, 10, 16. [Google Scholar] [CrossRef]
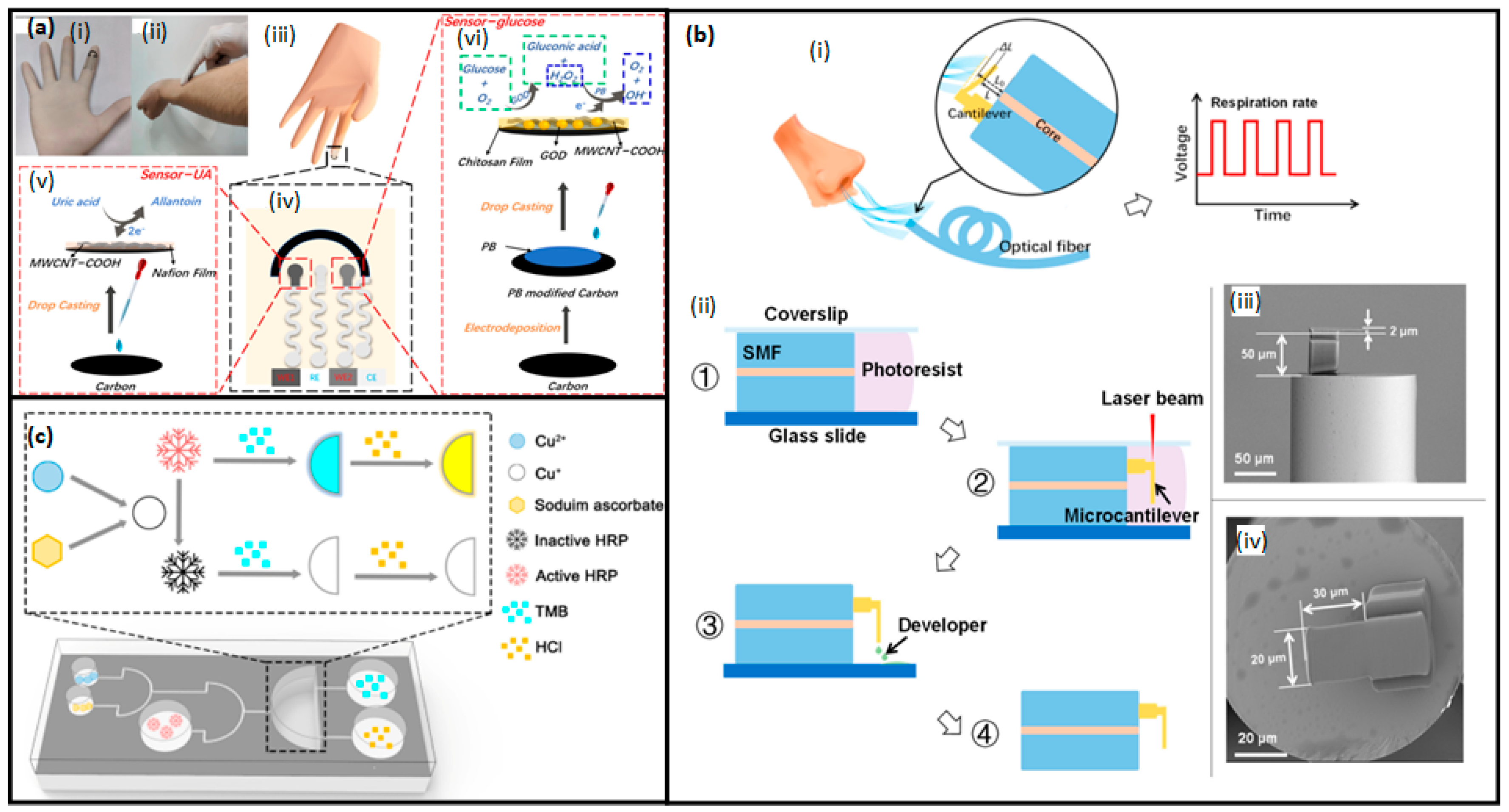
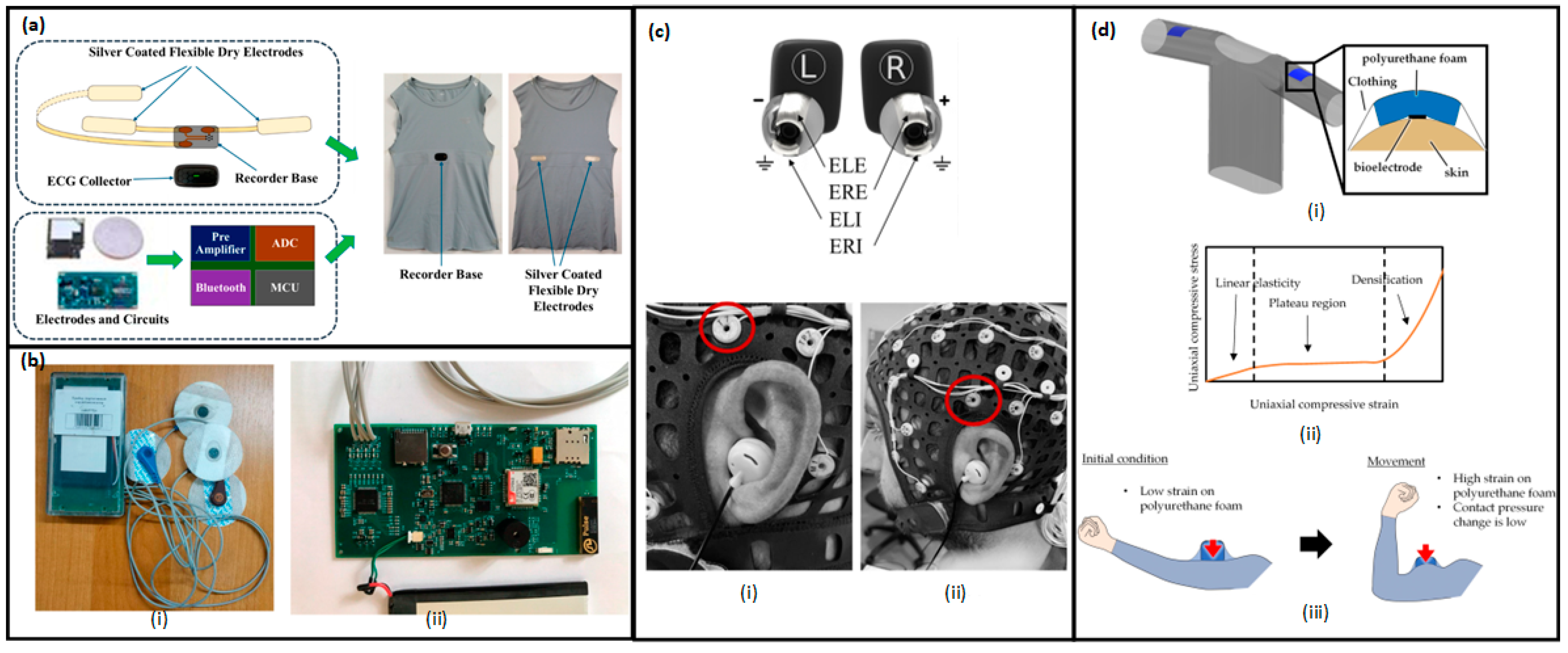
| Property | PDMS | Polyimide | Parylene C | PMMA | Polyurethane | Polypyrrole |
|---|---|---|---|---|---|---|
| Tensile strength (MPa) | 3.51 to 5.13 MPa | 78.3 ± 7.3 | 70 | 60–70 | 18 | 64 |
| Elongation at break (%) | <100 | 7.4 ± 1.0 | 200 | 0.5–5 | >140 | 5% |
| Young’s modulus (GPa) | 360–870 | 2.5 | 4.5 | 3.3 | 3.6–88.8 | >1 |
| Mechanical flexibility | Very high | Moderate | Moderate | Low | Very high | Low |
| Hardness (Shore D) | 41–43 | 1.21 | Moderate | 96 | 70.1 | -- |
| Density (g/cm3) | 0.965 | 1.33 | 1.289 | 1.17 | 1.12 | 1.6 |
| Water absorption (%) after 24 h | <0.05 | -- | 0.1 | -- | -- | -- |
| Biodegradability | No | -- | Yes | No | Yes | No |
| Biocompatibility | Excellent | Good (when pure) | Excellent | Good | Excellent (depends on formulation) | Moderate |
| Cytocompatibility | Yes | Yes | Yes | Yes | Yes | Yes |
| Glass transition temperature (°C) | −127 | 325 | 35–80 | 105 | −35 | 65–95 |
| Melting point (°C) | −49.9–−40 | None, decompose instead melt | 290 | 166 | -- | >300 |
| Thermal conductivity (W/m·K) | 0.2–0.27 | 8.04 × 10−4 | 1.0 | 0.22 | 0.19 ± 0.03 | 0.9 |
| Dielectric constant (1 MHz) | 2.3–2.8 | 3.4 | 2.95 | 3.5 | 4 | -- |
| Electrical conductivity (S/m) | 4 × 10−14 | Insulator | Insulator | 1 × 10−19 | Insulator | 105 |
| Property | Values |
|---|---|
| Density (gm/cm3) | 1.07–1.2 |
| Average molecular weight (kDa) | 50–130 |
| Tensile elastic modulus (MPa) | 250–440 |
| Tensile strength (MPa) | 10–30 |
| Yield stress (MPa) | 8–18 |
| Melting temperature (°C) | 56–65 |
| Glass transition temperature (°C) | (−65)–(−60) |
| Strain at yield (%) | 2–7 |
| Strain at break (%) | 80–800 |
| Mechanical flexibility | High flexibility and toughness |
| Thermal conductivity (W/mK) | 0.2–0.4 |
| Electrical conductivity (S/m) | Non-conductive |
| Biocompatibility | Excellent |
| Properties | PHB | PHV |
|---|---|---|
| Crystallinity (%) | 60 | 53 |
| Glass transition temperature (°C) | 5 | −5 |
| Yield stress (MPa) | 35 | 25 |
| Elongation at break (%) | 10 | 20 |
| Mechanical flexibility | Brittle | More flexible than PHB |
