A Review on AC-Dielectrophoresis of Nanoparticles
Abstract
1. Introduction
2. Theory of AC-DEP
3. DEP of Nanoparticles
3.1. Metallic Nanostructures
| Serial No. | Nanoparticle | Electric Field Signal | Electrode Information | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
| NP Type | Size (Diameter/Length) | Material | Voltage | Frequency | Materials | Types | Length Scales (Gap/Length/Width/Height) | ||
| 1 | Metal NPs | 20 nm | Au | 1–20 V | 100 Hz–10 MHz | Al | Parallel, arrowhead | 100 µm | [91] |
| 2 | Metal NPs | 5, 10, and 20 nm | Au | 2–3 V | 1 MHz | Au | Nanogap | 20 nm gap electrode | [100] |
| 3 | Metal NPs | 20–30 nm | Au | 10 Vpp | 1 MHz | Ti/Au | Interdigitated | 1 µm/-/1 µm/5 nm, 200 nm | [71] |
| 4 | Metal NPs | 60 nm | Ag | 10 v | 20 MHz | Cr/Au | Gap electrode | 5 µm/-/-/50 nm, 150 nm | [97] |
| 5 | Metal NPs | 50 nm | Ag | 1–20 Vpp | 1 Hz–1 MHz | Cr/Au | Quadrapole | -/-/-/5 nm, 100 nm | [4] |
| 6 | Metal NPs | 40 nm | Au | 10 Vpp | 1 MHz | Au | Nanopore | 20 µm gap/70 nm Dia/5 nm Au coating | [94] |
| 7 | Metal NPs | 15–100 nm | Ag/Au | 1–20 Vpp | 1 MHz | Au | Nanoelectrode array | 80 nm/-/-/50 nm | [74] |
| 8 | Metal NPs | 40 nm | Ag | 7–8 V | 1–10 MHz | Au | Si Tips/Au | - | [85] |
| 9 | Metal NPs | 40 nm | Ag | 16 Vpp | 2.5 MHz | Au | - | -/-/-/100 nm | [101] |
| 10 | Metal NPs | 2, 10, and 100 nm | Au | 20 Vpp | 5 MHz | SiC/SWCNTS | Nanotip | 540 ± 140 nm | [98] |
| 11 | Metal NPs | 35, 120 nm | Au | 0.6–6 Vrms | 600 kHz | Cr/Ag | Nanoprobe | (150–500 nm in diameter, 2–150 µm in length) | [56] |
| 12 | Metal NPs | 150 nm | Au | 1.9–15 Vrms | 100 kHz | Au | Gap electrode | 10 µm/ | [93] |
| 13 | Metal NPs | 60 nm | Au | 0.7–2.5 V | 1 kHz–1 MHz | Au | Rounded/Rectangular | 40–100 nm, 1 µm, 10 µm/-/-/100 nm | [102] |
| 14 | Metal NPs | 100 nm–200 µm | Silver coated Silica | 100–600 V | 200 kHz | Al | Needle-shaped electrode | - | [5] |
| 15 | Metal NPs | 20 nm | Au | 3 V | 10 kHz–1 MHz | Cr/Pd | Triangular planar electrode | 3 µm/-/-/10 nm, 70 nm | [10] |
| 16 | Metal NPs | 40 nm | DNA coated Au | 2.5–2.8 V | 4 MHz | Ti/Au | Nanogap electrode | 13 nm/-/-/- | [103] |
| 17 | Metal NPs | 10 nm | Au | 9–13 V | 1 kHz–1 MHz | Cr/Au | Triangular planar electrode | 10 µm/-/-/20 nm, 120 nm | [104] |
| 18 | Metal NPs | 80, 100, 150 nm | Au | <10 Vpp | 1–5 MHz | ITO | Thin-film electrode | 20 µm/-/-/- | [99] |
| 19 | Metal NPs | 20 nm | Au | 3 Vpp | 1 MHz | Cr/Au | Nanogap electrode | 200 nm/-/-/5 nm, 30 nm | [95] |
| 20 | Metal + nonmetal NPs | 5, 10, 22 nm | Au, Cu, W, Al, Si, PSL | 12–20 Vpp | 30–70 kHz | Cr/Au | Parallel electrode | -/-/-/2 nm, 120 nm | [73] |
| 21 | Metal NP + Ligands | 10 nm | Au | 4 Vpp | 1 MHz | Ti/Pd | Nanogap electrode | 50 nm/-/100 nm/10, 40 nm | [105] |
| 22 | Metal NPs | 15 nm | Pt | 5 V | 500 kHz | Au | Microgap electrode | -/-/-/4 µm | [92] |
| 23 | Metal NPs | 2–4 nm | Pd | 1–4 Vpp | 500 kHz–2 MHz | Ti/Au | Coplanar electrode | 4 µm/-/-/10, 200 nm | [76] |
3.2. Non-Conducting Nanostructures
| Serial No. | Nanoparticle | Electric Field Signal | Electrode Information | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
| NP Type | Size | Material | Voltage | Frequency | Materials | Types | Length Scales (Gap/Length/Width/Height) | ||
| 1 | NPs | 63, 160, 200, and 410 nm | polystyrene | 10 Vpp | 30 MHz | Au/Cr | Coplanar parallel electrodes | 27 µm/22 mm/-/0.2 µm | [108] |
| 2 | NPs/ QDs | 100, 25 nm | Polystyrene/PEG coated CdSe/Zn QDs | 8 Vpp | 3 MHz–50 MHz | Au | Nanowire electrodes | 10 µm/-/100 nm/- | [83] |
| 3 | NPs | 10–30 nm | Aluminum oxide | 10 Vpp | 1 kHz–10 MHz | ITO | Planar electrodes | (4 mm × 4 mm reservoir)/1 cm/1 cm/1 mm | [109] |
| 4 | NPs | 20 nm, 210 nm, and 1 µm | polystyrene | 7 Vrms | 1 kHz–1 MHz | Carbon nanofiber mat/ITO | Electrospun nanofiber electrode | 150 µm gap/Mat–80 µm thick and 3 mm wide | [86] |
| 5 | NPs | 10–50 nm | polystyrene | 10 Vpp | 5 kHz–20 MHz | Au | Quadrupole microelectrodes | 20 µm gap/-/-/- | [72] |
| 6 | NPs/DNA molecules | 20, 10 nm | polystyrene | 2 Vrms | 100 kHz–50 MHz | Au/Cr | Co-axial probe electrodes | - | [111] |
| 7 | NPs | 0.8 µm | biotin/avidin-conjugated polystyrene | 10 Vpp | 80–100 kHz | Au | Interdigitated electrode | 25 μm/-/25 μm gap/- | [115] |
| 8 | NPs | 20, 100 nm | polystyrene | 500 V | 600 Hz | - | Electrodeless | - | [116] |
| 9 | NPs/ DNAmolecules | 200 nm, 10 µm | polystyrene/genomic hmw-DNA | 160 Vpp/80 Vpp | 10 kHz/3 kHz | Pt/Au | Nanowire electrode/ring type counter electrode | - | [84] |
| 10 | NPs | 220, 80 nm | Polystyrene/ tungsten trioxide | 15 V | 250 kHz–20 MHz | Au/Cr | Curved electrodes | 20–80 nm/270 nm/30 nm/200 nm | [121] |
| 11 | NPs | 150 nm | Polystyrene | 0.18 V/µm | 10 kHz | Au/ITO | Nanopillar electrode | 120 nm height/150 nm radius | [122] |
| 12 | NPs/ Bioparticles | 190 nm | Polystyrene/BSA | 10 Vpp/6 Vpp | 1 kHz–10 MHz | Au/ITO | Nanohole array/planar electrode | Hole diameter—140 nm/periodicity—600 nm | [112] |
| 13 | NPs | 100 nm to 2 µm | Polystyrene | 1.7 to 13.7 Vrms | 15 kHz | W, Si/ITO | Vertical and conical array/planar electrode | Gap—2 µm/W—500 nm dia, Si—50 nm dia./Height—40 nm | [124] |
| 14 | NPs/DNA molecules | 40 nm | polystyrene/DNA | 20 Vpp | 10 kHz | Pt | Microarray electrodes | 80 µm dia/Dimensions—2 mm × 2 mm | [120] |
| 15 | NPs/ DNA molecules | 200 nm | polystyrene/ λ-DNA | 12 Vpp | 6 kHz | Au/Ni | Interdigitated circular electrodes | 100 µm/-/-/3 to 5 µm | [119] |
| 16 | NPs | 5, 20, 40, and 80 nm | Si/Au | 12 V | 1 MHz/10 MHz | Au, PMMA/Au | Planar electrodes | -/-/-/235 nm | [126] |
| 17 | Polymer/DNA | 116 nm | H1/DNA plasmids | 8 Vpp | 20 MHz | - | Interdigitated electrodes | - | [127] |
| 18 | Micro/NPs | 0.5, 3, and 4.5 µm | Polystyrene/Graphite | 150–600 Vrms | 1 kHz–15 kHz | Stainless steel | Parallel planar electrodes | 8 mm/18 mm/8 mm/29 mm | [125] |
| 19 | NPs | 190 nm, 2 µm, | Polystyrene | 10 Vpp | 10 kHz–10 MHz | Au/ITO | Pyramid tip/Planar electrode | 70 µm/-/-/- | [113] |
| 20 | NPs/ DNA molecules | 40 nm | polystyrene/DNA | 20 Vpp/14 Vpp | 10 kHz | Pt | Microarray electrodes | 80 µm dia, Patch dimensions—200 µm | [123] |
| 21 | NPs | 300 nm | PMMA | 10 Vpp | 200 kHz | Au/ITO | Fish-bone type/Planar electrode | 50 µm/-/30 µm/- | [118] |
| 22 | NPs | 300 nm | PMMA | 10 Vpp | 200 kHz | Au/ITO | Planar electrode | 30 µm/2000 µm/30 µm/- | [78] |
| 23 | NPs | 50 nm/40 nm/50 nm | Anti-FITC/polystyrene/Au | 50/100/300 V | 50/500/260 kHz | Si | Electrodeless | 150 nm/-/-/- | [117] |
| 24 | NPs/Bacteria | 5 µm and 20 nm | Polystyrene/Staphylococcus aureus and Pseudomonas aeruginosa | 15 Vpp | 100 kHz–1.2 MHz | Au/Ti | Quadruple electrode array | -/-/-/235 nm | [128] |
| 25 | NPs | 200 nm | Polystyrene | 1 V | 1–4 MHz | Pt | Castellated arrays | 5 µm/-/5 µm/100 nm | [129] |
| 26 | Polymer/nanomedicine particles | 100–200 nm | Polymer, Silica, Liposome | 18 Vpp,15 Vpp, 8 Vpp, 12 Vpp | 15 kHz | Pt | Circular electrode array | 60 µm diameter | [130] |
| 27 | NPs/ QDs | 100, 20 nm | Polystyrene/QDs | 8 Vpp | 1 MHz | Ti/Au | Microelectrode | 10 µm/-/-/55 nm | [131] |
