1. Introduction
Scaffolds have been a key element in tissue engineering and regenerative medicine as regenerative templates from the very beginning. Scaffold-based tissue engineering strategies offer a rapid opportunity for clinical transplantation of tissue engineering under the current medical device approval policy. However, after 40 years of tissue engineering research, there are still great technical barriers to the clinical translation of the scaffold concept, except for some simple tissue regeneration [
1]. As an ideal scaffold for bioartificial tissue regeneration, the scaffold should simulate the geometric structure and biophysical and biochemical properties of the extracellular matrix (ECM) in vivo and have complex physical and chemical structural information [
2]. Hollister summarized biomanufacturing and proposed the 4F principles for scaffold design and manufacturing: form, fixation, function, and formation [
3]. Due to the requirement for multiple interconnected properties, the manufacturing of scaffolds that meet all properties has not yet been successful.
Biological materials for tissue engineering scaffolds can generally be divided into two major categories: natural polymers and synthetic polymers [
4]. Natural biopolymers have good cell compatibility but poor mechanical properties. Collagen, the main structural protein of the extracellular matrix (ECM) in vertebrate connective tissue, has good biocompatibility and is the preferred material for current tissue engineering scaffolds, with type I collagen being the most commonly used. Many collagen-based products have been applied in clinical surgery. In addition to simple collagen medical products, obtaining collagen scaffolds with controllable structures and excellent mechanical properties through bioprinting has always been considered a promising method for clinical application. However, it is difficult to meet the mechanical requirements of scaffolds using only collagen materials, so the composite manufacturing of collagen and synthetic polymers is considered. The printing technology for single synthetic polymers has been mature, but the 3D printing of collagen and the mixed printing of collagen and synthetic polymers have not been well achieved yet.
The printing of collagen–synthetic polymer hybrid scaffolds mainly faces two problems. First, pure collagen has poor printability. Although methods such as low-temperature printing [
5], embedded printing [
6,
7], or doping with thickeners (such as alginate [
8], hyaluronic acid [
9], etc.) can improve it, these methods all have some problems, such as nozzle clogging, filament bonding issues, and reduced biocompatibility. A Lee [
10] used the embedded printing method and successfully printed collagen heart scaffolds with lower concentrations in a gelatin microsphere support bath. The support bath can melt at 37 degrees, ensuring the structural integrity of the scaffold. However, this method is not suitable for multi-material printing, especially the printing of collagen and synthetic polymers together, and it is difficult to complete the formation of hybrid scaffolds. Another problem is that it is difficult to meet the preparation and printing conditions for both collagen and synthetic polymers (such as temperature, cross-linking conditions, etc.) at the same time. The common method is to print porous polymer scaffolds first and then cast collagen to form the scaffold [
11]. However, these scaffolds often fail to meet the optimal pore conditions, which is not conducive to cell proliferation and differentiation. YoungWon Koo [
12] used temperature-controlled technology to prepare collagen/PCL hybrid scaffolds. However, this method could only retain the formation of interpenetrating pores in PCL single-material printing. When preparing hybrid scaffolds, collagen/PCL would collapse, losing the characteristic of lateral pores.
