Applications and Recent Advances of Low-Temperature Multicomponent Solders in Electronic Packaging: A Review
Abstract
1. Introduction
2. High-Entropy Alloys and Multicomponent Solders
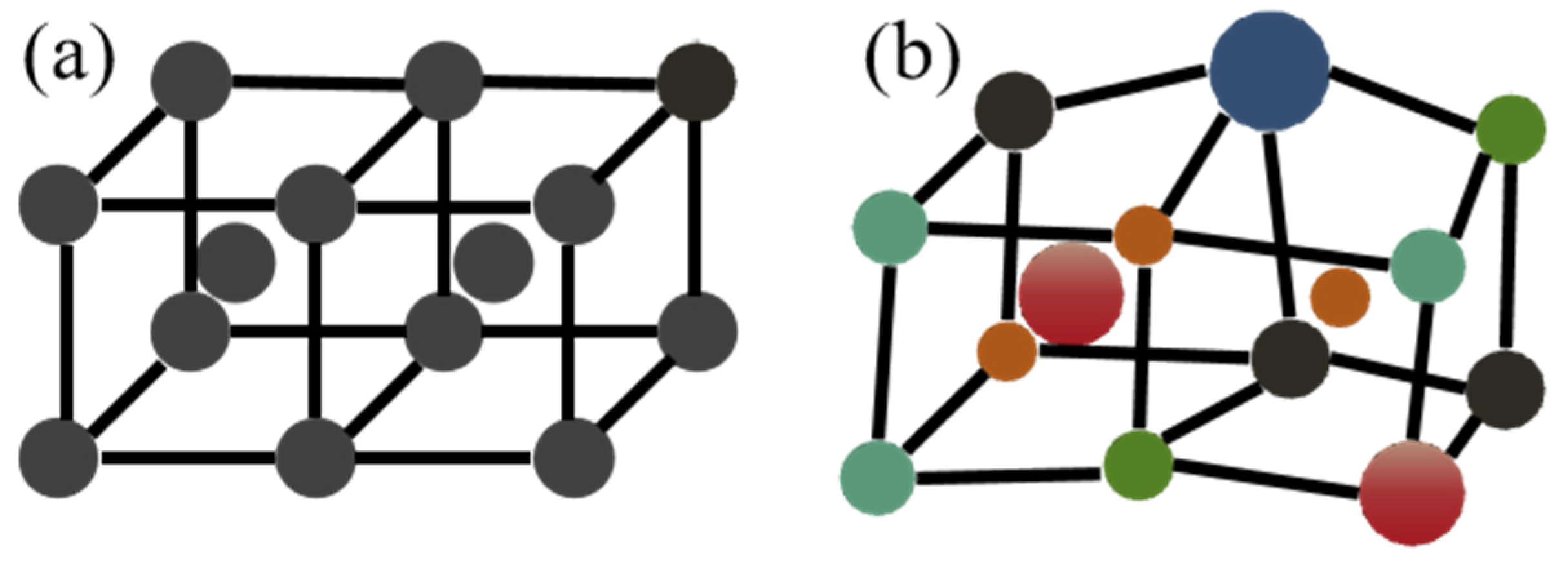
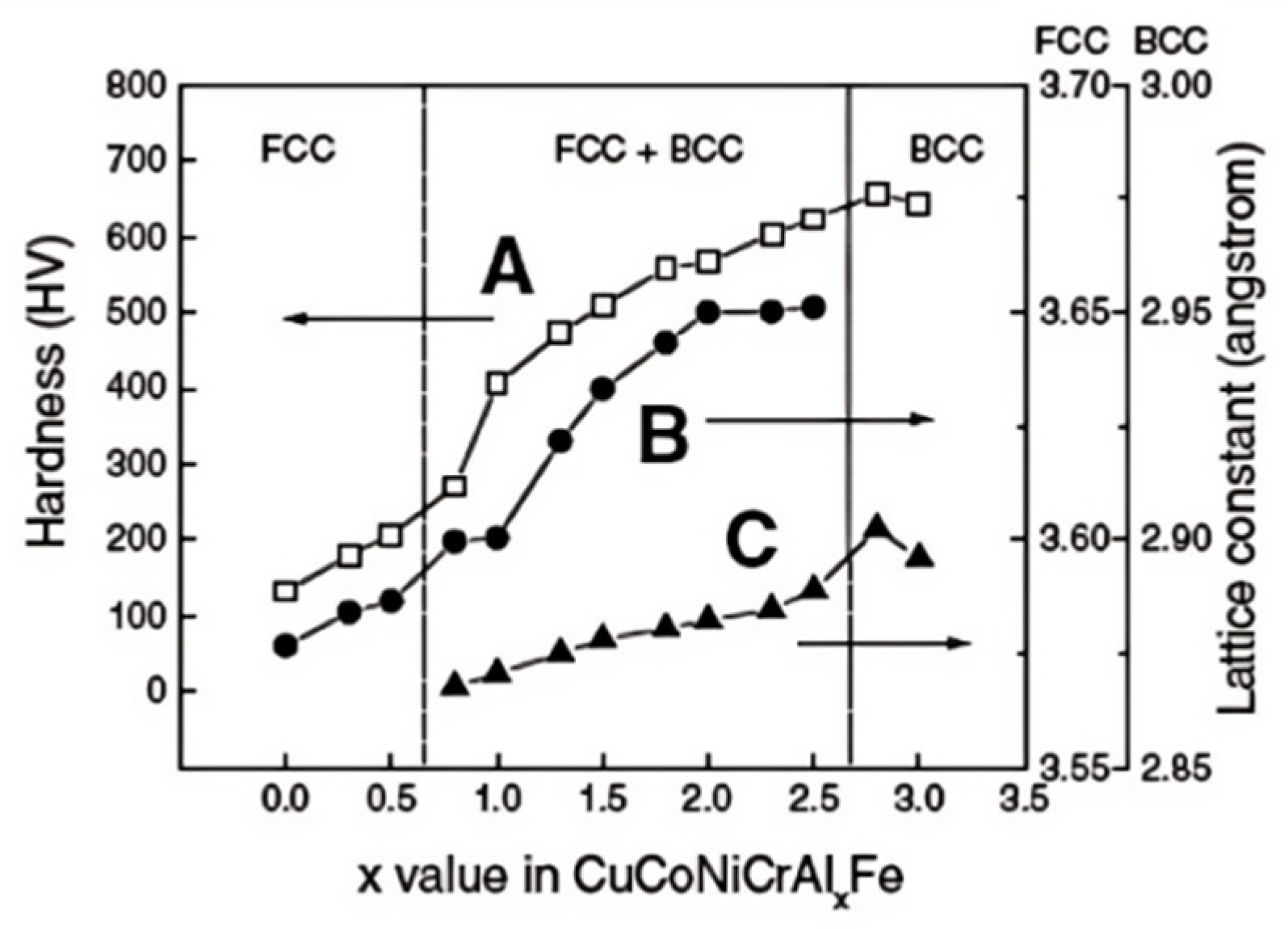
2.1. High-Entropy Effect of HEAs and Multicomponent Solders
2.2. Lattice Distortion Effect of HEAs and Multicomponent Solders
2.3. Sluggish Diffusion Effect of HEAs and Multicomponent Solders
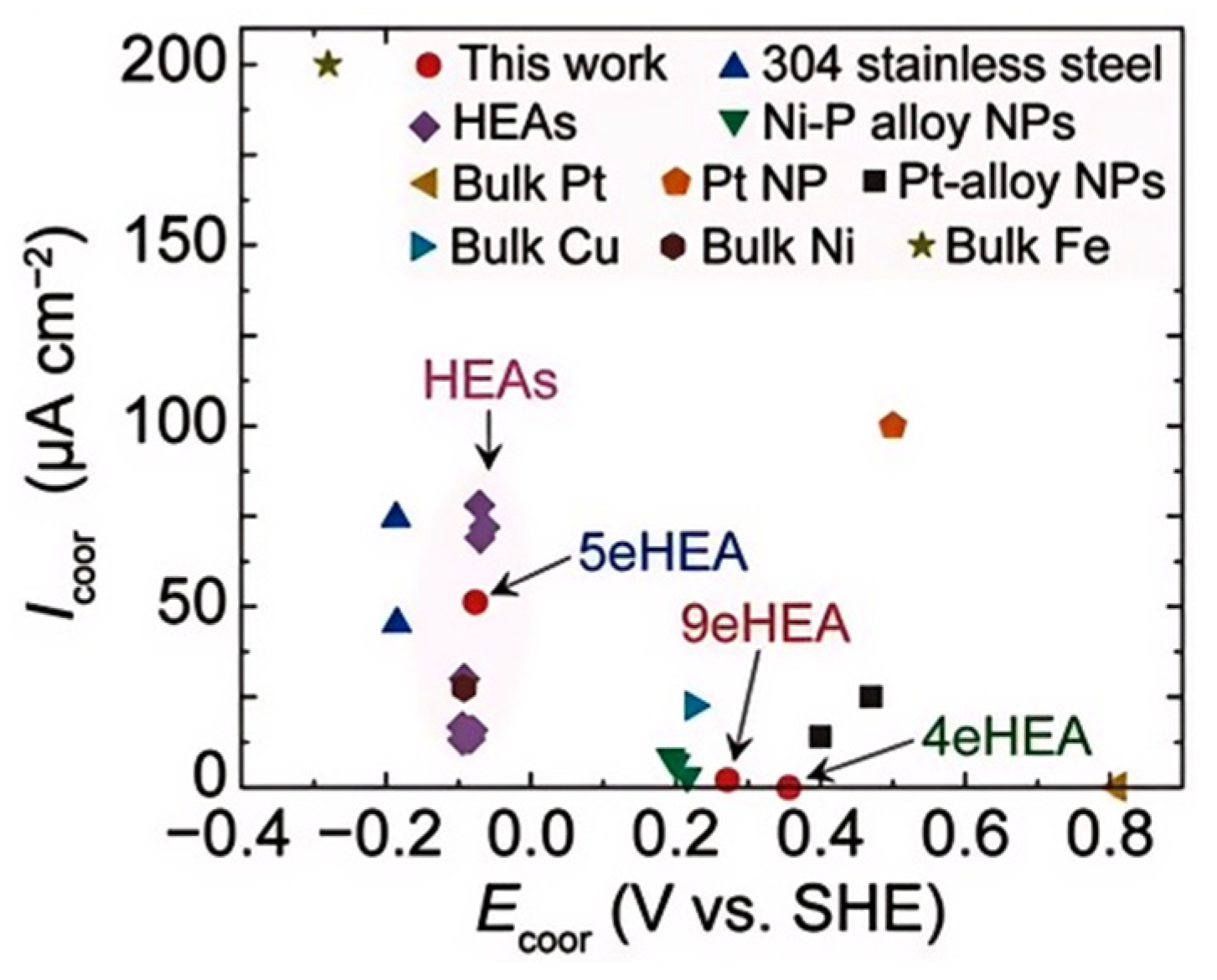
2.4. “Cocktail Effect” of HEAs and Multicomponent Solders
2.5. Research Significance and Application Prospects of Low-Temperature Multicomponent Solders in Electronic Packaging
3. Wettability of Low-Temperature Multicomponent Solders
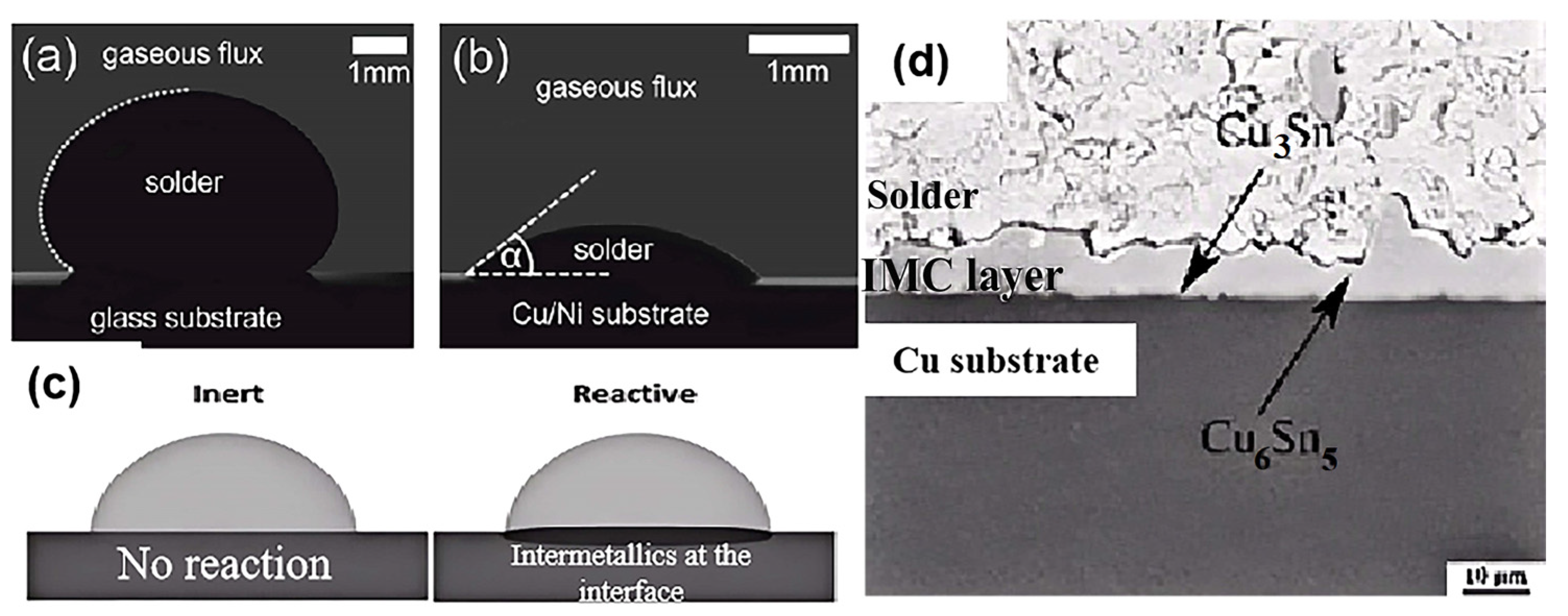
3.1. Interfacial Wetting Behavior
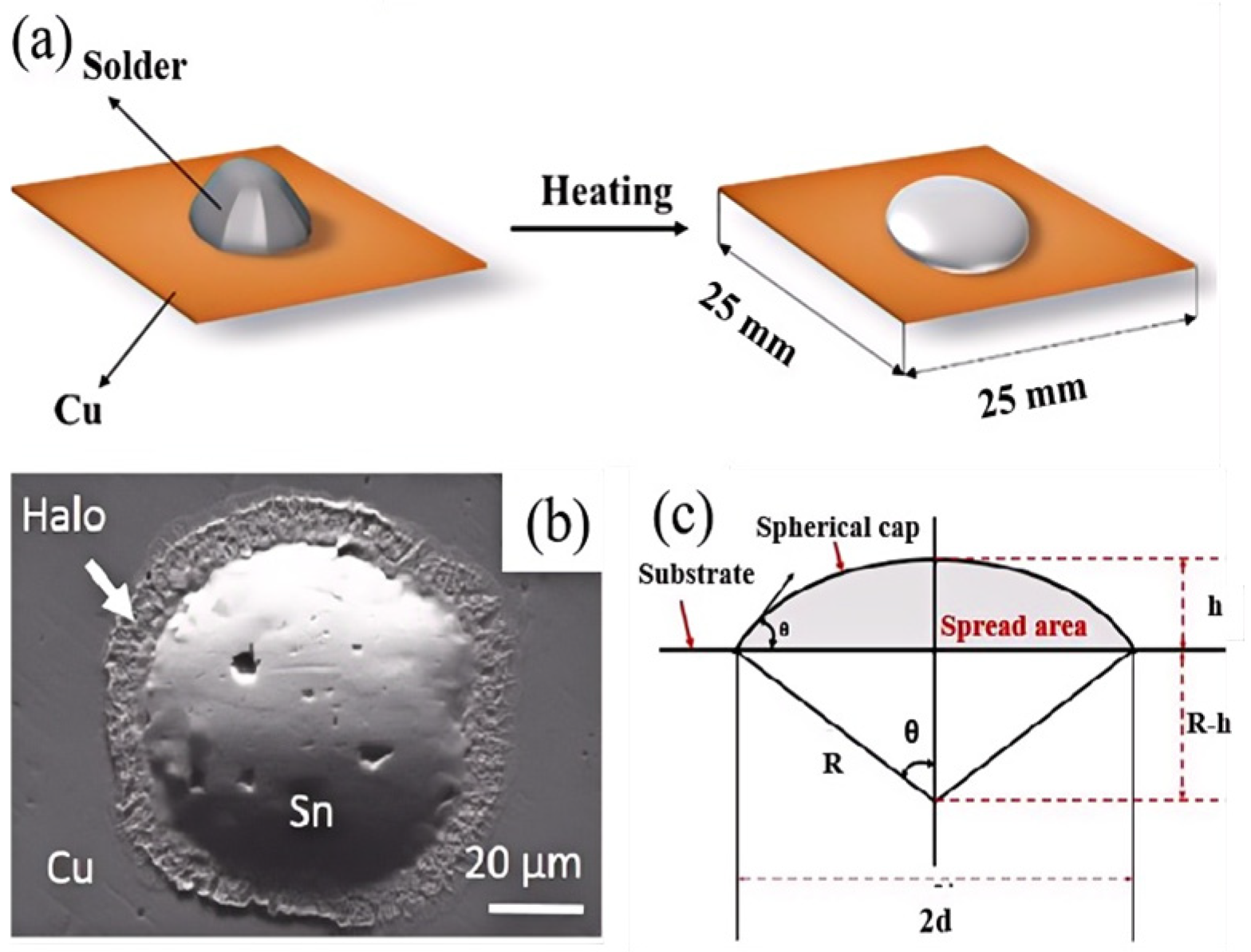
3.2. Wettability Analysis Method
3.3. Main Factors Affecting Wettability
4. Preparation of Low-Temperature Multicomponent Solders
4.1. Composition Design
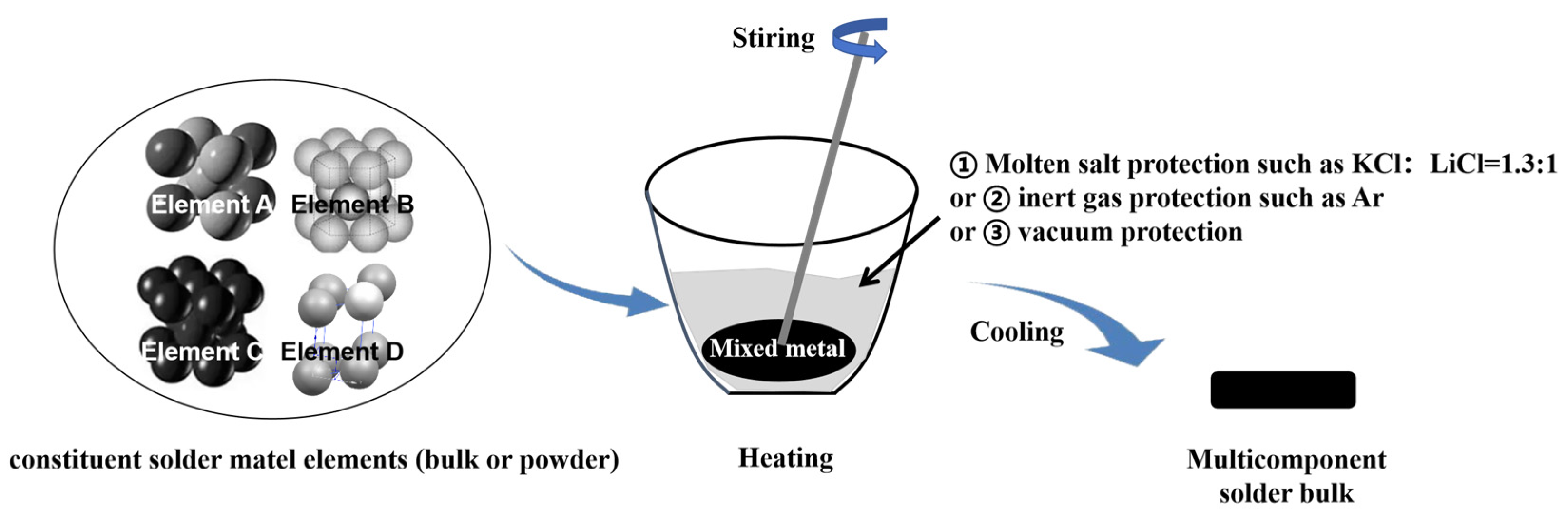
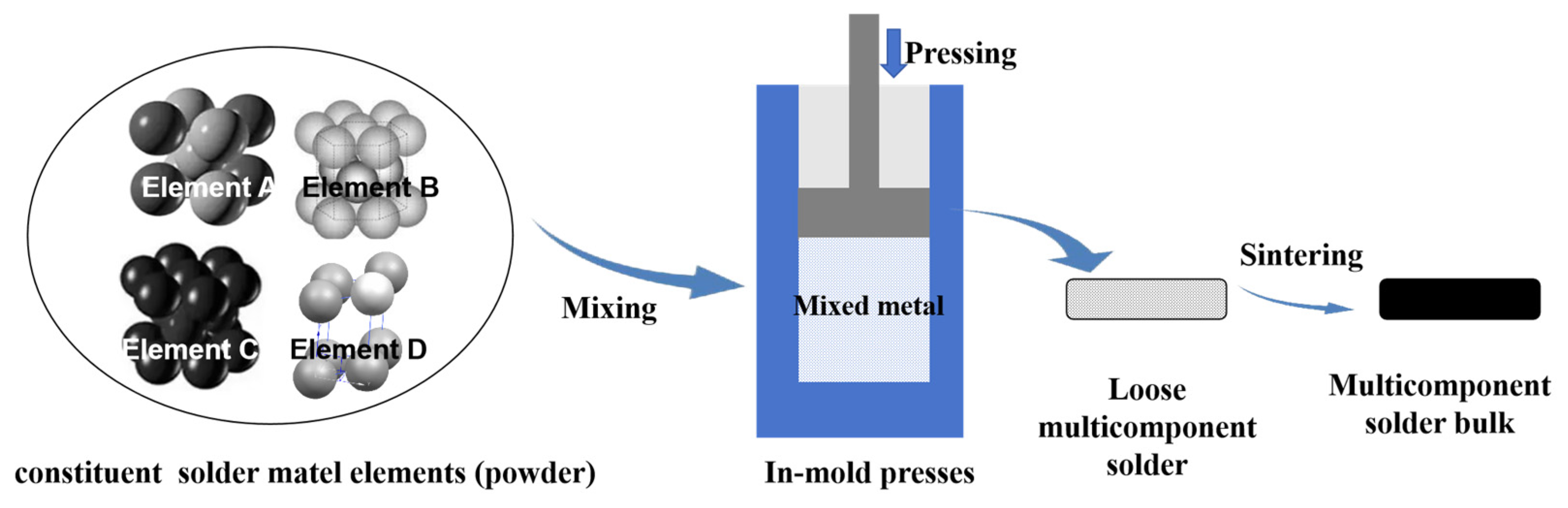
4.2. Multicomponent Solder Preparation Techniques
- (1)
- Melting Technique
- (2)
- Powder Metallurgy
5. Current Research on Low-Temperature Multicomponent Solders: Interface Reactions, Microstructure Characterization, and Performance Review
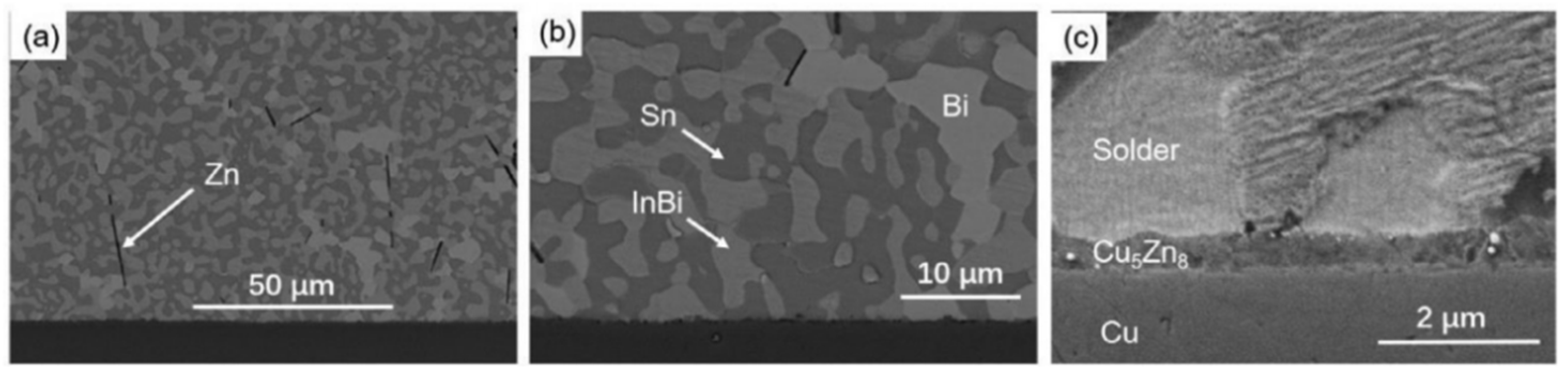
5.1. Four-Element Low-Temperature Multicomponent Solders
5.2. Five-Element Low-Temperature Multicomponent Solders
6. Future Research Directions and Prospects for Low-Temperature Multicomponent Solders
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| RoHS | Restriction of Hazardous Substances |
| WEEE | Waste Electrical and Electronic Equipment Directive |
References
- Wan, Y.-J.; Li, G.; Yao, Y.-M.; Zeng, X.-L.; Zhu, P.-L.; Sun, R. Recent advances in polymer-based electronic packaging materials. Compos. Commun. 2020, 19, 154–167. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, Y.; Yin, Y. Reliability of wide band gap power electronic semiconductor and packaging: A review. Energies 2022, 15, 6670. [Google Scholar] [CrossRef]
- Šír, M.; Feňo, I. Cooling of minimized surface-mount packages in power electronics applications. Przegląd Elektrotechniczny 2020, 96, 151–154. [Google Scholar] [CrossRef]
- Yang, Y.; Dorn-Gomba, L.; Rodriguez, R.; Mak, C.; Emadi, A. Automotive power module packaging: Current status and future trends. IEEE Access 2020, 8, 160126–160144. [Google Scholar] [CrossRef]
- Long, X.; Jia, Q.; Shen, Z.; Liu, M.; Guan, C. Strain rate shift for constitutive behaviour of sintered silver nanoparticles under nanoindentation. Mech. Mater. 2021, 158, 103881. [Google Scholar] [CrossRef]
- Zhang, P.; Xue, S.; Wang, J. New challenges of miniaturization of electronic devices: Electromigration and thermomigration in lead-free solder joints. Mater. Des. 2020, 192, 108726. [Google Scholar] [CrossRef]
- He, H.; Peng, W.; Liu, J.; Chan, X.Y.; Liu, S.; Lu, L.; Le Ferrand, H. Microstructured BN composites with internally designed high thermal conductivity paths for 3D electronic packaging. Adv. Mater. 2022, 34, 2205120. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, C.; Wen, Y.; Xue, Z.; Zhou, X.; Shi, D.; Hu, G.-H.; Xie, X. Novel micro-nano epoxy composites for electronic packaging application: Balance of thermal conductivity and processability. Compos. Sci. Technol. 2021, 209, 108760. [Google Scholar] [CrossRef]
- Li, H.; Moon, K.-S.; Li, Y.; Fan, L.; Xu, J.; Wong, C. Reliability enhancement of electrically conductive adhesives in thermal shock environment [electronics packaging]. In Proceedings of the 2004 54th Electronic Components and Technology Conference (IEEE Cat. No. 04CH37546), Las Vegas, NV, USA, 4 June 2004; pp. 165–169. [Google Scholar]
- Islam, M.; Chan, Y.; Rizvi, M.; Jillek, W. Investigations of interfacial reactions of Sn–Zn based and Sn–Ag–Cu lead-free solder alloys as replacement for Sn–Pb solder. J. Alloys Compd. 2005, 400, 136–144. [Google Scholar] [CrossRef]
- Directive, E. Restriction of the use of certain hazardous substances in electrical and electronic equipment (RoHS). Off. J. Eur. Communities 2013, 46, 19–23. [Google Scholar]
- Maxianova, K.; Rusche, T.M. Restriction of Hazardous Substances: On the Need for and the Limits of Comitology. Rev. Eur. Community Int. Environ. Law 2006, 15, 202–210. [Google Scholar] [CrossRef]
- Azizi, D.D.S.; Hanafiah, M.M.; Woon, K.S. Material flow analysis in WEEE management for circular economy: A content review on applications, limitations, and future outlook. Sustainability 2023, 15, 3505. [Google Scholar] [CrossRef]
- Lu, Y.; Pei, W.; Peng, K. State of the art of automatic disassembly of WEEE and perspective towards intelligent recycling in the era of Industry 4.0. Int. J. Adv. Manuf. Technol. 2023, 128, 2825–2843. [Google Scholar] [CrossRef]
- Long, X.; Lu, C.; Su, Y.; Dai, Y. Machine learning framework for predicting the low cycle fatigue life of lead-free solders. Eng. Fail. Anal. 2023, 148, 107228. [Google Scholar] [CrossRef]
- El-Taher, A.; Ali, H.E.; Algarni, H. Enhancing performance of Sn–Ag–Cu alloy through germanium additions: Investigating microstructure, thermal characteristics, and mechanical properties. Mater. Today Commun. 2024, 38, 108315. [Google Scholar] [CrossRef]
- Huang, J.; Wang, W.; Xiang, Q.; Qin, S.; Wang, P.; Mitsuzaki, N.; Chen, Z. Effect of deposition potential on electrodeposition of Sn-Ag-Cu ternary alloy solderable coating in deep eutectic solvent. J. Electroanal. Chem. 2023, 943, 117613. [Google Scholar] [CrossRef]
- Wakamoto, K.; Namazu, T. Mechanical Characterization of Sintered Silver Materials for Power Device Packaging: A Review. Energies 2024, 17, 4105. [Google Scholar] [CrossRef]
- Nafis, B.M. Capabilities of Sintered Silver as a High Temperature Packaging Material. Ph.D. Thesis, University of Arkansas, Fayetteville, AR, USA, 2023. [Google Scholar]
- Castillo, E.; Pasha, A.F.; Larson, Z.I.; Dimitrov, N. New generation copper-based interconnection from nanoporous CuSn alloy film sintered at low temperatures. Mater. Adv. 2024, 5, 2285–2295. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, F.; Li, Z.; Liu, H.; Tang, R.; Yin, H.; Zhang, J.; Yang, S.; Dong, D. Dual-cluster interpretation of Au–Sn binary eutectics and solders. AIP Adv. 2024, 14, 035010. [Google Scholar] [CrossRef]
- Nguyen, P.N.; Kim, W. Enhancing the electrochemical performance of Sn-Zn alloy anode foil for lithium-ion batteries through microstructure design via accumulative roll bonding technique. J. Power Sources 2024, 594, 233988. [Google Scholar] [CrossRef]
- Zhao, J.; Ji, X.; Jia, Q.; Wang, Y.; Ma, L.; Wang, Y.; Guo, F. Effect of electric pulse incubation melt on solidified microstructures and mechanical properties of Sn58Bi alloy. J. Mater. Sci. Mater. Electron. 2023, 34, 2033. [Google Scholar] [CrossRef]
