Pump-Free Insulin Delivery via an SLA-Printed Hollow Microneedle Patch with an Integrated Self-Sealing Reservoir
Abstract
1. Introduction
2. Methodology
2.1. Device Design and Materials
2.2. Stereolithography Additive Manufacturing and Post-Processing
2.3. Characterisation and Functional Testing
2.4. Surface-Response Design of Experiments
3. Results
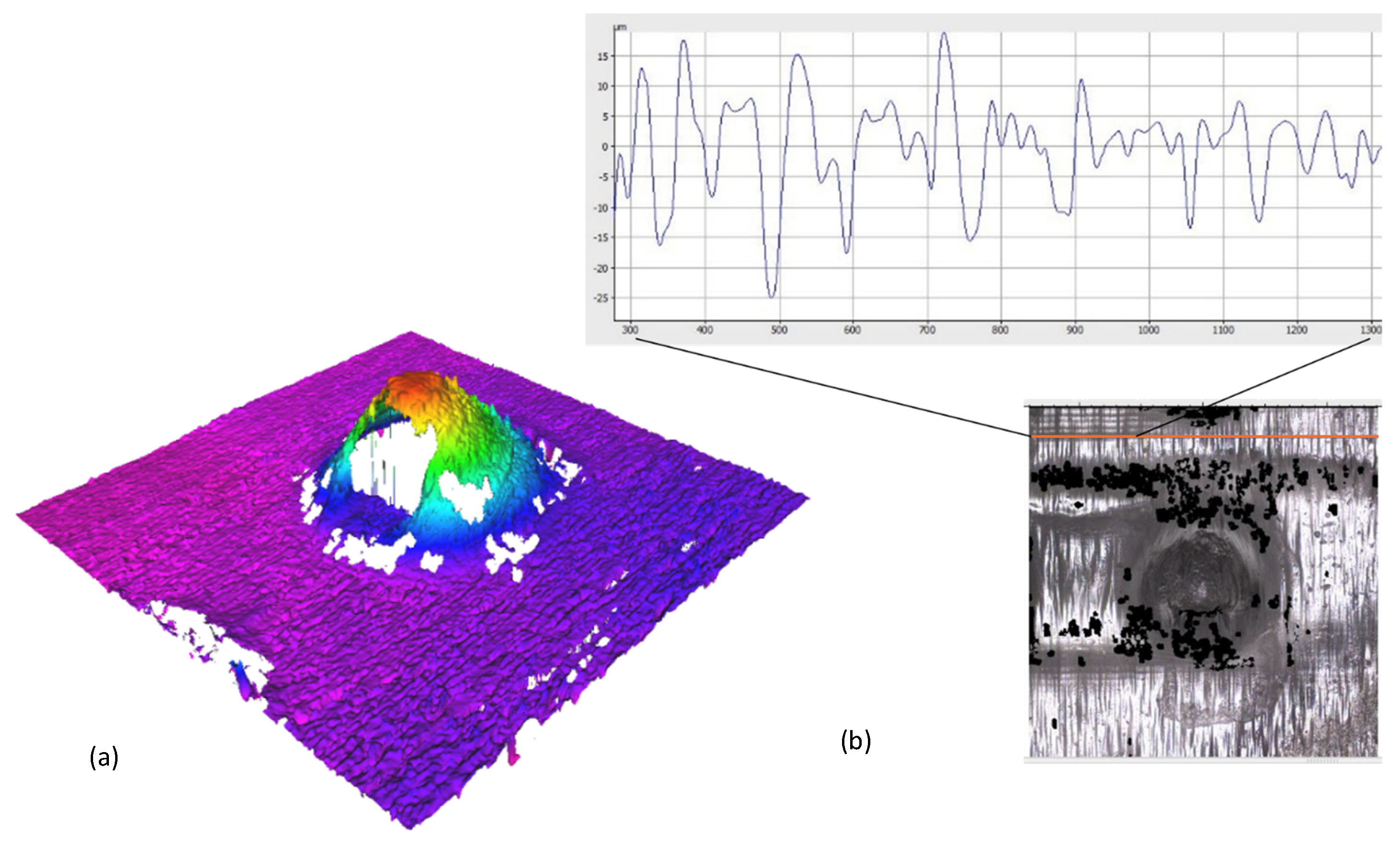
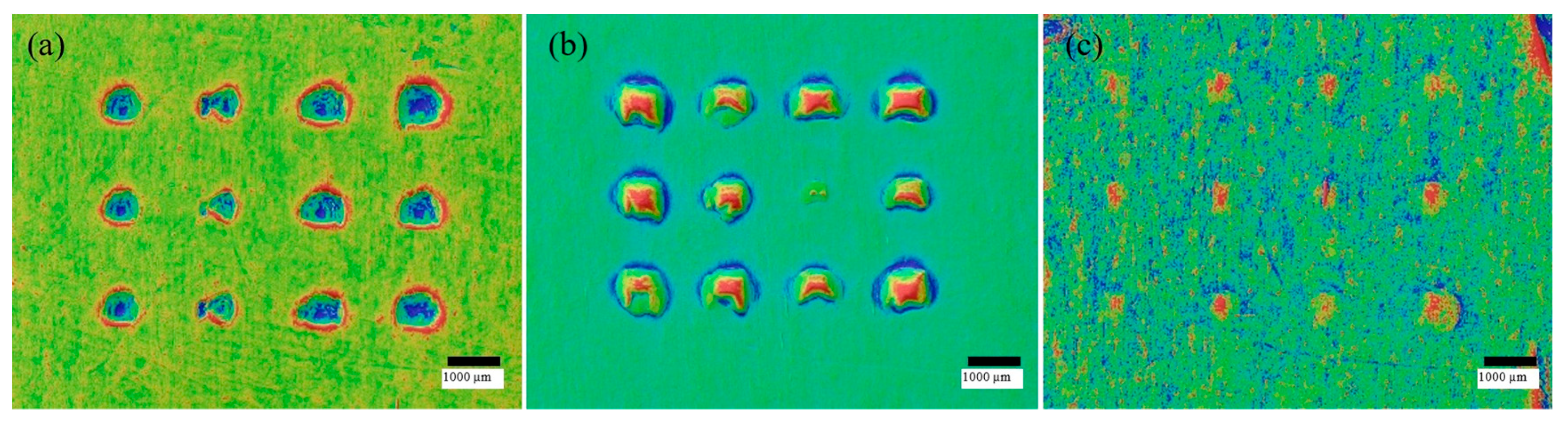
3.1. Geometric Fidelity and Orifice Patency
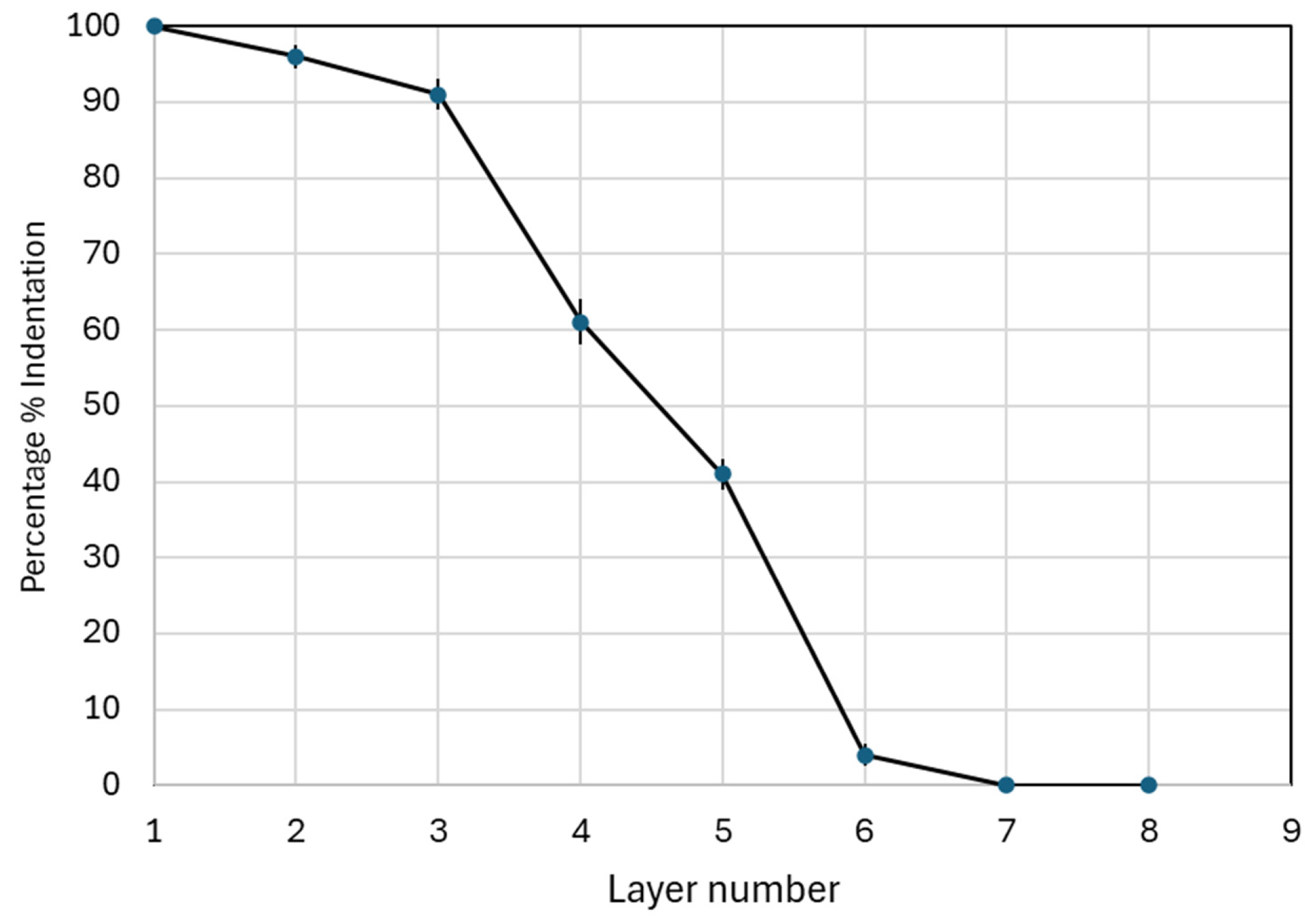
3.2. Insertion Mechanics and Penetration in Parafilm
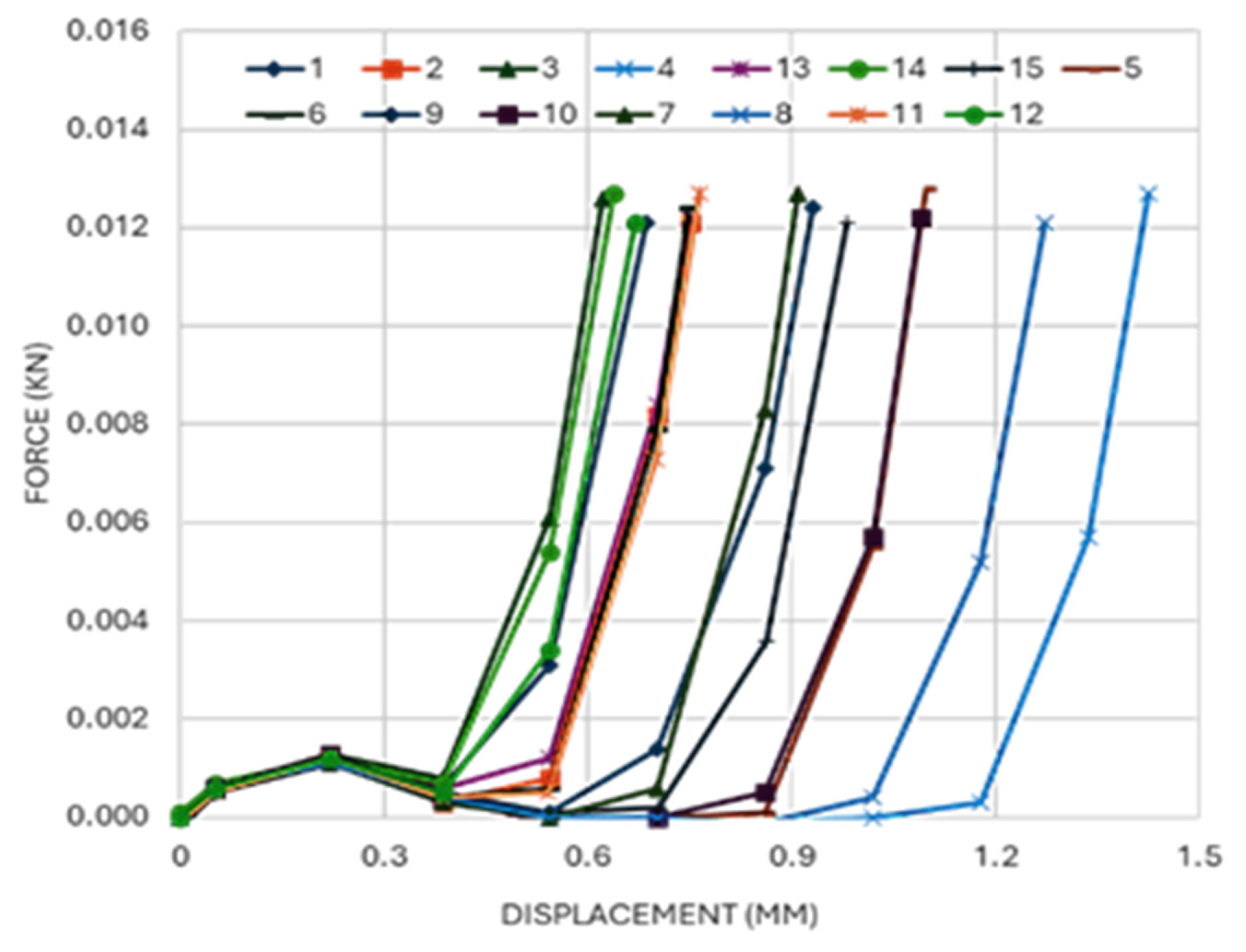
