Carbon Materials in Voltammetry: An Overview of Versatile Platforms for Antidepressant Drug Detection
Abstract
1. Introduction
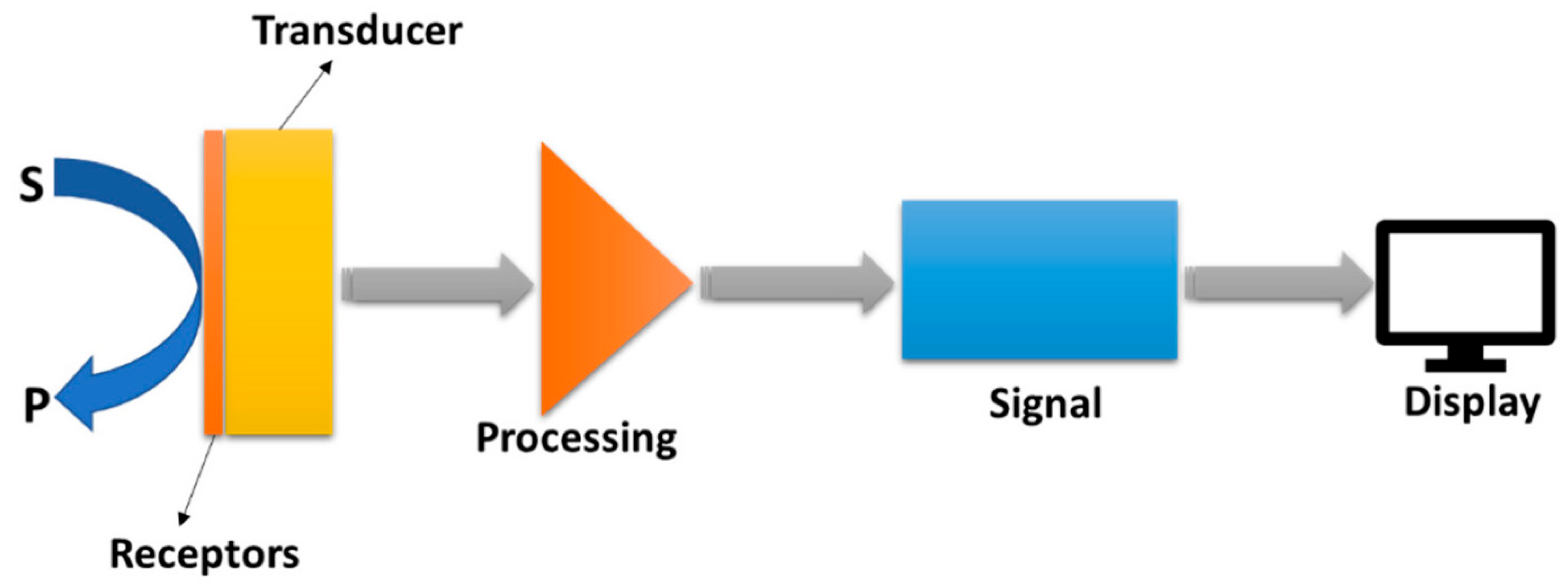
2. Principle of Electrochemical Sensor
3. Carbon-Based Electrochemical Sensors of Antidepressant Drugs
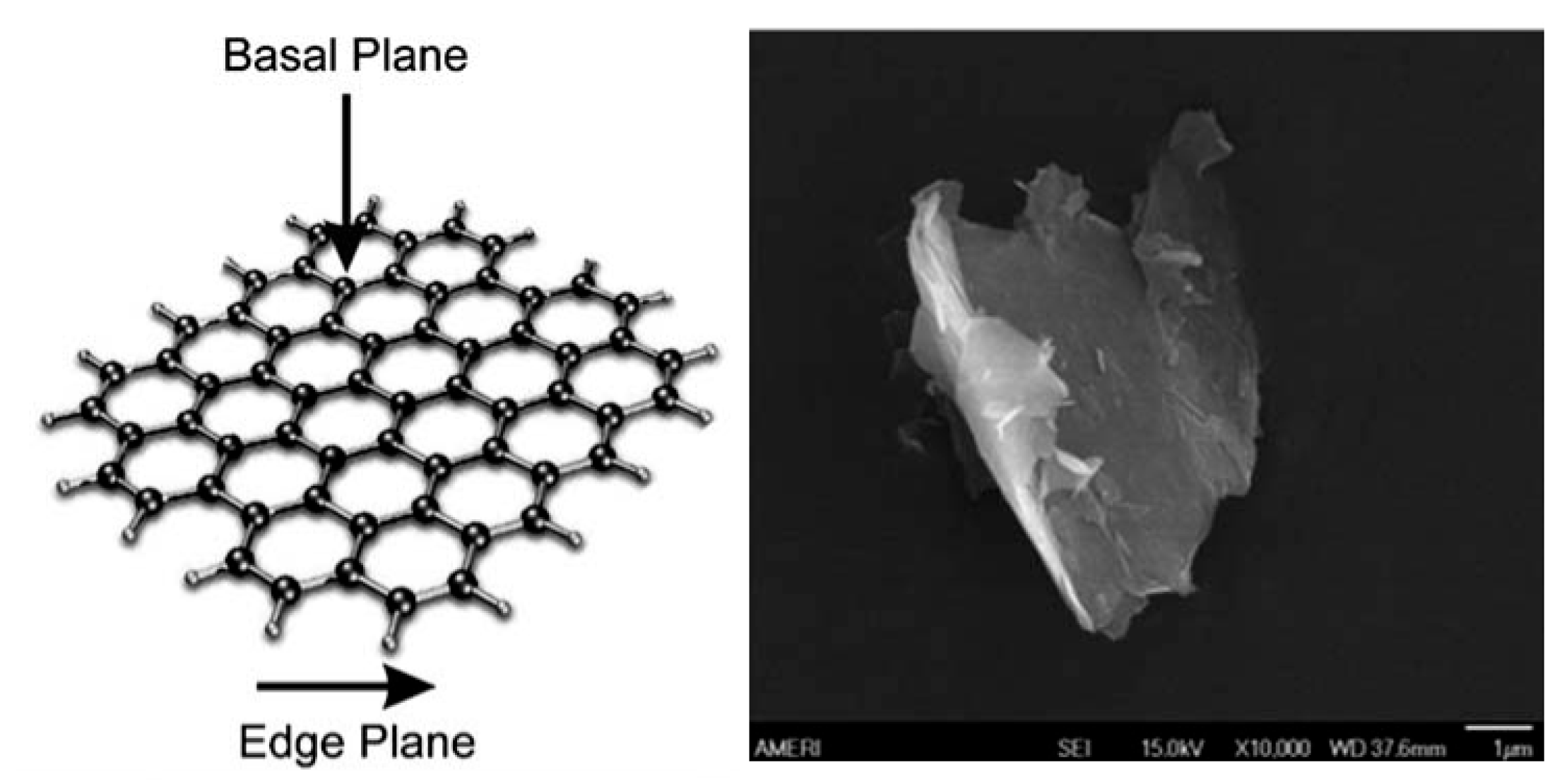
3.1. Glassy Carbon Electrode
3.2. Boron-Doped Diamond Electrode
4. Carbon Nanoparticle (CNP)-Modified Electrodes
4.1. Graphene and Graphene Oxide
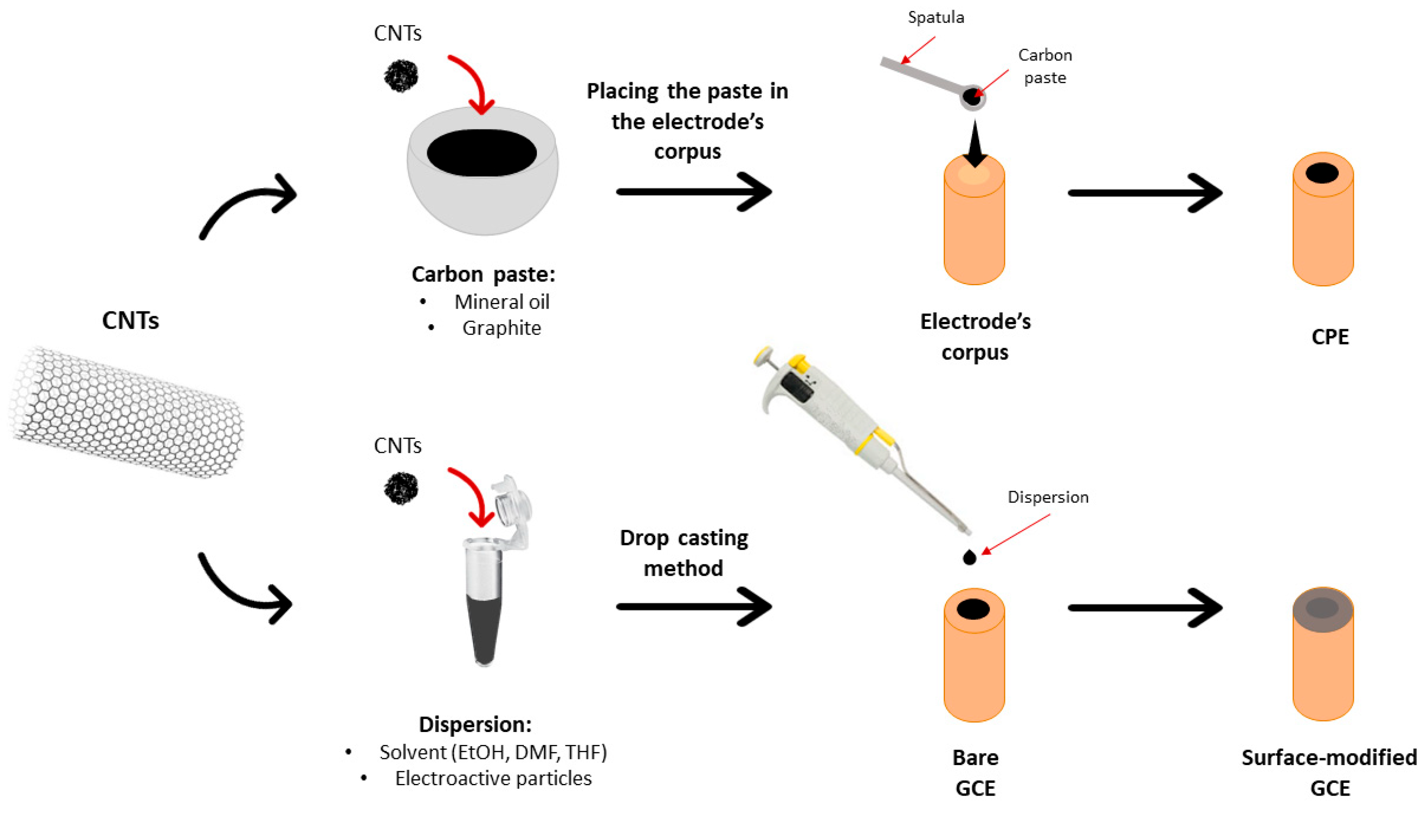
4.2. Carbon Nanotubes
| Electrode | Analyte | Technique | Linear Range | LOD | Sample | Ref. |
|---|---|---|---|---|---|---|
| 1 CNTPE | Amitriptyline | DPV | 0.0–30.0 µmol L−1 | 1.61 µmol L−1 | Pharmaceutical formulation | [94] |
| 2 MWCNTs/C/T/MCPE | Agomelatine | DPV | 3.0 × 10−9–0.5 × 10−6 mol L−1 1.0 × 10−6–5.0 × 10−4 mol L−1 | 5.26 × 10−10 mol L−1 | Tablets, urine | [95] |
| 3 ZnO-MWCNT/CPE | Citalopram | ASWV | 0.012–1.54 µmol L−1 | 0.005 µmol L−1 | Human serum, urine, pharmaceutical | [96] |
| 4 MWCNTs/ZnO-NPs/CPE | Clonazepam | DPV | 0.39–7.70 µg mL−1 | 0.17 µg mL−1 | Drug products, human urine | [97] |
| Desvenlafaxine | 0.66–8.42 µg mL−1 | 0.28 µg mL−1 | Drug products, human urine | |||
| 5 βCD-CNT-PE | Nifedipine | DPAdSV | 4.77 × 10−8–2.00 × 10−5 mol L−1 | 1.48 × 10−8 mol L−1 | Pharmaceutical formulation, biological fluid | [98] |
| 6 CNT/CsMCPE/SDS | Sertaline | SWV | 60 nM–15.0 µM | 9.2 × 10−9 M | Biological fluid | [99] |
4.2.1. Carbon Paste Electrodes Based on CNTs
4.2.2. Glassy Carbon Electrodes Modified with CNTs
4.2.3. Other Sensors Based on CNTs
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Woody, C.A.; Ferrari, A.J.; Siskind, D.J.; Whiteford, H.A.; Harris, M.G. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. J. Affect. Disord. 2017, 219, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Evans-Lacko, S.; Aguilar-Gaxiola, S.; Al-Hamzawi, A.; Alonso, J.; Benjet, C.; Bruffaerts, R.; Chiu, W.T.; Florescu, S.; de Girolamo, G.; Gureje, O.; et al. Socio-economic variations in the mental health treatment gap for people with anxiety, mood, and substance use disorders: Results from the WHO World Mental Health (WMH) surveys. Psychol. Med. 2018, 48, 1560–1571. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.; Campo, J.V. Depression in Adolescents. N. Engl. J. Med. 2021, 385, 445–449. [Google Scholar] [CrossRef]
- Cuijpers, P.; Oud, M.; Karyotaki, E.; Noma, H.; Quero, S.; Cipriani, A.; Arroll, B.; Furukawa, T.A. Psychologic treatment of depression compared with pharmacotherapy and combined treatment in primary care: A network meta-analysis. Ann. Fam. Med. 2021, 19, 262–270. [Google Scholar] [CrossRef]
- Chang, J.P.C.; Zamparelli, A. Antidepressant Drugs: Mechanisms of Action and Side Effects. In Encyclopedia of Behavioral Neuroscience, 2nd ed.; ACM Press: Cambridge, MA, USA, 2021; pp. 613–626. [Google Scholar] [CrossRef]
- Marasine, N.R.; Sankhi, S.; Lamichhane, R.; Marasini, N.R.; Dangi, N.B. Use of Antidepressants among Patients Diagnosed with Depression: A Scoping Review. BioMed Res. Int. 2021, 2021, 6699028. [Google Scholar] [CrossRef]
- Hulanicki, A.; Glab, S.; Ingman, F. Chemical sensors definitions and classification. Pure Appl. Chem. 1991, 63, 1247–1250. [Google Scholar] [CrossRef]
- Fan, Z.H. Chemical Sensors and Microfluidics. J. Biosens. Bioelectron. 2013, 4, 1–2. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Gatto, G.; Broncova, G. Electrochemical Sensors and Their Applications: A Review. Chemosensors 2022, 10, 363. [Google Scholar] [CrossRef]
- Saputra, H.A. Electrochemical sensors: Basic principles; engineering, and state of the art. Monatshefte Fur Chem. 2023, 154, 1083–1100. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R.; Swain, E.; Robey, C. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons. Inc.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Tomac, I.; Adam, V.; Labuda, J. Advanced chemically modified electrodes and platforms in food analysis and monitoring. Food Chem. 2024, 460, 140548. [Google Scholar] [CrossRef]
- Edwards, G.A.; Bergren, A.J.; Porter, M.D. Handbook of Electrochemistry; Elsevier B.V.: New Amsterdam, The Netherlands, 2007. [Google Scholar] [CrossRef]
- Curulli, A. Nanomaterials in electrochemical sensing area: Applications and challenges in food analysis. Molecules 2020, 25, 5759. [Google Scholar] [CrossRef] [PubMed]
- Alsaiari, N.S.; Katubi, K.M.M.; Alzahrani, F.M.; Siddeeg, S.M.; Tahoon, M.A. The application of nanomaterials for the electrochemical detection of antibiotics: A review. Micromachines 2021, 12, 308. [Google Scholar] [CrossRef] [PubMed]
- Barhoum, A.; Hamimed, S.; Slimi, H.; Othmani, A.; Abdel-Haleem, F.M.; Bechelany, M. Modern designs of electrochemical sensor platforms for environmental analyses: Principles, nanofabrication opportunities, and challenges. Trends Environ. Anal. Chem. 2023, 38, e00199. [Google Scholar] [CrossRef]