- Qiu, Z.; Shen, X.; Zhao, Z. Development Trends and Prospects of Semiconductor Devices and Technology. Highlights Sci. Eng. Technol. 2024, 81, 374–380. [Google Scholar] [CrossRef]
- Jayaram, V.; Gupte, O.; Bhangaonkar, K.; Nair, C. A review of low-temperature solders in microelectronics packaging. IEEE Trans. Compon. Packag. Manuf. Technol. 2023, 13, 570–579. [Google Scholar] [CrossRef]
- Wang, S.; Chen, X.; Luo, K.; Zhou, H.; Li, R.; He, P.; Paik, K.-W.; Zhang, S. The design of low-temperature solder alloys and the comparison of mechanical performance of solder joints on ENIG and ENEPIG interface. J. Mater. Res. Technol. 2023, 27, 5332–5339. [Google Scholar] [CrossRef]
- Mahmood, S.; Ahamad, N.; Kant, C.; Khan, A.; Wu, P.-W.; Jian, W.-B.; Chu, C.W.; Katiyar, M.; Lin, H.-C. Self-cleaning and fully polymer-based super-moisture-resistant gas barrier coating films with 2D polymers for flexible electronic devices and packaging applications. J. Mater. Chem. C 2023, 11, 15907–15917. [Google Scholar] [CrossRef]
- Tang, M.; Jiang, Z.; Wang, Z.; Qin, Y.; Jiang, Y.; Wu, L.; Li, Z. High-adhesion PDMS/Ag conductive composites for flexible hybrid integration. Chem. Eng. J. 2023, 451, 138730. [Google Scholar] [CrossRef]
- Wang, R.; Qu, C.; Wang, D.; Zhao, L.; Fan, X.; Sun, Q.; Liu, S.; Tan, L.; Cui, X.; Zhang, S. A study of the residual stress behavior of rigid and flexible epoxy adhesives during thermal cycle aging for electronics packaging. J. Adhes. Sci. Technol. 2024, 38, 517–532. [Google Scholar] [CrossRef]
- Skafi, Z.; Castriotta, L.A.; Taheri, B.; Matteocci, F.; Fahland, M.; Jafarzadeh, F.; Joseph, E.; Chakraborty, A.; Singh, V.; Mottaghitalab, V. Flexible Perovskite Solar Cells on Polycarbonate Film Substrates. Adv. Energy Mater. 2024, 14, 2400912. [Google Scholar] [CrossRef]
- Azli, D.A.B.M.; Ramli, M.B.; Zakaria, M.S.B.; Omar, G.B.; Dziaudin, A.F.B. Validation of Thermoplastic Urethane Substrates for Stretchable Circuits: Experimental and Simulation Approach. In Proceedings of the 9th International Conference and Exhibition on Sustainable Energy and Advanced Materials, Putrajaya, Malaysia, 14 September 2023; pp. 413–417. [Google Scholar]
- Lall, P.; Kulkarni, S.; Miller, S. Performance Analysis of Screen-Printed Functional Circuits on Biodegradable PET Substrates Using Low-Temperature ECA for SMD Component Attachment. In Proceedings of the 2024 23rd IEEE Intersociety Conference on Thermal and Thermomechanical Phenomena in Electronic Systems (ITherm), Aurora, CO, USA, 28–31 May 2024; pp. 1–10. [Google Scholar]
- Huang, Y.-C.; Hu, H.-W.; Liu, Y.-H.; Hsieh, H.-C.; Chen, K.-N. Investigation of Photosensitive Polyimide with Low Coefficient of Thermal Expansion and Excellent Adhesion Strength for Advanced Packaging Applications. IEEE J. Electron. Devices Soc. 2024, 12, 96–103. [Google Scholar] [CrossRef]
- Ding, Z.; Li, Y.; Jiang, H.; Zhou, Y.; Wan, H.; Qiu, J.; Jiang, F.; Tan, J.; Du, W.; Chen, Y.A.; et al. The integral role of high-entropy alloys in advancing solid-state hydrogen storage. Interdiscip. Mater. 2024, 4, 75–108. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, J.; Qin, Y.; Kong, Y.; Zhou, S.; Li, Q.; Sun, Q.; Chen, B.; Xie, P.; Wei, Z. Recent Progress in High-Entropy Alloy Electrocatalysts for Hydrogen Evolution Reaction. Adv. Mater. Interfaces 2024, 11, 2301020. [Google Scholar] [CrossRef]
- Abdullah, M.R.; Peng, Z. Review and perspective on additive manufacturing of refractory high entropy alloys. Mater. Today Adv. 2024, 22, 100497. [Google Scholar] [CrossRef]
- Nagini, M.; Murty, B. Advanced high-entropy alloys: A next generation materials. Trans. Indian Natl. Acad. Eng. 2024, 9, 541–557. [Google Scholar] [CrossRef]
- Hsu, W.-L.; Tsai, C.-W.; Yeh, A.-C.; Yeh, J.-W. Clarifying the four core effects of high-entropy materials. Nat. Rev. Chem. 2024, 8, 471–485. [Google Scholar] [CrossRef]
- Han, X.; Ling, Y.; Yang, Y.; Wu, Y.; Gao, Y.; Wei, B.; Lv, Z. Utilizing high entropy effects for developing chromium-tolerance cobalt-free cathode for solid oxide fuel cells. Adv. Funct. Mater. 2023, 33, 2304728. [Google Scholar] [CrossRef]
- Wang, H.; He, Q.; Gao, X.; Shang, Y.; Zhu, W.; Zhao, W.; Chen, Z.; Gong, H.; Yang, Y. Multifunctional high entropy alloys enabled by severe lattice distortion. Adv. Mater. 2024, 36, 2305453. [Google Scholar] [CrossRef] [PubMed]
- Gu, G.H.; Kwon, H.; Kim, Y.; Haftlang, F.; Heo, Y.-U.; Kim, H.S. Unprecedented bake hardening responses of interstitial high-entropy alloy by synergistic effect with lattice distortion. Mater. Des. 2023, 233, 112289. [Google Scholar] [CrossRef]
- Verma, V.; Belcher, C.H.; Apelian, D.; Lavernia, E.J. Diffusion in high entropy alloy systems—A review. Prog. Mater. Sci. 2024, 142, 101245. [Google Scholar] [CrossRef]
- Sen, S.; Zhang, X.; Rogal, L.; Wilde, G.; Grabowski, B.; Divinski, S.V. ‘Anti-sluggish’Ti diffusion in HCP high-entropy alloys: Chemical complexity vs. lattice distortions. Scr. Mater. 2023, 224, 115117. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Lu, X.; Wu, F.; Sun, X.; Zhao, H.; Li, Q. Surprising cocktail effect in high entropy alloys on catalyzing magnesium hydride for solid-state hydrogen storage. Chem. Eng. J. 2023, 465, 142766. [Google Scholar] [CrossRef]
- Khan, F.; Rajendran, S.H.; Jung, J.P. Recent Advances in High Entropy Alloy Fillers for Brazing Similar and Dissimilar Materials: A Review. Met. Mater. Int. 2024, 30, 1145–1169. [Google Scholar] [CrossRef]
- Ujah, C.O.; Von Kallon, D.V. Characteristics of phases and processing techniques of high entropy alloys. Int. J. Lightweight Mater. Manuf. 2024, 7, 809–824. [Google Scholar] [CrossRef]
- Ye, X.; Diao, Z.; Lei, H.; Wang, L.; Li, Z.; Li, B.; Feng, J.; Chen, J.; Liu, X.; Fang, D. Multi-phase FCC-based composite eutectic high entropy alloy with multi-scale microstructure. Mater. Sci. Eng. A 2024, 889, 145815. [Google Scholar] [CrossRef]
- Cabrera, M.; Oropesa, Y.; Sanhueza, J.P.; Tuninetti, V.; Oñate, A. Multicomponent alloys design and mechanical response: From high entropy alloys to complex concentrated alloys. Mater. Sci. Eng. R. Rep. 2024, 161, 100853. [Google Scholar] [CrossRef]
- Afifi, M.A.; Nazir, Z.; Khan, M.A. Characterization and properties of high-entropy alloys materials. In High-Entropy Alloys; Elsevier: Amsterdam, The Netherlands, 2024; pp. 57–86. [Google Scholar]
- Wang, X.; Luo, G.; Wei, Q.; Sun, Y.; Huang, W.; Peng, J.; Zhang, J.; Shen, Q. Superior high-temperature strength of a carbide-reinforced high-entropy alloy with ultrafine eutectoid structure. Scr. Mater. 2025, 255, 116393. [Google Scholar] [CrossRef]
- Gu, J.; Duan, F.; Liu, S.; Cha, W.; Lu, J. Phase engineering of nanostructural metallic materials: Classification, structures, and applications. Chem. Rev. 2024, 124, 1247–1287. [Google Scholar] [CrossRef]