| Elastic modulus (MPa) | 1700 | 1200 |
| Impact strength notched (KJ/mm2) | 3 | 6 |
| Impact strength without notch | Break | Break |
| Thermal conductivity (W/mK) | 0.2–0.3 | 0.2–0.3 |
| Electrical conductivity (S/m) | Non-conductive | Non-conductive |
| Biocompatibility | Excellent | Excellent |
| Device Type | Application | Material/Platform | Sensitivity | LOD | Response Time | Reference |
|---|---|---|---|---|---|---|
| Wearable fiber-optic breath sensor | Respiration rate monitoring | Fiber-tip microcantilever + FP interferometer | 0.8 nm/(m/s) | NA | 300 ms (rise), 500 ms (fall) | [323] |
| Flexible printed biosensor (H2O2, AA) | H2O2 and ascorbic acid detection | Prussian Blue/reduced graphene oxide (PB/RGO) ink | 31.65 μA·mM−1·cm−2 (H2O2)/58.7 μA·mM−1·cm−2 (AA) | 1.78 μM (H2O2)/99 μM (AA) | ~60 s | [371] |
| Dual-function wearable sweat biosensor | Glucose and uric acid monitoring | MWCNT-COOH/Prussian Blue/GOD ink-printed electrodes on gloves | 1.64 µA/mM (UA), 1.32 µA/mM (glucose) | 3.58 µM (UA), 9.10 µM (glucose) | 60 s | [322] |
| Wearable sweat-based hormone biosensor | Cortisol, IL-6, IL-10, TNF-α, NPY (in sweat) | Nanoporous ZnO SPEs and graphene-based FETs (SLOCK, SWEATSENSER) | ~30 kΩ–5 kΩ (impedance range) | IL-8 ~2 pg/mL (LOD)/Cortisol ~1 ng/mL | ~1–5 min (depending on platform) | [319] |
| Hollow microneedle glucose patch | Continuous glucose monitoring (ISF) | Screen-printed GOx/PB/NiHCF sensor + PEEK microneedle + Nafion coating | −26.1 nA·mM−1 (ex vivo) | NA | 2 min (ISF to readout) | [320] |
| Ultra-small wearable sweat biosensor | Glucose, lactate, Na+, K+ monitoring | Screen-printed flexible sensor array on PI + MS02 processor chip | 2.05 nA/μM (glucose); 25 nA/mM (lactate); 43.76 and 57.38 mV/log [Na+]/[K+] | Not stated | ~1–2 min | [321] |
| Self-powered TENG biosensor | Human motion and muscle monitoring; | Scotch tape–Al/PET triboelectric layer | Comparable to EMG sensors | NA | <100 ms | [372] |
| Printed core–shell biosensor | vitamin C, Trp, CK, and drug monitoring | MIP/NiHCF inkjet-printed nanoparticle ink | 185 (CY), 253 (BU), 536 (MPA) in nA/mm2/decade | ~5–10 µM (AA) | <1 min (DPV mode) | [373] |