- Uslu, B.; Ozkan, S.A. Electroanalytical application of carbon based electrodes to the pharmaceuticals. Anal. Lett. 2007, 40, 817–853. [Google Scholar] [CrossRef]
- de Souza Vieira, L. A review on the use of glassy carbon in advanced technological applications. Carbon 2022, 186, 282–302. [Google Scholar] [CrossRef]
- Harris, P.J.F. Fullerene-related structure of commercial glassy carbons. Philos. Mag. 2004, 84, 3159–3167. [Google Scholar] [CrossRef]
- Jurkiewicz, K.; Duber, S.; Fischer, H.E.; Burian, A. Modelling of glass-like carbon structure and its experimental verification by neutron and X-ray diffraction. J. Appl. Crystallogr. 2017, 50, 36–48. [Google Scholar] [CrossRef]
- Uskoković, V. A historical review of glassy carbon: Synthesis, structure, properties and applications. Carbon Trends 2021, 5, 100116. [Google Scholar] [CrossRef]
- Sharma, S. Glassy Carbon: A promising material for microand nanomanufacturing. Materials 2018, 11, 1857. [Google Scholar] [CrossRef]
- Turhan, E.; Uslu, B. Electroanalytical determination of opipramol in pharmaceutical preparations and biological fluids. Anal. Lett. 2008, 41, 2013–2032. [Google Scholar] [CrossRef]
- Merli, D.; Dondi, D.; Ravelli, D.; Tacchini, D.; Profumo, A. Electrochemistry and analytical determination of aripiprazole and octoclothepin at glassy carbon electrode. J. Electroanal. Chem. 2013, 711, 1–7. [Google Scholar] [CrossRef]
- Aşangil, D.; Taşdemir, I.H.; Klç, E. Adsorptive stripping voltammetric methods for determination of aripiprazole. J. Pharm. Anal. 2012, 2, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Madej, M.; Kochana, J.; Baś, B. Selective and Highly Sensitive Voltammetric Determination of Citalopram with Glassy Carbon Electrode. J. Electrochem. Soc. 2019, 166, H359–H369. [Google Scholar] [CrossRef]
- Ozkan, S.A.; Dogan, B.; Uslu, B. Voltammetric analysis of the novel atypical antipsychotic drug quetiapine in human serum and urine. Microchim. Acta 2006, 153, 27–35. [Google Scholar] [CrossRef]
- Muzyka, K.; Sun, J.; Fereja, T.H.; Lan, Y.; Zhang, W.; Xu, G. Boron-doped diamond: Current progress and challenges in view of electroanalytical applications. Anal. Methods 2019, 11, 397–414. [Google Scholar] [CrossRef]
- Einaga, Y. Boron-Doped Diamond Electrodes: Fundamentals for Electrochemical Applications. Acc. Chem. Res. 2022, 55, 3605–3615. [Google Scholar] [CrossRef]
- Macpherson, J.V. A practical guide to using boron doped diamond in electrochemical research. Phys. Chem. Chem. Phys. 2015, 17, 2935–2949. [Google Scholar] [CrossRef]
- Manrique, G.R.P.; Salamanca-Neto, C.A.R.; Moraes, J.T.; Sartori, E.R. Fast surface water quality analysis based on an ultra-sensitive determination of the antidepressant drug duloxetine hydrochloride on a diamond electrode by voltammetry. Int. J. Environ. Anal. Chem. 2022, 102, 5680–5694. [Google Scholar] [CrossRef]
- Ramos, D.L.O.; Freitas, J.M.; Munoz, R.A.A.; Richter, E.M. Simple and rapid voltammetric method for the detection of the synthetic adulterant fluoxetine in weight loss products. J. Electroanal. Chem. 2021, 882, 115028. [Google Scholar] [CrossRef]
- Brycht, M.; Skrzypek, S.; Karadas-Bakirhan, N.; Smarzewska, S.; Bozal-Palabiyik, B.; Ozkan, S.A.; Uslu, B. Voltammetric behavior and determination of antidepressant drug paroxetine at carbon-based electrodes. Ionics 2015, 21, 2345–2354. [Google Scholar] [CrossRef]
- Dhanjai; Sinha, A.; Lu, X.; Wu, L.; Tan, D.; Li, Y.; Chen, J.; Jain, R. Voltammetric sensing of biomolecules at carbon based electrode interfaces: A review. TrAC—Trends Anal. Chem. 2018, 98, 174–189. [Google Scholar] [CrossRef]
- Asadian, E.; Ghalkhani, M.; Shahrokhian, S. Electrochemical sensing based on carbon nanoparticles: A review. Sens. Actuators B Chem. 2019, 293, 183–209. [Google Scholar] [CrossRef]
- Gibi, C.; Liu, C.H.; Barton, S.C.; Anandan, S.; Wu, J.J. Carbon Materials for Electrochemical Sensing Application—A Mini Review. J. Taiwan Inst. Chem. Eng. 2024, 154, 105071. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef]
- Gao, H.; Duan, H. 2D and 3D graphene materials: Preparation and bioelectrochemical applications. Biosens. Bioelectron. 2015, 65, 404–419. [Google Scholar] [CrossRef]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.R.; Geim, A.K. Fine Structure Constant Defines Visual Transparency of Graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef]
- Bolotin, K.I.; Sikes, K.J.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H.L. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008, 146, 351–355. [Google Scholar] [CrossRef]