| 28 | Core–shell NP | 220 and 400 nm | poly-L-lysine shell NPs | 5 Vpp | 1 kHz–80 MHz | Ti/Au | Quadrupole microelectrodes | 25 µm/-/-/100 nm | [132] |
| 29 | NPs/Bioparticles | 200 nm | Polystyrene/BSA | 10 Vpp | 10 kHz | platinum−iridium/ITO | Tip and Planar electrode | Tip: Size—20 nm, height—15 nm, length—225 μm | [110] |
| 30 | NPs | 47 nm, 1 µm | Polystyrene | 20 Vpp | 10 kHz–1 MHz | Ti/Au | Zig-zag/face-to-face | Placement angle—60°. Zig-Zag gap—6 µm. Face-to-face gap—5 µm. Height—110 nm | [133] |
| 31 | NPs | 780 nm | Polystyrene | 1 V | 2 MHz–10 MHz | Pt | Crosswise configuration | 1 µm/-/-/60 nm | [134] |
| 32 | NPs/ QDs/nanodiamond | 30, 10, 190 nm | Polystyrene/Nanodiamond | 300 mV/750 mV/400 mV to 600 mV | 1 MHz to 10 MHz/1 MHz/100 kHz | Au | Nanogap electrode | 1–10 nm/0.8 mm/20 µm/150 nm | [114] |
3.3. Nanowires/Nanotubes/Nanorings and Similar
| Serial No. | Nanoparticle | Electric Field Signal | Electrode Information | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
| NP Type | Size (Diameter/Length) | Material | Voltage | Frequency | Materials | Types | Length Scales (Gap/Length/Width/Height) | ||
| 1 | Metal NPs/NWs | 100 nm (NPs), 60 nm (NWs) | Ag | 10–30 Vpp | 10 kHz–1 MHz | ITO | Interdigitated, pillars, and pits | - | [138] |
| 2 | NWs/NTs | 200 nm/3 µm | ZnO, CNT | 5 V | 1 MHz | Ti/Au | Narrow gap | 1 µm, 4 µm, 5 µm/-/-/5 nm, 30 nm | [140] |
| 3 | sc-SWCNTs | - | CNT | 10 V | 500 kHz | ITO | Rectangular gap electrode | 10 µm/995 µm/-/0.14 µm | [141] |
| 4 | MWCNTs/NWs | 5 nm/200 nm–4 µm | ZnO/TiO2/VOX/CNT | 2 V | 1 MHz | Au | Microelectrode pair | 2 µm/-/-/50 nm | [142] |
| 5 | NWs | 220 nm/30 µm | CuO | 6 V | 20 kHz | Au | Gap electrode | 2.25 µm/-/-/- | [152] |
| 6 | NWs | 60 nm/40 µm | Ag | - | 19–37 MHz | Cr/Au | Gap electrode | 75 µm/-/75 µm/50 Å, 500 Å) | [144] |
| 7 | NWs | 300 nm/2.5 µm | SiO2 coated Ag | 2–6 V | 750 kHz | Ti/Au | Cylindrical post | -/-/-/15 nm, 30 nm | [154] |
| 8 | NWs | 100 nm/200 µm | Ag | 0.6–5 V | 10 kHz–10 MHz | Ag | Dot-matrix electrode | 50 µm diameter/150 µm gap | [155] |
| 9 | NWs | 75 nm/22 µm | Si | 10 Vpp | 1 kHz–20 MHz | Au | 3D-well electrode | 150 µm/-/-/70 Cu, 150 Polyamide | [139] |
| 10 | NWs | 2 nm/- | Au | 2–10 Vpp | 2–20 MHz | Cr/Au | Parallel probe electrode | 3 µm/5 nm, 50 nm | [146] |
| 11 | NWs | 30 nm/20, 60 µm | Ag | 5–70 Vpp | 1 MHz | Interdigitated electrode | 36, 65 µm/-/36, 184 µm/- | [149] | |
| 12 | SWCNT | -/600 nm | CNT | 5 Vpp | 400 kHz | Ti/Pd/Au | Surface microelectrodes | 100 nm/-/100 nm/0.2, 15, 15 nm | [11] |
| 13 | NWs | 50 nm/4–5 µm | Si/InAs/ZnO | 3.2 Vpp | 50 kHz | Cr | Interdigitated electrode | 2, 4, and 5 µm/-/-/100 nm | [148] |
| 14 | NWs | 30 nm/1–20 µm | Si | 5–20 Vpp | 5 kHz–5 MHz | Ti/Au | Parallel electrode-bars/Interdigitated electrode | 10, 20 µm/-/-/2 nm, 50 nm | [151] |
| 15 | NWs | 240 nm/18 µm | Si | 7 Vrms | 500 Hz | Al | Isolated and sparse electrode | -/12 µm/2 µm/50 nm | [147] |
| 16 | NWs | 151 nm/5.8 µm | Au | 2–8 Vpp | 10–50 MHz | Au/Ni/Au | Quadrupole microelectrode | -/-/-/6 nm, 100 nm, 100 nm | [150] |
| 17 | NWs | - | Au/MWCNT | 4 Vpp | 10 kHz–1 MHz | Cr/Au | Array of electrodes | 100 nm/-/150 nm/85 nm | [143] |
| 18 | NWs | 130 nm/- | Si | 0.3–5 V | 1 MHz | Ti | Tapered electrode | 4 µm/-/-/- | [159] |
| 19 | Nanobelt | 1–2 µm length/size | SnO2 | 70 Vpp | 5 Hz–10 MHz | Au | Castellated microelectrodes | 20 µm/-/-/250 nm | [158] |
| 20 | Nanomotor | 250 nm/5 µm | Pt–Au | 0–50 Vpp | 10 kHz–10 MHz | Au | Quadruple microelectrode | -/-/-/500 µm | [145] |
| 21 | NWs/NTs | 50–150 nm/4–6 µm | Ni, ZnO, Au, Ag, Sn, Fe2O3 | 0.6–6 Vpp | 50–200 kHz | Au | Parallel plate electrodes | -/-/-/400 nm | [153] |
| 22 | NWs | 23 nm/- | Pt | 4 V | 100 kHz | Pt/Au | Interdigitated electrode | 2–4 µm/-/-/3, 17 nm | [77] |
| 23 | NTs | 1–3 nm/ | CNT | 5–20 Vpp | 300 kHz | W-Ti/Pt | Interdigitated electrode | -/-/-/120 nm | [156] |
3.4. Other Semiconductive Nanostructures
| Serial No. | Nanoparticle | Electric Field Signal | Electrode Information | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
| Type | Size (Diameter/Length) | Material | Voltage | Frequency | Materials | Types | Length scales(Gap/Length/Width/Height) | ||
| 1 | Nanostructure + NPs | 1 nm GO/15 nm Pt | Graphene Oxide/Platinum | 2, 5, and 10 Vpp | 100, 500, 1000 kHz | Ti/Au | Microgap electrode | 4 µm/-/-/- | [164] |
| 2 | Nano-onions | 5 nm | Carbon | 3–20 Vpp | 1, 100, and 1000 kHz | Au | Interdigitated microelectrode | 5 µm/6760 µm/5 µm/- | [173] |
| 3 | NPs | 21.4 nm | Strontium titanate | 5 V | 1 MHz | Al | Probe electrodes | 100 nm/-/-/60 nm | [168] |
| 4 | NPs | 80 nm& 210 nm | Tungsten trioxide and Polystyrene | 15 V | 100 kHz–20 MHz | Cr/Au | Curved microelectrode | -/-/-/50, 100 nm | [121] |
| 5 | Nps | 4.2 nm/- | Cadmium telluride | 4–10 V | 100 kHz | Cr/Au | Microgap electrode | 2 µm/-/-/- | [166] |
| 6 | Nanorods | 500 nm/2.5 µm | Indium gallium nitride/Gallium nitride | 2.8–21 Vrms | 100–950 kHz | Au | Interdigitated microelectrode | 2.5 µm/-/3 µm/- | [169] |
| 7 | NPs | 20–100 nm | Zinc oxide | 10 V | 300 kHz | Ti/Pd | Gap electrode | 800 nm/200 nm/45 nm/5, 40 nm | [170] |
| 8 | CBNPs | 30 nm/- | Carbon black | 12–225 V | 10 Hz–10 kHz | Cu | Triangular planar electrode | 2–30 mm/-/-/88 µm | [171] |
| 9 | MWCNTs | 40–60 nm/5–15 µm | Multiwall carbon nanotube | 20 Vpp | 5 MHz | Cr/Au | Parallel electrode | 1–10 µm/10 mm/5 mm/30, 100 nm | [172] |
| 10 | Nanorods | 500 nm/3 µm | Indium gallium nitride/Gallium nitride | 21 Vrms | 950 kHz | Ti/Au | Interdigitated microelectrode | 3 µm/0.7 cm/0.6 cm/20, 200 nm | [167] |
| 11 | NPs | 300 nm/15 µm | Zinc oxide | 40 Vrms | 1 kHz | Au | In-plane electrode | 150 µm/-/-/- | [177] |
| 12 | NPs | 1 nm/5.69 µm | Graphene Oxide | 20 Vpp | 10 kHz | ITO | Interlaced electrode | 1 mm/-/1 mm/- | [178] |
| 13 | NPs | 450 nm, 80 nm/- | silica and tungsten trioxide | 15 Vpp | 0.1–250 MHz | Cr/Au | Curved microelectrode | 20 µm/17 mm/-/- | [175] |
| 14 | NPs | 60 nm, 109 nm, 10–20 nm/10 nm | Polystyrene/Nanoliposomes/Micelles/DNA strands | 2–18 Vpp | 15 kHz | Pt | Microelectrode | 218 µm gap/60 µm diameter | [130] |
| 15 | NPs | 10 nm QD/300 nm, 5 µm Au Nanowires | Cadmium Selenide, Zinc Sulfide QDs/Gold NWs | 20 Vpp | 50–700 kHz | - | Parallel microelectrode | 20–50 µm/-/-/- | [176] |
| 16 | NPs | 3–8 nm | Platinum-palladium | 10 Vpp | 1 MHz | Ti/Au | Triangular probe electrodes | 2 µm/-/-/5 nm, 90 nm | [165] |
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, F.; Josephson, D.P.; Stein, A. Colloidal assembly: The road from particles to colloidal molecules and crystals. Angew. Chem. Int. Ed. 2011, 50, 360–388. [Google Scholar] [CrossRef]
- Velev, O.D.; Gupta, S. Materials fabricated by micro-and nanoparticle assembly–the challenging path from science to engineering. Adv. Mater. 2009, 21, 1897–1905. [Google Scholar] [CrossRef]
- Arpin, K.A.; Mihi, A.; Johnson, H.T.; Baca, A.J.; Rogers, J.A.; Lewis, J.A.; Braun, P.V. Multidimensional architectures for functional optical devices. Adv. Mater. 2010, 22, 1084–1101. [Google Scholar] [CrossRef] [PubMed]