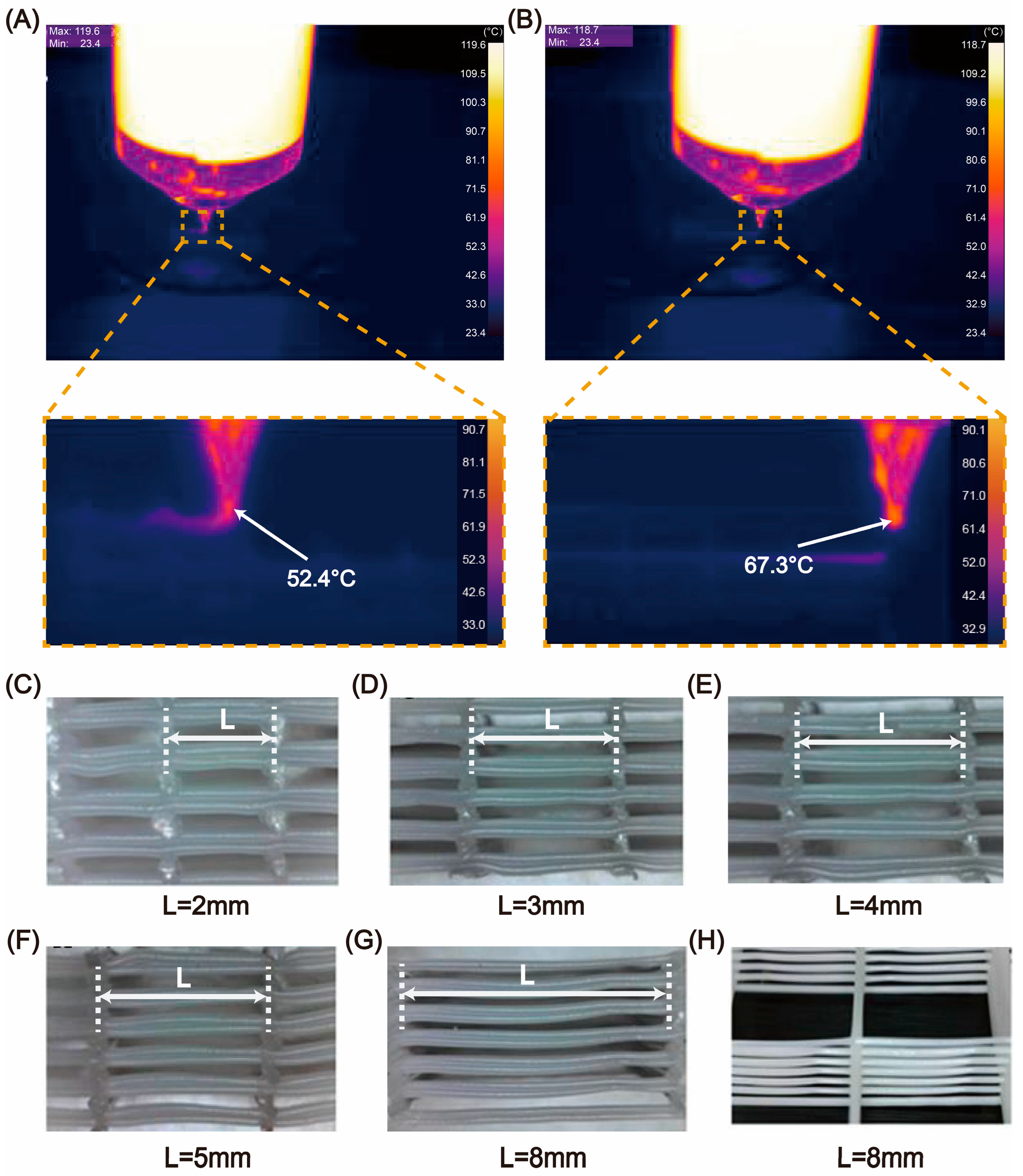
In the clinical application of tissue engineering scaffolds, various factors affecting the scaffold’s functionality should be considered comprehensively. This study developed a new printing technology. Through rheological analysis, we identified the collagen preparation conditions suitable for room-temperature printing in the air. Using a precise temperature-control system, we can complete the large-span printing of PCL and prepare collagen-synthetic polymer hybrid scaffolds with interconnected pores. Moreover, by adjusting the ratio of collagen and PCL, the mechanical properties of the hybrid scaffold can be regulated to meet different tissue regeneration needs. Additionally, we used fibroblasts and bone marrow mesenchymal stem cells as experimental cells for soft tissue and hard tissue regeneration, respectively, to assess the biocompatibility and mechanical properties of the hybrid scaffolds. Based on these results, the proposed hybrid scaffold printing method holds great clinical application value in tissue regeneration.
2. Materials and Methods
2.1. Preparation and Rheology of Bioinks
Type I collagen molecules were extracted from bovine tendons using a classical procedure. The brief process is as follows: Fresh tendons were cut into small pieces and soaked in a 0.8% sodium carbonate solution for 12 h to remove fat. After being washed multiple times with deionized water (dH2O), the tendon pieces were dissolved in a solution containing 0.2 M acetic acid and 0.25% (w/v) pepsin and reacted under stirring for 84 h. The crude solution was centrifuged, and the supernatant was selectively precipitated with 1 M NaCl. The precipitated type I collagen was dissolved in 0.2 M acetic acid, desalted by dialysis against 0.2 M acetic acid, and adjusted to the final concentration by dialysis against polyethylene glycol. During dialysis, dH2Oshould be changed every 24 h, and the pH of dH2Oshould be monitored with a pH meter. Dialysis was terminated when the pH reached the desired value. The solution then was lyophilized for 1 week to generate a white porous foam and stored at 4 °C. A predetermined mass of collagen foam was weighed and dissolved in phosphate buffer saline (PBS) to prepare collagen solutions at varying concentrations before printing.