- Wang, D.; Li, F.; Zhao, J.; Ren, W.; Chen, Z.; Tan, J.; Wu, Z.; Gentle, I.; Lu, G.Q.; Cheng, H. Fabrication of Graphene/Polyaniline Composite Paper via In Situ Anodic Electropolymerization for High-Performance Flexible Electrode. ACS Nano 2009, 3, 1745–1752. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.L.; Tian, Z.; Simon, G.P.; Li, D. Scalable production of graphene via wet chemistry: Progress and challenges. Mater. Today 2015, 18, 73–78. [Google Scholar] [CrossRef]
- Chandra, V.; Park, J.; Chun, Y.; Lee, J.W.; Hwang, I.; Kim, K.S. Water-Dispersible Magnetite-Reduced Graphene Oxide Composites for Arsenic Removal. ACS Nano 2010, 4, 3979–3986. [Google Scholar] [CrossRef] [PubMed]
- Lazar, P.; Karlický, F.; Jurecka, P.; Kocman, M.; Otyepková, E.; Šafářová, K.; Otyepka, M. Adsorption of small organic molecules on graphene. J. Am. Chem. Soc. 2013, 135, 6372–6377. [Google Scholar] [CrossRef]
- Brownson, D.A.C.; Banks, C.E. Graphene electrochemistry: An overview of potential applications. Analyst 2010, 135, 2768–2778. [Google Scholar] [CrossRef]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M.S.; Jing, K. Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett. 2009, 9, 30–35. [Google Scholar] [CrossRef]
- Guo, H.L.; Wang, X.F.; Qian, Q.Y.; Wang, F.B.; Xia, X.H. A green approach to the synthesis of graphene nanosheets. ACS Nano 2009, 3, 2653–2659. [Google Scholar] [CrossRef]
- Chacón-Torres, J.C.; Wirtz, L.; Pichler, T. Raman spectroscopy of graphite intercalation compounds: Charge transfer, strain, and electron-phonon coupling in graphene layers. Phys. Status Solidi Basic Res. 2014, 251, 2337–2355. [Google Scholar] [CrossRef]
- Madhu, R.; Dinesh, B.; Chen, S.M.; Saraswathi, R.; Mani, V. An electrochemical synthesis strategy for composite based ZnO microspheres-Au nanoparticles on reduced graphene oxide for the sensitive detection of hydrazine in water samples. RSC Adv. 2015, 5, 54379–54386. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, F.; Li, Q.; Shou, Q.; Cheng, J.; Zhang, L.; Nelson, B.J.; Zhang, X. Transition metal oxide and graphene nanocomposites for high-performance electrochemical capacitors. Phys. Chem. Chem. Phys. 2012, 14, 16331–16337. [Google Scholar] [CrossRef]
- Wang, H.W.; Hu, Z.A.; Chang, Y.Q.; Chen, Y.L.; Zhang, Z.Y.; Yang, Y.Y.; Wu, H.Y. Preparation of reduced graphene oxide/cobalt oxide composites and their enhanced capacitive behaviors by homogeneous incorporation of reduced graphene oxide sheets in cobalt oxide matrix. Mater. Chem. Phys. 2011, 130, 672–679. [Google Scholar] [CrossRef]
- Gnana, K.G.; Christy, M.; Jang, H.; Nahm, K.S. Cobaltite oxide nanosheets anchored graphene nanocomposite as an efficient oxygen reduction reaction (ORR) catalyst for the application of lithium-air batteries. J. Power Sources 2015, 288, 451–460. [Google Scholar] [CrossRef]
- Ahamed, A.J.; Kumar, P.V.; Srikesh, G. Low Temperature Synthesis and Characterization of rGO-CoO Nanocomposite with Efficient Electrochemical Properties. J. Environ. Nanotechnol. 2015, 4, 01–08. [Google Scholar] [CrossRef]
- Dinesh, B.; Veeramani, V.; Chen, S.M.; Saraswathi, R. In situ electrochemical synthesis of reduced graphene oxide-cobalt oxide nanocomposite modified electrode for selective sensing of depression biomarker in the presence of ascorbic acid and dopamine. J. Electroanal. Chem. 2017, 786, 169–176. [Google Scholar] [CrossRef]
- Ali, M.F.B.; El-Zahry, M.R. A comparative study of different electrodeposited NiCo2O4 microspheres anchored on a reduced graphene oxide platform: Electrochemical sensor for anti-depressant drug venlafaxine. RSC Adv. 2019, 9, 31609–31620. [Google Scholar] [CrossRef]
- Cincotto, F.H.; Golinelli, D.L.C.; Machado, S.A.S.; Moraes, F.C. Electrochemical sensor based on reduced graphene oxide modified with palladium nanoparticles for determination of desipramine in urine samples. Sens. Actuators B Chem. 2017, 239, 488–493. [Google Scholar] [CrossRef]
- Phua, C.H.; Saisahas, K.; Soleh, A.; Promsuwan, K.; Saichanapan, J.; Limbut, W. Electrode modified with reduced graphene oxide decorated with gold nanoparticles co-induced by laser for electrochemical alprazolam sensor. Microchem. J. 2023, 195, 109380. [Google Scholar] [CrossRef]
- Oghli, A.H.; Soleymanpour, A. Polyoxometalate/reduced graphene oxide modified pencil graphite sensor for the electrochemical trace determination of paroxetine in biological and pharmaceutical media. Mater. Sci. Eng. C 2020, 108, 110407. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Atty, S.A.; Asran, A.M.; Boukherroub, R. One-pot green synthesis of reduced graphene oxide decorated with β-Ni(OH)2 -nanoflakes as an efficient electrochemical platform for the determination of antipsychotic drug sulpiride. Microchem. J. 2019, 147, 555–563. [Google Scholar] [CrossRef]