- Dies, H.; Raveendran, J.; Escobedo, C.; Docoslis, A. In situ assembly of active surface-enhanced Raman scattering substrates via electric field-guided growth of dendritic nanoparticle structures. Nanoscale 2017, 9, 7847–7857. [Google Scholar] [CrossRef] [PubMed]
- Rozynek, Z.; Han, M.; Dutka, F.; Garstecki, P.; Józefczak, A.; Luijten, E. Formation of printable granular and colloidal chains through capillary effects and dielectrophoresis. Nat. Commun. 2017, 8, 15255. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yang, X.; Wang, W.; Sun, R.; Cui, J.; Fu, Y.; Li, K.; Zhang, M.; Liu, C.; Zhu, H.; et al. High-Performance Small Molecule Organic Solar Cells Enabled by a Symmetric-Asymmetric Alloy Acceptor with a Broad Composition Tolerance. Adv. Mater. 2023, 35, 2300531. [Google Scholar] [CrossRef]
- Wang, T.; Huang, D.; Yang, Z.; Xu, S.; He, G.; Li, X.; Hu, N.; Yin, G.; He, D.; Zhang, L. A review on graphene-based gas/vapor sensors with unique properties and potential applications. Nano-Micro Lett. 2016, 8, 95–119. [Google Scholar] [CrossRef]
- Kopperger, E.; List, J.; Madhira, S.; Rothfischer, F.; Lamb, D.C.; Simmel, F.C. A self-assembled nanoscale robotic arm controlled by electric fields. Science 2018, 359, 296–301. [Google Scholar] [CrossRef]
- Bornhoeft, L.R.; Castillo, A.C.; Smalley, P.R.; Kittrell, C.; James, D.K.; Brinson, B.E.; Rybolt, T.R.; Johnson, B.R.; Cherukuri, T.K.; Cherukuri, P. Teslaphoresis of carbon nanotubes. ACS Nano 2016, 10, 4873–4881. [Google Scholar] [CrossRef]
- Ding, H.; Shao, J.; Ding, Y.; Liu, W.; Tian, H.; Li, X. One-dimensional Au–ZnO heteronanostructures for ultraviolet light detectors by a two-step dielectrophoretic assembly method. ACS Appl. Mater. Interfaces 2015, 7, 12713–12718. [Google Scholar] [CrossRef]
- Cao, Q.; Han, S.-J.; Tulevski, G.S. Fringing-field dielectrophoretic assembly of ultrahigh-density semiconducting nanotube arrays with a self-limited pitch. Nat. Commun. 2014, 5, 5071. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Wägli, P.; Paeder, V.; Homsy, A.; Hvozdara, L.; van der Wal, P.; Di Francesco, J.; de Rooij, N.F.; Herzig, H.P. Cocaine detection by a mid-infrared waveguide integrated with a microfluidic chip. Lab A Chip 2012, 12, 3020–3023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Petit, T.; Lu, Y.; Kratochvil, B.E.; Peyer, K.E.; Pei, R.; Lou, J.; Nelson, B.J. Controlled propulsion and cargo transport of rotating nickel nanowires near a patterned solid surface. ACS Nano 2010, 4, 6228–6234. [Google Scholar] [CrossRef]
- Wang, W.; Castro, L.A.; Hoyos, M.; Mallouk, T.E. Autonomous motion of metallic microrods propelled by ultrasound. ACS Nano 2012, 6, 6122–6132. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.G.; Zhao, Y.-P. Autonomously motile catalytic nanomotors by bubble propulsion. Appl. Phys. Lett. 2009, 94, 163104. [Google Scholar] [CrossRef]
- Neuman, K.C.; Nagy, A. Single-molecule force spectroscopy: Optical tweezers, magnetic tweezers and atomic force microscopy. Nat. Methods 2008, 5, 491–505. [Google Scholar] [CrossRef]
- McGrath, J.; Jimenez, M.; Bridle, H. Deterministic lateral displacement for particle separation: A review. Lab A Chip 2014, 14, 4139–4158. [Google Scholar] [CrossRef]
- Munaz, A.; Shiddiky, M.J.A.; Nguyen, N.-T. Recent advances and current challenges in magnetophoresis based micro magnetofluidics. Biomicrofluidics 2018, 12, 031501. [Google Scholar] [CrossRef]
- Robert, D.; Pamme, N.; Conjeaud, H.; Gazeau, F.; Iles, A.; Wilhelm, C. Cell sorting by endocytotic capacity in a microfluidic magnetophoresis device. Lab A Chip 2011, 11, 1902–1910. [Google Scholar] [CrossRef]
- Pesce, G.; Jones, P.H.; Maragò, O.M.; Volpe, G. Optical tweezers: Theory and practice. Eur. Phys. J. Plus 2020, 135, 949. [Google Scholar] [CrossRef]
- Hettiarachchi, S.; Cha, H.; Ouyang, L.; Mudugamuwa, A.; An, H.; Kijanka, G.; Kashaninejad, N.; Nguyen, N.-T.; Zhang, J. Recent microfluidic advances in submicron to nanoparticle manipulation and separation. Lab A Chip 2023, 23, 982–1010. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, K.-K. Optical tweezers for single cells. J. R. Soc. Interface 2008, 5, 671–690. [Google Scholar] [CrossRef] [PubMed]
- Salafi, T.; Zeming, K.K.; Zhang, Y. Advancements in microfluidics for nanoparticle separation. Lab A Chip 2017, 17, 11–33. [Google Scholar] [CrossRef] [PubMed]
- Pohl, H. Dielectrophoresis: The Behavior of Neutral Matter in Nonuniform Electric Fields; Cambridge University Press: Cambridge, UK, 1978. [Google Scholar]
- Jones, T.B. Electromechanics of Particles; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Morgan, H.; Green, N.G. AC Electrokinetics: Colloids and Nanoparticles; Research Studies Press: Baldock, UK, 2003. [Google Scholar]
- Sarno, B.; Heineck, D.; Heller, M.J.; Ibsen, S.D. Dielectrophoresis: Developments and applications from 2010 to 2020. Electrophoresis 2021, 42, 539–564. [Google Scholar] [CrossRef]
- Tu, Y.; Peng, F.; Wilson, D.A. Motion manipulation of micro-and nanomotors. Adv. Mater. 2017, 29, 1701970. [Google Scholar] [CrossRef]
- Li, J.; Rozen, I.; Wang, J. Rocket science at the nanoscale. ACS Nano 2016, 10, 5619–5634. [Google Scholar] [CrossRef]
- Demircan, Y.; Özgür, E.; Külah, H. Dielectrophoresis: Applications and future outlook in point of care. Electrophoresis 2013, 34, 1008–1027. [Google Scholar] [CrossRef]
- Martinez-Duarte, R. Microfabrication technologies in dielectrophoresis applications—A review. Electrophoresis 2012, 33, 3110–3132. [Google Scholar] [CrossRef]
- Zhang, C.; Khoshmanesh, K.; Mitchell, A.; Kalantar-Zadeh, K. Dielectrophoresis for manipulation of micro/nano particles in microfluidic systems. Anal. Bioanal. Chem. 2010, 396, 401–420. [Google Scholar] [CrossRef]
- Kuzyk, A. Dielectrophoresis at the nanoscale. Electrophoresis 2011, 32, 2307. [Google Scholar] [CrossRef]
- Edwards, T.D.; Bevan, M.A. Controlling colloidal particles with electric fields. Langmuir 2014, 30, 10793–10803. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Wan, K.; Wu, M.; Guo, L.; Liu, X.; Wei, G. On the design, functions, and biomedical applications of high-throughput dielectrophoretic micro-/nanoplatforms: A review. Nanoscale 2021, 13, 4330–4358. [Google Scholar] [CrossRef] [PubMed]
- Pesch, G.R.; Du, F. A review of dielectrophoretic separation and classification of non-biological particles. Electrophoresis 2021, 42, 134–152. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, K.; Xiang, N.; Ni, Z. Dielectrophoretic manipulation of nanomaterials: A review. Electrophoresis 2019, 40, 873–889. [Google Scholar] [CrossRef]
- Pethig, R. Dielectrophoresis Tutorial: Inspired by Hatfield’s 1924 Patent and Boltzmann’s Theory and Experiments of 1874. Electrophoresis 2025. [Google Scholar] [CrossRef]
- Abd Rahman, N.; Ibrahim, F.; Yafouz, B. Dielectrophoresis for biomedical sciences applications: A review. Sensors 2017, 17, 449. [Google Scholar] [CrossRef]
- Pethig, R. Dielectrophoresis: Status of the theory, technology, and applications. Biomicrofluidics 2010, 4, 022811. [Google Scholar] [CrossRef]
- Hughes, M.P.; Morgan, H.; Flynn, M.F. The dielectrophoretic behavior of submicron latex spheres: Influence of surface conductance. J. Colloid Interface Sci. 1999, 220, 454–457. [Google Scholar] [CrossRef]
