Enhanced Performance of Novel Amorphous Silicon Carbide Microelectrode Arrays in Rat Motor Cortex
Abstract
1. Introduction
2. Materials and Methods
2.1. Microelectrode Arrays
2.2. Flexural Rigidity and Stiffness Calculations
2.3. MEA Implantation
2.4. Neural Recordings
2.5. Data Processing
2.6. Immunohistochemistry
2.7. Image Analysis
2.8. Statistical Analysis
3. Results
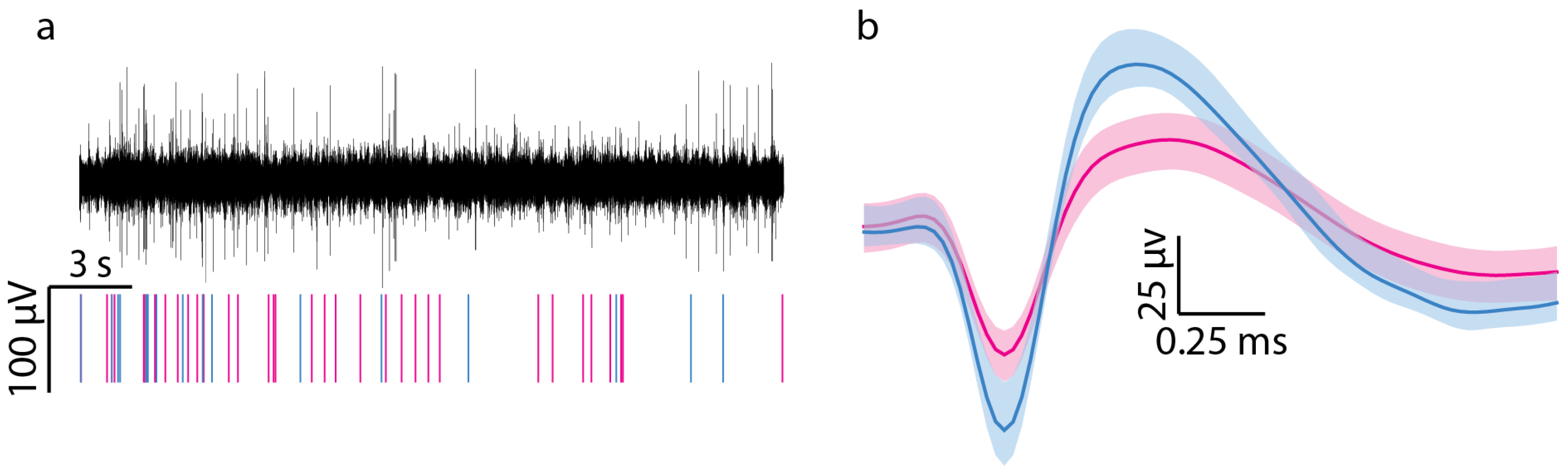
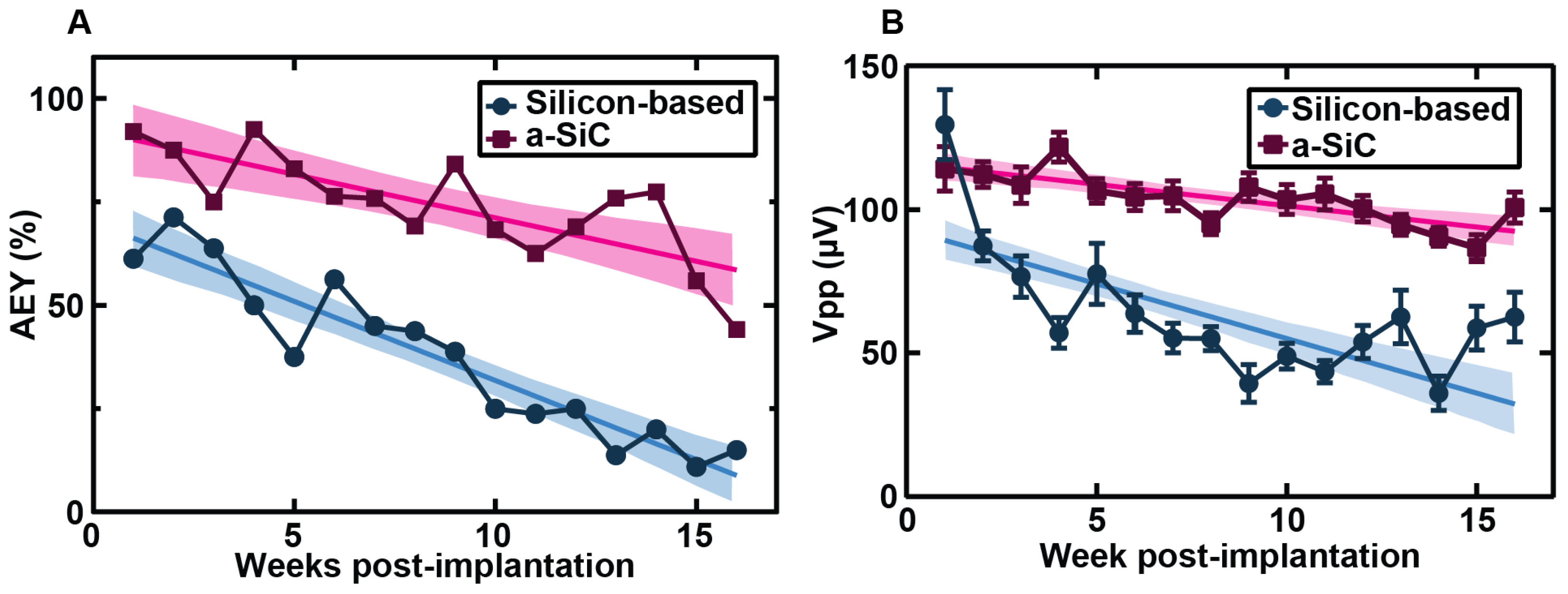
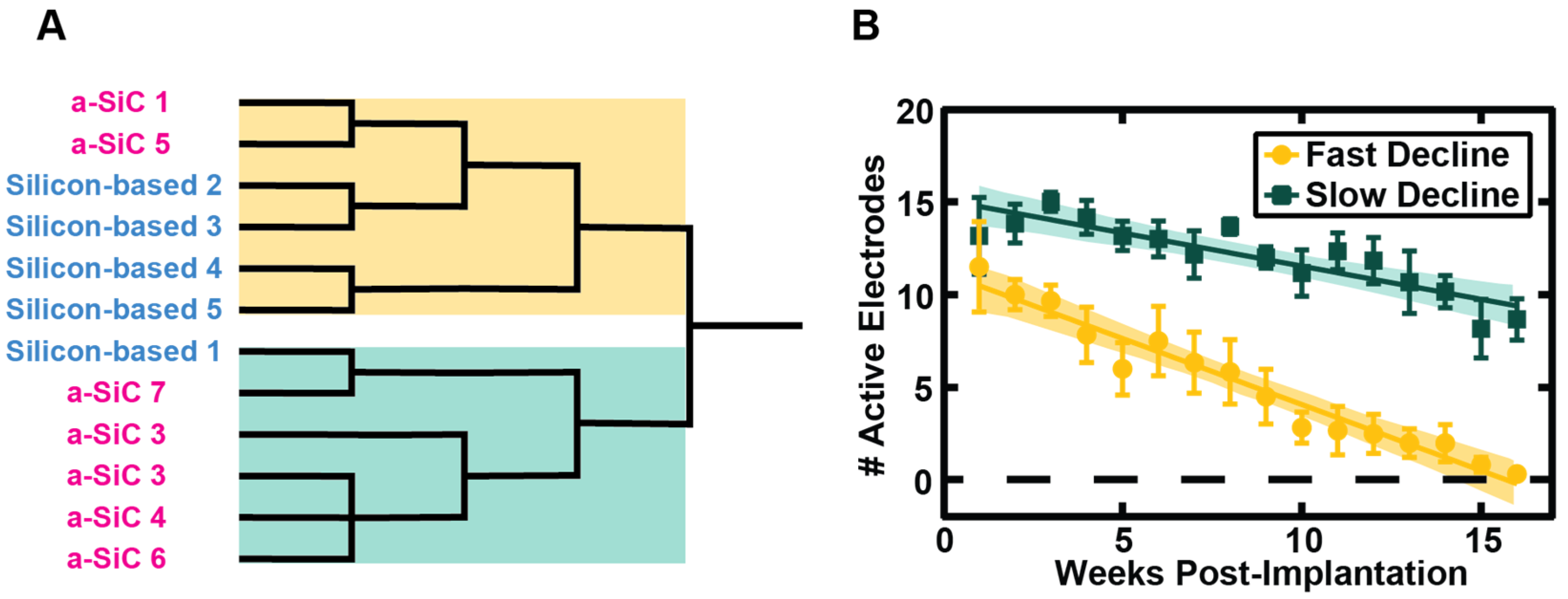
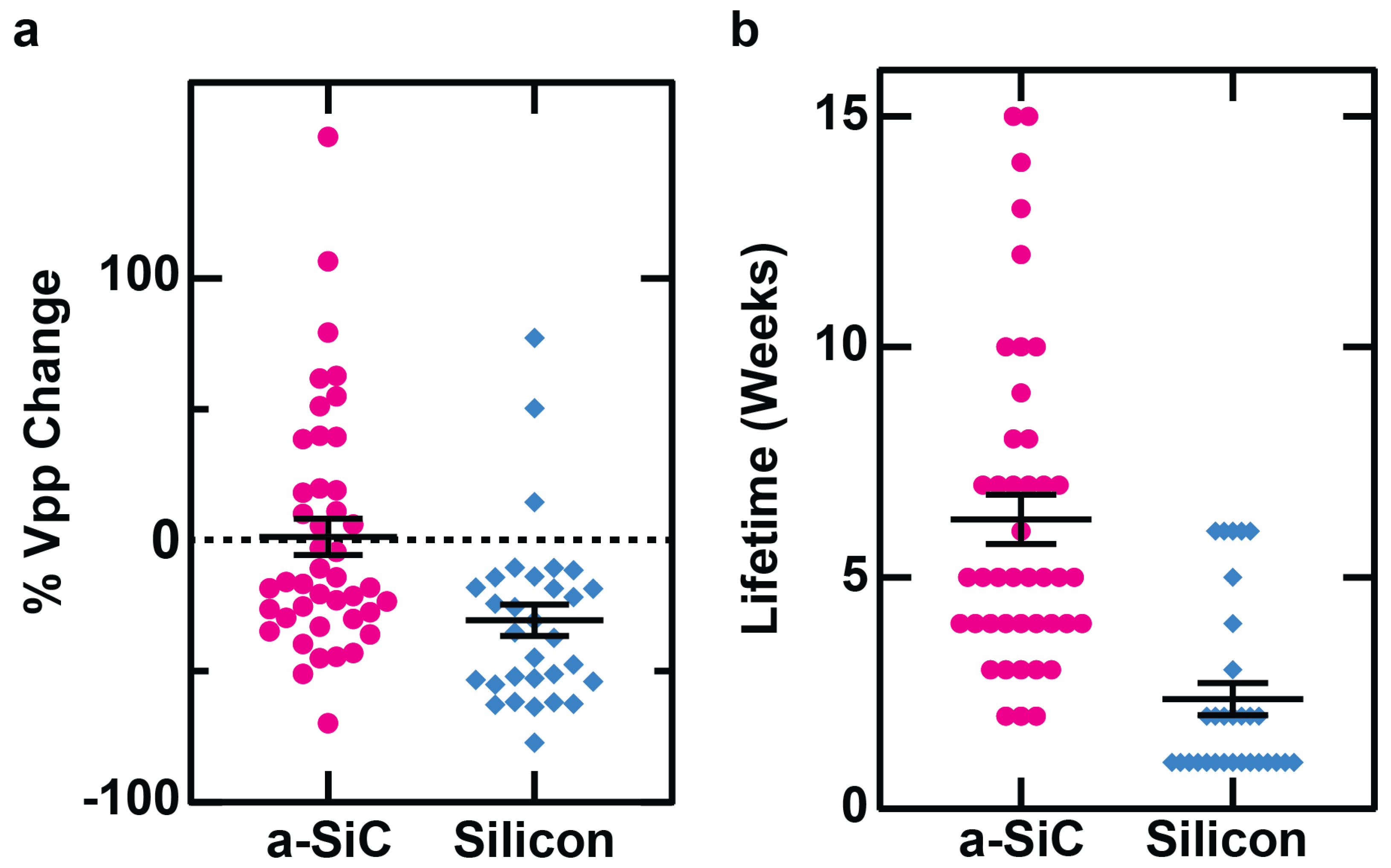
3.1. Neural Recording
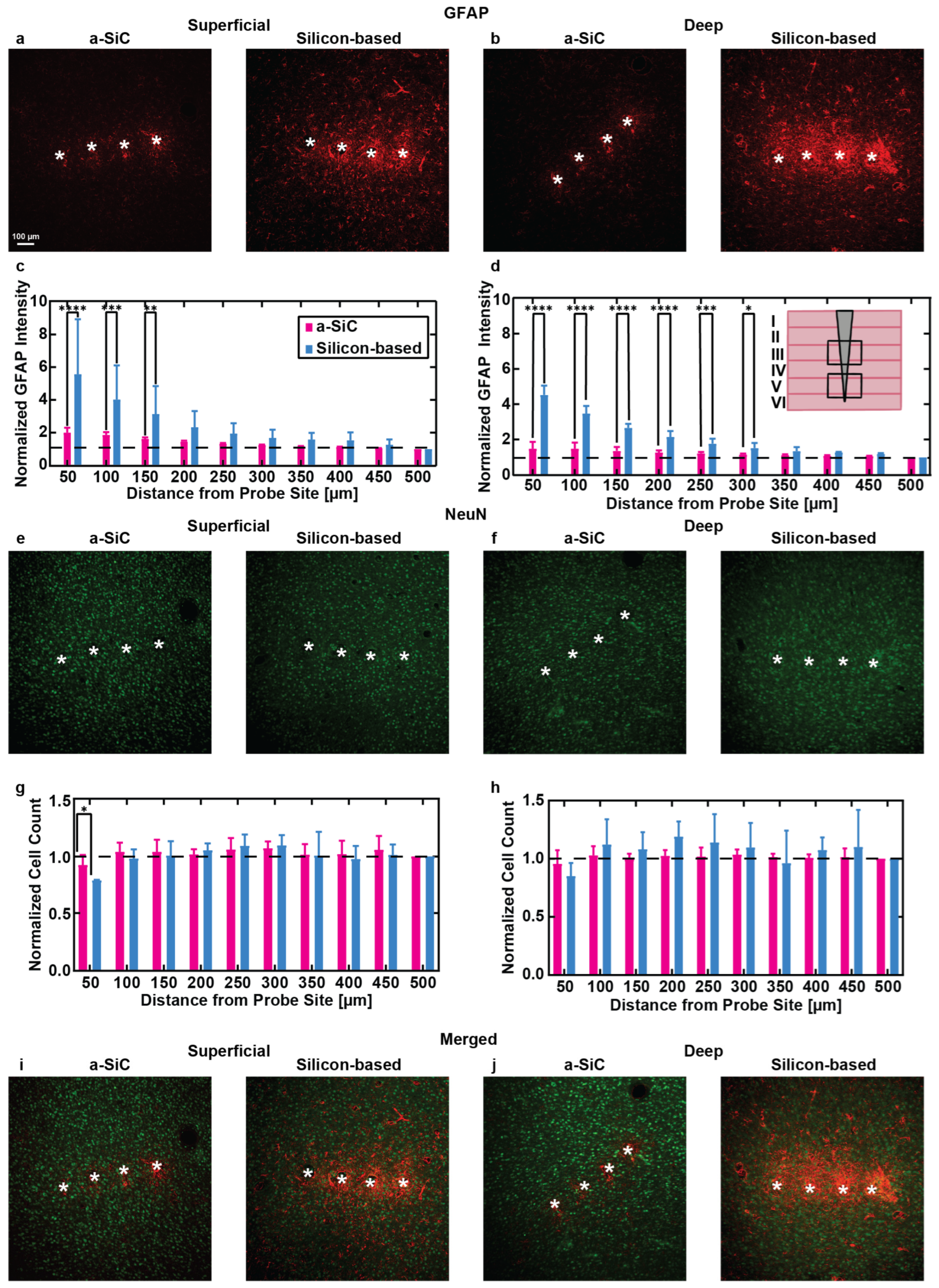
3.2. Immunohistochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dadarlat, M.C.; Canfield, R.A.; Orsborn, A.L. Neural Plasticity in Sensorimotor Brain-Machine Interfaces. Annu. Rev. Biomed. Eng. 2023, 25, 51–76. [Google Scholar] [CrossRef] [PubMed]
- Tayebi, H.; Azadnajafabad, S.; Maroufi, S.F.; Pour-Rashidi, A.; Khorasanizadeh, M.; Faramarzi, S.; Slavin, K.V. Applications of brain-computer interfaces in neurodegenerative diseases. Neurosurg. Rev. 2023, 46, 131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, X.; Zhang, X.; Wang, Y.; Pei, W. Implantable intracortical microelectrodes: Reviewing the present with a focus on the future. Microsyst. Nanoeng. 2023, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Ethier, C.; Oby, E.R.; Bauman, M.J.; Miller, L.E. Restoration of grasp following paralysis through brain-controlled stimulation of muscles. Nature 2012, 485, 368–371. [Google Scholar] [CrossRef]