- Ateş, A.K.; Er, E.; Çelikkan, H.; Erk, N. The fabrication of a highly sensitive electrochemical sensor based on AuNPs@graphene nanocomposite: Application to the determination of antidepressant vortioxetine. Microchem. J. 2019, 148, 306–312. [Google Scholar] [CrossRef]
- Chen, A.; Wei, Y.; Tuo, D.; Zhou, C.; Shi, S.; Tang, N.; He, Q.; Liu, J. Submicromolar electrochemical sensing platform for fast Fluoxetine quantification based on Nb2CTx MXene and nitrogen-doped graphene oxide nanocomposites. J. Alloys Compd. 2024, 970, 172557. [Google Scholar] [CrossRef]
- Urcuk, A.; Karadurmus, L.; Bakirhan, N.K.; Ozkan, S.A. Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride. Open Chem. 2021, 19, 228–236. [Google Scholar] [CrossRef]
- Nigović, B.; Mornar, A.; Sertić, M. Graphene nanocomposite modified glassy carbon electrode for voltammetric determination of the antipsychotic quetiapine. Microchim. Acta 2016, 183, 1459–1467. [Google Scholar] [CrossRef]
- Milani-Hosseini, M.-R.; Karamdoust, S.; Bahman, M.; Motaharian, A.; Mohammadsadegh, S. Molecularly Imprinted Polymer (MIP) Electrochemical Sensor based on Graphene Modified Platinum Electrode for Sertraline Determination. Anal. Bioanal. Electrochem. 2020, 12, 128–140. [Google Scholar]
- Daneshvar, L.; Rounaghi, G.H.; Es’haghi, Z.; Chamsaz, M.; Tarahomi, S. Fabrication a new modified electrochemical sensor based on Au–Pd bimetallic nanoparticle decorated graphene for citalopram determination. Mater. Sci. Eng. C 2016, 69, 653–660. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Balamurugan, T.S.T.; Chen, S.-M.; Chen, T.-W.; Ali, M.A.; Al-Hemaid, F.M.A.; Elshikh, M.S. An Amperometric Sensor for Low Level Detection of Antidepressant Drug Carbamazepine Based on Graphene Oxide-g-C3N4 Composite Film Modified Electrode. J. Electrochem. Soc. 2018, 165, B160–B166. [Google Scholar] [CrossRef]
- Boroujerdi, R.; Abdelkader, A.; Paul, R. Highly Sensitive and Selective Detection of the Antidepressant Amitriptyline Using a Functionalised Graphene-Based Sensor. ChemNanoMat 2022, 8, e202200209. [Google Scholar] [CrossRef]
- Tseliou, F.; Pappas, P.; Spyrou, K.; Hrbac, J.; Prodromidis, M.I. Lab-on-a-screen-printed electrochemical cell for drop-volume voltammetric screening of flunitrazepam in untreated, undiluted alcoholic and soft drinks. Biosens. Bioelectron. 2019, 132, 136–142. [Google Scholar] [CrossRef]
- Karikalan, N.; Sundaresan, P.; Na, J.H.; Lee, T.Y. Simultaneous in-situ extraction and electrochemical detection of antidepressant drug imipramine and its active metabolite in human biofluid samples. Sens. Actuators B Chem. 2022, 365, 131960. [Google Scholar] [CrossRef]
- Habibi, B.; Pashazadeh, S.; Saghatforoush, L.A.; Pashazadeh, A. Direct electrochemical synthesis of the copper based metal-organic framework on/in the heteroatoms doped graphene/pencil graphite electrode: Highly sensitive and selective electrochemical sensor for sertraline hydrochloride. J. Electroanal. Chem. 2021, 888, 115210. [Google Scholar] [CrossRef]
- Chen, Y.; Dai, W.; Zhou, S.; Liu, X.; Chen, W.; Zheng, Y.; Chen, X.; Sun, G. An electrochemical biosensor based on graphene oxide for determination of sertraline hydrochloride as an antidepressant drug. Alex. Eng. J. 2023, 78, 213–223. [Google Scholar] [CrossRef]
- Khosrokhavar, R.; Motaharian, A.; Hosseini, M.R.M.; Mohammadsadegh, S. Screen-printed carbon electrode (SPCE) modified by molecularly imprinted polymer (MIP) nanoparticles and graphene nanosheets for determination of sertraline antidepressant drug. Microchem. J. 2020, 159, 105348. [Google Scholar] [CrossRef]
- Fekry, A.M.; Mohamed, G.G.; Attia, F.M.A.; Ibrahim, N.S.; Azab, S.M. A nanoparticle modified carbon paste sensor for electrochemical determination of the antidepressant agent vilazodone. J. Electroanal. Chem. 2019, 848, 113305. [Google Scholar] [CrossRef]
- Alizadeh, T.; Azizi, S. Graphene/graphite paste electrode incorporated with molecularly imprinted polymer nanoparticles as a novel sensor for differential pulse voltammetry determination of fluoxetine. Biosens. Bioelectron. 2016, 81, 198–206. [Google Scholar] [CrossRef]
- Hassanein, A.M.; Moharram, Y.I.; Oraiby, N.F.; Ebied, S.E. Trace Determination of Duloxetine HCl in Formulation and Spiked Human Serum at a Carbon Paste Electrode. Am. J. Anal. Chem. 2017, 08, 708–725. [Google Scholar] [CrossRef]
- Attia, A.K.; Mohamed, M.A.; Fekry, A.M. Electroanalytical determination of escitalopram oxalate using nickel nanoparticles modified carbon paste sensor. Acta Chim. Slov. 2017, 64, 415–421. [Google Scholar] [CrossRef]
- Motaharian, A.; Naseri, K.; Mehrpour, O.; Shoeibi, S. Electrochemical determination of atypical antipsychotic drug quetiapine using nano-molecularly imprinted polymer modified carbon paste electrode. Anal. Chim. Acta 2020, 1097, 214–221. [Google Scholar] [CrossRef]