- Zhuo, L.; Xie, Y.; Chen, B. A review on recent progress of refractory high entropy alloys: From fundamental research to engineering applications. J. Mater. Res. Technol. 2024, 33, 1097–1129. [Google Scholar] [CrossRef]
- Xiong, W.; Guo, A.X.; Zhan, S.; Liu, C.-T.; Cao, S.C. Refractory high-entropy alloys: A focused review of preparation methods and properties. J. Mater. Sci. Technol. 2023, 142, 196–215. [Google Scholar] [CrossRef]
- Aksöz, S.; Esener, P.A.; Öztürk, E.; Maraşlı, N. Effects of Bi content on thermal, microstructure and mechanical properties of Sn-Bi-In-Zn solder alloy systems. J. Mater. Sci. Mater. Electron. 2022, 33, 11–26. [Google Scholar] [CrossRef]
- Jiang, N.; Bian, H.; Song, X.; Kim, H.S.; Lin, D.; Long, W.; Zhong, S.; Jia, L.; Hu, D. Microstructure and mechanical property of Zr-3/CoCrFeMnNi high-entropy alloys joints brazed using a novel ZrCu alloys. Mater. Charact. 2024, 217, 114411. [Google Scholar] [CrossRef]
- Song, X.; Jiang, N.; Bian, H.; Kim, H.S.; Lin, D.; Long, W.; Zhong, S.; Jia, L.; Hu, D. Microstructure evolution and strengthening mechanism of CoCrFeMnNi HEA/Zr-3 brazed joints reinforced by fine-grained BCC HEA and HCP Zr. J. Mater. Sci. Technol. 2024, 185, 32–47. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Lv, Z.; Wang, J.; Tian, F.; Zhang, L.; Zhang, W.; Chen, H.; Li, M. An innovative high-entropy alloy solder for high-reliability low-temperature bonding in 3D electronic packaging–based on nano InSnBiZnAg particles. J. Mater. Res. Technol. 2024, 30, 5622–5631. [Google Scholar] [CrossRef]
- He, Q.; Yang, Y. On lattice distortion in high entropy alloys. Front. Mater. 2018, 5, 42. [Google Scholar] [CrossRef]
- Yeh, J.-W. Recent progress in high entropy alloys. Ann. Chim. Sci. Mat 2006, 31, 633–648. [Google Scholar] [CrossRef]
- Wang, S.; Tian, R.; Wen, J.; Wang, W.; Feng, J.; Wang, S.; Tian, Y. SnPbInBiSb high-entropy solder joints with inhibited interfacial IMC growth and high shear strength. Intermetallics 2025, 176, 108551. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, Y.; Ling, Z.; Yuan, Z.; Shi, J. Advancements in diffusion barrier layers based on heterogeneous connection of electrode/thermoelectric materials. J. Alloys Compd. 2024, 1001, 175185. [Google Scholar] [CrossRef]
- Zhao, R.; Ma, L.; Zuo, Y.; Liu, S.; Guo, F. Retarding electromigration in lead-free solder joints by alloying and composite approaches. J. Electron. Mater. 2013, 42, 280–287. [Google Scholar] [CrossRef]
- He, B.; Zu, Y.; Mei, Y. Design of advanced electrocatalysts for the high-entropy alloys: Principle, progress, and perspective. J. Alloys Compd. 2023, 958, 170479. [Google Scholar] [CrossRef]
- Anamu, U.; Ayodele, O.; Olorundaisi, E.; Babalola, B.; Odetola, P.; Ogunmefun, A.; Ukoba, K.; Jen, T.-C.; Olubambi, P. Fundamental design strategies for advancing the development of high entropy alloys for thermo-mechanical application: A critical review. J. Mater. Res. Technol. 2023, 27, 4833–4860. [Google Scholar] [CrossRef]
- Lin, Q.; Wang, C.; Xie, K.; Wang, L.; Sui, R. Comparative study on wetting of smooth and rough silica surface by molten Sn–3.5 Ag–2Ti alloys. Ceram. Int. 2021, 47, 29205–29212. [Google Scholar] [CrossRef]
- Griffiths, S.; Wedi, A.; Schmitz, G. Work of adhesion and reactive wetting in SnPb/Cu, Ni and SnBi/Cu, Ni soldering systems. Mater. Charact. 2021, 178, 111304. [Google Scholar] [CrossRef]
- Li, P.; Zhang, J.; Yang, T.; Zhang, T.; Zhang, J.; Lin, J.; Yan, Y.; Li, C.; Si, X.; Cao, J. Characteristics, applications and perspective of high entropy alloys for interfacial joining: A review. J. Manuf. Process. 2024, 110, 303–317. [Google Scholar] [CrossRef]
- Gui, Z.; Hu, X.; Jiang, X.; Li, Y.; Wang, H. Interfacial reaction, wettability, and shear strength of ultrasonic-assisted lead-free solder joints prepared using Cu–GNSs-doped flux. J. Mater. Sci. Mater. Electron. 2021, 32, 24507–24523. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Wang, X.; Guo, Y.-H.; Sun, L.; Liu, Y.-X.; Chen, C.; Lu, X. Growth behavior and reliability of interfacial IMC for Sn58Bi/Cu and Sn58Bi–AlN/Cu solder joints applied in IGBT modules. J. Mater. Res. Technol. 2022, 21, 4263–4280. [Google Scholar] [CrossRef]
- Griffith, S.; Siddiqui, F.N.; Schmitz, G. Effect of surface roughness and droplet size on solder wetting angles. ACS Appl. Mater. Interfaces 2023, 15, 24999–25008. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.K.; Gergely, G.; Koncz-Horváth, D.; Gácsi, Z. Investigation of microstructure and wetting behavior of Sn–3.0 Ag–0.5 Cu (SAC305) lead-free solder with additions of 1.0 wt% SiC on copper substrate. Intermetallics 2021, 128, 106991. [Google Scholar] [CrossRef]
- Sehirli, E.; Erer, A.M.; Turan, M.K. A new approach for measuring the wetting angles of lead-free solder alloys from digital images. Eng. Sci. Technol. Int. J. 2022, 36, 101279. [Google Scholar] [CrossRef]
- Silva, B.L.; Gouveia, G.L.; Cheung, N.; Garcia, A.; Spinelli, J.E. Analysis of extensive wetting angle vs. cooling rate data in Bi-, Zn-and Sn-based solder alloys. Microelectron. Reliab. 2022, 135, 114593. [Google Scholar] [CrossRef]
- Shen, B.; Yang, S.; Xu, M.; Zhao, J.; Liu, G.; Xie, M.; Zhang, Q. Effect of in Addition on Thermodynamic Properties, Wettability, Interface Microstructure, and Soldering Performance of SnBiAg–xIn/Cu Solder Joints. Metals 2022, 12, 1594. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Pu, L.; Yang, Y.; He, Q.-F.; Zhou, Z.; Tan, C.; Zhao, X.; Zhang, Q.; Tu, K. A high-entropy alloy as very low melting point solder for advanced electronic packaging. Mater. Today Adv. 2020, 7, 100101. [Google Scholar] [CrossRef]
- Xie, J.; Tang, L.; Gao, P.; Zhang, Z.; Li, L. Effects of Ni addition on wettability and interfacial microstructure of Sn-0.7 Cu-xNi solder alloy. Solder. Surf. Mt. Technol. 2024; ahead-of-print. [Google Scholar] [CrossRef]
- Nadia, A.; Haseeb, A. Understanding the effects of addition of copper nanoparticles to Sn-3.5 Ag solder. Solder. Surf. Mt. Technol. 2011, 23, 68–74. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, Q.; Li, Z. Effect of Surface Finishes on the Welding of Sn58Bi Solder. J. Electron. Mater. 2022, 51, 1106–1115. [Google Scholar] [CrossRef]
- Shi, H.; Xian, A. Effect of organic acid in Isopropyl alcohol fluxes on wetting of Sn-Bi solder on Cu surface. In Proceedings of the 2011 12th International Conference on Electronic Packaging Technology and High Density Packaging, Shanghai, China, 8–11 August 2011; pp. 1–5. [Google Scholar]
- He, S.; Gao, R.; Li, J.; Shen, Y.-A.; Nishikawa, H. In-situ observation of fluxless soldering of Sn-3.0 Ag-0.5 Cu/Cu under a formic acid atmosphere. Mater. Chem. Phys. 2020, 239, 122309. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Liang, H.-T.; Chang, K.-C.; Wu, G.-W.; Tseng, T.-T.; Chen, Y. Partial Segregation of Bi and Microvoid Formation on a Pure Cu Substrate After Solid–Solid Reactions. J. Electron. Mater. 2023, 52, 4000–4010. [Google Scholar] [CrossRef]