- Chestek, C.A.; Gilja, V.; Nuyujukian, P.; Foster, J.D.; Fan, J.M.; Kaufman, M.T.; Churchland, M.M.; Rivera-Alvidrez, Z.; Cunningham, J.P.; Ryu, S.I.; et al. Long-term stability of neural prosthetic control signals from silicon cortical arrays in rhesus macaque motor cortex. J. Neural Eng. 2011, 8, 045005. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T.D.; Du, Z.; Gugel, Z.V.; Smith, M.A.; Chase, S.M.; Bodily, L.M.; Caparosa, E.M.; Friedlander, R.M.; Cui, X.T. Comprehensive chronic laminar single-unit, multi-unit, and local field potential recording performance with planar single shank electrode arrays. J. Neurosci. Methods 2015, 242, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.; Sharma, D.; Ross, D.; Zhao, F. A Critical Review of Microelectrode Arrays and Strategies for Improving Neural Interfaces. Adv. Healthc. Mater. 2019, 8, e1900558. [Google Scholar] [CrossRef] [PubMed]
- Szarowski, D.H.; Andersen, M.D.; Retterer, S.; Spence, A.J.; Isaacson, M.; Craighead, H.G.; Turner, J.N.; Shain, W. Brain responses to micro-machined silicon devices. Brain Res. 2003, 983, 23–35. [Google Scholar] [CrossRef]
- Bjornsson, C.S.; Oh, S.J.; Al-Kofahi, Y.A.; Lim, Y.J.; Smith, K.L.; Turner, J.N.; De, S.; Roysam, B.; Shain, W.; Kim, S.J. Effects of insertion conditions on tissue strain and vascular damage during neuroprosthetic device insertion. J. Neural Eng. 2006, 3, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Thelin, J.; Jorntell, H.; Psouni, E.; Garwicz, M.; Schouenborg, J.; Danielsen, N.; Linsmeier, C.E. Implant size and fixation mode strongly influence tissue reactions in the CNS. PLoS ONE 2011, 6, e16267. [Google Scholar] [CrossRef] [PubMed]
- Edell, D.J.; Toi, V.V.; McNeil, V.M.; Clark, L.D. Factors influencing the biocompatibility of insertable silicon microshafts in cerebral cortex. IEEE Trans. Biomed. Eng. 1992, 39, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Subbaroyan, J.; Martin, D.C.; Kipke, D.R. A finite-element model of the mechanical effects of implantable microelectrodes in the cerebral cortex. J. Neural Eng. 2005, 2, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Sharafkhani, N.; Kouzani, A.Z.; Adams, S.D.; Long, J.M.; Lissorgues, G.; Rousseau, L.; Orwa, J.O. Neural tissue-microelectrode interaction: Brain micromotion, electrical impedance, and flexible microelectrode insertion. J. Neurosci. Methods 2022, 365, 109388. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.N.; Shain, W.; Szarowski, D.H.; Andersen, M.; Martins, S.; Isaacson, M.; Craighead, H. Cerebral astrocyte response to micromachined silicon implants. Exp. Neurol. 1999, 156, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Roitbak, T.; Sykova, E. Diffusion barriers evoked in the rat cortex by reactive astrogliosis. Glia 1999, 28, 40–48. [Google Scholar] [CrossRef]