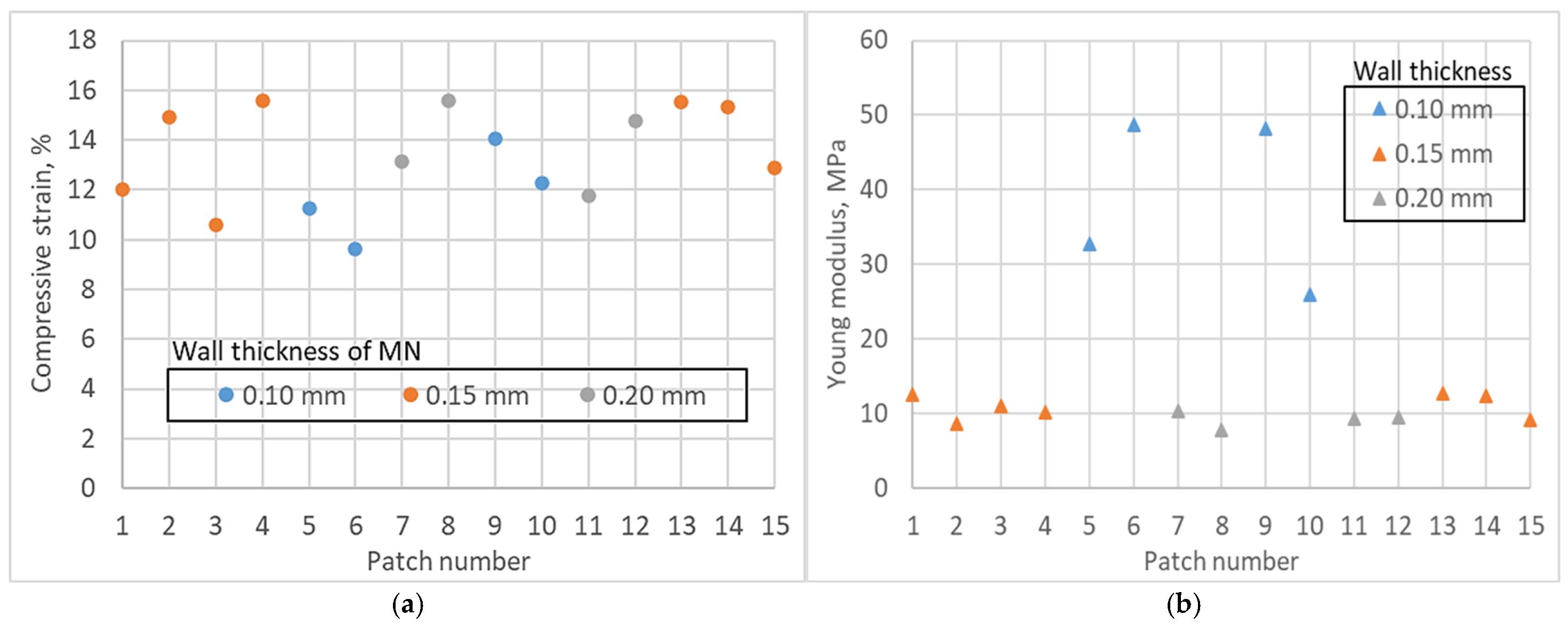
3.3. Axial Compression
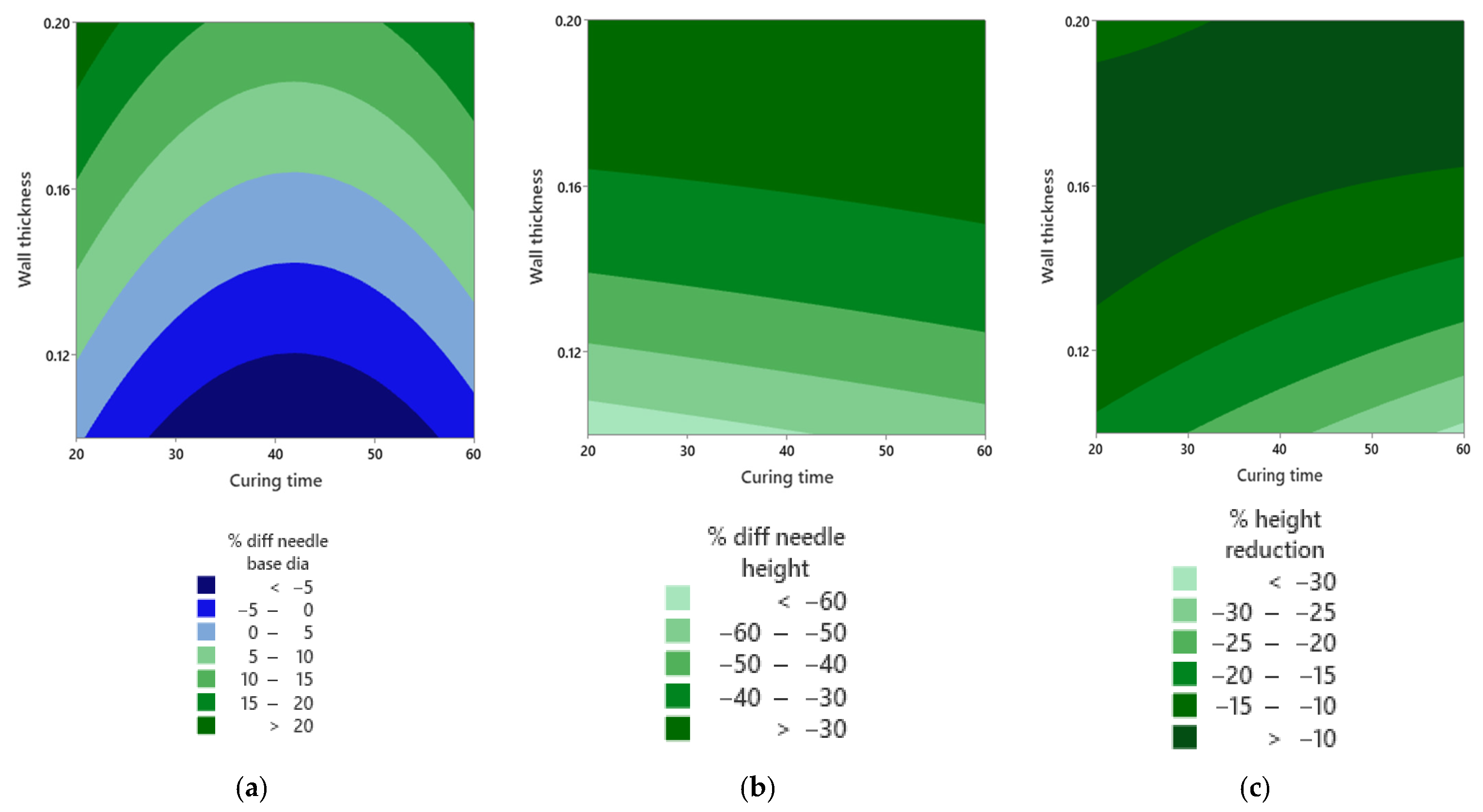
3.4. Factor Effects from the Response-Surface Design
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Diabetes: Fact Sheet. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 19 October 2025).