- Plevachuk, Y.; Poverzhuk, V.; Svec Sr, P.; Svec, P.; Janotová, I.; Janickovic, D.; Rud, A. Electrical resistivity of lead-free solders reinforced by carbon nanospheres. Int. J. Thermophys. 2024, 45, 31. [Google Scholar] [CrossRef]
- Huang, B. Handbook of Nonferrous Metal Materials; Chemical Industry Press: Beijing, China, 2009. [Google Scholar]
- Morando, C.; Fornaro, O.; Garbellini, O.; Palacio, H. Thermal properties of Sn-based solder alloys. J. Mater. Sci. Mater. Electron. 2014, 25, 3440–3447. [Google Scholar] [CrossRef]
- Dele-Afolabi, T.; Ansari, M.; Hanim, M.A.; Oyekanmi, A.; Ojo-Kupoluyi, O.; Atiqah, A. Recent advances in Sn-based lead free solder interconnects for microelectronics packaging: Materials and technologies. J. Mater. Res. Technol. 2023, 25, 4231–4263. [Google Scholar] [CrossRef]
- Lu, M.; Shih, D.-Y.; Lauro, P.; Goldsmith, C.; Henderson, D.W. Effect of Sn grain orientation on electromigration degradation mechanism in high Sn-based Pb-free solders. Appl. Phys. Lett. 2008, 92, 211909. [Google Scholar] [CrossRef]
- Liashenko, O.Y.; Lay, S.; Hodaj, F. On the initial stages of phase formation at the solid Cu/liquid Sn-based solder interface. Acta Mater. 2016, 117, 216–227. [Google Scholar] [CrossRef]
- Feng, W.; Wang, C.; Morinaga, M. Electronic structure mechanism for the wettability of Sn-based solder alloys. J. Electron. Mater. 2002, 31, 185–190. [Google Scholar] [CrossRef]
- Fazio, C.; Sobolev, V.; Aerts, A.; Gavrilov, S.; Lambrinou, K.; Schuurmans, P.; Gessi, A.; Agostini, P.; Ciampichetti, A.; Martinelli, L. Handbook on Lead-Bismuth Eutectic Alloy and Lead Properties, Materials Compatibility, Thermal-Hydraulics and Technologies—2015 Edition; Organisation for Economic Co-Operation and Development: Paris, France, 2015. [Google Scholar]
- Wang, F.; Chen, H.; Huang, Y.; Liu, L.; Zhang, Z. Recent progress on the development of Sn–Bi based low-temperature Pb-free solders. J. Mater. Sci. Mater. Electron. 2019, 30, 3222–3243. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.-Q. Inhibition of intermetallic compounds growth at Sn–58Bi/Cu interface bearing CuZnAl memory particles (2–6 μm). J. Mater. Sci. Mater. Electron. 2020, 31, 2466–2480. [Google Scholar] [CrossRef]
- Raza, M.; Shewchenko, L.; Olofinjana, A.; Kent, D.; Mata, J.; Haque, R. The effects of Bi substitution for Sn on mechanical properties of Sn-based lead-free solders. J. Mater. Sci. Mater. Electron. 2021, 32, 22155–22167. [Google Scholar] [CrossRef]
- Matahir, M.; Chin, L.; Tan, K.; Olofinjana, A. Mechanical strength and its variability in Bi-modified Sn-Ag-Cu solder alloy. J. Achiev. Mater. Manuf. Eng. 2011, 46, 50–56. [Google Scholar]
- Yang, S.; Ho, C.; Chang, C.; Kao, C. Strong Zn concentration effect on the soldering reactions between Sn-based solders and Cu. J. Mater. Res. 2006, 21, 2436–2439. [Google Scholar] [CrossRef]
- Liu, S.; Xue, S.-B.; Xue, P.; Luo, D.-X. Present status of Sn–Zn lead-free solders bearing alloying elements. J. Mater. Sci. Mater. Electron. 2015, 26, 4389–4411. [Google Scholar] [CrossRef]
- Yu, C.-Y.; Duh, J.-G. Stabilization of hexagonal Cu6(Sn, Zn)5 by minor Zn doping of Sn-based solder joints. Scr. Mater. 2011, 65, 783–786. [Google Scholar] [CrossRef]
- Gunn, G. Critical Metals Handbook; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Liu, Y.; Tu, K. Low melting point solders based on Sn, Bi, and In elements. Mater. Today Adv. 2020, 8, 100115. [Google Scholar] [CrossRef]
- Deshpande, M.C.; Chaudhari, R.; Narayanan, R.; Kale, H. Study of mechanical properties of indium-based solder alloys for cryogenic applications. Solder. Surf. Mt. Technol. 2022, 34, 212–221. [Google Scholar] [CrossRef]
- Wang, C.-H.; Li, K.-T.; Yen, Y.-W. Effects of Minor Ga Addition on Interfacial Reactions Between Sn-Ga Solders and Cu. JOM 2024, 76, 2731–2740. [Google Scholar] [CrossRef]
- Yang, X.; He, J.; Xu, S.; Zhang, D.; Fu, L.; Zhang, S.; Kai, X.; Zhang, X.; Pi, L.; Mao, Y. Microstructure and brazing properties of a novel Ag–Cu-Ga solder. J. Mater. Res. Technol. 2023, 23, 1515–1527. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Z.; Yang, S.; Fan, M.; Cheng, X. Microstructure and mechanical properties of Sn-xGa alloys and solder joints. J. Mater. Res. Technol. 2023, 26, 3830–3839. [Google Scholar] [CrossRef]
- Chen, T.; Dutta, I. Effect of Ag and Cu concentrations on the creep behavior of Sn-based solders. J. Electron. Mater. 2008, 37, 347–354. [Google Scholar] [CrossRef]
- Huang, M.; Loeher, T.; Ostmann, A.; Reichl, H. Role of Cu in dissolution kinetics of Cu metallization in molten Sn-based solders. Appl. Phys. Lett. 2005, 86, 181908. [Google Scholar] [CrossRef]
- Shen, Y.-A.; Yang, X.-M.; Tsai, C.-Y.; Ouyang, Y.-H.; Tsai, M.-H.; Shun, T.-T. Effect of Cu on the interfacial reaction between Sn-based solders and FeCoNiCu alloys. Intermetallics 2022, 144, 107530. [Google Scholar] [CrossRef]
- Smith, D.R.; Fickett, F. Low-temperature properties of silver. J. Res. Natl. Inst. Stand. Technol. 1995, 100, 119. [Google Scholar] [CrossRef]
- Hadian, F.; Schoeller, H.; Cotts, E. Correlation between the growth of voids and Ni 3 Sn 4 intermetallic compounds at SnAg/Ni and SnAgCuBiSbNi/Ni interfaces at temperatures up to 200 C. J. Electron. Mater. 2020, 49, 226–240. [Google Scholar] [CrossRef]
- Amagai, M.; Watanabe, M.; Omiya, M.; Kishimoto, K.; Shibuya, T. Mechanical characterization of Sn–Ag-based lead-free solders. Microelectron. Reliab. 2002, 42, 951–966. [Google Scholar] [CrossRef]
- Bui, Q.; Nam, N.; Noh, B.I.; Kar, A.; Kim, J.G.; Jung, S.B. Effect of Ag addition on the corrosion properties of Sn-based solder alloys. Mater. Corros. 2010, 61, 30–33. [Google Scholar] [CrossRef]
- Adetunji, O.R.; Ashimolowo, R.A.; Aiyedun, P.O.; Adesusi, O.M.; Adeyemi, H.O.; Oloyede, O.R. Tensile, hardness and microstructural properties of Sn-Pb solder alloys. Mater. Today Proc. 2021, 44, 321–325. [Google Scholar] [CrossRef]
- Li, M.; Lee, K.; Olsen, D.; Chen, W.T.; Tan, B.T.C.; Mhaisalkar, S. Microstructure, joint strength and failure mechanisms of SnPb and Pb-free solders in BGA packages. IEEE Trans. Electron. Packag. Manuf. 2002, 25, 185–192. [Google Scholar]
- Lin, Q.; Ye, C.; Sui, R. Wetting of Ni-based amorphous and crystalline alloys by Sn and Sn-based solders. Microelectron. Reliab. 2020, 111, 113722. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, Y.; Huang, J.; Zhao, W.; Liu, X.; Li, M.; Ji, H. Ultrasonic-accelerated metallurgical reaction of Sn/Ni composite solder: Principle, kinetics, microstructure, and joint properties. Ultrason. Sonochem. 2020, 66, 105090. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Li, M.; Ma, S.; Li, M. Ni3Sn4-composed die bonded interface rapidly formed by ultrasonic-assisted soldering of Sn/Ni solder paste for high-temperature power device packaging. Mater. Des. 2016, 108, 590–596. [Google Scholar] [CrossRef]