| Wireless cortisol sweat sensor | Stress monitoring (sweat cortisol) | Laser-induced graphene + immunosensing (GS4) | ~3.7 nA/mm2/ng/mL | 0.08 ng/mL | <1 min (DPV) | [374] |
| Universal wearable sweat biosensor | Glucose, uric acid, lactate monitoring | Pt-NPs on N-doped mesoporous carbon/rGO (PNGO) + enzyme ink | 15.33 µA·mM−1·cm−2 (glucose); 103.2 (UA); 219.1 (lactate) | 10.83 µM (glucose); 3.21 µM (UA); 5.27 µM (lactate) | ~1 min | [327] |
| Flexible SERS-based sweat sensor | Drug and biomolecule monitoring | Heart-shaped gold NP dimers on PDMS (F3S metasurface) | SERS EF: up to 1011 | Single molecule | ~1–2 min (Raman) | [343] |
| Wearable scalable SERS sensor | Sweat biomarkers, drugs, microplastics | Gold nanomesh on skin/fabric/plastic | SERS EF ≈ 108 | 10 nM (R6G, AA) | ~20 s (Raman scan) | [344] |
| Origami paper-based sweat biosensor | Glucose, lactate, UA, Mg2+, pH, cortisol | 3D wax-patterned origami chip with enzymatic colorimetry + MIP sensor | R2 > 0.99 (colorimetry); electrochemical MIP for cortisol | 1 nM (cortisol) | 10–15 min (analyte-specific) | [345] |
| Soft epidermal microfluidic sensor | Sweat rate, pH, lactate, glucose, Cl− | Stretchable PDMS microchannels + colorimetric assay + NFC | 0.2 mM (glucose), 0.3 mM (lactate), 0.1 mM (Cl−), 0.5 pH units | ~1–2 mM (glucose); 1.5 mM (lactate) | <1 min (colorimetric) | [346] |
| Wireless self-powered acetone sensor | Breath acetone sensing for prediabetes | Chitosan–RGO film + triboelectric nanogenerator (WET) | 27.89% @10 ppm acetone | NA | ~1 min | [360] |
| Graphene–QD photodetector patch | HR, SpO2, RR, UV exposure monitoring | Graphene–PbS QD on PET/PI + NFC + Bluetooth | ~105 A/W responsivity | ~3.7 × 10−11 W/cm2 NEI | 50 µs (optical), <1 min (PPG) | [353] |
| Device | Function | Technology | Clinical Application | Manufacturer |
|---|---|---|---|---|
| Abbott FreeStyle Libre | Glucose monitoring | Electrochemical biosensor | Diabetes management | Abbott Laboratories |
| Senseonics Eversense | Glucose monitoring | Fluorescence-based biosensor | Diabetes management | Senseonics |
| CardioMEMS HF System | Hemodynamic monitoring | MEMS-based wireless pressure sensor | Heart failure | Abbott |