- International Diabetes Federation. Diabetes Facts and Figures. Available online: https://idf.org/about-diabetes/diabetes-facts-figures/ (accessed on 19 October 2025).
- Magliano, D.J.; Boyko, E.J. IDF Diabetes Atlas 10th Edition Scientific Committee. Global picture. In IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK581940/ (accessed on 19 October 2025).
- Duncanson, E.; Le Leu, R.K.; Shanahan, L.; Macauley, L.; Bennett, P.N.; Weichula, R.; McDonald, S.; Burke, A.L.J.; Collins, K.L.; Chur-Hansen, A.; et al. The prevalence and evidence-based management of needle fear in adults with chronic disease: A scoping review. PLoS ONE 2021, 16, e0253048. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Kim, M.-S.; Cho, J.; Kim, S.; Kwon, E.K.; Kim, Y.; Kang, D.; Cho, S.Y. Development and validation of a distress measurement for insulin injections among patients with diabetes. Sci. Rep. 2023, 13, 11725. [Google Scholar] [CrossRef]
- Xu, X.H.; Carvalho, V.; Wang, X.; Qiu, S.; Yang, H.; Sun, Z. Lipohypertrophy: Prevalence, clinical consequence, and pathogenesis. Chin. Med. J. 2020, 134, 47–49. [Google Scholar] [CrossRef]
- Mader, J.K.; Fornengo, R.; Hassoun, A.; Heinemann, L.; Kulzer, B.; Monica, M.; Nguyen, T.; Sieber, J.; Renard, E.; Reznik, Y.; et al. Risk factors for lipohypertrophy in people with insulin-treated diabetes: A systematic meta-analysis. Diabetes Sci. Technol. 2025, 19322968251325569. [Google Scholar] [CrossRef]
- Kim, Y.C.; Park, J.H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef]
- Gill, H.S.; Denson, D.D.; Burris, B.A.; Prausnitz, M.R. Effect of microneedle design on pain in human volunteers. Clin. J. Pain 2008, 24, 585–594. [Google Scholar] [CrossRef]
- Kaushik, S.; Hord, A.H.; Denson, D.D.; McAllister, D.V.; Smitra, S.; Allen, M.G.; Prausnitz, M.R. Lack of pain associated with microfabricated microneedles. Anesth. Analg. 2001, 92, 502–504. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Park, S.; Bondy, B.; Felner, E.I.; Prausnitz, M.R. Minimally invasive insulin delivery in subjects with type 1 diabetes using hollow microneedles. Diabetes Technol. Ther. 2009, 11, 329–337. [Google Scholar] [CrossRef]
- Kochba, E.; Levin, Y.; Raz, I.; Cahn, A. Improved insulin pharmacokinetics using a novel microneedle device for intradermal delivery in patients with type 2 diabetes. Diabetes Technol. Ther. 2016, 18, 525–531. [Google Scholar] [CrossRef]
- Cárcamo-Martínez, Á.; Mallon, B.; Anjani, Q.K.; Domínguez-Robles, J.; Utomo, E.; Vora, L.K.; Tekko, I.A.; Larrañeta, E.; Donnelly, R.F. Hollow microneedles: A perspective in biomedical applications. Int. J. Pharm. 2021, 99, 120455. [Google Scholar] [CrossRef] [PubMed]
- Detamornrat, U.; Parrilla, M.; Domínguez-Robles, J.; Anjani, Q.K.; Larrañeta, E.; De Wael, K.; Donnelly, R.F. Transdermal on-demand drug delivery based on an iontophoretic hollow microneedle array system. Lab A Chip 2023, 23, 2304–2315. [Google Scholar] [CrossRef]