- Wu, C.; Yu, D.; Law, C.; Wang, L. Properties of lead-free solder alloys with rare earth element additions. Mater. Sci. Eng. R. Rep. 2004, 44, 1–44. [Google Scholar] [CrossRef]
- Guo, F.; Zhao, M.; Xia, Z.; Lei, Y.; Li, X.; Shi, Y. Lead-free solders with rare earth additions. JOM 2009, 61, 39–44. [Google Scholar] [CrossRef]
- Hao, H.; Tian, J.; Shi, Y.; Lei, Y.; Xia, Z. Properties of Sn3. 8Ag0. 7Cu solder alloy with trace rare earth element Y additions. J. Electron. Mater. 2007, 36, 766–774. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, X.-Y.; Guo, Y.-H.; He, C.-W. Properties enhancement of SnAgCu solders containing rare earth Yb. Mater. Des. 2014, 57, 646–651. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, Y.; Zhang, J.; Liu, Y.; Hu, X.; Jiang, X. Effect of rare earth Ce on the thermal behavior, microstructure and mechanical properties of Zn–30Sn–2Cu high temperature lead-free solder alloy. J. Mater. Sci. Mater. Electron. 2020, 31, 16437–16447. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Dai, J.; Jing, Y.; Ge, J.; Zhang, N. Microstructure, interfacial IMC and mechanical properties of Sn–0.7 Cu–xAl (x = 0–0.075) lead-free solder alloy. Mater. Des. 2015, 67, 209–216. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, L.; Zhang, Y.; Liu, P.; Zhang, N.; Zhou, S.; Jiang, L. Microstructure and reliability of Mo nanoparticle reinforced Sn–58Bi-based lead-free solder joints. Mater. Sci. Technol. 2018, 34, 992–1002. [Google Scholar] [CrossRef]
- Liu, H.; Guo, W.; Xue, H.; Zhang, X. Effect of Active Al on the Microstructure and Mechanical Properties of a Mo/Sn-Based Solder Interface: First-Principles Calculation and Experimental Study. J. Electron. Mater. 2020, 49, 6754–6762. [Google Scholar] [CrossRef]
- Fan, J.; Liu, Z.; Zhai, H.; Wang, X.; Wang, Y.; Li, Y.; Zhou, X.; Wu, S.; Liu, J. Effect of Co content on the microstructure, spreadability, conductivity and corrosion resistance of Sn-0.7 Cu alloy. Microelectron. Reliab. 2020, 107, 113615. [Google Scholar] [CrossRef]
- Koleňák, R.; Kostolný, I.; Drápala, J.; Kusý, M.; Pašák, M. Research on soldering AlN ceramics with Cu substrate using Sn-Ag-Ti solder. Solder. Surf. Mt. Technol. 2019, 31, 93–101. [Google Scholar] [CrossRef]
- Mu, D.; McDonald, S.; Read, J.; Huang, H.; Nogita, K. Critical properties of Cu6Sn5 in electronic devices: Recent progress and a review. Curr. Opin. Solid State Mater. Sci. 2016, 20, 55–76. [Google Scholar] [CrossRef]
- Xian, J.; Belyakov, S.; Ollivier, M.; Nogita, K.; Yasuda, H.; Gourlay, C. Cu6Sn5 crystal growth mechanisms during solidification of electronic interconnections. Acta Mater. 2017, 126, 540–551. [Google Scholar] [CrossRef]
- Kumar, S.; Jung, J. Mechanical and electronic properties of Ag3Sn intermetallic compound in lead free solders using ab initio atomistic calculation. Mater. Sci. Eng. B 2013, 178, 10–21. [Google Scholar] [CrossRef]
- Min, Z.; Qiu, Y.; Hu, X.; Wang, H. Effect of Cu6Sn5 nanoparticles size on the properties of Sn0.3Ag0.7Cu nano-composite solders and joints. J. Mater. Sci. Mater. Electron. 2019, 30, 14726–14735. [Google Scholar] [CrossRef]
- Hu, X.; Qiu, Y.; Jiang, X.; Li, Y. Effect of Cu6Sn5 nanoparticle on thermal behavior, mechanical properties and interfacial reaction of Sn3.0Ag0.5Cu solder alloys. J. Mater. Sci. Mater. Electron. 2018, 29, 15983–15993. [Google Scholar] [CrossRef]
- Guo, B.; Kunwar, A.; Zhao, N.; Chen, J.; Wang, Y.; Ma, H. Effect of Ag3Sn nanoparticles and temperature on Cu6Sn5 IMC growth in Sn-xAg/Cu solder joints. Mater. Res. Bull. 2018, 99, 239–248. [Google Scholar] [CrossRef]
- Kınacı, A.; Haskins, J.B.; Sevik, C.; Çağın, T. Thermal conductivity of BN-C nanostructures. Phys. Rev. B—Condens. Matter Mater. Phys. 2012, 86, 115410. [Google Scholar] [CrossRef]
- Naclerio, A.E.; Kidambi, P.R. A review of scalable hexagonal boron nitride (h-BN) synthesis for present and future applications. Adv. Mater. 2023, 35, 2207374. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, Z.; Xu, Y.; Li, Y.; Zhong, J.; Wang, X.; Zhang, Y. Effect of trace boron nitride nanoparticles on the microstructure and shear properties of Sn58Bi solder joint. J. Mater. Eng. Perform. 2024, 33, 9336–9345. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, H.; Sun, F.; Zhang, H.; Kong, X.; Xin, T. Microstructure and mechanical properties of as-reflowed Sn58Bi composite solder pastes. J. Mater. Process. Technol. 2016, 238, 290–296. [Google Scholar] [CrossRef]
- Sharma, B.; Kumar, M.; Kumar, V.; Sharma, A. Boron nitride nanotubes modified on a lead-free solder alloy for microelectromechanical packaging. ACS Appl. Nano Mater. 2022, 5, 13626–13636. [Google Scholar] [CrossRef]
- Robertson, J. Realistic applications of CNTs. Mater. Today 2004, 7, 46–52. [Google Scholar] [CrossRef]
- Soni, S.K.; Thomas, B.; Kar, V.R. A comprehensive review on CNTs and CNT-reinforced composites: Syntheses, characteristics and applications. Mater. Today Commun. 2020, 25, 101546. [Google Scholar] [CrossRef]
- Ibrahim, K.S. Carbon nanotubes-properties and applications: A review. Carbon Lett. 2013, 14, 131–144. [Google Scholar] [CrossRef]
- Tian, R.; Wang, C.; Huang, Y.; Guo, X. Effects of nanoparticle addition on the reliability of Sn-based Pb-free solder joints under various conditions: A review. Nano 2023, 18, 2330001. [Google Scholar] [CrossRef]
- Li, L.; Qin, W.; Mai, B.; Qi, D.; Yang, W.; Feng, J.; Zhan, Y. Effect of Carbon Nanotubes on the Mechanical, Thermal, and Electrical Properties of Tin-Based Lead-Free Solders: A Review. Crystals 2023, 13, 789. [Google Scholar] [CrossRef]
- Xu, K.-K.; Zhang, L.; Jiang, N. Effect of CNTs on the intermetallic compound growth between Sn solder and Cu substrate during aging and reflowing. J. Mater. Sci. Mater. Electron. 2021, 32, 2655–2666. [Google Scholar] [CrossRef]
- Rao, C.; Biswas, K.; Subrahmanyam, K.; Govindaraj, A. Graphene, the new nanocarbon. J. Mater. Chem. 2009, 19, 2457–2469. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Sahoo, S.; Wang, N.; Huczko, A. Graphene research and their outputs: Status and prospect. J. Sci. Adv. Mater. Devices 2020, 5, 10–29. [Google Scholar] [CrossRef]
- Li, Y.; Yu, S.; Li, L.; Song, S.; Qin, W.; Qi, D.; Yang, W.; Zhan, Y. A review on the development of adding graphene to Sn-based lead-free solder. Metals 2023, 13, 1209. [Google Scholar] [CrossRef]