- Kijlstra, J.; van Leeuwen, H.P.; Lyklema, J. Effects of surface conduction on the electrokinetic properties of colloids. J. Chem. Soc. Faraday Trans. 1992, 88, 3441–3449. [Google Scholar] [CrossRef]
- Cui, L.; Holmes, D.; Morgan, H. The dielectrophoretic levitation and separation of latex beads in microchips. Electrophoresis 2001, 22, 3893–3901. [Google Scholar] [CrossRef]
- White, C.M.; Holland, L.A.; Famouri, P. Application of capillary electrophoresis to predict crossover frequency of polystyrene particles in dielectrophoresis. Electrophoresis 2010, 31, 2664–2671. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.K.; Gencoglu, A.; Minerick, A.R. DC insulator dielectrophoretic applications in microdevice technology: A review. Anal. Bioanal. Chem. 2011, 399, 301–321. [Google Scholar] [CrossRef] [PubMed]
- Bruus, H. Theoretical Microfluidics; Oxford University Press: Oxford, UK, 2007; Volume 18. [Google Scholar]
- Castellanos, A.; Ramos, A.; Gonzalez, A.; Green, N.G.; Morgan, H. Electrohydrodynamics and dielectrophoresis in microsystems: Scaling laws. J. Phys. D Appl. Phys. 2003, 36, 2584. [Google Scholar] [CrossRef]
- Kirby, B.J. Micro-and Nanoscale Fluid Mechanics: Transport in Microfluidic Devices; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Williams, S.J. Enhanced electrothermal pumping with thin film resistive heaters. Electrophoresis 2013, 34, 1400–1408. [Google Scholar] [CrossRef]
- Chang, H.-C.; Yeo, L.Y. Electrokinetically Driven Microfluidics and Nanofluidics; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Ramos, A.; Morgan, H.; Green, N.G.; Castellanos, A. Ac electrokinetics: A review of forces in microelectrode structures. J. Phys. D Appl. Phys. 1998, 31, 2338. [Google Scholar] [CrossRef]
- Salari, A.; Navi, M.; Lijnse, T.; Dalton, C. AC Electrothermal Effect in Microfluidics: A Review. Micromachines 2019, 10, 762. [Google Scholar] [CrossRef]
- Cao, J.; Cheng, P.; Hong, F. Applications of electrohydrodynamics and Joule heating effects in microfluidic chips: A review. Sci. China Ser. E Technol. Sci. 2009, 52, 3477–3490. [Google Scholar] [CrossRef]
- Ramos, A. Electrokinetics and Electrohydrodynamics in Microsystems; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011; Volume 530. [Google Scholar]
- Bazant, M.Z.; Squires, T.M. Induced-Charge Electrokinetic Phenomena: Theory and Microfluidic Applications. Phys. Rev. Lett. 2004, 92, 066101. [Google Scholar] [CrossRef]
- Wood, N.R.; Wolsiefer, A.I.; Cohn, R.W.; Williams, S.J. Dielectrophoretic trapping of nanoparticles with an electrokinetic nanoprobe. Electrophoresis 2013, 34, 1922–1930. [Google Scholar] [CrossRef]
- Hyler, A.R.; Hong, D.; Davalos, R.V.; Swami, N.S.; Schmelz, E.M. A novel ultralow conductivity electromanipulation buffer improves cell viability and enhances dielectrophoretic consistency. Electrophoresis 2021, 42, 1366–1377. [Google Scholar] [CrossRef]
- Luna, R.; Heineck, D.; Hinestrosa, J.P.; Dobrovolskaia, I.; Hamilton, S.; Malakian, A.; Gustafson, K.T.; Huynh, K.T.; Kim, S.; Ware, J.; et al. Enhancement of dielectrophoresis-based particle collection from high conducting fluids due to partial electrode insulation. Electrophoresis 2023, 44, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Rashed, M.Z.; Green, N.G.; Williams, S.J. Scaling law analysis of electrohydrodynamics and dielectrophoresis for isomotive dielectrophoresis microfluidic devices. Electrophoresis 2020, 41, 148–155. [Google Scholar] [CrossRef]
- Zhao, H. Double-layer polarization of a non-conducting particle in an alternating current field with applications to dielectrophoresis. Electrophoresis 2011, 32, 2232–2244. [Google Scholar] [CrossRef]
- Holzel, R. Dielectric and dielectrophoretic properties of DNA. IET Nanobiotechnology 2009, 3, 28–45. [Google Scholar] [CrossRef]
- Camacho-Alanis, F.; Ros, A. Protein dielectrophoresis and the link to dielectric properties. Bioanalysis 2015, 7, 353–371. [Google Scholar] [CrossRef]
- Martinez-Duarte, R.; Renaud, P.; Madou, M.J. A novel approach to dielectrophoresis using carbon electrodes. Electrophoresis 2011, 32, 2385–2392. [Google Scholar] [CrossRef]
- Hughes, M.P. Strategies for dielectrophoretic separation in laboratory-on-a-chip systems. Electrophoresis 2002, 23, 2569–2582. [Google Scholar] [CrossRef]
- Adekanmbi, E.O.; Srivastava, S.K. Dielectrophoretic applications for disease diagnostics using lab-on-a-chip platforms. Lab A Chip 2016, 16, 2148–2167. [Google Scholar] [CrossRef]
- Biswas, A.; Bayer, I.S.; Biris, A.S.; Wang, T.; Dervishi, E.; Faupel, F. Advances in top–down and bottom–up surface nanofabrication: Techniques, applications & future prospects. Adv. Colloid Interface Sci. 2012, 170, 2–27. [Google Scholar] [CrossRef]
- Cui, Z. Nanofabrication; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Wiley, B.J.; Qin, D.; Xia, Y. Nanofabrication at High Throughput and Low Cost. ACS Nano 2010, 4, 3554–3559. [Google Scholar] [CrossRef]
- Tahir, U.; Shim, Y.B.; Kamran, M.A.; Kim, D.-I.; Jeong, M.Y. Nanofabrication techniques: Challenges and future prospects. J. Nanosci. Nanotechnol. 2021, 21, 4981–5013. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Hu, W.; Zhu, D. Nanogap Electrodes. Adv. Mater. 2010, 22, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Cherukulappurath, S.; Lee, S.H.; Campos, A.; Haynes, C.L.; Oh, S.-H. Rapid and Sensitive in Situ SERS Detection Using Dielectrophoresis. Chem. Mater. 2014, 26, 2445–2452. [Google Scholar] [CrossRef]
- Froude, V.E.; Godfroy, J.I.; Wang, S.; Dombek, H.; Zhu, Y. Anomalous Dielectrophoresis of Nanoparticles: A Rapid and Sensitive Characterization by Single-Particle Laser Spectroscopy. J. Phys. Chem. C 2010, 114, 18880. [Google Scholar] [CrossRef]
- Yilmaz, C.; Cetin, A.E.; Goutzamanidis, G.; Huang, J.; Somu, S.; Altug, H.; Wei, D.; Busnaina, A. Three-dimensional crystalline and homogeneous metallic nanostructures using directed assembly of nanoparticles. ACS Nano 2014, 8, 4547–4558. [Google Scholar] [CrossRef]
- Leiterer, C.; Deckert-Gaudig, T.; Singh, P.; Wirth, J.; Deckert, V.; Fritzsche, W. Dielectrophoretic positioning of single nanoparticles on atomic force microscope tips for tip-enhanced Raman spectroscopy. Electrophoresis 2015, 36, 1142–1148. [Google Scholar] [CrossRef]
- Sun, T.; Morgan, H.; Green, N.G. Analytical solutions of ac electrokinetics in interdigitated electrode arrays: Electric field, dielectrophoretic and traveling-wave dielectrophoretic forces. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2007, 76, 046610. [Google Scholar] [CrossRef]
- Rathi, S.; Kwak, Y.; Jing, L.; Yi, K.S.; Baik, J.M.; Kim, G.-H. Programmed dielectrophoretic assembly of Pd nanoparticles for conductance control in VO2 nanowires. Curr. Appl. Phys. 2017, 17, 351–357. [Google Scholar] [CrossRef]
- Nerowski, A.; Poetschke, M.; Bobeth, M.; Opitz, J.; Cuniberti, G. Dielectrophoretic Growth of Platinum Nanowires: Concentration and Temperature Dependence of the Growth Velocity. Langmuir 2012, 28, 7498–7504. [Google Scholar] [CrossRef]