- McConnell, G.C.; Rees, H.D.; Levey, A.I.; Gutekunst, C.A.; Gross, R.E.; Bellamkonda, R.V. Implanted neural electrodes cause chronic, local inflammation that is correlated with local neurodegeneration. J. Neural Eng. 2009, 6, 056003. [Google Scholar] [CrossRef] [PubMed]
- Lempka, S.F.; Johnson, M.D.; Moffitt, M.A.; Otto, K.J.; Kipke, D.R.; McIntyre, C.C. Theoretical analysis of intracortical microelectrode recordings. J. Neural Eng. 2011, 8, 045006. [Google Scholar] [CrossRef]
- McCreery, D.; Cogan, S.; Kane, S.; Pikov, V. Correlations between histology and neuronal activity recorded by microelectrodes implanted chronically in the cerebral cortex. J. Neural Eng. 2016, 13, 036012. [Google Scholar] [CrossRef] [PubMed]
- Gori, M.; Vadala, G.; Giannitelli, S.M.; Denaro, V.; Di Pino, G. Biomedical and Tissue Engineering Strategies to Control Foreign Body Reaction to Invasive Neural Electrodes. Front. Bioeng. Biotechnol. 2021, 9, 659033. [Google Scholar] [CrossRef]
- Carnicer-Lombarte, A.; Chen, S.T.; Malliaras, G.G.; Barone, D.G. Foreign Body Reaction to Implanted Biomaterials and Its Impact in Nerve Neuroprosthetics. Front. Bioeng. Biotechnol. 2021, 9, 622524. [Google Scholar] [CrossRef] [PubMed]
- Abbott, J.R.; Jeakle, E.N.; Haghighi, P.; Usoro, J.O.; Sturgill, B.S.; Wu, Y.; Geramifard, N.; Radhakrishna, R.; Patnaik, S.; Nakajima, S.; et al. Planar amorphous silicon carbide microelectrode arrays for chronic recording in rat motor cortex. Biomaterials 2024, 308, 122543. [Google Scholar] [CrossRef] [PubMed]