Medical-grade polycaprolactone (PCL) with an average molecular weight of 15,000 and an intrinsic viscosity ranging from 1.75 to 2.25 dL/g was purchased from ShenZhen Polymtek Co., Ltd. in Shenzhen, China. PCL pellets were introduced into a planetary ball mill (XQM-2A, purchased from Shanghai, China) and ground for 1 h at a rotational speed of 300 rpm. Following the grinding process, the PCL powder was transferred to a filament extruder (Wellzoom Desktop Filament Extruder B-pro, purchased from Shanghai, China), where it was extruded into PCL rods via a screw mechanism. The PCL rods can be stored at room temperature and, prior to printing, were loaded into the printing chamber and heated to a molten state.
The rheological properties of the collagen solution were measured using an Anton Paar MCR 501 rheometer (Anton Paar GmbH, Ostfildern, Germany). The PP25 parallel plate measuring system was employed, with a gap setting of 0.3 mm and a temperature of 25 °C.
2.2. Preparation of the Hybrid Scaffold
The scaffold was accomplished using a bioprinter (SIA Bioprinter Pro, developed by our research team,
Figure 1). In previous work, we have utilized this printer for the bioprinting of various elastomers and hydrogels [
13,
14,
15,
16]. High-precision extrusion-based printing of collagen and PCL was achieved using an electric linear actuator. The temperature control accuracy of the nozzle and the working platform was within 1 degree. During the printing process, stainless steel needles with diameters of 150 μm, 200 μm, 500 μm, and 600 μm were used. The printing paths for multiple nozzles were generated using Matlab(R2023b) software. To prevent collagen from drying, the printing environment was maintained at 90% relative humidity (RH). After printing, different post-treatment methods were used. For the freeze-drying group, the scaffold was cooled to −80 °C and then freeze-dried using a freeze-dryer (Alpha 1–2 LD plus, Christ, Osterode, Germany).
2.3. Overall Preparation Process of the Hybrid Scaffold
The overall fabrication process of the hybrid scaffold is shown in
Figure 2. Its three basic fabrication stages can be summarized as follows:
2.4. Physicochemical Tests of Scaffold
2.4.1. Macroscopic and Microscopic Structures
The macroscopic and microscopic structures of the scaffold were observed using a scanning electron microscope (SEM). First, the scaffold was treated with genipin and then freeze-dried. A layer of ultra-thin Aurum/Platinum (Au/Pt) was applied to the freeze-dried scaffold for sample preparation and SEM imaging.
2.4.2. Mechanical Testing
Collagen/PCL scaffolds with different ratios were fabricated into a uniform size of 15 × 15 × 5 mm (L × W ×H). Compressive tests were performed using a mechanical analyzer (CT3 texture analyzer, 100 g/1500 g, AMETEK Brookfield, Middleboro, MA, USA).
2.4.3. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS–PAGE)
SDS-PAGE was performed using 6% (w/v) normal collagen prepared in the laboratory and 6% (w/v) collagen from the hybrid scaffold that had been in contact with PCL. The collagen protein was dissolved in a 0.02 M sodium phosphate buffer (pH 7.2) containing 1% (w/v) SDS and 3.5 M urea to obtain a final concentration of 2 mg/mL. It was then mixed with an equal volume of sample buffer (0.5 M Tris-HCl, pH 6.8, containing 4% (w/v) SDS and 20% (v/v) glycerol). Subsequently, 20 μL of the sample (20 μg of protein) was loaded into each well. High-molecular-weight markers were used to estimate the molecular weight of the bands. After gel electrophoresis, the gel was stained with 0.1% (w/v) Coomassie Brilliant Blue R–250 in a solution of 50% (v/v) methanol and 6.8% (v/v) glacial acetic acid for 5 h. It was then destained with a solution of 7.5% (v/v) glacial acetic acid and 5% (v/v) methanol for about 9 h, with the solution changed every 3 h.