- Chuang, C.-H.; Huang, Y.-W. Multistep manipulations of poly(methyl-methacrylate) submicron particles using dielectrophoresis. Electrophoresis 2013, 34, 3111–3118. [Google Scholar] [CrossRef]
- Lifeng, Z.; Shengdong, L.; Burke, P.J.; Brody, J.P. Towards single molecule manipulation with dielectrophoresis using nanoelectrodes. In Proceedings of the 2003 Third IEEE Conference on Nanotechnology, San Francisco, CA, USA, 12–14 August 2003; Volume 432, pp. 437–440. [Google Scholar]
- Zheng, L.; Li, S.; Brody, J.P.; Burke, P.J. Manipulating nanoparticles in solution with electrically contacted nanotubes using dielectrophoresis. Langmuir 2004, 20, 8612–8619. [Google Scholar] [CrossRef] [PubMed]
- Tuukkanen, S.; Toppari, J.J.; Kuzyk, A.; Hirviniemi, L.; Hytönen, V.P.; Ihalainen, T.; Törmä, P. Carbon nanotubes as electrodes for dielectrophoresis of DNA. Nano Lett. 2006, 6, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.-S.; Lee, H.; Lee, S.-M.; Kim, J.; Kim, T.; Lee, J.; Kim, C.; Seo, M.; Kim, J.H.; Byun, Y.T.; et al. Precise capture and dynamic relocation of nanoparticulate biomolecules through dielectrophoretic enhancement by vertical nanogap architectures. Nat. Commun. 2020, 11, 2804. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, S.; Francis Suh, J.-K.; Ho Park, J.; Shin, H.-J. Active control of dielectrophoretic force at nanowire electrode for ultrahigh single nanoparticle manipulation yield. Appl. Phys. Lett. 2013, 102, 063105. [Google Scholar] [CrossRef]
- Song, Y.; Sonnenberg, A.; Heaney, Y.; Heller, M.J. Device for dielectrophoretic separation and collection of nanoparticles and DNA under high conductance conditions. Electrophoresis 2015, 36, 1107–1114. [Google Scholar] [CrossRef]
- Leiterer, C.; Wünsche, E.; Singh, P.; Albert, J.; Köhler, J.M.; Deckert, V.; Fritzsche, W. High precision attachment of silver nanoparticles on AFM tips by dielectrophoresis. Anal. Bioanal. Chem. 2016, 408, 3625–3631. [Google Scholar] [CrossRef]
- Mondal, T.K.; West, J.H.; Williams, S.J. An electrospun nanofiber mat as an electrode for AC-dielectrophoretic trapping of nanoparticles. Nanoscale 2023, 15, 18241–18249. [Google Scholar] [CrossRef]
- West, J.H.; Mondal, T.K.; Williams, S.J. Electrokinetic particle trapping in microfluidic wells using conductive nanofiber mats. Electrophoresis, 2024; Early View. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.; Wang, C.; Liu, Q.; Chen, W.-T.; Gao, S.; Hu, G. Recent advances in designable nanomaterial-based electrochemical sensors for environmental heavy-metal detection. Nanoscale 2025, 17, 2386–2407. [Google Scholar] [CrossRef]
- Choi, J.-R.; Shin, D.-M.; Song, H.; Lee, D.; Kim, K. Current achievements of nanoparticle applications in developing optical sensing and imaging techniques. Nano Converg. 2016, 3, 30. [Google Scholar] [CrossRef]
- Georgeous, J.; AlSawaftah, N.; Abuwatfa, W.H.; Husseini, G.A. Review of Gold Nanoparticles: Synthesis, Properties, Shapes, Cellular Uptake, Targeting, Release Mechanisms and Applications in Drug Delivery and Therapy. Pharmaceutics 2024, 16, 1332. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.-H.; Kang, S.-H.; Lee, Y.J.; Kim, J.-H.; Ahn, E.-Y.; Park, Y.; Cho, S. Fabrication of nanoribbons by dielectrophoresis assisted cold welding of gold nanoparticles on mica substrate. Sci. Rep. 2019, 9, 3629. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Rathi, S.; Singh, B.; Lee, I.; Maeng, S.; Joh, H.-I.; Kim, G.-H. Dielectrophoretic assembly of Pt nanoparticle-reduced graphene oxide nanohybrid for highly-sensitive multiple gas sensor. Sens. Actuators B Chem. 2015, 220, 755–761. [Google Scholar] [CrossRef]
- Koshi, T.; Nakajima, Y.; Iwase, E. Voltage and current conditions for nanoparticle chain formation using dielectrophoresis. Micro Nano Lett. 2017, 12, 532–535. [Google Scholar] [CrossRef]
- Freedman, K.J.; Crick, C.R.; Albella, P.; Barik, A.; Ivanov, A.P.; Maier, S.A.; Oh, S.-H.; Edel, J.B. On-Demand Surface- and Tip-Enhanced Raman Spectroscopy Using Dielectrophoretic Trapping and Nanopore Sensing. ACS Photon. 2016, 3, 1036–1044. [Google Scholar] [CrossRef]
- Khondaker, S.I.; Luo, K.; Yao, Z. The fabrication of single-electron transistors using dielectrophoretic trapping of individual gold nanoparticles. Nanotechnology 2010, 21, 095204. [Google Scholar] [CrossRef]
- Almeida, G.B.; Poppi, R.J.; da Silva, J.A.F. Trapping of Au nanoparticles in a microfluidic device using dielectrophoresis for surface enhanced Raman spectroscopy. Analyst 2017, 142, 375–379. [Google Scholar] [CrossRef]
- Chrimes, A.F.; Khoshmanesh, K.; Stoddart, P.R.; Kayani, A.A.; Mitchell, A.; Daima, H.; Bansal, V.; Kalantar-zadeh, K. Active Control of Silver Nanoparticles Spacing Using Dielectrophoresis for Surface-Enhanced Raman Scattering. Anal. Chem. 2012, 84, 4029–4035. [Google Scholar] [CrossRef]
- Yeo, W.-H.; Kopacz, A.M.; Kim, J.-H.; Chen, X.; Wu, J.; Gao, D.; Lee, K.-H.; Liu, W.-K.; Chung, J.-H. Dielectrophoretic concentration of low-abundance nanoparticles using a nanostructured tip. Nanotechnology 2012, 23, 485707. [Google Scholar] [CrossRef]
- Midelet, C.; Le Pioufle, B.; Werts, M.H.V. Brownian Motion and Large Electric Polarizabilities Facilitate Dielectrophoretic Capture of Sub-200 nm Gold Nanoparticles in Water. ChemPhysChem 2019, 20, 3354–3365. [Google Scholar] [CrossRef]
- Cheon, D.; Kumar, S.; Kim, G.-H. Assembly of gold nanoparticles of different diameters between nanogap electrodes. Appl. Phys. Lett. 2010, 96, 013101. [Google Scholar] [CrossRef]
- Salemmilani, R.; Piorek, B.D.; Mirsafavi, R.Y.; Fountain, A.W., III; Moskovits, M.; Meinhart, C.D. Dielectrophoretic Nanoparticle Aggregation for On-Demand Surface Enhanced Raman Spectroscopy Analysis. Anal. Chem. 2018, 90, 7930–7936. [Google Scholar] [CrossRef] [PubMed]
- Leiterer, C.; Berg, S.; Eskelinen, A.-P.; Csaki, A.; Urban, M.; Törmä, P.; Fritzsche, W. Assembling gold nanoparticle chains using an AC electrical field: Electrical detection of organic thiols. Sens. Actuators B Chem. 2013, 176, 368–373. [Google Scholar] [CrossRef]
- Strobel, S.; Sperling, R.A.; Fenk, B.; Parak, W.J.; Tornow, M. Dielectrophoretic trapping of DNA-coated gold nanoparticles on silicon based vertical nanogap devices. Phys. Chem. Chem. Phys. 2011, 13, 9973–9977. [Google Scholar] [CrossRef]
- Liu, W.; Wang, C.; Ding, H.; Shao, J.; Ding, Y. AC electric field induced dielectrophoretic assembly behavior of gold nanoparticles in a wide frequency range. Appl. Surf. Sci. 2016, 370, 184–192. [Google Scholar] [CrossRef]
- Fu, K.; Chen, S.; Zhao, J.; Willis, B.G. Dielectrophoretic assembly of gold nanoparticles in nanoscale junctions for rapid, miniature chemiresistor vapor sensors. Acs Sens. 2016, 1, 444–450. [Google Scholar] [CrossRef]
- Rashed, M.Z.; Williams, S.J. Advances and applications of isomotive dielectrophoresis for cell analysis. Anal. Bioanal. Chem. 2020, 412, 3813–3833. [Google Scholar] [CrossRef]
- Chen, Q.; Yuan, Y.J. A review of polystyrene bead manipulation by dielectrophoresis. RSC Adv. 2019, 9, 4963–4981. [Google Scholar] [CrossRef]