- Brassard, D.; El Khakani, M.A. Dielectric properties of amorphous hydrogenated silicon carbide thin films grown by plasma-enhanced chemical vapor deposition. J. Appl. Phys. 2003, 93, 4066–4071. [Google Scholar] [CrossRef]
- Knaack, G.L.C.; Hamid; Cogan, S.F.; Joseph, J.P. Silicon Carbide Biotechnology, 2nd ed.; Saddow, S.E., Ed.; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Cogan, S.F.; Edell, D.J.; Guzelian, A.A.; Ping Liu, Y.; Edell, R. Plasma-enhanced chemical vapor deposited silicon carbide as an implantable dielectric coating. J. Biomed. Mater. Res. Part A 2003, 67A, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Jeakle, E.N.; Abbott, J.R.; Usoro, J.O.; Wu, Y.; Haghighi, P.; Radhakrishna, R.; Sturgill, B.S.; Nakajima, S.; Thai, T.T.D.; Pancrazio, J.J.; et al. Chronic Stability of Local Field Potentials Using Amorphous Silicon Carbide Microelectrode Arrays Implanted in the Rat Motor Cortex. Micromachines 2023, 14, 680. [Google Scholar] [CrossRef]
- Luan, L.; Wei, X.; Zhao, Z.; Siegel, J.J.; Potnis, O.; Tuppen, C.A.; Lin, S.; Kazmi, S.; Fowler, R.A.; Holloway, S.; et al. Ultraflexible nanoelectronic probes form reliable, glial scar-free neural integration. Sci. Adv. 2017, 3, e1601966. [Google Scholar] [CrossRef] [PubMed]
- Hierarchical Cluster: Group Observations Using a Tree of Clusters. Available online: https://www.jmp.com/support/help/en/18.1/index.shtml#page/jmp/hierarchical-cluster.shtml (accessed on 24 July 2024).