2.5. Post-Treatment Methods of the Scaffold
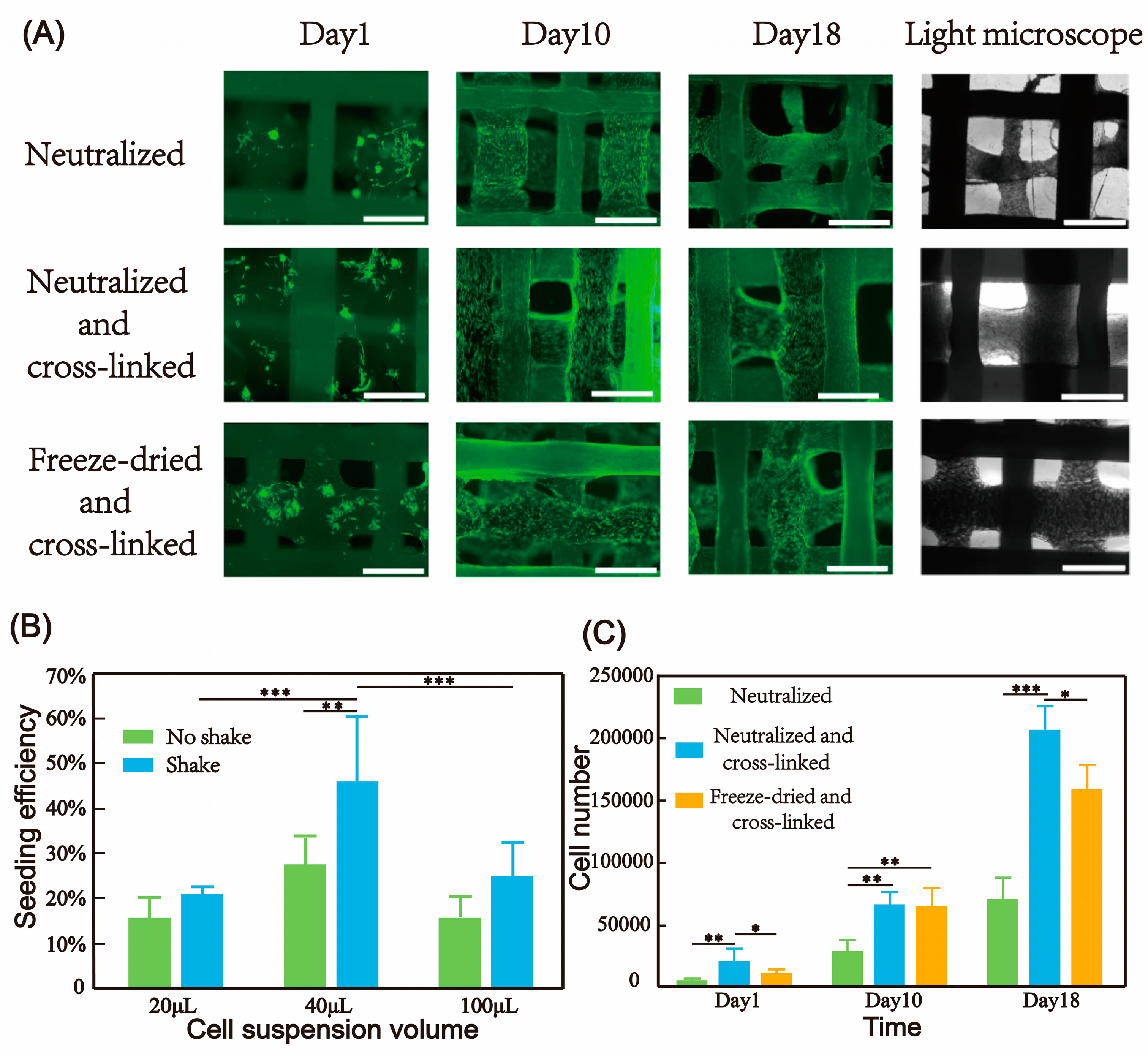
Three methods were used to treat collagen/polycaprolactone hybrid scaffolds. In the first method, after printing, the collagen scaffolds were frozen at −80 °C and then freeze-dried. They were incubated in a 1% (w/v) genipin ethanol solution for 12 h to cross-link the collagen. After washing the scaffolds three times with ethanol and distilled water, they were blocked with a 1% (w/v) glycine solution to neutralize residual cross-linking agents. This group was named the freeze-dried and cross-linked group.