- Öztürk, F.; Yüksel, E.; Erden, P.E.; Kiliç, E. Electrochemical determination of aripiprazole based on aluminium oxide nanoparticles modified carbon paste electrode. Turk. J. Chem. 2023, 47, 126–136. [Google Scholar] [CrossRef]
- Norizan, M.N.; Moklis, M.H.; Demon, S.Z.N.; Halim, N.A.; Samsuri, A.; Mohamad, I.S.; Knight, V.F.; Abdullah, N. Carbon nanotubes: Functionalisation and their application in chemical sensors. RSC Adv. 2020, 10, 43704–43732. [Google Scholar] [CrossRef]
- Rahman, G.; Najaf, Z.; Mehmood, A.; Bilal, S.; Shah, A.U.; Mian, S.A.; Ali, G. An Overview of the Recent Progress in the Synthesis and Applications of Carbon Nanotubes. C 2019, 5, 3. [Google Scholar] [CrossRef]
- Gupta, N.; Gupta, S.M.; Sharma, S.K. Carbon nanotubes: Synthesis, properties and engineering applications. Carbon Lett. 2019, 29, 419–447. [Google Scholar] [CrossRef]
- Shoukat, R.; Khan, M.I. Carbon nanotubes: A review on properties, synthesis methods and applications in micro and nanotechnology. Microsyst. Technol. 2021, 27, 4183–4192. [Google Scholar] [CrossRef]
- Rathinavel, S.; Priyadharshini, K.; Panda, D. A review on carbon nanotube: An overview of synthesis, properties, functionalization, characterization, and the application, Mater. Sci. Eng. B 2021, 268, 115095. [Google Scholar] [CrossRef]
- Manikandan, N.; Kumar, V.P.S.; Murugan, S.S.; Rathis, G.; Saran, K.V.; Shabariganesh, T.K. Carbon nanotubes and their properties-The review. Mater. Today Proc. 2021, 47, 4682–4685. [Google Scholar] [CrossRef]
- Fu, S.; Chen, X.; Liu, P. Preparation of CNTs/Cu composites with good electrical conductivity and excellent mechanical properties. Mater. Sci. Eng. A 2020, 771, 138656. [Google Scholar] [CrossRef]
- Vairavapandian, D.; Vichchulada, P.; Lay, M.D. Preparation and modification of carbon nanotubes: Review of recent advances and applications in catalysis and sensing. Anal. Chim. Acta 2008, 626, 119–129. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Chen, W.-H.; Chang, Y.-H. Preparation and properties of chitosan/carbon nanotube nanocomposites using poly(styrene sulfonic acid)-modified CNTs. Carbohydr. Polym. 2009, 76, 232–238. [Google Scholar] [CrossRef]
- Aslam, M.M.; Kuo, H.-W.; Den, W.; Usman, M.; Sultan, M.; Ashraf, H. Functionalized Carbon Nanotubes (CNTs) for Water and Wastewater Treatment: Preparation to Application. Sustainability 2021, 13, 5717. [Google Scholar] [CrossRef]
- Jacobs, C.B.; Peairs, M.J.; Venton, B.J. Review: Carbon nanotube based electrochemical sensors for biomolecules. Anal. Chim. Acta 2010, 662, 105–127. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Sheikh-Mohseni, M.A. Carbon Nanotubes in Electrochemical Sensors. In Carbon Nanotubes-Growth and Applications; BoD: Norderstedt, Germany, 2011. [Google Scholar] [CrossRef]
- Duarte, E.H.; Santos, W.P.D.; Hudari, F.F.; Neto, J.L.B.; Sartori, E.R.; Dallantonia, L.H.; Pereira, A.C.; Tarley, C.R.T. A highly improved method for sensitive determination of amitriptyline in pharmaceutical formulations using an unmodified carbon nanotube electrode in the presence of sulfuric acid. Talanta 2014, 127, 26–32. [Google Scholar] [CrossRef]
- Fekry, A.M.; Azab, S.M.; Attia, F.M.A.; Ibrahim, N.S.; Mohamed, G.G. An innovative sensor for the electrochemical determination of the new melatonergic antidepressant drug agomelatine. Meas. J. Int. Meas. Confed. 2021, 186, 110160. [Google Scholar] [CrossRef]
- Ghaedi, H.; Afkhami, A.; Madrakian, T.; Soltani-Felehgari, F. Construction of novel sensitive electrochemical sensor for electro-oxidation and determination of citalopram based on zinc oxide nanoparticles and multi-walled carbon nanotubes. Mater. Sci. Eng. C 2016, 59, 847–854. [Google Scholar] [CrossRef]
- Rizk, M.; Taha, E.A.; El-Alamin, M.M.A.; Hendawy, H.A.M.; Sayed, Y.M. Highly Sensitive Carbon Based Sensors Using Zinc Oxide Nanoparticles Immobilized Multiwalled Carbon Nanotubes for Simultaneous Determination of Desvenlafaxine Succinate and Clonazepam. J. Electrochem. Soc. 2018, 165, H333–H341. [Google Scholar] [CrossRef]
- Gaichore, R.R.; Srivastava, A.K. Voltammetric determination of nifedipine using a β-cyclodextrin modified multi-walled carbon nanotube paste electrode. Sens. Actuators B Chem. 2013, 188, 1328–1337. [Google Scholar] [CrossRef]
- Atty, S.A.; Ibrahim, A.H.; Ibrahim, H.; Abdelzaher, A.M.; Abdel-Raoof, A.M.; Fouad, F.A. Simultaneous voltammetric detection of anti-depressant drug, sertraline HCl and paracetamol in biological fluid at CNT-cesium modified electrode in micellar media. Microchem. J. 2020, 159, 105524. [Google Scholar] [CrossRef]