- Huang, H.; Ou-Yang, H.D. A novel dielectrophoresis potential spectroscopy for colloidal nanoparticles. Electrophoresis 2017, 38, 1609–1616. [Google Scholar] [CrossRef]
- Chuang, C.H.; Hung, C.H. AFM manipulation of nanoparticles based on dielectrophoresis. In Proceedings of the 2011 6th IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Kaohsiung, Taiwan, 20–23 February 2011; pp. 923–927. [Google Scholar]
- Zhou, P.; Yu, H.; Yang, W.; Wen, Y.; Wang, Z.; Li, W.J.; Liu, L. Spatial Manipulation and Assembly of Nanoparticles by Atomic Force Microscopy Tip-Induced Dielectrophoresis. ACS Appl. Mater. Interfaces 2017, 9, 16715–16724. [Google Scholar] [CrossRef]
- Tao, Y.; Kumar Wickramasinghe, H. Coaxial atomic force microscope probes for dielectrophoresis of DNA under different buffer conditions. Appl. Phys. Lett. 2017, 110, 073701. [Google Scholar] [CrossRef]
- Barik, A.; Otto, L.M.; Yoo, D.; Jose, J.; Johnson, T.W.; Oh, S.-H. Dielectrophoresis-Enhanced Plasmonic Sensing with Gold Nanohole Arrays. Nano Lett. 2014, 14, 2006–2012. [Google Scholar] [CrossRef] [PubMed]
- Jose, J.; Kress, S.; Barik, A.; Otto, L.M.; Shaver, J.; Johnson, T.W.; Lapin, Z.J.; Bharadwaj, P.; Novotny, L.; Oh, S.-H. Individual Template-Stripped Conductive Gold Pyramids for Tip-Enhanced Dielectrophoresis. ACS Photon. 2014, 1, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Barik, A.; Chen, X.; Oh, S.-H. Ultralow-power electronic trapping of nanoparticles with sub-10 nm gold nanogap electrodes. Nano Lett. 2016, 16, 6317–6324. [Google Scholar] [CrossRef]
- Park, S.; Yossifon, G. Combining dielectrophoresis and concentration polarization-based preconcentration to enhance bead-based immunoassay sensitivity. Nanoscale 2019, 11, 9436–9443. [Google Scholar] [CrossRef]
- Viefhues, M.; Eichhorn, R.; Fredrich, E.; Regtmeier, J.; Anselmetti, D. Continuous and reversible mixing or demixing of nanoparticles by dielectrophoresis. Lab A Chip 2012, 12, 485–494. [Google Scholar] [CrossRef]
- Chiou, C.-H.; Chien, L.-J.; Kuo, J.-N. Nanoconstriction-based electrodeless dielectrophoresis chip for nanoparticle and protein preconcentration. Appl. Phys. Express 2015, 8, 085201. [Google Scholar] [CrossRef]
- Chuang, C.H.; Huang, Y.W.; Wu, H.P.; Lin, S.Y. Microfluidic device for manipulations of PMMA nanoparticles based on dielectrophoresis. In Proceedings of the 2011 6th IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Kaohsiung, Taiwan, 20–23 February 2011; pp. 1012–1015. [Google Scholar]
- Noh, S.; Song, Y. Dielectrophoretic Trapping for Nanoparticles, High-Molecule-Weight DNA, and SYBR Gold Using Polyimide-Based Printed Circuit Board. IEEE Sens. J. 2021, 21, 18451–18458. [Google Scholar] [CrossRef]
- Sonnenberg, A.; Marciniak, J.Y.; Krishnan, R.; Heller, M.J. Dielectrophoretic isolation of DNA and nanoparticles from blood. Electrophoresis 2012, 33, 2482–2490. [Google Scholar] [CrossRef]
- Chrimes, A.F.; Kayani, A.A.; Khoshmanesh, K.; Stoddart, P.R.; Mulvaney, P.; Mitchell, A.; Kalantar-zadeh, K. Dielectrophoresis–Raman spectroscopy system for analysing suspended nanoparticles. Lab A Chip 2011, 11, 921–928. [Google Scholar] [CrossRef]
- Zaman, M.A.; Padhy, P.; Hansen, P.C.; Hesselink, L. Dielectrophoresis-assisted plasmonic trapping of dielectric nanoparticles. Phys. Rev. A 2017, 95, 023840. [Google Scholar] [CrossRef]
- McCanna, J.P.; Sonnenberg, A.; Heller, M.J. Low level epifluorescent detection of nanoparticles and DNA on dielectrophoretic microarrays. J. Biophotonics 2013, 7, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Knigge, X.; Wenger, C.; Bier, F.F.; Hölzel, R. Dielectrophoretic immobilisation of nanoparticles as isolated singles in regular arrays. J. Phys. D Appl. Phys. 2018, 51, 065308. [Google Scholar] [CrossRef]
- Lorenz, M.; Malangré, D.; Du, F.; Baune, M.; Thöming, J.; Pesch, G.R. High-throughput dielectrophoretic filtration of sub-micron and micro particles in macroscopic porous materials. Anal. Bioanal. Chem. 2020, 412, 3903–3914. [Google Scholar] [CrossRef]
- Chai, Z.; Yilmaz, C.; Busnaina, A.A.; Lissandrello, C.A.; Carter, D.J. Directed assembly-based printing of homogeneous and hybrid nanorods using dielectrophoresis. Nanotechnology 2017, 28, 475303. [Google Scholar] [CrossRef]
- Yang, S.-M.; Yao, H.; Zhang, D.; Li, W.J.; Kung, H.-F.; Chen, S.-C. Droplet-based dielectrophoresis device for on-chip nanomedicine fabrication and improved gene delivery efficiency. Microfluid. Nanofluidics 2015, 19, 235–243. [Google Scholar] [CrossRef]
- Cheng, I.-F.; Chen, T.-Y.; Lu, R.-J.; Wu, H.-W. Rapid identification of bacteria utilizing amplified dielectrophoretic force-assisted nanoparticle-induced surface-enhanced Raman spectroscopy. Nanoscale Res. Lett. 2014, 9, 324. [Google Scholar] [CrossRef]
- Bakewell, D.J.; Bailey, J.; Holmes, D. Real-time dielectrophoretic signaling and image quantification methods for evaluating electrokinetic properties of nanoparticles. Electrophoresis 2015, 36, 1443–1450. [Google Scholar] [CrossRef]
- Ibsen, S.; Sonnenberg, A.; Schutt, C.; Mukthavaram, R.; Yeh, Y.; Ortac, I.; Manouchehri, S.; Kesari, S.; Esener, S.; Heller, M.J. Recovery of drug delivery nanoparticles from human plasma using an electrokinetic platform technology. Small 2015, 11, 5088–5096. [Google Scholar] [CrossRef]
- Kim, J.; Hwang, K.S.; Lee, S.; Park, J.H.; Shin, H.-J. Selective nanomanipulation of fluorescent polystyrene nano-beads and single quantum dots at gold nanostructures based on the AC-dielectrophoretic force. Nanoscale 2015, 7, 20277–20283. [Google Scholar] [CrossRef]
- Yang, C.; Wu, C.-J.; Ostafin, A.E.; Thibaudeau, G.; Minerick, A.R. Size and medium conductivity dependence on dielectrophoretic behaviors of gas core poly-l-lysine shell nanoparticles. Electrophoresis 2015, 36, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Dimaki, M.; Olsen, M.H.; Rozlosnik, N.; Svendsen, W.E. Sub–100 nm Nanoparticle Upconcentration in Flow by Dielectrophoretic Forces. Micromachines 2022, 13, 866. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Tsutsui, M.; Theodore, H.; Yuhui, H.; Arima, A.; Tsuji, T.; Doi, K.; Kawano, S.; Taniguchi, M.; Kawai, T. Tailoring particle translocation via dielectrophoresis in pore channels. Sci. Rep. 2016, 6, 31670. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, J.; Chen, D.; Shen, G. Flexible electronics based on inorganic nanowires. Chem. Soc. Rev. 2015, 44, 161–192. [Google Scholar] [CrossRef]
- Hochbaum, A.I.; Yang, P. Semiconductor Nanowires for Energy Conversion. Chem. Rev. 2010, 110, 527–546. [Google Scholar] [CrossRef]
- Huang, Y.; Duan, X.; Lieber, C.M. Nanowires for Integrated Multicolor Nanophotonics. Small 2005, 1, 142–147. [Google Scholar] [CrossRef]
- Kataoka, R.; Tokita, H.; Uchida, S.; Sano, R.; Nishikawa, H. Frequency dependence and assembly characteristics of silver nanomaterials trapped by dielectrophoresis. J. Phys. Conf. Ser. 2015, 646, 012005. [Google Scholar] [CrossRef]
- Constantinou, M.; Hoettges, K.F.; Krylyuk, S.; Katz, M.B.; Davydov, A.; Rigas, G.-P.; Stolojan, V.; Hughes, M.P.; Shkunov, M. Rapid determination of nanowires electrical properties using a dielectrophoresis-well based system. Appl. Phys. Lett. 2017, 110, 133103. [Google Scholar] [CrossRef]