- Dušek, K.; Veselý, P.; Bušek, D.; Petráč, A.; Géczy, A.; Illés, B.; Krammer, O. Influence of Flux and Related Factors on Intermetallic Layer Growth within SAC305 Solder Joints. Materials 2021, 14, 7909. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xiu, Z.; Wu, G.; Tian, Y.; He, P.; Gu, X.; Long, W. Improving shear strength of Sn-3.0 Ag-0.5 Cu/Cu joints and suppressing intermetallic compounds layer growth by adding graphene nanosheets. Mater. Lett. 2016, 169, 262–264. [Google Scholar] [CrossRef]
- Liu, X.; Han, Y.; Jing, H.; Wei, J.; Xu, L. Effect of graphene nanosheets reinforcement on the performance of Sn Ag Cu lead-free solder. Mater. Sci. Eng. A 2013, 562, 25–32. [Google Scholar] [CrossRef]
- Ahmad, I.; Nazeri, M.F.M.; Salleh, N.A.; Kheawhom, S.; Erer, A.M.; Kurt, A.; Mohamad, A.A. Selective electrochemical etching of the Sn-3Ag-0.5 Cu/0.07 wt% graphene nanoparticle composite solder. Arab. J. Chem. 2021, 14, 103392. [Google Scholar] [CrossRef]
- Gain, A.K.; Zhang, L. The effects of TiO2 nanoparticles addition on the thermal shock resistance, shear strength and IMC layer growth of SAC305 alloy. Materialia 2018, 3, 64–73. [Google Scholar] [CrossRef]
- Gharaibeh, A.; Dayoub, A.; Medgyes, B. Understanding Electrochemical Migration Behavior in Fe2O3-Enhanced SAC Lead-Free Alloys. In Proceedings of the 2024 IEEE 30th International Symposium for Design and Technology in Electronic Packaging (SIITME), Sibiu, Romania, 16–18 October 2024; pp. 314–319. [Google Scholar]
- Bachok, Z.; Saad, A.; Abas, M.; Ali, M.; Fakpan, K. Structural analysis on nanocomposites lead free solder using nanoindentation. J. Adv. Manuf. Technol. (JAMT) 2022, 16, 15–28. [Google Scholar]
- Shuai, W.; FENG, J.-y.; Wei, W.; CAO, W.-c.; Xin, D.; Shang, W.; TIAN, Y.-h. Growth kinetics of interfacial intermetallic compounds formed in SnPbInBiSb high entropy alloy soldered joints on Cu substrates. Trans. Nonferrous Met. Soc. China 2024, 34, 3650–3661. [Google Scholar]
- Hirata, Y.; Yang, C.-H.; Lin, S.-K.; Nishikawa, H. Improvements in mechanical properties of Sn–Bi alloys with addition of Zn and In. Mater. Sci. Eng. A 2021, 813, 141131. [Google Scholar] [CrossRef]
- Wang, S.; Feng, J.; Sa, Z.; Wang, Y.; Wang, S.; Tian, Y. Interfacial reaction of Cu/SnPbInBiSb/Cu sandwich solder joints. In Proceedings of the 2022 23rd International Conference on Electronic Packaging Technology (ICEPT), Dalian, China, 10–13 August 2022; pp. 1–5. [Google Scholar]
- Shen, Y.-A.; Lin, C.-M.; Li, J.; He, S.; Nishikawa, H. Effect of FeCoNiCrCu0.5 high-entropy-alloy substrate on Sn grain size in Sn-3.0 Ag-0.5 Cu solder. Sci. Rep. 2019, 9, 3658. [Google Scholar]
- Akinwekomi, A.D.; Bamisaye, O.S.; Bodunrin, M.O. Powder metallurgy processing of high entropy alloys: Bibliometric analysis and systematic review. Rev. Adv. Mater. Sci. 2024, 63, 20230188. [Google Scholar] [CrossRef]
- Kafali, M.; Doleker, K.M.; Erdogan, A.; Sunbul, S.E.; Icin, K.; Yildiz, A.; Gok, M.S. Wear, corrosion and oxidation characteristics of consolidated and laser remelted high entropy alloys manufactured via powder metallurgy. Surf. Coat. Technol. 2023, 467, 129704. [Google Scholar] [CrossRef]
- Torralba, J.M.; Alvaredo, P.; García-Junceda, A. High-entropy alloys fabricated via powder metallurgy. A critical review. Powder Metall. 2019, 62, 84–114. [Google Scholar] [CrossRef]
- Shanthar, R.; Chen, K.; Abeykoon, C. Powder-based additive manufacturing: A critical review of materials, methods, opportunities, and challenges. Adv. Eng. Mater. 2023, 25, 2300375. [Google Scholar] [CrossRef]
- Rajendran, S.H.; Jung, D.H.; Jung, J.P. Investigating the physical, mechanical, and reliability study of high entropy alloy reinforced Sn–3.0 Ag–0.5 Cu solder using 1608 chip capacitor/ENIG joints. J. Mater. Sci. Mater. Electron. 2022, 33, 3687–3710. [Google Scholar] [CrossRef]
- Chen, B.; Zou, M.; Chen, W.; Zhang, H.; Wang, J.; Jiang, L.; Zhang, Z.; Zhou, J.; Hu, X.; Li, Q. Influence of CrFeCoNiCu high-entropy alloy and ultrasonic stirring on the thermal, electrochemical and mechanical properties of Zn-30Sn high-temperature solder alloy. Mater. Charact. 2023, 201, 112977. [Google Scholar] [CrossRef]
- Shen, Y.-A.; Hsieh, H.-M.; Chen, S.-H.; Li, J.; Chen, S.-W.; Nishikawa, H. Investigation of FeCoNiCu properties: Thermal stability, corrosion behavior, wettability with Sn-3.0 Ag-0.5 Cu and interlayer formation of multi-element intermetallic compound. Appl. Surf. Sci. 2021, 546, 148931. [Google Scholar] [CrossRef]
- Ma, G.; Li, Z.; Ye, H.; He, C.; Zhang, H.; Hu, Z. Wetting and interface phenomena in the molten Sn/CuFeNiCoCr high-entropy alloy system. Appl. Surf. Sci. 2015, 356, 460–466. [Google Scholar] [CrossRef]
- Liu, Y.; Pu, L.; Gusak, A.; Zhao, X.; Tan, C.; Tu, K. Ultra-thin intermetallic compound formation in microbump technology by the control of a low Zn concentration in solder. Materialia 2020, 12, 100791. [Google Scholar] [CrossRef]
- Zhang, T.-Y.; Cheng, Q.; Zhu, H.-Y.; Wei, Q.-Q.; Xu, X.-D. Microstructure characterization of (Sn1− xZnx) 57 (In0. 78Bi0. 22) 43 low melting point lead-free solder materials. Trans. Nonferrous Met. Soc. China 2023, 33, 201–208. [Google Scholar] [CrossRef]
- Pu, L.; He, Q.; Yang, Y.; Zhao, X.; Hou, Z.; Tu, K.; Liu, Y. The microstructure and mechanical property of the high entropy alloy as a low temperature solder. In Proceedings of the 2019 IEEE 69th Electronic Components and Technology Conference (ECTC), Las Vegas, NV, USA, 28–31 May 2019; pp. 1716–1721. [Google Scholar]
- Qiao, J.; Mao, X.; Tu, K.-N.; Liu, Y. Microstructure and Intermetallic Growth Characteristics of Sn-Bi-In-xGa Quaternary Low Melting Point Solders. In Proceedings of the 2024 International Conference on Electronics Packaging (ICEP), Toyama, Japan, 17–20 April 2024; pp. 13–14. [Google Scholar]
- Bai, J.; Jin, X.; Yang, H.; Qiao, J. A low melting high entropy alloy with conformal electroconductivity for flexible electronic circuits. J. Alloys Compd. 2022, 919, 165736. [Google Scholar] [CrossRef]
- Villarreal-Loya, R.; Garay-Reyes, C.; Mendoza-Duarte, J.; Hernández-Rivera, J.; Cruz-Rivera, J.; Estrada-Guel, I.; Martínez-Sánchez, R. Ultra-low-temperature lead-free multicomponent alloy solder for application in heat-sensitive electronic components. Mater. Lett. 2023, 343, 134342. [Google Scholar] [CrossRef]
- Kim, S.H.; Nam, Y.; Lee, H.; Back, S.; Park, M.-S.; Jang, G.J.; Choi, J.-P.; Aranas, C., Jr. Microstructural transformation and thermo-mechanical improvement of quinary Bi–Sn–In–Ga–Zn solder bumps on a flexible PET substrate. Mater. Sci. Eng. B 2017, 224, 93–102. [Google Scholar] [CrossRef]
- Wang, J.; Wen, L.; Zhou, J.; Chung, M. Mechanical properties and joint reliability improvement of Sn-Bi alloy. In Proceedings of the 2011 IEEE 13th Electronics Packaging Technology Conference, Singapore, 7–9 December 2011; pp. 492–496. [Google Scholar]
- Anuar, N.E.E.; Singh, A.; Mei Kit, M.L.; Choo, H.L.; Durairaj, R.; Janasekaran, S. Investigation on the Thermal and Wettability Properties Aided with Mechanical Test Simulation of Tin (Sn)-Bismuth (Bi) Solder Alloy at Low Reflow Temperatures. Key Eng. Mater. 2024, 982, 99–114. [Google Scholar] [CrossRef]