In the second method, 10 × PBS was used to induce fiber formation. The collagen scaffolds were soaked in 10 × PBS at 37 °C for 1 h. Then, the samples were taken out from the 10 × PBS solution and washed three times in 1 × PBS. The samples were stored in 1 × PBS until needed and named the neutralized group.
The third method involved immersing the 10 × PBS-treated scaffold material in a 1% (w/v) genipin solution for 12 h to stabilize the fiber structure and prolong degradation time. After washing the scaffolds three times with PBS, they were blocked with a 1% (w/v) glycine solution to neutralize residual cross-linking agents. This group was named the neutralized and cross-linked group.
2.6. Cell Culture Experiments
2.6.1. Rabbit Bone Mesenchymal Stem Cells (BMSCs)
Rabbit BMSCs were purchased from Cyagen Biosciences, Inc(Suzhou, China). The cells were expanded at 37 °C and 5% CO2 in Dulbecco’s modified eagle’s medium low glucose (DMEM; Gibco, Life Technologies, Shanghai, China) supplemented with 10% fetal calf serum (Gibco, Life Technologies), 100 U mL−1 penicillin, and 100 μg · mL−1 streptomycin (Gibco, Life Technologies). Cells in passage 6 were used for the experiments.
2.6.2. Human Dermal Fibroblasts (FBs)
Human FB cells were purchased from ScienCell Research Laboratories, Inc. (Carlsbad, CA, USA). The cells were expanded at 37 °C and 5% CO2 in Dulbecco’s modified eagle’s medium high glucose (DMEM; Gibco, Life Technologies) supplemented with 10% fetal calf serum (Gibco, Life Technologies), 100 U mL−1 penicillin, and 100 μg · mL−1 streptomycin (Gibco, Life Technologies). Cells in passage 6 were used for the experiments.
2.7. Cell Seeding in Collagen Scaffolds
The produced scaffolds were incubated in cell culture medium for 24 h and thereafter seeded at a certain amount of cells in culture medium in 24-well cell culture plate (Corning 3473 Ultra-Low Attachment, Corning, NY, USA). Osteogenic differentiation of BMSC was induced by addition of 100 nM dexamethasone, 3.5 mM β-glycerophosphate, and 0.05 mM ascorbic acid 2-phosphate (all from Sigma-Aldrich, St. Louis, MO, USA) to the cell culture medium. Induction of osteogenic differentiation was started 7 days after seeding.
2.8. Analysis of Cell Adhesion, Proliferation
Cell proliferation on the scaffolds was evaluated at 1, 10, and 21 days post-seeding. To quantify the attached cells, the scaffolds were treated with 0.25% trypsin-EDTA solution at 37 °C for 10 min to detach the cells. Subsequently, the cell suspension was collected for counting. For qualitative assessment, the samples were incubated with Calcein-AM solution (Life Technologies, Carlsbad, CA, USA) and visualized using a fluorescence microscope (Leica DMI8, Wetzlar, Germany).
2.9. Analysis of BMSC Osteogenesis Differentiation in Hybrid Scaffold
2.9.1. Alizarin Red S Stain
After washing twice with PBS, the samples were immersed in 4% (w/v) paraformaldehyde for 15 min and stained with 1% (w/v) alizarin red S (Sigma) for 20 min to observe under a light microscope.
2.9.2. Von Kossa Stain
After washing twice with PBS, the samples were immersed in 4% (w/v) paraformaldehyde for 15 min and soaked in 5% (w/v) silver nitrate solution under UV light for 10 min. After soaking in sodium thiosulfate for 10 min, scaffolds were observed under a light microscope.
2.9.3. Alkaline Phosphatase (ALP) Staining and Activity Assay
After induction for 21 days, the scaffolds were rinsed three times with PBS for ALP staining via a BCIP/NBT ALP color development kit (Beyotime, Shanghai, China). ALP assay kit (Beyotime, China) was used for quantitative detection of ALP activity. Each sample in 24-well plate was washed with PBS and incubated in 100 μL 1% (w/v) Triton X-100 in PBS for 30 min. A total of 100 μL of substrate solution was added to each well. After incubation at 37 °C for 30 min, the enzymatic reaction was stopped by addition of 1 M NaOH; pnitrophenolate (pNp) formation was quantified by absorbance measurement at 405 nm. The amount of pNp produced by each sample was calculated using ap-nitrophenol calibration line, and ALP activity (μmol pNp/30 min/one scaffold) was defined.