- Griss, P.; Stemme, G. Side-opened out-of-plane microneedles for microfluidic transdermal liquid transfer. J. Microelectromech. Syst. 2003, 12, 296–301. [Google Scholar] [CrossRef]
- Gardeniers, H.J.G.E.; Luttge, R.; Berenschot, E.J.W.; de Boer, M.J.; Yeshurun, S.Y.; Hefetz, M.; van ’t Oever, R.; van den Berg, A. Silicon micromachined hollow microneedles for transdermal liquid transport. J. Microelectromech. Syst. 2003, 12, 855–862. [Google Scholar] [CrossRef]
- Yan, Q.; Shen, S.; Wang, Y.; Weng, J.; Wan, A.; Yang, G.; Feng, L. The finite element analysis research on microneedle design strategy and transdermal drug delivery system. Pharmaceutics 2022, 14, 1625. [Google Scholar] [CrossRef]
- Shrestha, P.; Stoeber, B. Fluid absorption by skin tissue during intradermal injections through hollow microneedles. Sci. Rep. 2018, 8, 13749. [Google Scholar] [CrossRef]
- Bhuimali, T.; Sarifuddin Das, D.B.; Mandal, P.K. Modelling hollow microneedle-mediated drug delivery in skin considering drug binding. Pharmaceutics 2025, 17, 105. [Google Scholar] [CrossRef]
- Hassanin, H.; Jiang, K. Net shape manufacturing of ceramic micro parts with tailored graded layers. J. Micromech. Microeng. 2014, 24, 015018. [Google Scholar] [CrossRef]
- Hassanin, H.; Jiang, K. Fabrication and characterization of stabilised zirconia micro parts via slip casting and soft moulding. Scr. Mater. 2013, 69, 433–436. [Google Scholar] [CrossRef]
- Hassanin, H.; Jiang, K. Optimized process for the fabrication of zirconia micro parts. Microelectron. Eng. 2010, 87, 1617–1619. [Google Scholar] [CrossRef]
- Hassanin, H.; Jiang, K. Functionally graded microceramic components. Microelectron. Eng. 2010, 87, 1610–1613. [Google Scholar] [CrossRef]
- Hassanin, H.; Jiang, K. Multiple replication of thick PDMS micropatterns using surfactants as release agents. Microelectron. Eng. 2011, 88, 3275–3277. [Google Scholar] [CrossRef]
- Razzaghi, M.; Alexander Ninan, J.; Akbari, M. Advancements in materials for 3D-printed microneedle arrays: Enhancing performance and biocompatibility. Micromachines 2024, 15, 1433. [Google Scholar] [CrossRef] [PubMed]
- Jarrahian, C.; Myers, D.; Creelman, B.; Saxon, E.; Zehrung, D. Vaccine vial stopper performance for fractional dose delivery of vaccines. Hum. Vaccines Immunother. 2017, 13, 1666–1668. [Google Scholar] [CrossRef]
- Roxhed, N.; Samel, B.; Nordquist, L.; Griss, P.; Stemme, G. Painless drug delivery through microneedle-based transdermal patches featuring active infusion. IEEE Trans. Biomed. Eng. 2008, 55, 1063–1071. [Google Scholar] [CrossRef]
- Ünver, N.; Odabaş, S.; Demirel, G.B.; Gül, O.T. Hollow microneedle array fabrication using a rational design to prevent skin clogging in transdermal drug delivery. J. Mater. Chem. B. 2022, 10, 8419–8431. [Google Scholar] [CrossRef]