| Synchron Stentrode | Brain–computer interface | Endovascular neural implant | Assistive communication for paralysis | Synchron |
| Withings Omnia | Comprehensive health monitoring | Integrated health tracking technologies | General health monitoring | Withings |
| Circular Smart Ring | Vital sign monitoring | Wearable sensors for HRV, sleep stages, blood oxygen levels | General health monitoring | Circular |
| Novosound’s Ultrasound Blood Pressure Monitor | Blood pressure monitoring | Ultrasound technology | Hypertension management | Novosound |
| Peri AI-Enabled Perimenopause Tracker | Perimenopause symptom tracking | AI-driven wearable | Women’s health | Peri |
| eDoctor Respiratory Health Monitor | Respiratory health monitoring | IoT-enabled sensors for vital signs | Respiratory health | TEKTELIC |
| eBeat Continuous Health Monitoring Device | Personalized vital sign monitoring | IoT-enabled sensors for real-time data transmission | Chronic disease management, elderly care | TEKTELIC |
| Device | Function | Technology | Clinical Application | Manufacturer/Ref. |
|---|---|---|---|---|
| Medtronic DBS system | Deep brain stimulation | Electrical stimulation | Parkinson’s, essential tremor | Medtronic, Galway, Ireland |
| Vagus nerve stimulation (VNS) device | Neuromodulation | Electrical stimulation | Epilepsy, depression | LivaNova, London, UK |
| Spinal cord stimulator | Chronic pain relief | Electrical stimulation | Neuropathic pain | Boston Scientific, Marlborough, MA, USA |
| Magnetoelectric brain stimulator | Deep brain stimulation | Magnetoelectric metamaterial | movement disorders, psychiatric conditions | Robinson Lab (Company targeting 2025 FDA trial), Houston, TX, USA |
| Bioresorbable temporary pacemaker | Cardiac pacing | Bioresorbable electronics | Post-surgical cardiac care | Rogers Lab (Preclinical), Evanston, IL, USA |
| eCoin tibial neurostimulator | Overactive bladder management | Tibial nerve stimulation | Urological disorders | Valencia Technologies Corporation, Valencia, CA, USA |
| Boston Scientific Vercise DBS | Deep brain stimulation | Multi-target electrical stimulation | Dystonia, Parkinson’s | CE Mark (Boston Scientific), Marlborough, MA, USA |