- Sanguarnsak, C.; Promsuwan, K.; Saichanapan, J.; Soleh, A.; Saisahas, K.; Phua, C.H.; Limbut, W. Voltammetric Amitriptyline Determination Using a Metal-Free Electrode Based on Phosphorus-Doped Multi-Walled Carbon Nanotubes. J. Electrochem. Soc. 2022, 169, 017510. [Google Scholar] [CrossRef]
- Gholivand, M.B.; Akbari, A. A novel voltammetric sensor for citalopram based on multiwall carbon nanotube/(poly(p-aminobenzene sulfonic acid)/β-cyclodextrin). Mater. Sci. Eng. C 2016, 62, 480–488. [Google Scholar] [CrossRef]
- Keypour, H.; Saremi, S.G.; Veisi, H.; Noroozi, M. Electrochemical determination of citalopram on new Schiff base functionalized magnetic Fe3O4 nanoparticle/MWCNTs modified glassy carbon electrode. J. Electroanal. Chem. 2016, 780, 160–168. [Google Scholar] [CrossRef]
- Jat, M.S.; Meena, K.; Jhankal, K.K.; Sharma, D.K. Sensitive electro-chemical determination of antidepressant drug clomipramine at f-mwcnts nano clusters modified glassy carbon electrode. J. Pharm. Sci. Res. 2019, 11, 700–707. [Google Scholar]
- Ehzari, H.; Gholivand, M.; Shamsipur, M. A sensitive electrochemical sensor based on multiwall carbon nanotube-ionic liquid/nickel oxide nanoparticles for simultaneous determination of the antipsychotic drugs clozapine and sertraline. Adv. Nanochem. 2021, 3, 23–33. [Google Scholar] [CrossRef]
- Madrakian, T.; Soleimani, M.; Afkhami, A. Electrochemical determination of fluvoxamine on mercury nanoparticle multi-walled carbon nanotube modified glassy carbon electrode. Sens. Actuators B Chem. 2015, 210, 259–266. [Google Scholar] [CrossRef]
- Arvand, M.; Pourhabib, A. Surfactant-Assisted Voltammetric Determination of Olanzapine at Amine Functionalized TiO2/Multi-Walled Carbon Nanotubes Nanocomposite. J. Anal. Chem. 2019, 74, 1096–1103. [Google Scholar] [CrossRef]
- Piech, R.; Rumin, M.; Paczosa-Bator, B. High sensitive voltammetric determination of paroxetine on glassy carbon electrode modified with Nafion/MWCNTs. Int. J. Electrochem. Sci. 2014, 9, 7528–7539. [Google Scholar] [CrossRef]
- Becerra-Hernández, A.; Galindo-de-la-Rosa, J.; Martínez-Pimentel, Y.; Ledesma-García, J.; Álvarez-Contreras, L.; Guerra-Balcázar, M.; Aguilar-Elguezabal, A.; Álvarez, A.; Chávez-Ramírez, A.U.; Vallejo-Becerra, V. Novel biomaterial based on monoamine oxidase-A and multi-walled carbon nanotubes for serotonin detection. Biochem. Eng. J. 2019, 149, 107240. [Google Scholar] [CrossRef]
- Atta, N.F.; Ahmed, Y.M.; Galal, A. Electrochemical Determination of Neurotransmitters at Crown Ether Modified Carbon Nanotube Composite: Application for Sub-nano-sensing of Serotonin in Human Serum. Electroanalysis 2019, 31, 1204–1214. [Google Scholar] [CrossRef]
- Babaei, A.; Afrasiabi, M.; Yousefi, A. Fe3O4@MCM-48-SO3H/ Multi-Wall Carbon Nanotubes Composite Modified Glassy Carbon Electrode: An Efficient Sensor for Sensitive and Selective Simultaneous Determination of Serotonin and Sertraline in the presence of Uric Acid. Anal. Bioanal. Electrochem. 2019, 11, 1–18. [Google Scholar]
- Tang, S.; Liang, A.; Liu, M.; Wang, W.; Zhang, F.; Luo, A. A glassy carbon electrode modified with a composite consisting of electrodeposited chitosan and carboxylated multi-walled carbon nanotubes for simultaneous voltammetric determination of dopamine, serotonin and melatonin. Carbon Lett. 2023, 33, 2129–2139. [Google Scholar] [CrossRef]
- Liang, H.; Zhu, M.; Ye, H.; Zeng, C.; Wang, S.; Niu, Y. Carbon fiber microelectrode array loaded with the diazonium salt-single-walled carbon nanotubes composites for the simultaneous monitoring of dopamine and serotonin in vivo. Anal. Chim. Acta 2021, 1186, 339086. [Google Scholar] [CrossRef]
- Shoja, Y.; Rafati, A.A.; Ghodsi, J. Electropolymerization of Ni-LD metallopolymers on gold nanoparticles enriched multi-walled carbon nanotubes as nano-structure electrocatalyst for efficient voltammetric sertraline detection in human serum. Electrochim. Acta 2016, 203, 281–291. [Google Scholar] [CrossRef]
- Shahrokhian, S.; Ghalkhani, M.; Adeli, M.; Amini, M.K. Multi-walled carbon nanotubes with immobilised cobalt nanoparticle for modification of glassy carbon electrode: Application to sensitive voltammetric determination of thioridazine. Biosens. Bioelectron. 2009, 24, 3235–3241. [Google Scholar] [CrossRef]
- Hegde, R.N.; Shetti, N.P.; Nandibewoor, S.T. Electro-oxidation and determination of trazodone at multi-walled carbon nanotube-modified glassy carbon electrode. Talanta 2009, 79, 361–368. [Google Scholar] [CrossRef]
- Gholivand, M.B.; Godini, Z. Trace-level determination of trazodone with electrochemical sensor based on TiO2-cMWCNTs nanocomposite. Adv. Nanochem. 2021, 3, 88–96. [Google Scholar] [CrossRef]
- Sanghavi, B.J.; Srivastava, A.K. Adsorptive stripping differential pulse voltammetric determination of venlafaxine and desvenlafaxine employing Nafion-carbon nanotube composite glassy carbon electrode. Electrochim. Acta 2011, 56, 4188–4196. [Google Scholar] [CrossRef]