- Seymour, J.P.; Kipke, D.R. Neural probe design for reduced tissue encapsulation in CNS. Biomaterials 2007, 28, 3594–3607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Mandeville, E.T.; Xu, L.; Stary, C.M.; Lo, E.H.; Lieber, C.M. Ultraflexible endovascular probes for brain recording through micrometer-scale vasculature. Science 2023, 381, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Deku, F.; Cohen, Y.; Joshi-Imre, A.; Kanneganti, A.; Gardner, T.J.; Cogan, S.F. Amorphous silicon carbide ultramicroelectrode arrays for neural stimulation and recording. J. Neural Eng. 2018, 15, 016007. [Google Scholar] [CrossRef]
- Remy, A.; Lin, X.; Liu, J. Materials for flexible and soft brain-computer interfaces, a review. MRS Commun. 2024, 14, 827–834. [Google Scholar] [CrossRef]
- Geramifard, N.; Khajehzadeh, M.; Dousti, B.; Abbott, J.R.; Nguyen, C.K.; Hernandez-Reynoso, A.G.; Joshi-Imre, A.; Varner, V.D.; Cogan, S.F. Flexible and Extensible Ribbon-Cable Interconnects for Implantable Electrical Neural Interfaces. ACS Appl. Mater. Interfaces 2024, 16, 61621–61632. [Google Scholar] [CrossRef]
- Stiller, A.M.; Black, B.J.; Kung, C.; Ashok, A.; Cogan, S.F.; Varner, V.D.; Pancrazio, J.J. A Meta-Analysis of Intracortical Device Stiffness and Its Correlation with Histological Outcomes. Micromachines 2018, 9, 443. [Google Scholar] [CrossRef] [PubMed]
- Gregory, B.A.; Thompson, C.H.; Salatino, J.W.; Railing, M.J.; Zimmerman, A.F.; Gupta, B.; Williams, K.; Beatty, J.A.; Cox, C.L.; Purcell, E.K. Structural and functional changes of deep layer pyramidal neurons surrounding microelectrode arrays implanted in rat motor cortex. Acta Biomater. 2023, 168, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Hoeferlin, G.F.; Bajwa, T.; Olivares, H.; Zhang, J.; Druschel, L.N.; Sturgill, B.S.; Sobota, M.; Boucher, P.; Duncan, J.; Hernandez-Reynoso, A.G.; et al. Antioxidant Dimethyl Fumarate Temporarily but Not Chronically Improves Intracortical Microelectrode Performance. Micromachines 2023, 14, 1902. [Google Scholar] [CrossRef] [PubMed]
- Stiller, A.M.; Usoro, J.; Frewin, C.L.; Danda, V.R.; Ecker, M.; Joshi-Imre, A.; Musselman, K.C.; Voit, W.; Modi, R.; Pancrazio, J.J.; et al. Chronic Intracortical Recording and Electrochemical Stability of Thiol-ene/Acrylate Shape Memory Polymer Electrode Arrays. Micromachines 2018, 9, 500. [Google Scholar] [CrossRef]
- Sturgill, B.; Radhakrishna, R.; Thai, T.T.D.; Patnaik, S.S.; Capadona, J.R.; Pancrazio, J.J. Characterization of Active Electrode Yield for Intracortical Arrays: Awake versus Anesthesia. Micromachines 2022, 13, 480. [Google Scholar] [CrossRef] [PubMed]