- Sarker, S.; Wen, Z.; Acevedo, R.; Lamont, A.C.; Colton, A.; Muller, L.K.; Park, D.; Tubaldi, E.; Rand-Yadin, K.; Sochol, R.D. 3D nanoprinting of embryo microinjection needles with anti-clogging features. Microsyst. Nanoeng. 2025, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- Larrañeta, E.; Moore, J.; Vicente-Pérez, E.M.; González-Vázquez, P.; Lutton, R.; Woolfson, A.D.; Donnelly, R.F. A proposed model membrane and test method for microneedle insertion studies. Int. J. Pharm. 2014, 472, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Anjani, Q.K.; Nainggolan, A.D.C.; Li, H.; Miatmoko, A.; Larrañeta, E.; Donnelly, R.F. Parafilm® M and Strat-M® as skin simulants in in vitro permeation of dissolving microarray patches loaded with proteins. Int. J. Pharm. 2024, 655, 124071. [Google Scholar] [CrossRef] [PubMed]
- Fitaihi, R.; Abukhamees, S.; Chung, S.H.; Craig, D.Q.M. Optimization of stereolithography 3D printing of microneedle micro-molds for ocular drug delivery. Int. J. Pharm. 2024, 658, 124195. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Sharma, P.K.; Murty, U.S.; Mohan, N.H.; Thomas, R.; Dwivedy, S.K.; Banerjee, S. 3D-printed hollow microneedles array using stereolithography for efficient transdermal delivery of rifampicin. Int. J. Pharm. 2021, 605, 120815. [Google Scholar] [CrossRef]
- Zhang, S.; Sims, J.; Mehochko, I.; Zolovick, R.; Kwak, T.; Staples, A. Design and characterization of 3D-printed hollow microneedle arrays for transdermal insulin delivery. AIP Adv. 2024, 14, 065234. [Google Scholar] [CrossRef]
- Razzaghi, M.; Akbari, M. 3D printed hollow microneedles: The latest innovation in drug delivery. Expert Opin. Drug Deliv. 2025, 22, 1487–1507. [Google Scholar] [CrossRef]
- Smith, F.; Kotowska, A.M.; Fiedler, B.; Cerny, E.; Cheung, K.; Rutland, C.S.; Chowdhury, F.; Segal, J.; Rawson, F.J.; Marlow, M. Using oscillation to improve the insertion depth and consistency of hollow microneedles for transdermal insulin delivery with mechanistic insights. Mol. Pharm. 2025, 22, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xu, G.; Yao, X.; Zhou, H.; Lyu, B.; Pei, S.; Wen, P. Microneedle-based insulin transdermal delivery system: Current status and translation challenges. Drug Deliv. Transl. Res. 2022, 12, 2403–2427. [Google Scholar] [CrossRef]
- Economidou, S.; Pere, C.; Okereke, M.; Douroumis, D. Optimisation of design and manufacturing parameters of 3D-printed solid microneedles for improved strength, sharpness, and drug delivery. Micromachines 2021, 12, 117. [Google Scholar] [CrossRef]
- Economidou, S.N.; Pissinato Pere, C.P.; Reid, A.; Uddin, M.J.; Windmill, J.F.C.; Lamprou, D.A.; Douroumis, D. 3D-printed microneedle patches using stereolithography (SLA) for intradermal insulin delivery. Mater. Sci. Eng. C 2019, 102, 743–755. [Google Scholar] [CrossRef]
- Choo, S.; Jin, S.; Jung, J. Fabricating high-resolution and high-dimensional microneedle mold through the resolution improvement of stereolithography 3D printing. Pharmaceutics 2022, 14, 766. [Google Scholar] [CrossRef] [PubMed]
- Muldoon, K.; Song, Y.; Ahmad, Z.; Chen, X.; Chang, M.-W. High precision 3D printing for micro to nano scale biomedical and electronic devices. Micromachines 2022, 13, 642. [Google Scholar] [CrossRef]