2.10. Real-Time Polymerase Chain Reaction (PCR)
The ExicyclerTM 96 real-time PCR detection system (BIONEER, Daejeon, Republic of Korea) was used to perform RT–PCR experiments. Ribonucleic acid (RNA) was extracted from the printed samples cultured in osteoblastic medium for 21 days. The expression levels of osteogenesis–related genes, including alkaline phosphatase (ALP), bone sialoprotein (BSP), osteocalcin (OCN), and collagen type I alpha 1 (COLLA1), were used to represent the fold changes in target gene expression.
2.11. Statistical Analysis
All data were presented as means ± standard deviations. Statistical significance was determined by analysis of variance with Tukey honest significant difference post hoc as * p < 0.05, ** p < 0.01, and *** p < 0.001.
4. Discussion
In recent years, the progress in the field of tissue engineering has increasingly highlighted the crucial role of scaffolds. However, the issue of how to optimally integrate mechanical properties with biocompatibility remains unresolved. From the perspective of developmental biology, tissues originate from the differentiation and proliferation of stem cells, with their mechanical properties transitioning from a soft to a hardened state. In contrast, in adult tissue regeneration through engineering strategies, the scaffold must match the mechanical properties of the surrounding tissue while providing immediate structural stability and having an appropriate porosity to support cell activity [
21,
22,
23]. In most cases, the mechanical properties required for implantation differ from those needed for stem cell adhesion and differentiation. This approach essentially requires the development of composite materials, where one component ensures the stability of the implant, and the other promotes stem cell adhesion and differentiation. In this study, we chose a synthetic polymer to provide structural stability and collagen as the bioactive component.
Although low-temperature printing methods are widely used for the manufacturing of collagen scaffolds [
24,
25], they typically produce porous structures rather than nano-fiber structures that mimic the natural extracellular matrix. Extensive literature has demonstrated that nano-fiber structures significantly enhance biological functions [
26,
27], which is a major advantage of the collagen printing technology described in this paper. Adjusting the pH value to improve the printability of dialyzed collagen also avoids the issue of collagen fibers becoming too dense due to increased concentration, which can affect cell growth and proliferation. The transition of tissue-engineering scaffolds from research to clinical application is a lengthy process, mainly involving two key aspects: the selection of biomaterials and manufacturing methods. In this study, we used two clinically approved materials and developed a simple and practical manufacturing method. Avoiding the use of organic solvents, eliminating additional additives, and using clinical-grade materials together enhance the potential for clinical translation and accelerate the regulatory approval process.
Based on this method, the scaffold design can be further optimized to achieve mechanical properties and pore structures that are more suitable for specific tissue regeneration needs. In addition, before clinical transplantation, comprehensive and systematic experimental studies should be conducted, including using computer-aided design to accurately determine key scaffold parameters such as degradation kinetics, mechanical properties, and bioactive characteristics. This systematic approach will help optimize scaffold properties to meet specific clinical needs and ensure predictable in vivo performance.
5. Conclusions
In this paper, a versatile multi-materials printing method for hybrid scaffolds was developed, enabling the composite printing of collagen and thermoplastic elastomers at room temperature. The rheological properties and printability of pure collagen solutions were studied, and the impacts of various post-treatment methods on the bioactivity of collagen were explored, leading to the successful printing of collagen scaffolds with porous/nano-fiber morphologies of the extruded filaments. Through the development of PCL large-span printing technology, the hybrid printing of collagen and PCL was achieved. A continuous interconnected porous network was formed between the materials, which facilitated the exchange and permeation of nutrients and ensured uniform cell seeding and activity in subsequent long-term culture. The higher the proportion of collagen in the hybrid scaffold, the more cells were seeded. Depending on different tissue regeneration needs, the mechanical properties and cell seeding number of the hybrid scaffold could be adjusted by changing the ratio of collagen and synthetic polymer. The long-term three-dimensional culture of human fibroblasts on the hybrid scaffold showed that cells initially grew on the collagen fibers, and after about 10 days, cells were also able to cover the entire PCL fibers. BMSCs successfully achieved osteogenic differentiation on the hybrid scaffold, demonstrating the good biocompatibility of the scaffold. The culture of fibroblasts and BMSCs as test cells for soft tissue and hard tissue regeneration, respectively, revealed the great clinical application prospects of the collagen and synthetic polymer hybrid scaffold.