| NEVRO HF10 therapy | Chronic pain management | 10 kHz high-frequency stimulation | Chronic trunk/limb pain | NEVRO (FDA-Approved), Redwood City, CA , USA |
| Responsive neurostimulation (RNS) system | Seizure control | Closed-loop brain sensing + stimulation | Epilepsy | UCSF Epilepsy Center, San Francisco, CA 94143, USA |
| Optogenetic implant | Neural modulation | Light-activated ion channels | Neurological disorders | Research stage [488] |
| Ultrasound neuromodulation device | Non-invasive stimulation | Focused ultrasound waves | Chronic pain, depression | Research stage [489] |
| PrimeAdvanced SCS | Spinal cord stimulation | Rechargeable multi-waveform | Chronic pain | Medtronic, Galway, Ireland |
| Intellis™ neurostimulator | Adaptive pain relief | Closed-loop sensing + AI algorithms | Failed back surgery syndrome | Medtronic, Galway, Ireland |
| Biohybrid regenerative implant | Tissue repair + electrical stimulation | Cell-embedded conductive scaffolds | Cardiac/neural regeneration | Research stage [490] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abhinav, V.; Basu, P.; Verma, S.S.; Verma, J.; Das, A.; Kumari, S.; Yadav, P.R.; Kumar, V. Advancements in Wearable and Implantable BioMEMS Devices: Transforming Healthcare Through Technology. Micromachines 2025, 16, 522. https://doi.org/10.3390/mi16050522
Abhinav V, Basu P, Verma SS, Verma J, Das A, Kumari S, Yadav PR, Kumar V. Advancements in Wearable and Implantable BioMEMS Devices: Transforming Healthcare Through Technology. Micromachines. 2025; 16(5):522. https://doi.org/10.3390/mi16050522
Chicago/Turabian StyleAbhinav, Vishnuram, Prithvi Basu, Shikha Supriya Verma, Jyoti Verma, Atanu Das, Savita Kumari, Prateek Ranjan Yadav, and Vibhor Kumar. 2025. "Advancements in Wearable and Implantable BioMEMS Devices: Transforming Healthcare Through Technology" Micromachines 16, no. 5: 522. https://doi.org/10.3390/mi16050522
APA StyleAbhinav, V., Basu, P., Verma, S. S., Verma, J., Das, A., Kumari, S., Yadav, P. R., & Kumar, V. (2025). Advancements in Wearable and Implantable BioMEMS Devices: Transforming Healthcare Through Technology. Micromachines, 16(5), 522. https://doi.org/10.3390/mi16050522