- Song, Q.; Yang, W.; Li, Y.; Mao, J.; Qin, W.; Zhan, Y. Interfacial reaction and mechanical properties of Sn58Bi-XCr solder joints under isothermal aging conditions. Vacuum 2021, 194, 110559. [Google Scholar] [CrossRef]
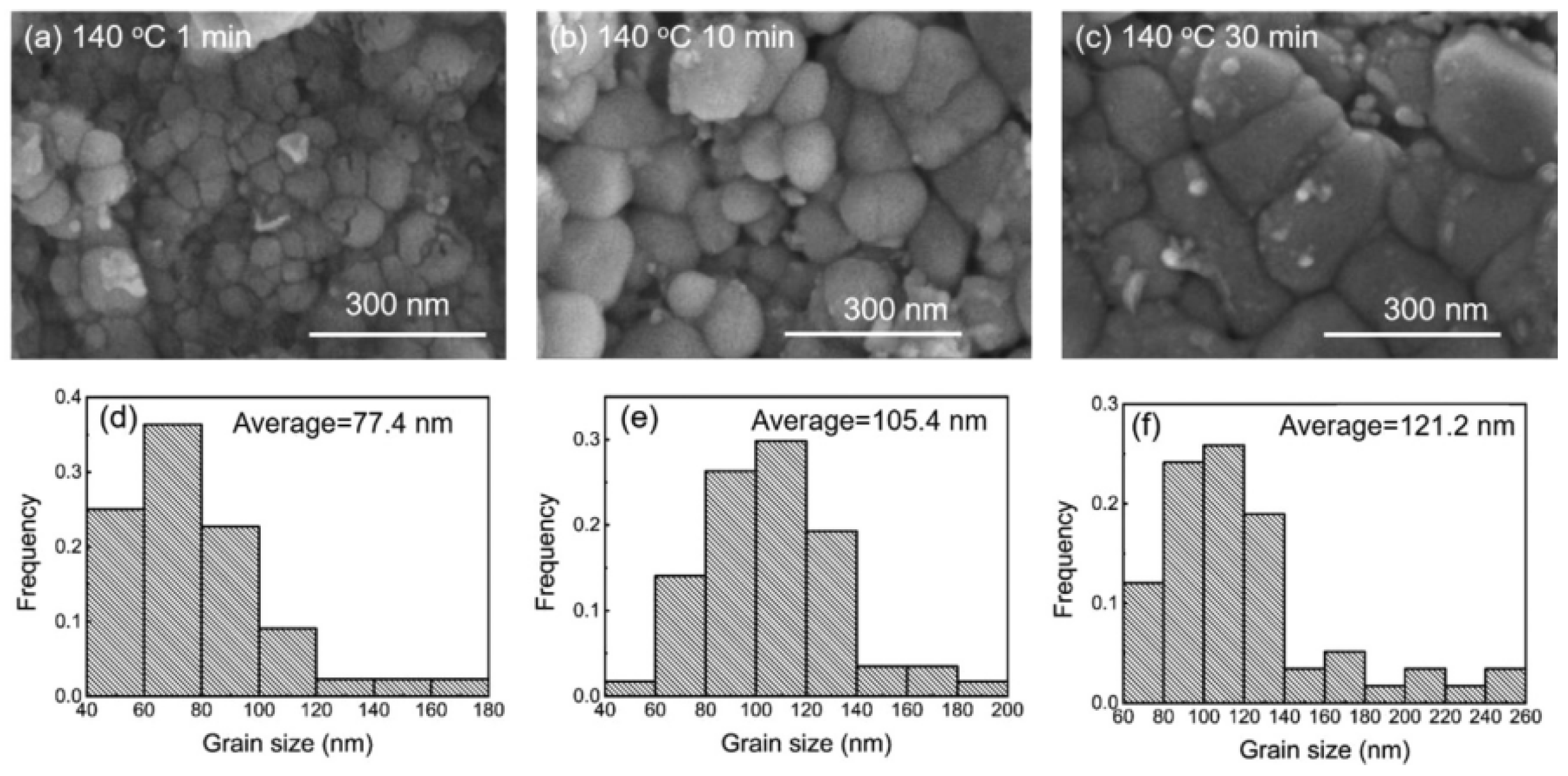
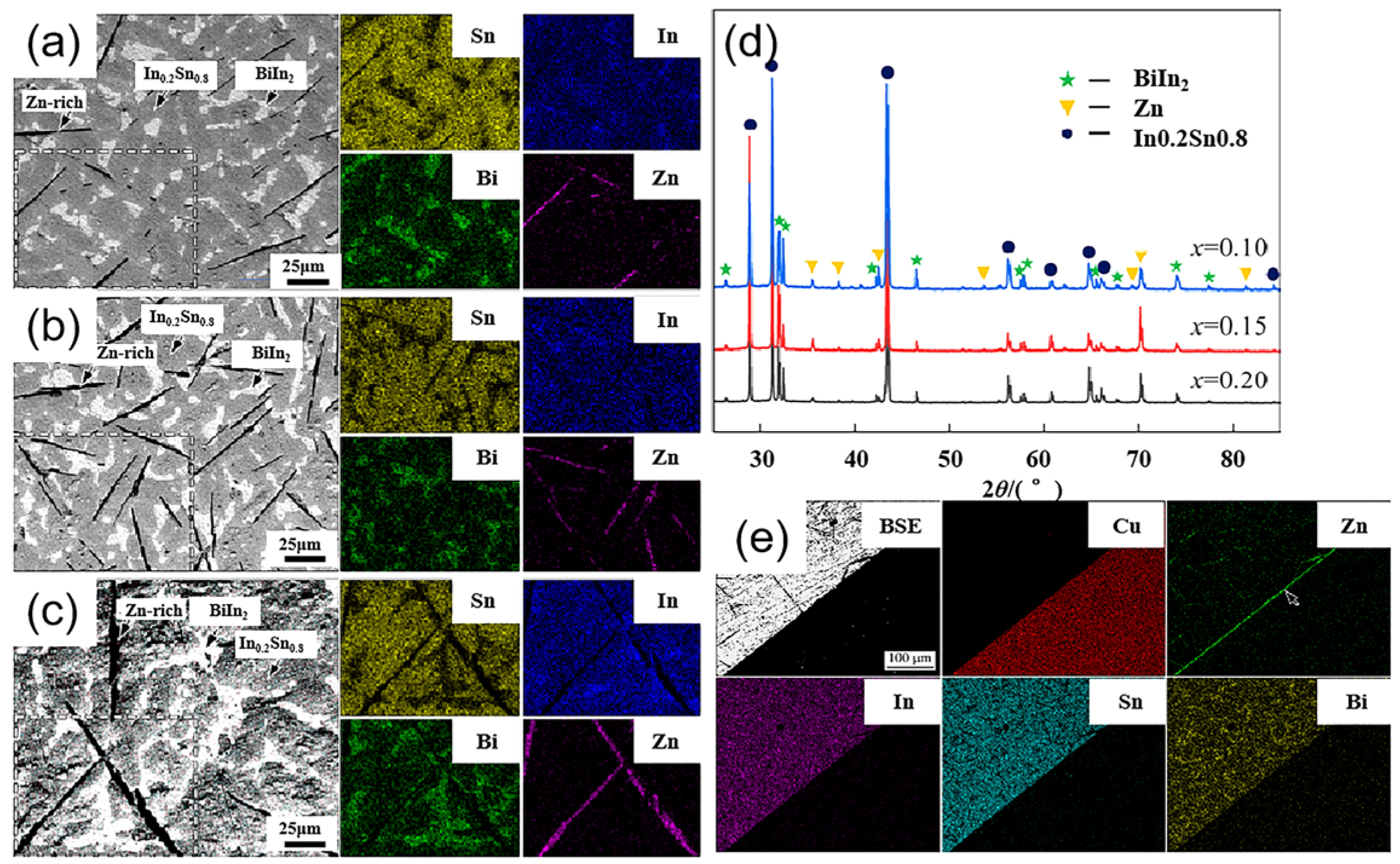

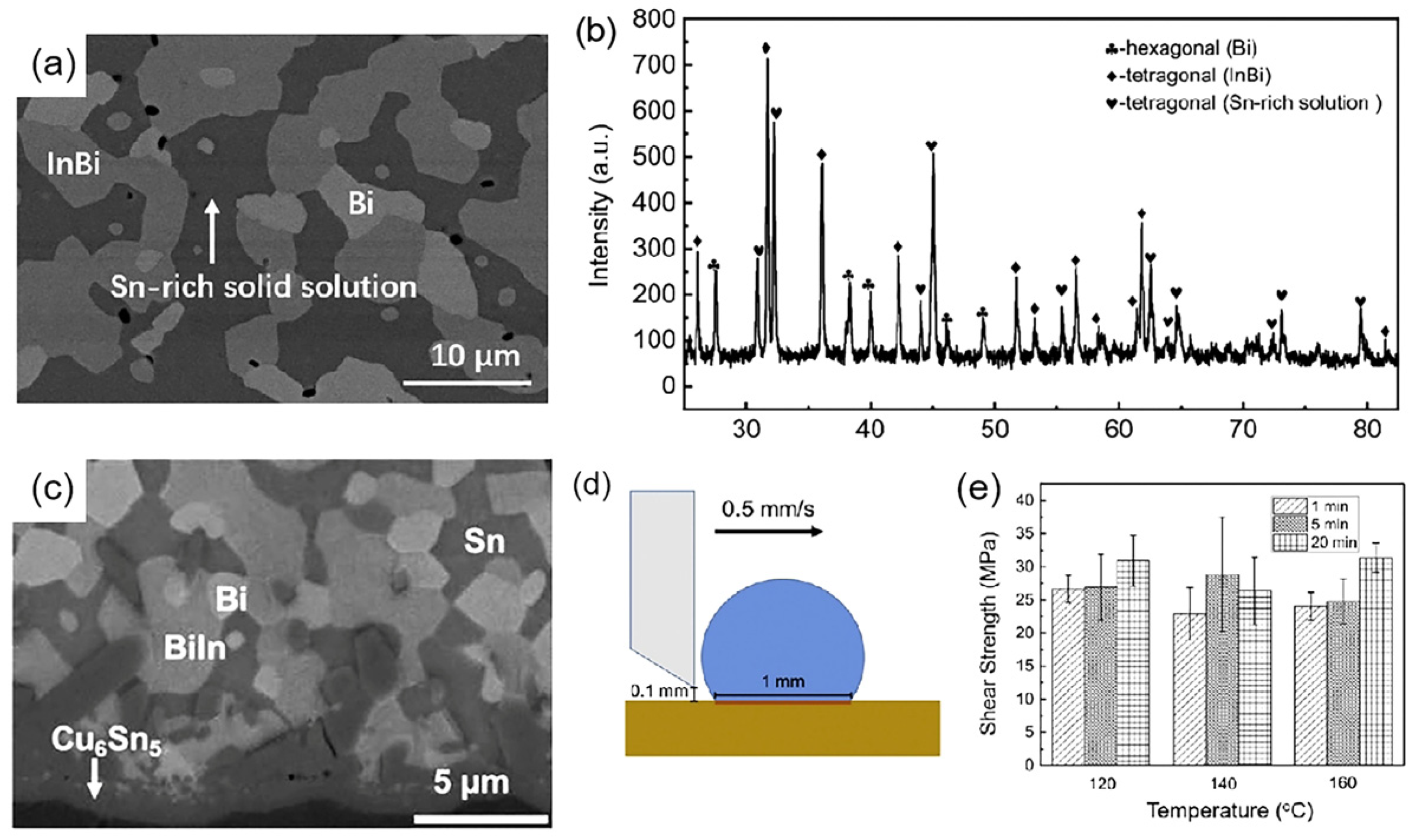
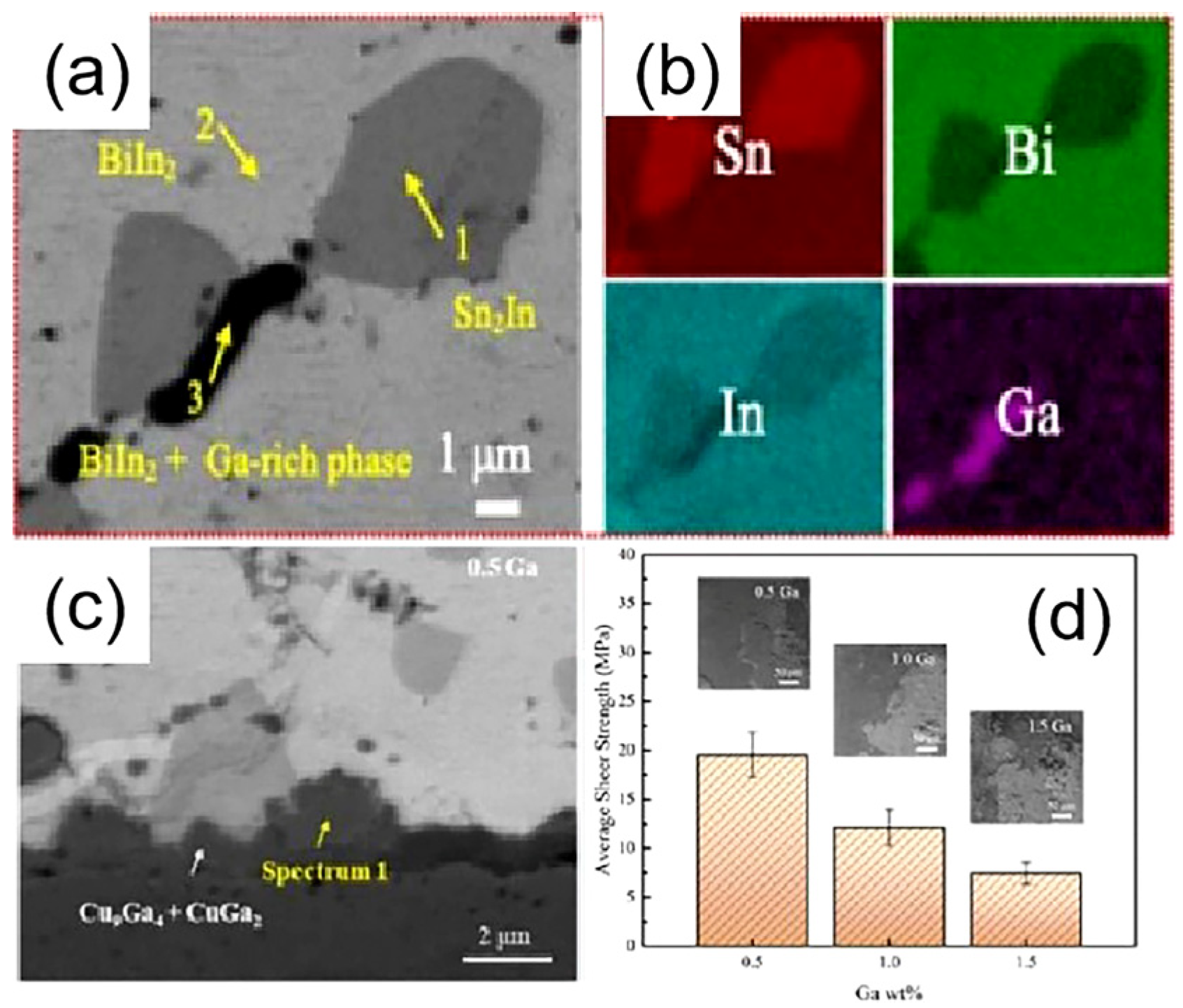

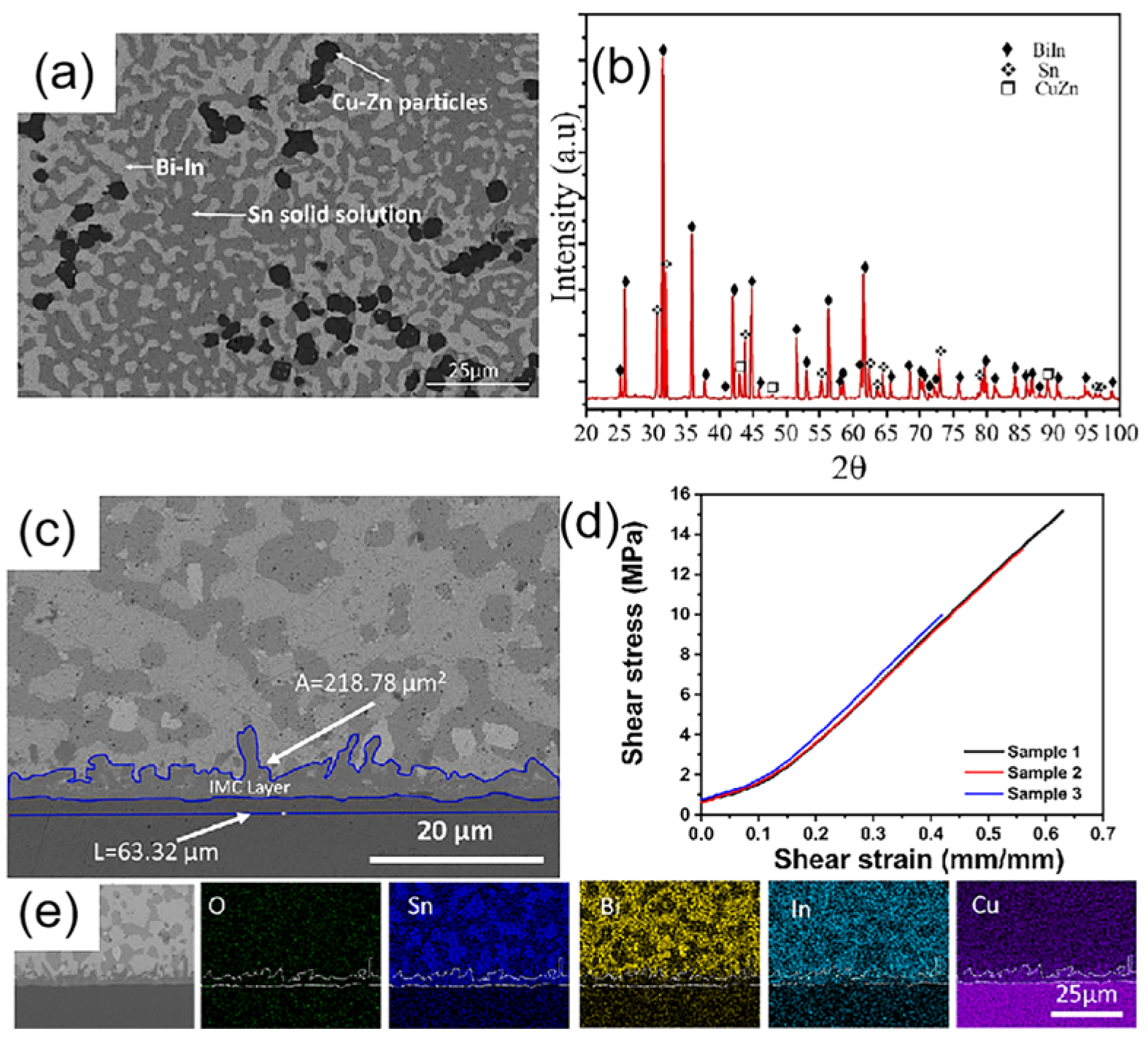
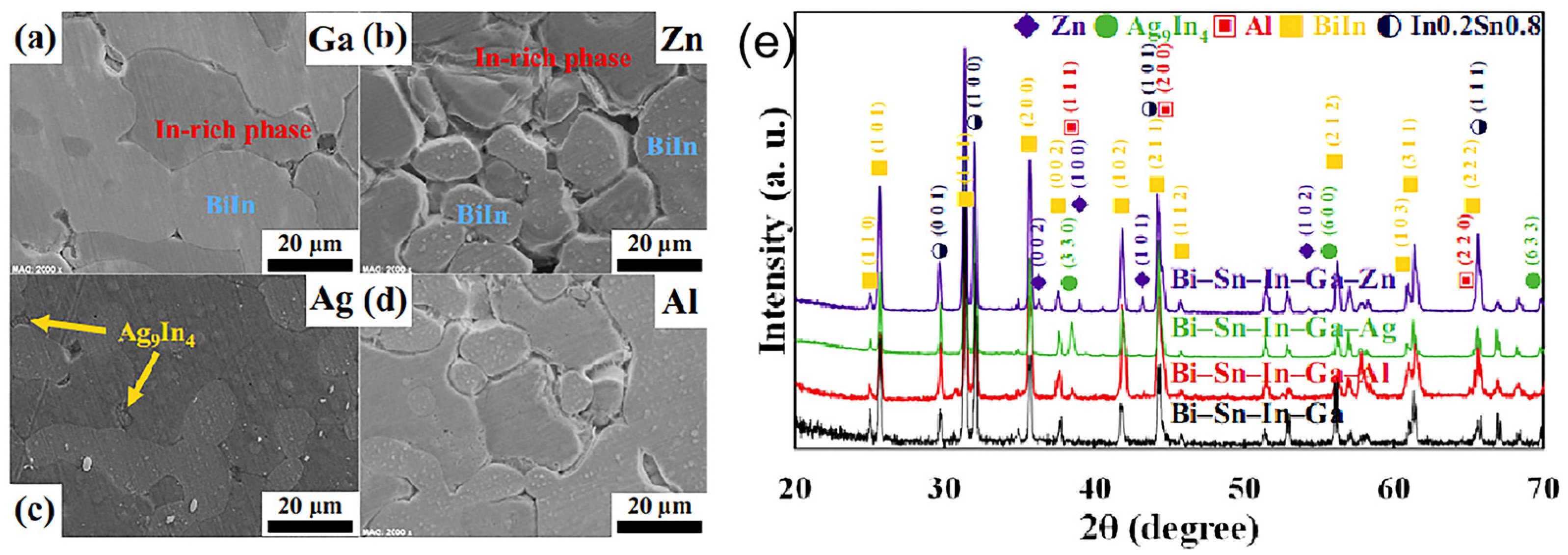

| Series | Elements | J/(mol·K) | Melting Point (K) | Tensile Strength (MPa) | Elongation (%) | Microhardness of Solder Matrix Phase HV (kgfmm−2) |
|---|---|---|---|---|---|---|
| 1 | 85Sn4Bi2In9Zn | 4.67 | 477.2 | 53.6 ± 0.05 | 22.18 | 20.7 ± 0.41 |
| 75Sn14Bi2In9Zn | 6.53 | 463.7 | 63.1 ± 0.06 | 20.73 | 27.10 ± 0.54 | |
| 65Sn24Bi2In9Zn | 7.48 | 444.5 | 64.3 ± 0.06 | 18.68 | 27.1 ± 0.54 | |
| 2 | 85Sn1Bi5In9Zn | 4.58 | 476.4 | 48.8 ± 0.05 | 38.25 | 28.4 ± 0.57 |
| 80Sn6Bi5In9Zn | 5.93 | 471.8 | 61.2 ± 0.06 | 26.25 | 31.7 ± 0.63 | |
| 76Sn10Bi5In9Zn | 6.70 | 469.0 | 63.8 ± 0.06 | 18.40 | 33.2 ± 0.66 |
| Elements | T | k | R | β | γ | Pmin | Specificities |
|---|---|---|---|---|---|---|---|
| Sn [83] | 231.9 | 66 | 11.5 | 22.0 | / | 19,506 | Sn is a cost-effective, low-melting-point metal widely used in low-temperature solder applications. It exhibits excellent compatibility with substrates like Cu and Ag, by forming stable IMCs. Due to its favorable properties, Sn is the most commonly utilized base material in low-temperature solder. Typical solder systems include Sn-Bi-based, Sn-Zn-based, and Sn-Ag-Cu-based alloys [84,85,86,87,88]. |
| Bi [89] | 271.4 | 8 | 115 | 13.4 | 300 | 6000 | Bi and Sn can combine to form the Sn58Bi eutectic alloy, which is widely recognized for its excellent low-temperature soldering properties. The addition of Bi significantly reduces the melting point of the Sn-based solder, making it suitable for low-temperature applications. Furthermore, Bi enhances the wetting performance of the solder on Cu substrate, promoting better adhesion and improved joint reliability. This combination of properties makes Sn58Bi alloys particularly advantageous in applications requiring efficient soldering at reduced temperatures [90,91,92,93]. |
| Zn [83] | 419.5 | 116 | 5.9 | 30.2 | 510 | 2831 | Zn exhibits a stronger reactivity with Cu and Ag substrates compared to elements like Sn. This heightened reactivity allows Zn to more readily form IMCs such as Cu5Zn8 and CuZn2 when interacting with Cu-based substrates. These IMCs are critical for ensuring robust metallurgical bonding at the solder joint. The pronounced reactivity of Zn with these substrates makes it an advantageous element in alloy design, particularly for applications requiring strong, durable solder joints [94,95,96]. |
| In [97] | 156.6 | 82 | 8.4 | 29.7 | 170 | 239,000 | The addition of In effectively reduces the melting point of the alloy, making it particularly suitable for low-temperature soldering applications. However, In has relatively low mechanical strength, and when added in large amounts, it can lead to alloys with insufficient structural integrity. During the melting process, In tends to form oxides rapidly, which can adversely affect the soldering quality. Moreover, an increase In content promotes continuous growth of IMCs, potentially compromising the reliability of the solder joint. Additionally, the high cost of In presents a significant economic challenge, limiting its widespread use in commercial applications [98,99]. |
| Ga [97] | 29.8 | 29 | 27.6 | 18.0 | / | 300,000 | The addition of Ga significantly reduces the melting point of multicomponent solders, making it highly advantageous for low-temperature soldering applications. Ga readily reacts with Cu substrates to form IMCs such as CuGa2, and Cu9Ga4. However, the inclusion of Ga also comes with drawbacks, as it tends to reduce the joint strength of the solder. This trade-off necessitates careful control of Ga content to balance the benefits of lower melting points and effective IMC formation against the potential decrease in the mechanical performance of the solder joint [100,101,102]. |
| Cu [83] | 1084.6 | 400 | 1.7 | 16.5 | 6746 | Adding an appropriate amount of Cu to low-temperature multicomponent solders can effectively enhance its tensile strength and elongation. Cu acts as a reinforcing element, contributing to the formation of robust IMCs, such as Cu6Sn5, and Cu3Sn. These IMCs improve the mechanical integrity and bonding reliability of the solder joints. Moreover, the addition of Cu helps to refine the microstructure of the solder, distributing stress more evenly under mechanical loads, thereby improving its overall ductility and resistance to cracking. This makes Cu a crucial component in designing solder alloys for applications requiring both strength and flexibility [103,104,105]. | |
| Ag [106] | 961.8 | 429 | 1.6 | 19.1 | 995 | 1,080,000 | Ag enhances the flow and wetting capabilities of the Sn-Zn alloy solder, allowing for better bonding between the solder and the substrate, which is crucial for achieving high-quality joints. Additionally, Ag improves the mechanical properties of the alloy, such as its strength, hardness, and fatigue resistance, making it more suitable for applications requiring reliable performance under stress. However, the main downside of incorporating Ag is its high cost, which can increase the overall price of the solder alloy. Despite this, the benefits of improved wetting and mechanical performance make Ag a valuable addition to high-performance solder materials [107,108,109]. |
| Pb [83,89] | 327.5 | 35 | 20.6 | 28.9 | / | 2311 | In the aerospace industry, Pb is sometimes added to solder alloys to improve both wettability and strength, which helps achieve strong, reliable bonds between the solder and substrates, particularly in complex components that require high precision. It also contributes to the alloy’s mechanical strength, enhancing the overall durability of the solder joints. However, the use of Pb in soldering materials has been heavily restricted in many industries due to environmental and health concerns, especially under regulations like RoHS. Despite these challenges, Pb remains useful in certain high-performance applications, such as aerospace, where its properties outweigh the potential drawbacks in specific scenarios. In these cases, Pb is carefully controlled and used in small quantities to balance performance and compliance with industry standards [110,111]. |