- Rolston, J.D.; Gross, R.E.; Potter, S.M. Common median referencing for improved action potential detection with multielectrode arrays. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2009, 2009, 1604–1607. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T.D.; Gugel, Z.; Li, X.; Gilgunn, P.J.; Khilwani, R.; Ozdoganlar, O.B.; Fedder, G.K.; Weber, D.J.; Cui, X.T. Chronic tissue response to carboxymethyl cellulose based dissolvable insertion needle for ultra-small neural probes. Biomaterials 2014, 35, 9255–9268. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T.D.; Li, X.; Bodily, L.M.; Caparosa, E.M.; Zenonos, G.A.; Carlisle, D.L.; Friedlander, R.M.; Cui, X.T. Effects of caspase-1 knockout on chronic neural recording quality and longevity: Insight into cellular and molecular mechanisms of the reactive tissue response. Biomaterials 2014, 35, 9620–9634. [Google Scholar] [CrossRef]
- Usoro, J.O.; Dogra, K.; Abbott, J.R.; Radhakrishna, R.; Cogan, S.F.; Pancrazio, J.J.; Patnaik, S.S. Influence of Implantation Depth on the Performance of Intracortical Probe Recording Sites. Micromachines 2021, 12, 1158. [Google Scholar] [CrossRef]
- Lanjakornsiripan, D.; Pior, B.J.; Kawaguchi, D.; Furutachi, S.; Tahara, T.; Katsuyama, Y.; Suzuki, Y.; Fukazawa, Y.; Gotoh, Y. Layer-specific morphological and molecular differences in neocortical astrocytes and their dependence on neuronal layers. Nat. Commun. 2018, 9, 1623. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, C. Numerical data on neocortical neurons in adult rat, with special reference to the GABA population. Brain Res. 1993, 609, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T.D.; Langhals, N.B.; Patel, P.R.; Deng, X.; Zhang, H.; Smith, K.L.; Lahann, J.; Kotov, N.A.; Kipke, D.R. Ultrasmall implantable composite microelectrodes with bioactive surfaces for chronic neural interfaces. Nat. Mater. 2012, 11, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Moshayedi, P.; Ng, G.; Kwok, J.C.; Yeo, G.S.; Bryant, C.E.; Fawcett, J.W.; Franze, K.; Guck, J. The relationship between glial cell mechanosensitivity and foreign body reactions in the central nervous system. Biomaterials 2014, 35, 3919–3925. [Google Scholar] [CrossRef] [PubMed]
- Darlot, F.; Villard, P.; Salam, L.A.; Rousseau, L.; Piret, G. Glial scarring around intra-cortical MEA implants with flexible and free microwires inserted using biodegradable PLGA needles. Front. Bioeng. Biotechnol. 2024, 12, 1408088. [Google Scholar] [CrossRef]
- Delbeke, J.; Haesler, S.; Prodanov, D. Failure Modes of Implanted Neural Interfaces. In Neural Interface Engineering: Linking the Physical World and the Nervous System; Guo, L., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 123–172. [Google Scholar]
- Weltman, A.; Yoo, J.; Meng, E. Flexible, Penetrating Brain Probes Enabled by Advances in Polymer Microfabrication. Micromachines 2016, 7, 180. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Forrest, A.M.; Burgos, G.G.; Kozai, T.D.Y. Neuronal functional connectivity is impaired in a layer dependent manner near chronically implanted intracortical microelectrodes in C57BL6 wildtype mice. J. Neural Eng. 2024, 21, 036033. [Google Scholar] [CrossRef]