- Tao, Q.; Jiang, M.; Li, G. Simulation and Experimental Study of Nanowire Assembly by Dielectrophoresis. IEEE Trans. Nanotechnol. 2014, 13, 517–526. [Google Scholar] [CrossRef]
- Abdulhameed, A.; Abdul Halin, I.; Mohtar, M.N.; Hamidon, M.N. The role of medium on the assembly of carbon nanotube by dielectrophoresis. J. Dispers. Sci. Technol. 2020, 41, 1576–1587. [Google Scholar] [CrossRef]
- Duchamp, M.; Lee, K.; Dwir, B.; Seo, J.W.; Kapon, E.; Forró, L.; Magrez, A. Controlled Positioning of Carbon Nanotubes by Dielectrophoresis: Insights into the Solvent and Substrate Role. ACS Nano 2010, 4, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Niroui, F.; Lang, J.H.; Bulović, V. Scalable Self-Limiting Dielectrophoretic Trapping for Site-Selective Assembly of Nanoparticles. Nano Lett. 2022, 22, 8258–8265. [Google Scholar] [CrossRef]
- Chen, Y.; Ding, X.; Steven Lin, S.-C.; Yang, S.; Huang, P.-H.; Nama, N.; Zhao, Y.; Nawaz, A.A.; Guo, F.; Wang, W. Tunable nanowire patterning using standing surface acoustic waves. ACS Nano 2013, 7, 3306–3314. [Google Scholar] [CrossRef]
- Guo, J.; Gallegos, J.J.; Tom, A.R.; Fan, D. Electric-field-guided precision manipulation of catalytic nanomotors for cargo delivery and powering nanoelectromechanical devices. ACS Nano 2018, 12, 1179–1187. [Google Scholar] [CrossRef]
- Venkatesh, R.; Kundu, S.; Pradhan, A.; Sai, T.P.; Ghosh, A.; Ravishankar, N. Directed assembly of ultrathin gold nanowires over large area by dielectrophoresis. Langmuir 2015, 31, 9246–9252. [Google Scholar] [CrossRef]
- Freer, E.M.; Grachev, O.; Duan, X.; Martin, S.; Stumbo, D.P. High-yield self-limiting single-nanowire assembly with dielectrophoresis. Nat. Nanotechnol. 2010, 5, 525–530. [Google Scholar] [CrossRef]
- Collet, M.; Salomon, S.; Klein, N.Y.; Seichepine, F.; Vieu, C.; Nicu, L.; Larrieu, G. Large-Scale Assembly of Single Nanowires through Capillary-Assisted Dielectrophoresis. Adv. Mater. 2015, 27, 1268–1273. [Google Scholar] [CrossRef]
- Wang, X.; Chen, K.; Liu, L.; Xiang, N.; Ni, Z. Dielectrophoresis-based multi-step nanowire assembly on a flexible superstrate. Nanotechnology 2017, 29, 025301. [Google Scholar] [CrossRef]
- Kim, K.; Zhu, F.Q.; Fan, D. Innovative Mechanisms for Precision Assembly and Actuation of Arrays of Nanowire Oscillators. ACS Nano 2013, 7, 3476–3483. [Google Scholar] [CrossRef]
- Constantinou, M.; Rigas, G.P.; Castro, F.A.; Stolojan, V.; Hoettges, K.F.; Hughes, M.P.; Adkins, E.; Korgel, B.A.; Shkunov, M. Simultaneous Tunable Selection and Self-Assembly of Si Nanowires from Heterogeneous Feedstock. ACS Nano 2016, 10, 4384–4394. [Google Scholar] [CrossRef]
- Wu, J.; Yin, B.; Wu, F.; Myung, Y.; Banerjee, P. Charge transport in single CuO nanowires. Appl. Phys. Lett. 2014, 105, 183506. [Google Scholar] [CrossRef]
- Maijenburg, A.W.; Maas, M.G.; Rodijk, E.J.B.; Ahmed, W.; Kooij, E.S.; Carlen, E.T.; Blank, D.H.A.; ten Elshof, J.E. Dielectrophoretic alignment of metal and metal oxide nanowires and nanotubes: A universal set of parameters for bridging prepatterned microelectrodes. J. Colloid Interface Sci. 2011, 355, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Boehm, S.J.; Lin, L.; Brljak, N.; Famularo, N.R.; Mayer, T.S.; Keating, C.D. Reconfigurable Positioning of Vertically-Oriented Nanowires Around Topographical Features in an AC Electric Field. Langmuir 2017, 33, 10898–10906. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, K.; Huang, D.; Wang, X.; Xiang, N.; Ni, Z. A novel ‘leadless’ dielectrophoresis chip with dot matrix electrodes for patterning nanowires. Nanotechnology 2017, 28, 285302. [Google Scholar] [CrossRef]
- Seichepine, F.; Rothe, J.; Dudina, A.; Hierlemann, A.; Frey, U. Dielectrophoresis-Assisted Integration of 1024 Carbon Nanotube Sensors into a CMOS Microsystem. Adv. Mater. 2017, 29, 1606852. [Google Scholar] [CrossRef]
- Suehiro, J. Fabrication and characterization of nanomaterial-based sensors using dielectrophoresis. Biomicrofluidics 2010, 4, 022804. [Google Scholar] [CrossRef]
- Kumar, S.; Peng, Z.; Shin, H.; Wang, Z.L.; Hesketh, P.J. Ac Dielectrophoresis of Tin Oxide Nanobelts Suspended in Ethanol: Manipulation and Visualization. Anal. Chem. 2010, 82, 2204–2212. [Google Scholar] [CrossRef]
- Leiterer, C.; Broenstrup, G.; Jahr, N.; Urban, M.; Arnold, C.; Christiansen, S.; Fritzsche, W. Applying contact to individual silicon nanowires using a dielectrophoresis (DEP)-based technique. J. Nanoparticle Res. 2013, 15, 1628. [Google Scholar] [CrossRef]
- Wang, J.; Maier, S.A.; Tittl, A. Trends in Nanophotonics-Enabled Optofluidic Biosensors. Adv. Opt. Mater. 2022, 10, 2102366. [Google Scholar] [CrossRef]
- Willner, M.R.; Vikesland, P.J. Nanomaterial enabled sensors for environmental contaminants. J. Nanobiotechnology 2018, 16, 95. [Google Scholar] [CrossRef]
- Fang, J.; Zhou, Z.; Xiao, M.; Lou, Z.; Wei, Z.; Shen, G. Recent advances in low-dimensional semiconductor nanomaterials and their applications in high-performance photodetectors. InfoMat 2020, 2, 291–317. [Google Scholar] [CrossRef]
- Chang, H.; Wu, H. Graphene-Based Nanomaterials: Synthesis, Properties, and Optical and Optoelectronic Applications. Adv. Funct. Mater. 2013, 23, 1984–1997. [Google Scholar] [CrossRef]
- Wang, J.; Rathi, S.; Singh, B.; Lee, I.; Joh, H.-I.; Kim, G.-H. Alternating current dielectrophoresis optimization of Pt-decorated graphene oxide nanostructures for proficient hydrogen gas sensor. ACS Appl. Mater. Interfaces 2015, 7, 13768–13775. [Google Scholar] [CrossRef]
- Peng, Y.; Ye, J.; Zheng, L.; Zou, K. The hydrogen sensing properties of Pt–Pd/reduced graphene oxide based sensor under different operating conditions. RSC Adv. 2016, 6, 24880–24888. [Google Scholar] [CrossRef]
- Jung, S.-H.; Chen, C.; Cha, S.-H.; Yeom, B.; Bahng, J.H.; Srivastava, S.; Zhu, J.; Yang, M.; Liu, S.; Kotov, N.A. Spontaneous Self-Organization Enables Dielectrophoresis of Small Nanoparticles and Formation of Photoconductive Microbridges. J. Am. Chem. Soc. 2011, 133, 10688–10691. [Google Scholar] [CrossRef]
- Eo, Y.J.; Yoo, G.Y.; Kang, H.; Lee, Y.K.; Kim, C.S.; Oh, J.H.; Lee, K.N.; Kim, W.; Do, Y.R. Enhanced DC-Operated Electroluminescence of Forwardly Aligned p/MQW/n InGaN Nanorod LEDs via DC Offset-AC Dielectrophoresis. ACS Appl. Mater. Interfaces 2017, 9, 37912–37920. [Google Scholar] [CrossRef]
- Budiman, F.; Kotooka, T.; Horibe, Y.; Eguchi, M.; Tanaka, H. Electric property measurement of free-standing SrTiO3 nanoparticles assembled by dielectrophoresis. Jpn. J. Appl. Phys. 2018, 57, 06HE07. [Google Scholar] [CrossRef]
- Park, H.K.; Yoon, S.W.; Eo, Y.J.; Chung, W.W.; Yoo, G.Y.; Oh, J.H.; Lee, K.N.; Kim, W.; Do, Y.R. Horizontally assembled green InGaN nanorod LEDs: Scalable polarized surface emitting LEDs using electric-field assisted assembly. Sci. Rep. 2016, 6, 28312. [Google Scholar] [CrossRef]
- Yan, W.; Mechau, N.; Hahn, H.; Krupke, R. Ultraviolet photodetector arrays assembled by dielectrophoresis of ZnO nanoparticles. Nanotechnology 2010, 21, 115501. [Google Scholar] [CrossRef]