- Gupta, J.; Felner, E.I.; Prausnitz, M.R. Rapid pharmacokinetics of intradermal insulin administered using microneedles in type 1 diabetes subjects. Diabetes Technol. Ther. 2011, 13, 451–456. [Google Scholar] [CrossRef]
- Alrimawi, B.H.; Lee, J.Y.; Ng, K.W.; Goh, C.F. In vitro evaluation of microneedle strength: A comparison of test configurations and experimental insights. R. Soc. Chem. Pharm. 2024, 1, 227. [Google Scholar] [CrossRef]
- Makvandi, P.; Kirkby, M.; Hutton, A.R.J.; Shabani, M.; Yiu, C.K.Y.; Baghbantaraghdari, Z.; Jamaledin, R.; Carlotti, M.; Mazzolai, B.; Mattoli, V.; et al. Engineering microneedle patches for improved penetration: Analysis, skin models and factors affecting needle insertion. Nano-Micro Lett. 2021, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- Tsuboko, Y.; Sakoda, H.; Okamoto, Y.; Nomura, Y.; Yamamoto, E. Mechanical characterization of individual needles in microneedle arrays: Factors affecting compression test results. Pharmaceutics 2024, 16, 1480. [Google Scholar] [CrossRef] [PubMed]
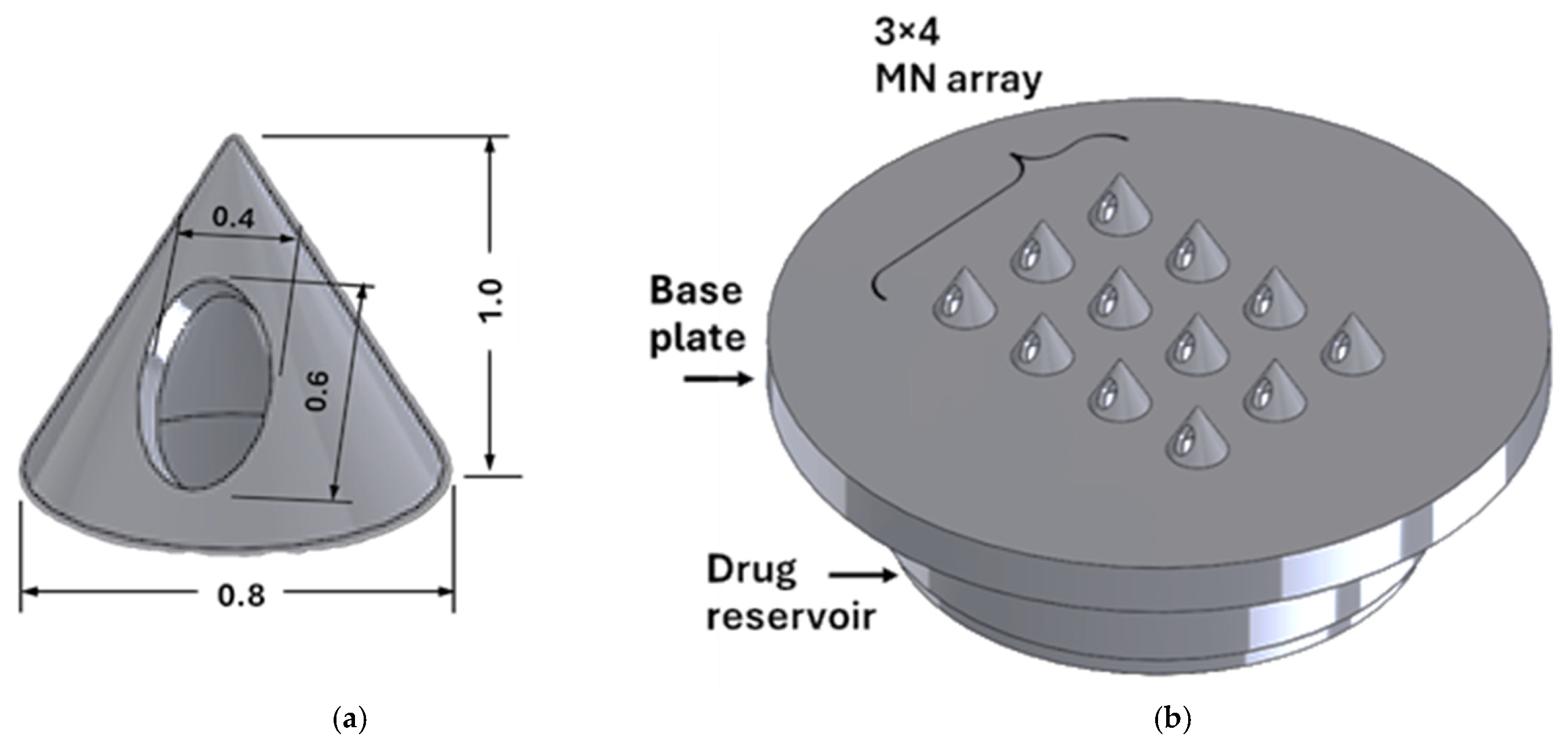
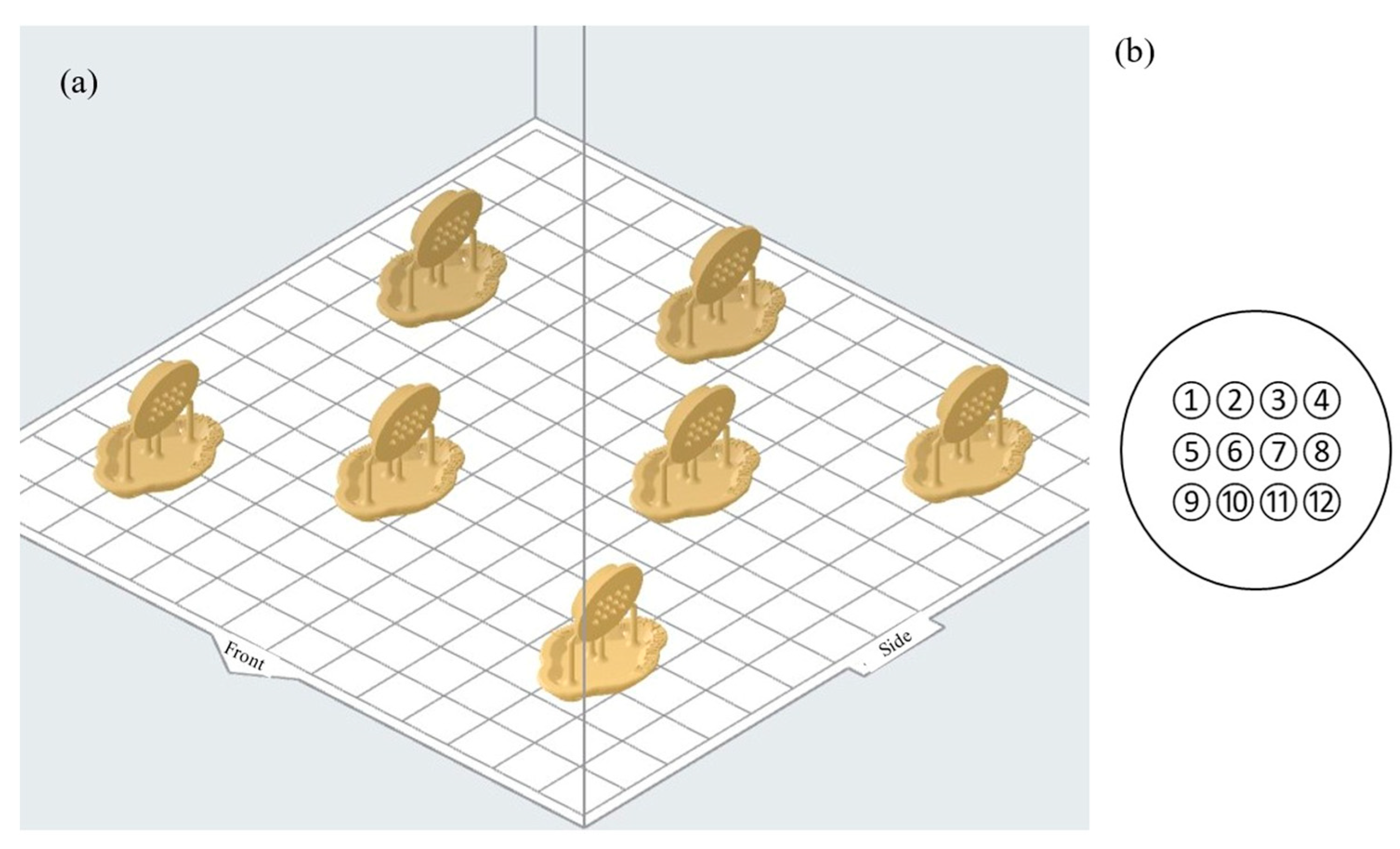
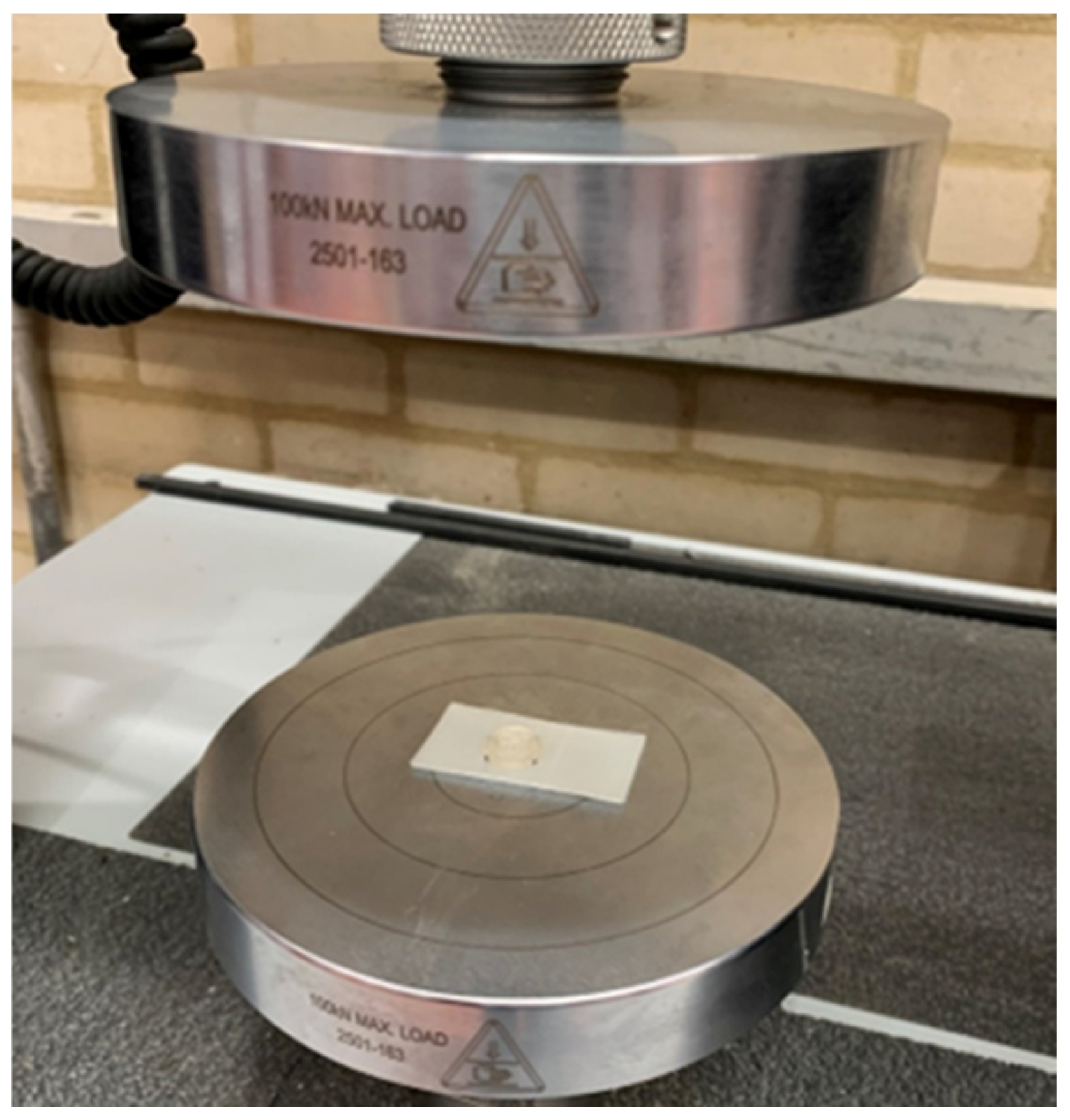
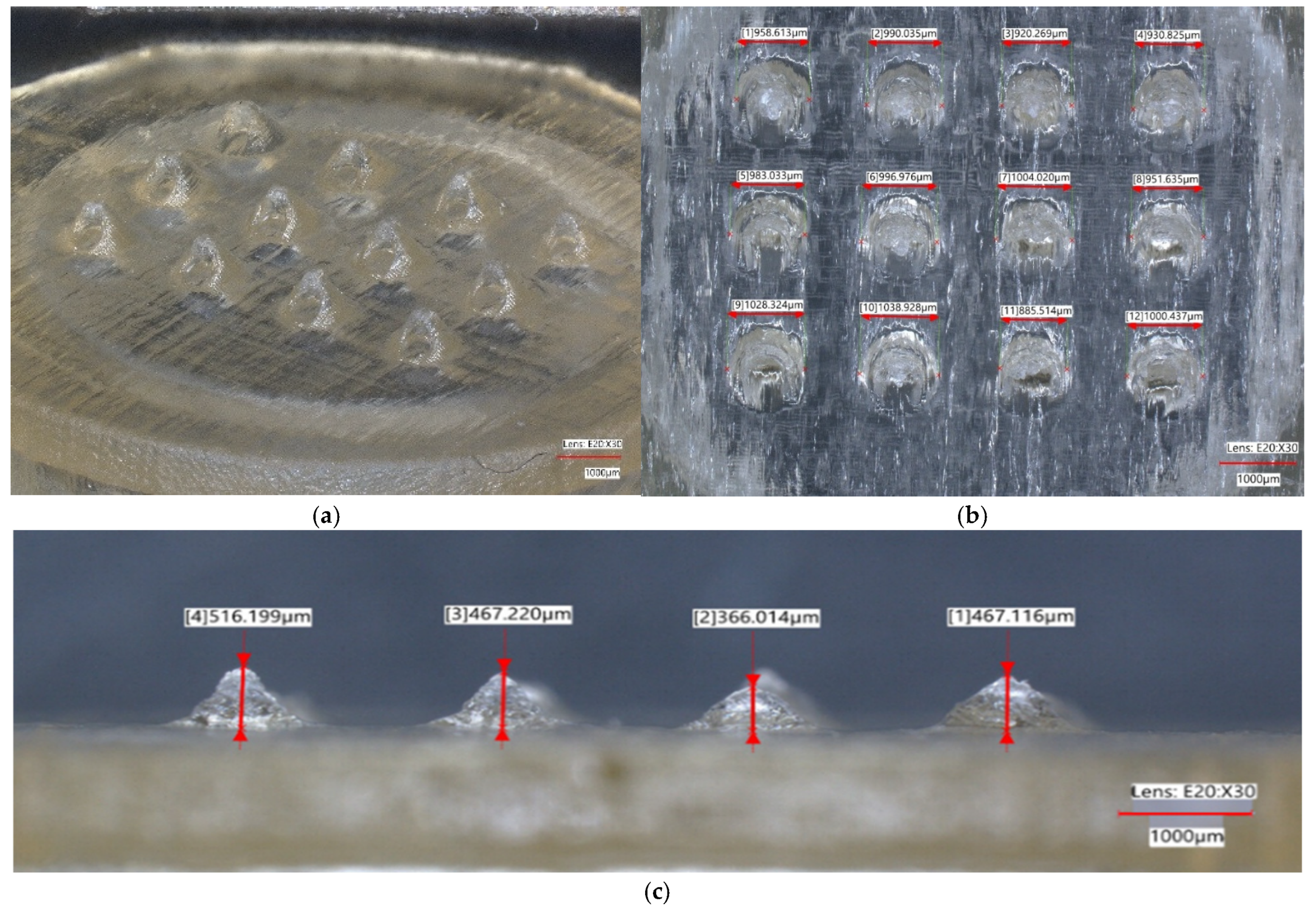
| Design Element | Rationale/Criteria |
|---|---|
| Through hole | An internal channel not blocked by resin to allow for efficient insulin flow [37]. |
| Solid cone-shaped base needle tip | A solid cone-shaped tip can withstand the highest compressional forces and provides the easiest insertion [38]. |
| Orifice located on needle shaft | A side-opened orifice greatly reduces clogging of the needle with skin tissue and hence improves drug flow [15]. |
| Outer diameter | 15 mm—to be compatible with the uPATCHTM universal MN applicator. |
| Reservoir | A cavity to store insulin is provided with chamfers at the needle entry for ease of fluid flow. Capacity to store at least 10 units of insulin. |
| Pitch-to-radius aspect ratio | 3.5. |
| Needle height | Preferably <1500 μm (revise the above discussion). |
| Size of opening | Small enough to avoid loss of drug in cases of partial insertion. |
| Base plate | Base plate is larger than the reservoir to secure the patch in place with an adhesive sticker. |
| Factor | Levels | Units | Notes |
|---|---|---|---|
| Wall thickness | 0.10, 0.15, 0.20 | mm | Manufacturable and lumen-patent levels. Pitch values tied to these levels to maintain pitch-to-radius of 3.5. |
| Post-cure time | 20, 40, 60 | min | Within curing unit capability and material guidance to expose time effects. |
| Post-cure temperature | 35, 60, 80 | °C | Within curing unit capability and material guidance to expose temperature effects. |
| Patch | % Diff Through-hole | Mean Ra (µm) | % Diff Needle Height | % Diff Needle Base | % Height Reduction | Insertion Gradient | Compressive Strain at Fmax (%) | Young’s Modulus (MPa) |
|---|---|---|---|---|---|---|---|---|
| 1 | 7.0272 | 2.15282 | −37.7059 | 20.0889 | −13.8386 | −2.1905 | 12.00 | 12.53 |
| 2 | 16.5541 | 4.77575 | −32.8250 | 10.0410 | −7.5159 | −2.0714 | 14.90 | 8.71 |
| 3 | 13.6077 | 4.24242 | −28.8122 | 13.0008 | −13.3020 | −2.1429 | 10.60 | 11.07 |