| Ni [83] | 1455 | 91 | 6.8 | 13.4 | / | 14,600 | Ni is commonly added to solder alloys to refine the weld seam’s microstructure and inhibit the growth of IMC at the interface, which can lead to improved mechanical strength and a stable interface. Additionally, Ni enhances the wettability of the solder, allowing it to better spread across the substrate and form a more uniform and reliable joint. This property is particularly valuable in high-performance applications, where the strength and durability of the solder joint are crucial. By controlling the amount of Ni in the alloy, it is possible to optimize both the mechanical properties and the wettability, making it a versatile element in soldering for electronics and other advanced materials [112,113,114]. |
| REEs: Sm, Sb [97] | 1072.0; 630.6 | 13; 24 | 150; 39.5 | 12.7; 11.0 | / | 6000, 11,800 | The addition of rare earth elements (REEs), such as Sm, Sb, La, Ce, and Y, to solder alloys can significantly improve both wettability and mechanical properties. These elements can lower the surface tension of the molten solder, leading to better wetting and spreading on the substrate. Additionally, REEs can refine the microstructure of the solder, contributing to improved mechanical strength, ductility, and oxidation resistance. The influence of REEs also extends to the formation of IMCs at the solder–substrate interface, where they can help control the growth of these compounds, leading to more stable and durable joints. As a result, the incorporation of REEs in low-temperature multicomponent solders is a promising approach to enhance the overall performance of solder joints, particularly in challenging applications where both high reliability and excellent wettability are required [115,116,117,118,119]. |
| Al, Co, Mn, Mo [83,97] | / | / | / | / | / | / | Elements like Mo, Co, and Ti have a minor effect on the melting point but play a key role in forming IMCs, strengthening grain boundaries and refining the grain size. However, they can reduce wettability. While improving strength and thermal stability, their presence requires careful optimization to balance both mechanical properties and wettability for reliable solder joints [120,121,122,123,124]. |
| IMC powder: Cu6Sn5, Sn3Ag [125,126,127] | / | 20–40 | 50–100 | 19–21 | / | / | The direct addition of IMC particles, like Cu6Sn5, refines the grain structure, improving toughness and suppressing aging IMC growth, enhancing mechanical properties, such as ductility and fracture resistance. For example, Sn3Ag strengthens the solder by reinforcing the matrix, forming a stable IMC with copper, and improving thermal stability. This makes the solder ideal for durable applications like electronics and aerospace [128,129,130]. |
| BN [131,132] | / | / | 1012 | 1–5 | / | / | BN, known for its mechanical properties and thermal stability, reinforces the solder matrix. It enhances tensile and shear strength, improving the bond between the solder and substrate. BN also improves thermal conductivity, which benefits temperature-sensitive components in electronics and aerospace. Additionally, BN refines the microstructure, reduces defects, and prevents grain coarsening, maintaining mechanical stability, particularly under thermal stresses [133,134,135]. |
| CNTs [136,137,138] | / | / | 1–10 | −1–2 | / | / | CNTs significantly enhance the tensile and shear strength of low-temperature multicomponent solders. CNTs reinforce the solder by preventing excessive deformation under stress and strengthening the interface with the substrate. They also inhibit crack propagation and improve ductility, making the solder joints more resilient under dynamic conditions. Additionally, CNTs enhance the thermal and electrical conductivity of the solder, improving its performance in high-demand applications like electronics, aerospace, and automotive industries [139,140,141]. |
| GNSs [142,143] | / | / | 10−6 | −6–0 | / | / | GNSs improve tensile strength and shear strength by reinforcing the solder matrix and enhancing the interface between the solder and substrate. GNSs also prevent crack propagation and improve ductility, allowing the solder joints to withstand dynamic stresses without failure. Additionally, GNSs can enhance thermal and electrical conductivity, making them ideal for applications requiring high performance and reliability in the electronics, aerospace, and automotive industries [144,145,146,147,148]. |
| Oxides TiO2, Fe2O2, SnO2, Al2O3, etc. | / | / | / | / | / | / | Incorporating various oxides such as TiO2, Fe2O3, SnO2, and Al2O3 into low-temperature multicomponent solders can effectively adjust the contact angle, improving wettability. These oxides also enhance the solder’s aging resistance, ensuring long-term reliability under thermal and mechanical stresses. Additionally, the inclusion of these oxides helps refine the solder’s microstructure, contributing to improved mechanical properties and stability over time [149,150,151]. |
| Component | Tonset (°C) | Toffset (°C) | ∆T (°C) | Ρ (µΩ·cm) |
|---|---|---|---|---|
| 39.3Bi28.5Sn22.6In4.6Ga5Zn | 58.9 | 65.8 | 6.9 | 44.27 ± 0.32 |
| 39.3Bi28.5Sn22.6In4.6Ga5Ag | 59.5 | 70.2 | 10.7 | 43.91 ± 0.30 |
| 39.3Bi28.5Sn22.6In4.6Ga5Al | 59.1 | 67.3 | 8.2 | 46.83 ± 0.47 |
| 41.4Bi30Sn23.8In4.8Ga | 61.6 | 69.2 | 7.6 | 43.88 ± 0.28 |
| Solder | Melting Point | Bonding Temperature | Wettability | Shear Strength |
|---|---|---|---|---|
| Sn58Bi | 139 °C [171] | 150 °C [75] | 28.1–50° [172] | Up to 68 MPa [173] |
| InZnSnBi [75] | 83 °C | 100–160 °C | 35–52° | Up to 31 MPa |
| 43In28Sn14Bi9Zn6Ag [57] | 62.8 °C | 85–145 °C | 23–55° | / |
| Comparisons | Melting point of multicomponent solder is lower | Multicomponent solder can be bonded at lower temperatures | Multicomponent solders remain their wettability at low bonding temperatures | Shear strength of multicomponent solder is lower than Sn58Bi |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, G.; Shen, J.; Zhou, D.; Khairi Faiz, M.; Wong, Y.H. Applications and Recent Advances of Low-Temperature Multicomponent Solders in Electronic Packaging: A Review. Micromachines 2025, 16, 300. https://doi.org/10.3390/mi16030300
Wu G, Shen J, Zhou D, Khairi Faiz M, Wong YH. Applications and Recent Advances of Low-Temperature Multicomponent Solders in Electronic Packaging: A Review. Micromachines. 2025; 16(3):300. https://doi.org/10.3390/mi16030300
Chicago/Turabian StyleWu, Guodong, Jingfang Shen, Ding Zhou, Muhammad Khairi Faiz, and Yew Hoong Wong. 2025. "Applications and Recent Advances of Low-Temperature Multicomponent Solders in Electronic Packaging: A Review" Micromachines 16, no. 3: 300. https://doi.org/10.3390/mi16030300
APA StyleWu, G., Shen, J., Zhou, D., Khairi Faiz, M., & Wong, Y. H. (2025). Applications and Recent Advances of Low-Temperature Multicomponent Solders in Electronic Packaging: A Review. Micromachines, 16(3), 300. https://doi.org/10.3390/mi16030300