- Slopek, R.P.; Gilchrist, J.F. Self-assembly of wires in acrylate monomer via nanoparticle dielectrophoresis. J. Phys. D Appl. Phys. 2010, 43, 045402. [Google Scholar] [CrossRef]
- Yang, B.; Yang, Z.; Zhao, Z.; Hu, Y.; Li, J. The assembly of carbon nanotubes by dielectrophoresis: Insights into the dielectrophoretic nanotube–nanotube interactions. Phys. E Low-Dimens. Syst. Nanostructures 2014, 56, 117–122. [Google Scholar] [CrossRef]
- Olariu, M.; Arcire, A.; Plonska-Brzezinska, M.E. Controlled trapping of onion-like carbon (OLC) via dielectrophoresis. J. Electron. Mater. 2017, 46, 443–450. [Google Scholar] [CrossRef]
- Chrimes, A.; Kayani, A.; Khoshmanesh, K.; Kalantar-zadeh, K. Dielectrophoresis-Raman Spectroscopy System for Analysing Suspended WO3 Nanoparticles. In Proceedings of the SPIE Defense, Security, and Sensing, Orlando, FL, USA, 25–29 April 2011; SPIE: Bellingham, WA, USA, 2011; Volume 8031. [Google Scholar]
- Kayani, A.A.; Chrimes, A.F.; Khoshmanesh, K.; Sivan, V.; Zeller, E.; Kalantar-zadeh, K.; Mitchell, A. Interaction of guided light in rib polymer waveguides with dielectrophoretically controlled nanoparticles. Microfluid. Nanofluidics 2011, 11, 93–104. [Google Scholar] [CrossRef]
- Liu, C.; Kim, K.; Fan, D. Location deterministic biosensing from quantum-dot-nanowire assemblies. Appl. Phys. Lett. 2014, 105, 083123. [Google Scholar] [CrossRef]
- Riahifar, R.; Marzbanrad, E.; Raissi, B.; Zamani, C.; Kazemzad, M.; Aghaei, A. Sorting ZnO particles of different shapes with low frequency AC electric fields. Mater. Lett. 2011, 65, 632–635. [Google Scholar] [CrossRef]
- Hong, S.H.; Shen, T.Z.; Lee, B.; Song, J.K. Dielectrophoretic condensation and tailored phase separation in graphene oxide liquid crystals. Part. Part. Syst. Charact. 2017, 34, 1600344. [Google Scholar] [CrossRef]
- Mathew, B.; Alazzam, A.; Khashan, S.; Abutayeh, M. Lab-on-chip for liquid biopsy (LoC-LB) based on dielectrophoresis. Talanta 2017, 164, 608–611. [Google Scholar] [CrossRef]
- Pakhira, W.; Kumar, R.; Ibrahimi, K.M.; Bhattacharjee, R. Design and analysis of a microfluidic lab-on-chip utilizing dielectrophoresis mechanism for medical diagnosis and liquid biopsy. J. Braz. Soc. Mech. Sci. Eng. 2022, 44, 482. [Google Scholar] [CrossRef]
- di Toma, A.; Brunetti, G.; Chiriacò, M.S.; Ferrara, F.; Ciminelli, C. A Novel Hybrid Platform for Live/Dead Bacteria Accurate Sorting by On-Chip DEP Device. Int. J. Mol. Sci. 2023, 24, 7077. [Google Scholar] [CrossRef]
- Thomas, D.E.; Kinskie, K.S.; Brown, K.M.; Flanagan, L.A.; Davalos, R.V.; Hyler, A.R. Dielectrophoretic Microfluidic Designs for Precision Cell Enrichments and Highly Viable Label-Free Bacteria Recovery from Blood. Micromachines 2025, 16, 236. [Google Scholar] [CrossRef]
- Hölzel, R.; Pethig, R. Protein dielectrophoresis: Key dielectric parameters and evolving theory. Electrophoresis 2021, 42, 513–538. [Google Scholar] [CrossRef] [PubMed]
- Nakano, A.; Ros, A. Protein dielectrophoresis: Advances, challenges, and applications. Electrophoresis 2013, 34, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Pethig, R.R. Dielectrophoresis: Theory, Methodology and Biological Applications; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Lin, Y.-Y.; Lo, Y.-J.; Lei, U. Measurement of the Imaginary Part of the Clausius-Mossotti Factor of Particle/Cell via Dual Frequency Electrorotation. Micromachines 2020, 11, 329. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Ding, Z.; Suehiro, J. Frequency-dependent conductance change of dielectrophoretic-trapped DNA-labeled microbeads and its application in DNA size determinations. Microfluid. Nanofluidics 2018, 22, 26. [Google Scholar] [CrossRef]
- Sherif, S.; Ghallab, Y.H.; El-Wakad, M.T.; Ismail, Y. A Novel Stimulation and impedance sensing Setup for Dielectrophoresis based Microfluidic Platform. Alex. Eng. J. 2023, 65, 189–207. [Google Scholar] [CrossRef]
- Oladokun, R.; Adekanmbi, E.O.; An, V.; Gangavaram, I.; Srivastava, S.K. Dielectrophoretic profiling of erythrocytes to study the impacts of metabolic stress, temperature, and storage duration utilizing a point-and-planar microdevice. Sci. Rep. 2023, 13, 17281. [Google Scholar] [CrossRef]
- Wu, J.; Fang, H.; Zhang, J.; Yan, S. Modular microfluidics for life sciences. J. Nanobiotechnology 2023, 21, 85. [Google Scholar] [CrossRef]
- Martinez-Duarte, R.; Mager, D.; Korvink, J.G.; Islam, M. Evaluating carbon-electrode dielectrophoresis under the ASSURED criteria. Front. Med. Technol. 2022, 4, 922737. [Google Scholar] [CrossRef]
- Wu, M.; Liu, Z.; Gao, Y. Design and Fabrication of Microelectrodes for Dielectrophoresis and Electroosmosis in Microsystems for Bio-Applications. Micromachines 2025, 16, 190. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Y.; Wang, D.; He, F.; Rotello, V.M.; Carter, K.R.; Watkins, J.J.; Nugen, S.R. UV-nanoimprint lithography as a tool to develop flexible microfluidic devices for electrochemical detection. Lab A Chip 2015, 15, 3086–3094. [Google Scholar] [CrossRef]
- Huang, X.; Liang, F.; Huang, B.; Luo, H.; Shi, J.; Wang, L.; Peng, J.; Chen, Y. On-chip real-time impedance monitoring of hiPSC-derived and artificial basement membrane-supported endothelium. Biosens. Bioelectron. 2023, 235, 115324. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Peng, R.; Li, D. Separation of nanoparticles by a nano-orifice based DC-dielectrophoresis method in a pressure-driven flow. Nanoscale 2016, 8, 18945–18955. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Mishra, R.; Kim, T. Review of micro/nanofluidic particle separation mechanisms: Toward combined multiple physical fields for nanoparticles. Sens. Actuators A Phys. 2023, 363, 114688. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, J.; Luo, Y. A Microfluidic Device for Nano-scale Extracellular Vesicles Differentiation via the Synergetic Effect of Deterministic Lateral Displacement and Dielectrophoresis. In Proceedings of the 2023 IEEE 18th International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Jeju Island, South Korea, 14–17 May 2023; pp. 174–177. [Google Scholar]
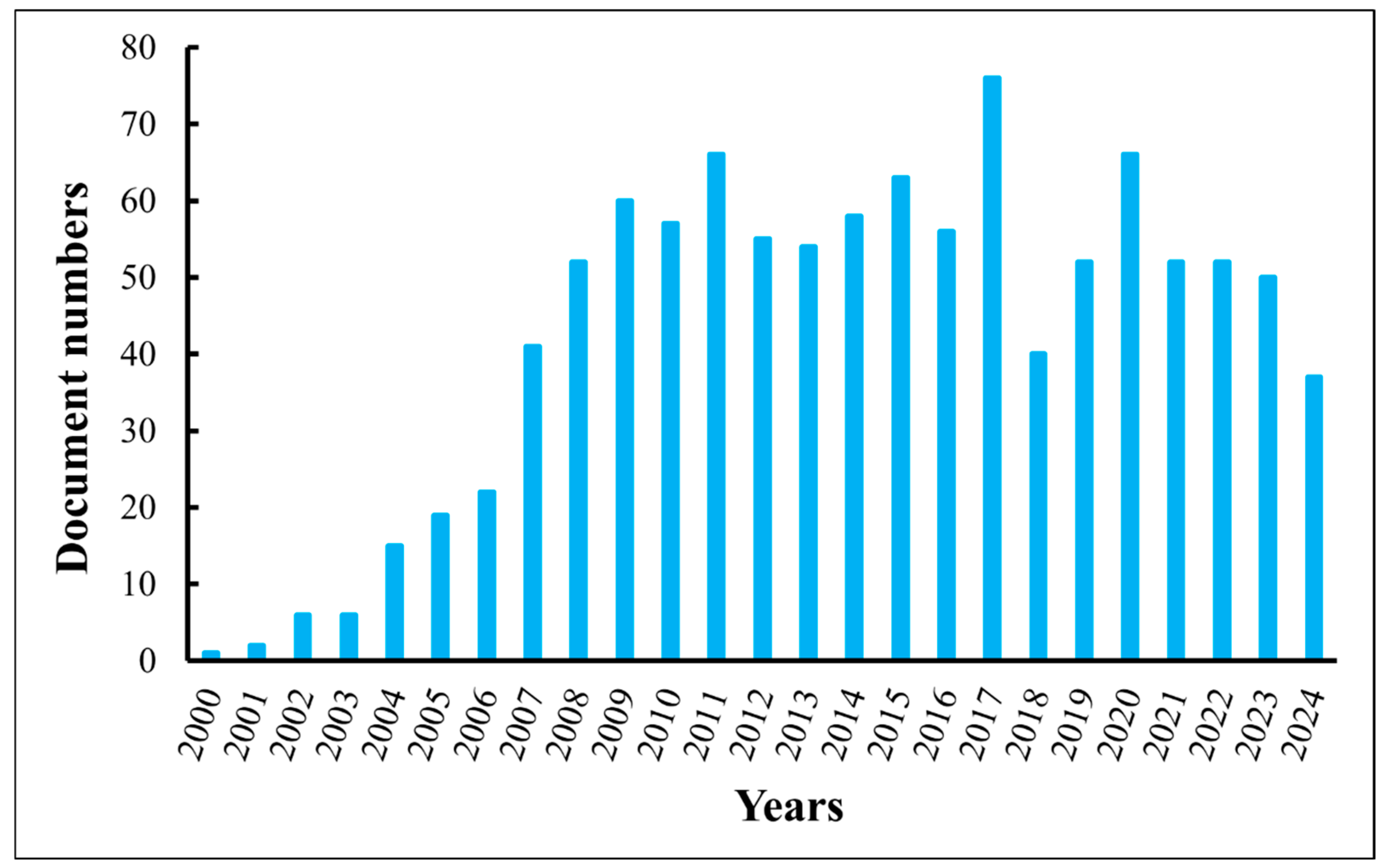
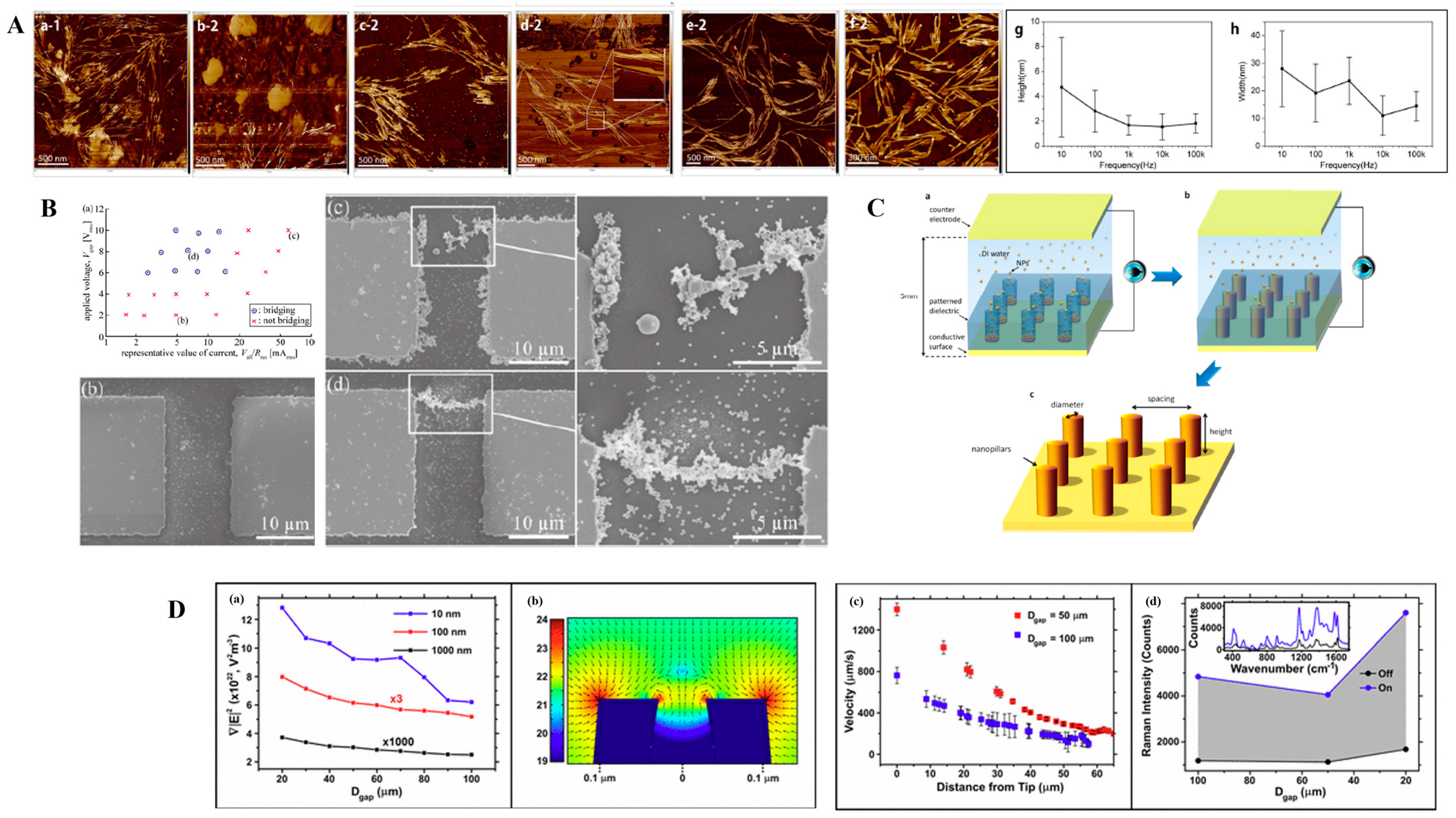
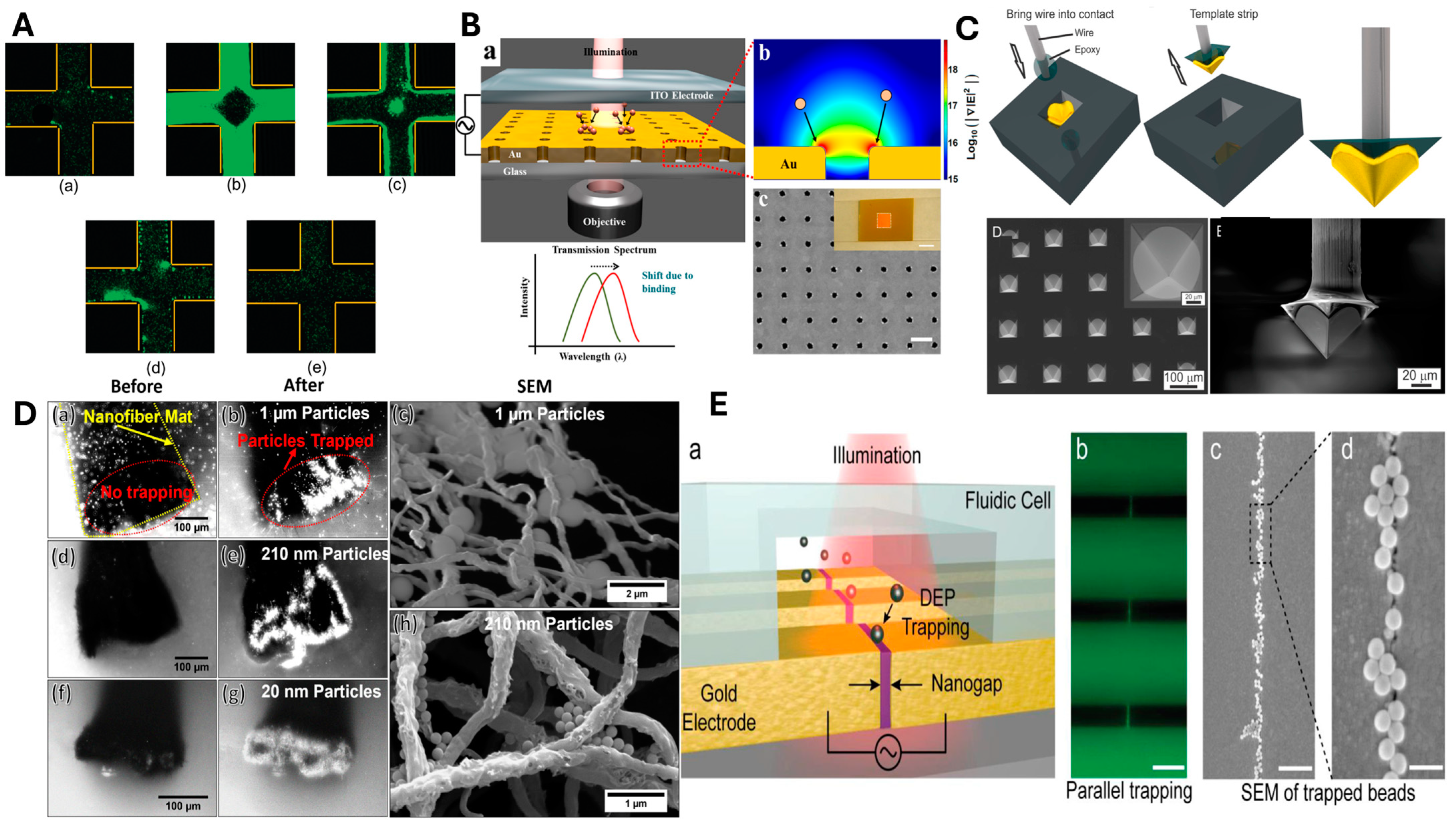
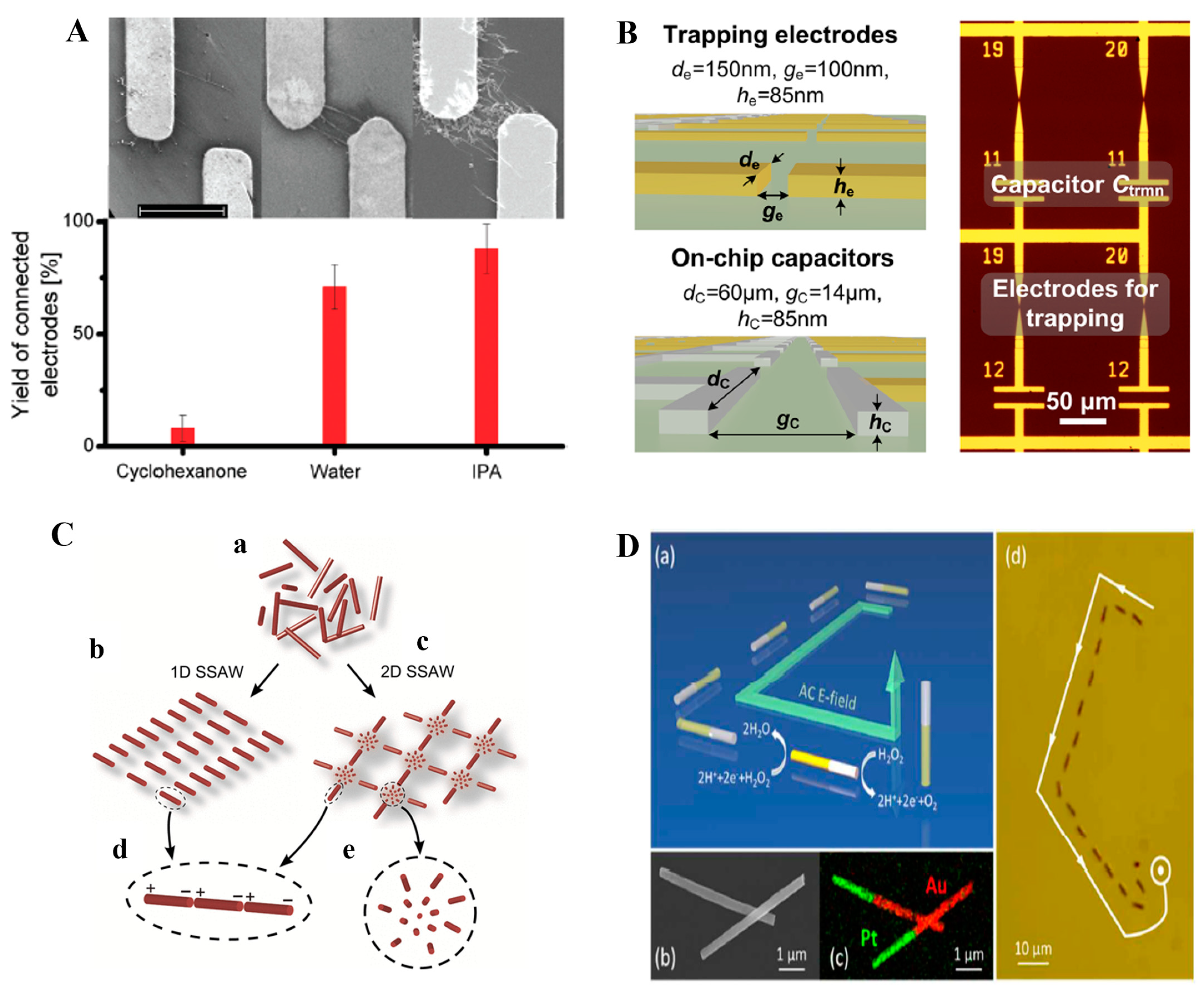
| Particle Size (nm) | Gradient of Field-Squared (V2/m3) |
|---|---|
| 10 | 1.5 × 1018 |
| 100 | 4.8 × 1015 |
| 1000 | 1.5 × 1013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondal, T.K.; Bangaru, A.V.B.; Williams, S.J. A Review on AC-Dielectrophoresis of Nanoparticles. Micromachines 2025, 16, 453. https://doi.org/10.3390/mi16040453
Mondal TK, Bangaru AVB, Williams SJ. A Review on AC-Dielectrophoresis of Nanoparticles. Micromachines. 2025; 16(4):453. https://doi.org/10.3390/mi16040453
Chicago/Turabian StyleMondal, Tonoy K., Aaditya V. B. Bangaru, and Stuart J. Williams. 2025. "A Review on AC-Dielectrophoresis of Nanoparticles" Micromachines 16, no. 4: 453. https://doi.org/10.3390/mi16040453
APA StyleMondal, T. K., Bangaru, A. V. B., & Williams, S. J. (2025). A Review on AC-Dielectrophoresis of Nanoparticles. Micromachines, 16(4), 453. https://doi.org/10.3390/mi16040453