| 4 | 8.1529 | 4.15740 | −32.4072 | 7.7731 | −14.4208 | −2.1429 | 15.57 | 10.19 |
| 5 | 1.1254 | 3.81850 | −66.3591 | −3.9035 | −10.3267 | −2.1905 | 11.24 | 32.80 |
| 6 | 2.0300 | 6.12222 | −50.4950 | 0.5727 | −32.7013 | −2.1071 | 9.65 | 48.79 |
| 7 | 8.9478 | 4.37838 | −27.5420 | 19.5655 | −7.2029 | −2.2500 | 13.16 | 10.47 |
| 8 | 10.5489 | 1.77180 | −25.6737 | 17.7654 | −9.5859 | −2.2024 | 15.60 | 7.80 |
| 9 | 14.9613 | 5.47717 | −64.5962 | −9.7717 | −31.6732 | −1.7500 | 14.05 | 48.20 |
| 10 | 5.0916 | 9.99875 | −62.6381 | −11.3968 | −20.5217 | −1.6548 | 12.30 | 26.02 |
| 11 | 12.0193 | 2.28385 | −24.5237 | 19.1022 | −8.4684 | −2.2024 | 11.76 | 9.35 |
| 12 | 1.5742 | 8.71082 | −28.1032 | 11.0312 | −10.9279 | −2.1905 | 14.77 | 9.48 |
| 13 | 8.0408 | 9.17765 | −32.5828 | −2.2769 | −11.4801 | −2.1429 | 15.53 | 12.85 |
| 14 | 7.7026 | 5.14190 | −28.9758 | −0.5369 | −6.1791 | −2.1667 | 15.32 | 12.43 |
| 15 | 6.3473 | 5.86445 | −34.9782 | 6.6710 | −8.1221 | −2.1548 | 12.91 | 9.12 |
| Response Variable | Significant Factor Term(s) | p Value | Practical Reading |
|---|---|---|---|
| Percent difference in needle height | Wall thickness | 0.00 | Thickness controls height accuracy. |
| Wall thickness squared | 0.003 | Curved thickness effect points to an interior optimum. | |
| Percent difference in needle base diameter | Wall thickness | 0.001 | Thickness influences base sizing error. |
| Curing time squared | 0.012 | Mid-curing times minimise error, matching the islands in Figure 12a. | |
| Percent height reduction | Wall thickness | 0.014 | Mid-thickness reduces post-compression height loss. |
| Insertion gradient | Wall thickness | 0.029 | Deeper penetration performance depends on thickness. |
| Young’s modulus | Wall thickness | 0.00 | Thin walls show higher axial stiffness. |
| Wall thickness squared | 0.004 | Stiffness varies non-linearly with thickness. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, E.; Alsaleh, N.A.; Ahmadein, M.; Elfar, A.A.; Hassanin, H.; Essa, K. Pump-Free Insulin Delivery via an SLA-Printed Hollow Microneedle Patch with an Integrated Self-Sealing Reservoir. Micromachines 2025, 16, 1322. https://doi.org/10.3390/mi16121322
Smith E, Alsaleh NA, Ahmadein M, Elfar AA, Hassanin H, Essa K. Pump-Free Insulin Delivery via an SLA-Printed Hollow Microneedle Patch with an Integrated Self-Sealing Reservoir. Micromachines. 2025; 16(12):1322. https://doi.org/10.3390/mi16121322
Chicago/Turabian StyleSmith, Evie, Naser A. Alsaleh, Mahmoud Ahmadein, Abdullah A. Elfar, Hany Hassanin, and Khamis Essa. 2025. "Pump-Free Insulin Delivery via an SLA-Printed Hollow Microneedle Patch with an Integrated Self-Sealing Reservoir" Micromachines 16, no. 12: 1322. https://doi.org/10.3390/mi16121322
APA StyleSmith, E., Alsaleh, N. A., Ahmadein, M., Elfar, A. A., Hassanin, H., & Essa, K. (2025). Pump-Free Insulin Delivery via an SLA-Printed Hollow Microneedle Patch with an Integrated Self-Sealing Reservoir. Micromachines, 16(12), 1322. https://doi.org/10.3390/mi16121322