- Druschel, L.N.; Kasthuri, N.M.; Song, S.S.; Wang, J.J.; Hess-Dunning, A.; Chan, E.R.; Capadona, J.R. Cell-specific spatial profiling of targeted protein expression to characterize the impact of intracortical microelectrode implantation on neuronal health. J. Mater. Chem. B 2024, 12, 12307–12319. [Google Scholar] [CrossRef] [PubMed]
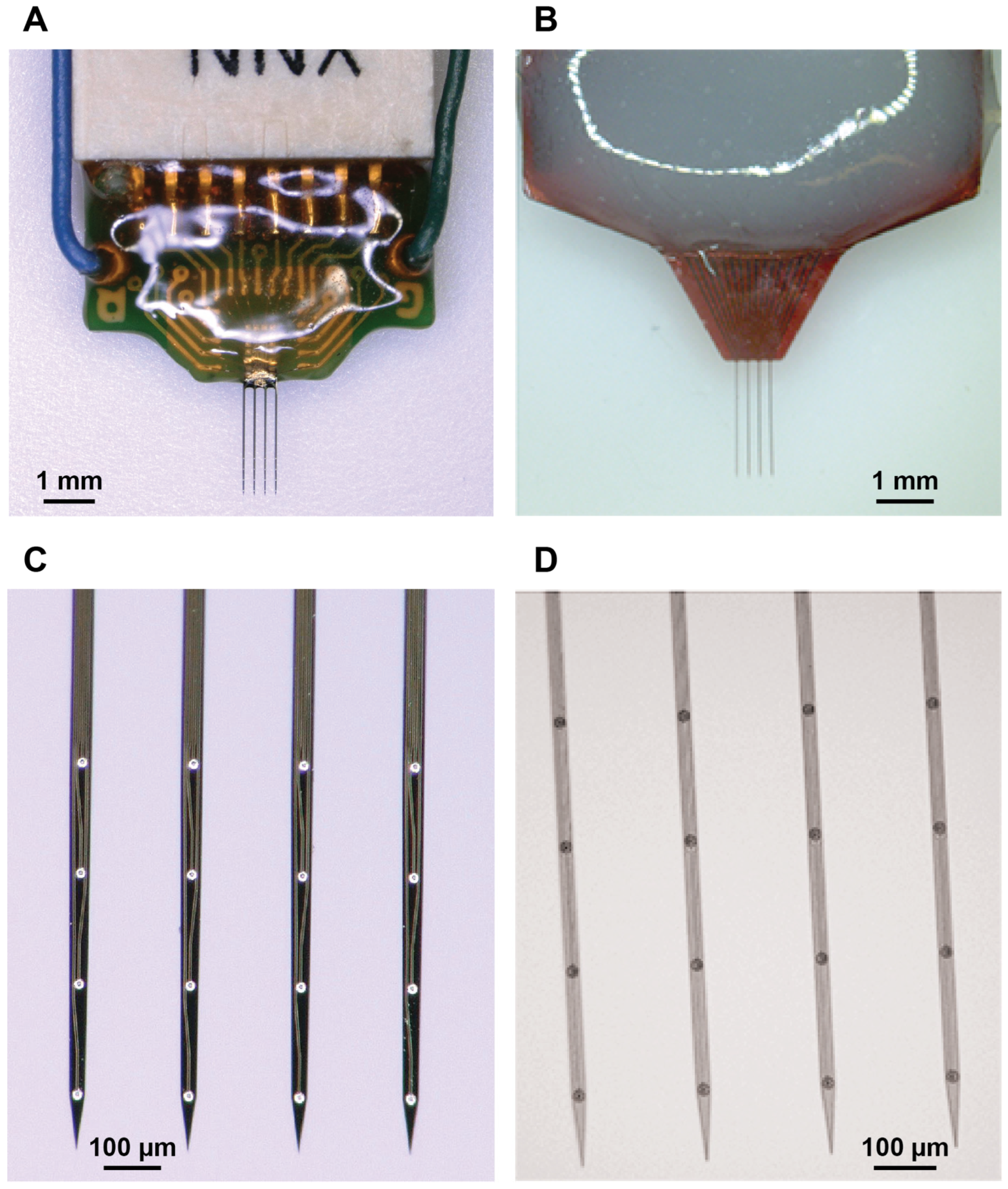
| Probe Type | Young’s Modulus (E) | Width (b) | Height (h) | Shank Length (L) |
|---|---|---|---|---|
| a-SiC | 75 [32] | 20 µm | 8 µm | 2 mm |
| Silicon-based | 190 [33] | 45 µm | 15 µm | 2 mm |
| Antibody | Target | Concentration | Supplier | Catalog Number |
|---|---|---|---|---|
| NeuN | Neuronal Nuclei | 1:500 | Abcam | ab4674 |
| GFAP | Astrocytes | 1:500 | Abcam | ab104224 |
| Alexa Fluor 555 | NeuN | 1:4000 | Abcam | ab150118 |
| Alexa Fluor 647 | GFAP | 1:4000 | Abcam | ab150171 |
| a-SiC (n = 7) | Silicon-Based (n = 3) | |
|---|---|---|
| Superficial (100–800 µm) | 17 slices stained with GFAP and NeuN | GFAP: 12 slices NeuN: 11 Slices |
| Deep (800–1200 µm) | 18 slices stained with GFAP and NeuN | 9 slices stained with GFAP and NeuN |
| a-SiC (n = 7) | Silicon-based (n = 3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haghighi, P.; Jeakle, E.N.; Sturgill, B.S.; Abbott, J.R.; Solis, E.; Devata, V.S.; Vijayakumar, G.; Hernandez-Reynoso, A.G.; Cogan, S.F.; Pancrazio, J.J. Enhanced Performance of Novel Amorphous Silicon Carbide Microelectrode Arrays in Rat Motor Cortex. Micromachines 2025, 16, 113. https://doi.org/10.3390/mi16020113
Haghighi P, Jeakle EN, Sturgill BS, Abbott JR, Solis E, Devata VS, Vijayakumar G, Hernandez-Reynoso AG, Cogan SF, Pancrazio JJ. Enhanced Performance of Novel Amorphous Silicon Carbide Microelectrode Arrays in Rat Motor Cortex. Micromachines. 2025; 16(2):113. https://doi.org/10.3390/mi16020113
Chicago/Turabian StyleHaghighi, Pegah, Eleanor N. Jeakle, Brandon S. Sturgill, Justin R. Abbott, Elysandra Solis, Veda S. Devata, Gayathri Vijayakumar, Ana G. Hernandez-Reynoso, Stuart F. Cogan, and Joseph J. Pancrazio. 2025. "Enhanced Performance of Novel Amorphous Silicon Carbide Microelectrode Arrays in Rat Motor Cortex" Micromachines 16, no. 2: 113. https://doi.org/10.3390/mi16020113
APA StyleHaghighi, P., Jeakle, E. N., Sturgill, B. S., Abbott, J. R., Solis, E., Devata, V. S., Vijayakumar, G., Hernandez-Reynoso, A. G., Cogan, S. F., & Pancrazio, J. J. (2025). Enhanced Performance of Novel Amorphous Silicon Carbide Microelectrode Arrays in Rat Motor Cortex. Micromachines, 16(2), 113. https://doi.org/10.3390/mi16020113