- Ding, L.; Li, L.; You, W.; Gao, Z.N.; Yang, T.L. Electrocatalytic oxidation of venlafaxine at a multiwall carbon nanotubes-ionic liquid gel modified glassy carbon electrode and its electrochemical determination. Croat. Chem. Acta 2015, 88, 81–87. [Google Scholar] [CrossRef]
- Eslami, E.; Farjami, F. Voltammetric Determination of Venlafaxine by Using Multiwalled Carbon Nanotube-Ionic Liquid Composite Electrode. J. Appl. Chem. Res. 2018, 12, 42–52. [Google Scholar]
- Jyoti; Żołek, T.; Maciejewska, D.; Gilant, E.; Gniazdowska, E.; Kutner, A.; Noworyta, K.R.; Kutner, W. Polytyramine Film-Coated Single-Walled Carbon Nanotube Electrochemical Chemosensor with Molecularly Imprinted Polymer Nanoparticles for Duloxetine-Selective Determination in Human Plasma. ACS Sens. 2022, 7, 1829–1836. [Google Scholar] [CrossRef]
- Mohammadi-Behzad, L.; Gholivand, M.B.; Shamsipur, M.; Gholivand, K.; Barati, A.; Gholami, A. Highly sensitive voltammetric sensor based on immobilization of bisphosphoramidate-derivative and quantum dots onto multi-walled carbon nanotubes modified gold electrode for the electrocatalytic determination of olanzapine. Mater. Sci. Eng. C 2016, 60, 67–77. [Google Scholar] [CrossRef]
- Al-dolaimy, F.; Tapia, N.E.F.; Hussein, T.K.; Kaur, M.; Alhameedi, D.Y.; Rasen, F.A.; Ramadan, M.F.; Khaleel, L.A.; Alsalamy, A.; Asiri, M.; et al. Green synthesis of copper(II) oxide nanoparticles covered on multiwalled carbon nanotubes modified screen-printed electrode as rapid electrochemical sensing platform for detection of doxepin. Results Chem. 2024, 7, 101526. [Google Scholar] [CrossRef]




| Electrode | Analyte | Technique | Linear Range | LOD | Sample | Ref. |
|---|---|---|---|---|---|---|
| GCE | Opipramol | DPV | 2 × 10−6–2 × 10−4 | 2.70 × 10−7 | Serum, urine | [23] |
| OSW | 3.10 × 10−7 | |||||
| GCE | Aripiprazole | LSV | 0.1–5 mg L−1 | 50 µg L−1 | Tablets, urine | [24] |
| AdSV | 4–40 µg L−1 | 1 µg L−1 | ||||
| GCE | Aripiprazole | DPV | 11.4–157 µM | 6.16 µM | Tablets | [25] |
| SWV | 5.49 µM | |||||
| DPAAdSV | 0.221–13.6 µM | 0.14 µM | Serum, urine | |||
| SWAAdSV | 0.11 µM | |||||
| GCE | Citalopram | DPV | 0.05–10.0 µM | 0.036 µM | Pharmaceuticals, tap, river, and wastewater | [26] |
| 10.0–115.0 µM | ||||||
| GCE | Quetiapine | DPV | 4 × 10−6–2 × 10−4 mol L−1 | 4.01 × 10−8 mol L−1 | Serum, urine | [27] |
| OSWV | 1.33 × 10−7 mol L−1 |
| Electrode | Analyte | Technique | Linear Range | LOD | Sample | Ref. |
|---|---|---|---|---|---|---|
| 1 CP-BDDE | Duloxetine | DPV | 0.030–0.333 µmol L−1 | 5.87 nmol L−1 | Lake, river, and tap water | [31] |
| SWV | 0.10–12.4 µmol L−1 | 42 nmol L−1 | ||||
| BDDE | Fluoxetine | SWV | 3.2–162 µmol L−1 | 0.3 µmol L−1 | Weight loss products in capsules | [32] |
| BDDE | Paroxetine | SWAdSV | 7.0 × 10−7–3.5 × 10−6 M | 6.95 × 10−9 M | Pharmaceutics | [33] |
| Electrode | Analyte | Technique | Linear Range | LOD | Sample | Ref. |
|---|---|---|---|---|---|---|
| 1 RGO/Co3O4/GCE | Serotonin | DPV | 1–51 µM | 48.7 nM | Serum | [57] |
| 2 in situ-NiCo2O4@rGO/PGE | Venlafaxine | SWV | 0.5–50 × 10−8 mol L−1 | 3.4 × 10−9 mol L−1 | Pharmaceutical formulations, human plasma | [58] |
| 3 RGO/PdNPs/GC | Desipramine | DPV | 0.3–2.5 µmol L−1 | 1.04 nmol L−1 | Urine | [59] |
| 4 LI-AuNP-rGO/GCE | Alprazolam | DPAdCSV | 0.001–0.10 mg L−1 0.10–8.0 mg L−1 | 0.3 µg L−1 | Beverage samples | [60] |
| 5 rGO/PWA/PGE | Paroxetine | DPV | 8.0 × 10−9–1.0 × 10−6 M | 9.0 × 10−10 M | Tablets, human serum, urine | [61] |
| 6 Ni(OH)2 × Gr-IL/CPE | Sulpiride | SWV | 1.0 × 10−9–2.0 × 10−4 mol L−1 | 2.50 × 10−10 mol L−1 | Biological fluids, pharmaceutical dosage form | [62] |
| 7 AuNPs@GRP/GCE | Vortioxetine | AdsDPVs | 0.1–1.0 µM 1.0–6.0 µM | 0.050 µM | Tablets | [63] |
| 8 Nb2CTx/NRGO/GCE | Fluoxetine | SWV | 1.0–10 µM 10–100 µM | 0.34 µM | Human serum and urine | [64] |
| 9 GO/β-CD/PGE | Sulpiride | AdSSWV | 1.0 × 10−7–5.0 × 10−5 M | 2.83 × 10−9 M | Tablet, capsule, urine | [65] |
| 10 GnPs-Naf/GCE | Quetiapine | DPAdSV | 1 × 10−7–1 × 10−5 M | 2.2 × 10−8 M | Tablets, human urine | [66] |
| 11 MIP−Graphene Pt | Sertraline | DPV | 1.0 × 10−8–1.0 × 10−6 mol L−1 | 7.0 × 10−9 mol L−1 | Human serum | [67] |
| 12 Au–PdNPs-GR/AuE | Citalopram | SWV | 0.5–50 µM | 0.049 µM | Tablet, plasma | [68] |
| 13 GO/g-C3N4/GCE | Carbamazepine | amperometry | 0.092–266 µM | 10.5 nM | Human urine, pharmaceutical samples | [69] |
| 14 rGO-D2 | Amitriptyline | CV | 1–80 µg/mL | 1 ng/mL | - | [70] |
| 15 SPCE/AGO-Cu | Flunitrazepam | DPV | 0.4–140 µM | 0.13 µM | Fruit juice | [71] |
| 16 HSA-FeM-rGO/SPCE | Imipramine | DPV | 10–756 ng/mL | 4 ± 2 ng/mL | Plasma, serum | [72] |
| 17 Cu-MOF/SNDGr/PGE | Sertraline | DPV | 0.05–2.67 µM | 0.038 µM | Tablet, human serum | [73] |
| 18 AgVO3/c-GO/GCE | Sertraline | DPV | 0–1600 µM | 25 μM | Pharmaceutical sample | [74] |
| 19 MIP/Gr-SPCE | Sertraline | SWV | 5.0 × 10−9–7.5 × 10−7 M | 1.99 × 10−9 M | Tablet, human serum | [75] |
| 20 C/T/Pd/MCPE | Vilazodone | DPV | 2.5 × 10−8–2 × 10−4 M | 8 × 10−10 M | Pharmaceutical dosage forms, urine | [76] |
| 21 nano-MIP/G-CP | Fluoxetine | DPV | 0.006–0.1 µM | 0.0015 µM | Plasma, pharmaceutical samples | [77] |
| 22 CPE | Duloxetine | SW-AdASV | 1.0 × 10−8–1.0 × 10−6 M | 3.0 × 10−9 M | Pharmaceutical formulation, human serum | [78] |
| 23 NiCACP | Escitalopram | DPV | 1.0 × 10−6–7.0 × 10−5 M | 2.0 × 10−7 M | Dosage form, urine | [79] |
| 24 MIP-CPE | Quetiapine | SWV | 1.6 × 10−8–2.5 × 10−6 M | 5.04 × 10−9 M | Pharmaceutical formulation, human urine | [80] |
| 25 Al2O3NP-CPE | Aripiprazole | SWAdSV | 0.03–8.0 µM | 0.006 µM | Pharmaceutical formulations, human serum | [81] |
| Electrode | Analyte | Technique | Linear Range | LOD | Sample | Ref. |
|---|---|---|---|---|---|---|
| 1 P-MWCNTs/GCE | Amitriptyline | AdSV | 0.50–10 µg mL−1 10–40 µg mL−1 | 0.15 µg mL−1 | Pharmaceutical tablets | [100] |
| 2 p(p-ABSA)/β-CD/MWCNT/GC | Citalopram | DPV | 90 nM–1 µM 1–11 µM 11–100 µM | 44 nM | Pharmaceutical, human body fluids | [101] |
| 3 Fe3O4@[(EtO)3Si-L]/MWCNTs/GCE | Citalopram | DPV | 0.02–62 µM | 32.2 nM | Human blood serum, pharmaceuticals | [102] |
| 4 f-MWCNTs/GCE | Clomipramine | DP AAdSV | 1.45 × 10−5–4.52 × 10−3 mol L−1 | 1.315 × 10−8 g mL−1 | Drug tablets | [103] |
| 5 MWCNT-IL/NiONPs/GCE | Clozapine | DP AdSV | 0.5–67 µM | 0.052 µM | Human serum, pharmaceuticals | [104] |
| Sertraline | 0.21–85 µM | 0.047 µM | ||||
| 6 HgNPs/MWCNTs/GCE | Fluvoxamine | DPV | 0.020–1.750 µmol L−1 | 0.01 µmol L−1 | Tablets, urine | [105] |
| 7 ISS-NH2-TiO2-MWCNTs/GCE | Olanzapine | SWV | 0.05–0.1 µM 0.1–10 µM | 8 nM | Tablets, blood serum | [106] |
| 8 Nafion/MWCNTs/GCE | Paroxetine | DPV | 0.1–2.5 µM | 8 nM | Tablets, urine | [107] |
| 9 MWCNTs/MAO-A/GCE | Serotonin | DPV | 5.67 × 10−7–2.26 × 10−6 M | 2 × 10−7 M | - | [108] |
| 10 GC/CNTs-ILC/Crown | Serotonin | DPV | 0.005–100 µM | 2.02 × 10−10 mol L−1 | Human serum, | [109] |
| 11 Fe3O4@MCM-48-SO3H/MWCNTs/GCE | Serotonin | DPV | 0.05–100 µM | 0.015 µM | Human serum, urine | [110] |
| Sertraline | 0.1–85 µM | 0.025 µM | ||||
| 12 e-CS/MWCNTs/GCE | Serotonin | DPV | 9–1000 µmol L−1 | 10 µmol L−1 | Human saliva | [111] |
| 13 DS-SWCNTs/CFMEA | Serotonin | DPV | 0.07–0.9 µm | 4.6 nM | Mouse striatum | [112] |
| 14 Ni(II)-LD/AuNPs/MWCNT/GCE | Sertraline | DPV | 0.05–5.5 µM | 95 nM | Human serum | [113] |
| 15 CoNP/MWCNT/GCE | Thioridazine | DPV | 5.0 × 10−7–1.0 × 10−4 M | 5.0 × 10−8 M | Human blood serum, | [114] |
| 16 MWCNTs/GCE | Trazodone | DPV | 0.2–10 µM | 24 nM | Urine | [115] |
| 17 TiO2-cMWCNTs/GCE | Trazodone | DPASV | 6–100 nM 100–1000 nM | 5 nM | Pharmaceutical formulation, human serum | [116] |
| 18 NAF-CNT-GCE | Venlafaxine | DPAdSV | 3.81 × 10−8–6.22 × 10−5 M | 1.24 × 10−8 M | Pharmaceutical formulation, urine, blood serum | [117] |
| 19 MWCNTs-RTIL/GC | Venlafaxine | SWV | 2.0 × 10−6–2.0 × 10−3 M | 1.69 × 10−6 | Pharmaceutical | [118] |
| 20 MWCNT-CILE | Venlafaxine | CV | 10.0–500.0 µM | 0.47 µM | Pharmaceutical formulation, urine, blood serum | [119] |
| Electrode | Analyte | Technique | Linear Range | LOD | Sample | Ref. |
|---|---|---|---|---|---|---|
| 1 nanoMIPs-SWCNTs@polytyramine film-coated Au electrode | Duloxetine | DPV | 10 pM–676 nM | 1.6 pM | Human plasma | [120] |
| 2 BMBPBP/CdS-QDs/MWCNTs | Olanzapine | Amerometric | 20 nM–100 µM | 6nM | Pharmaceutical, human serum | [121] |
| 3 CuO/MWCNTs/SPE | Doxepin | Amperometric | 0.001–400 µM | 0.17 nM | Tablets, urine samples | [122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smajdor, J.; Fendrych, K.; Górska-Ratusznik, A. Carbon Materials in Voltammetry: An Overview of Versatile Platforms for Antidepressant Drug Detection. Micromachines 2025, 16, 423. https://doi.org/10.3390/mi16040423
Smajdor J, Fendrych K, Górska-Ratusznik A. Carbon Materials in Voltammetry: An Overview of Versatile Platforms for Antidepressant Drug Detection. Micromachines. 2025; 16(4):423. https://doi.org/10.3390/mi16040423
Chicago/Turabian StyleSmajdor, Joanna, Katarzyna Fendrych, and Anna Górska-Ratusznik. 2025. "Carbon Materials in Voltammetry: An Overview of Versatile Platforms for Antidepressant Drug Detection" Micromachines 16, no. 4: 423. https://doi.org/10.3390/mi16040423
APA StyleSmajdor, J., Fendrych, K., & Górska-Ratusznik, A. (2025). Carbon Materials in Voltammetry: An Overview of Versatile Platforms for Antidepressant Drug Detection. Micromachines, 16(4), 423. https://doi.org/10.3390/mi16040423








