Interfacial Engineering of Co3O4@MXene for Superior Charge Storage: A Route to High-Capacitance Supercapacitors
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Pristine Co3O4 via Hydrothermal Method
2.3. Synthesis of MXene
2.4. Synthesis of Co3O4/MXene Composite
2.5. Co3O4/MXene Composite Electrode Fabrication
2.6. Electrochemical Measurements
2.7. Characterization Techniques
3. Characterization Analysis
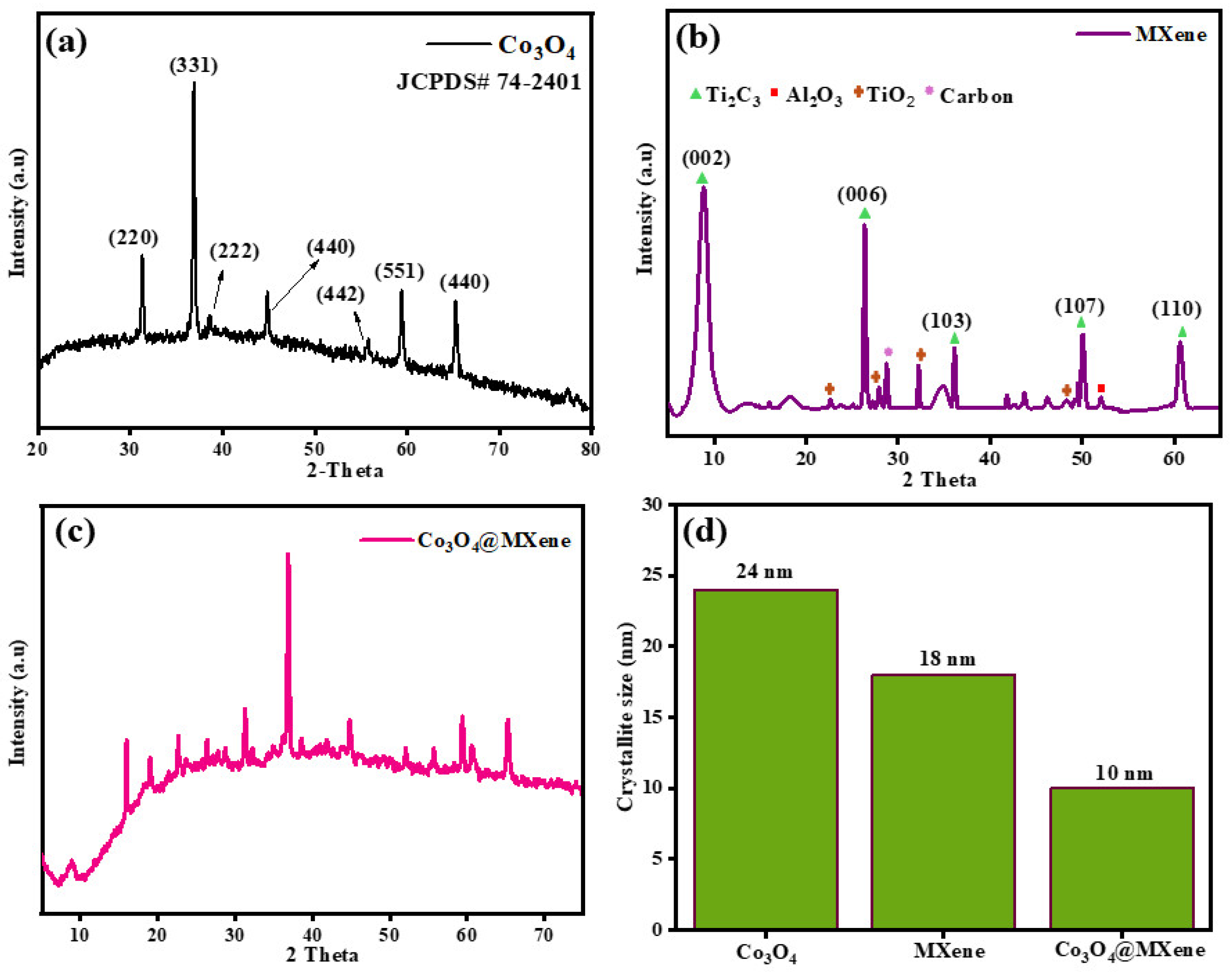
3.1. XRD Analysis
3.2. FTIR Spectroscopic Analysis
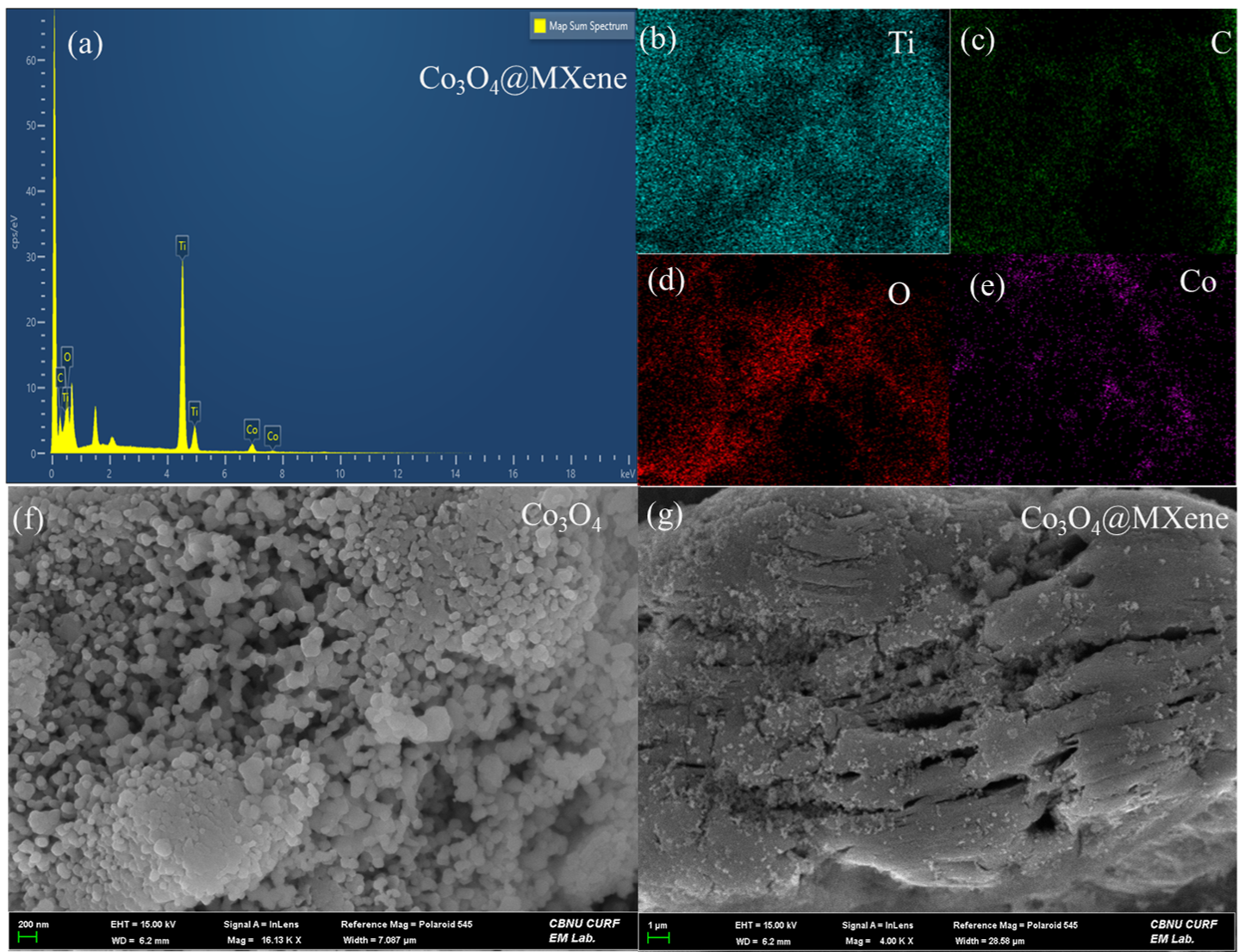
3.3. Morphological Analysis
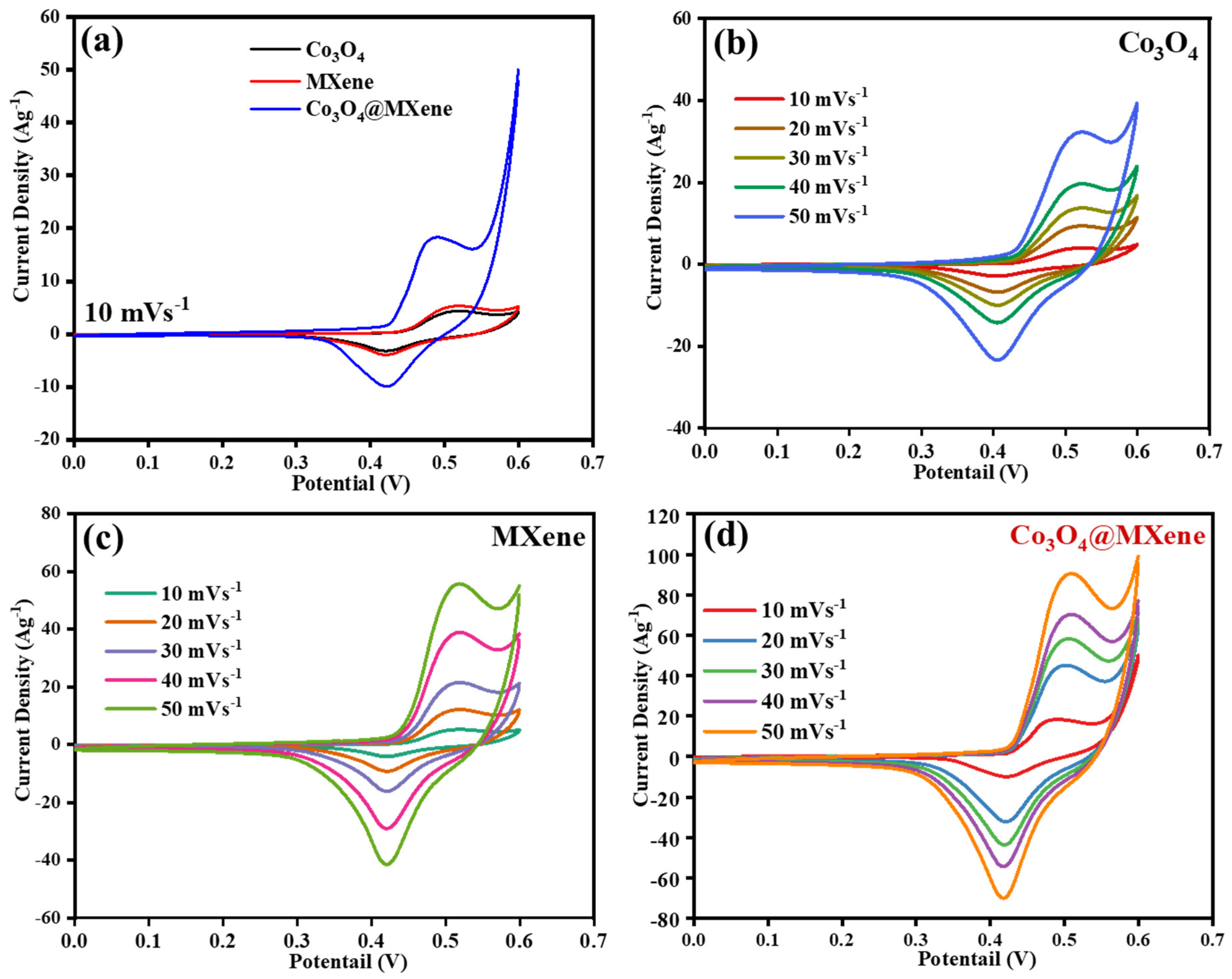
3.4. Cyclic Voltammetry
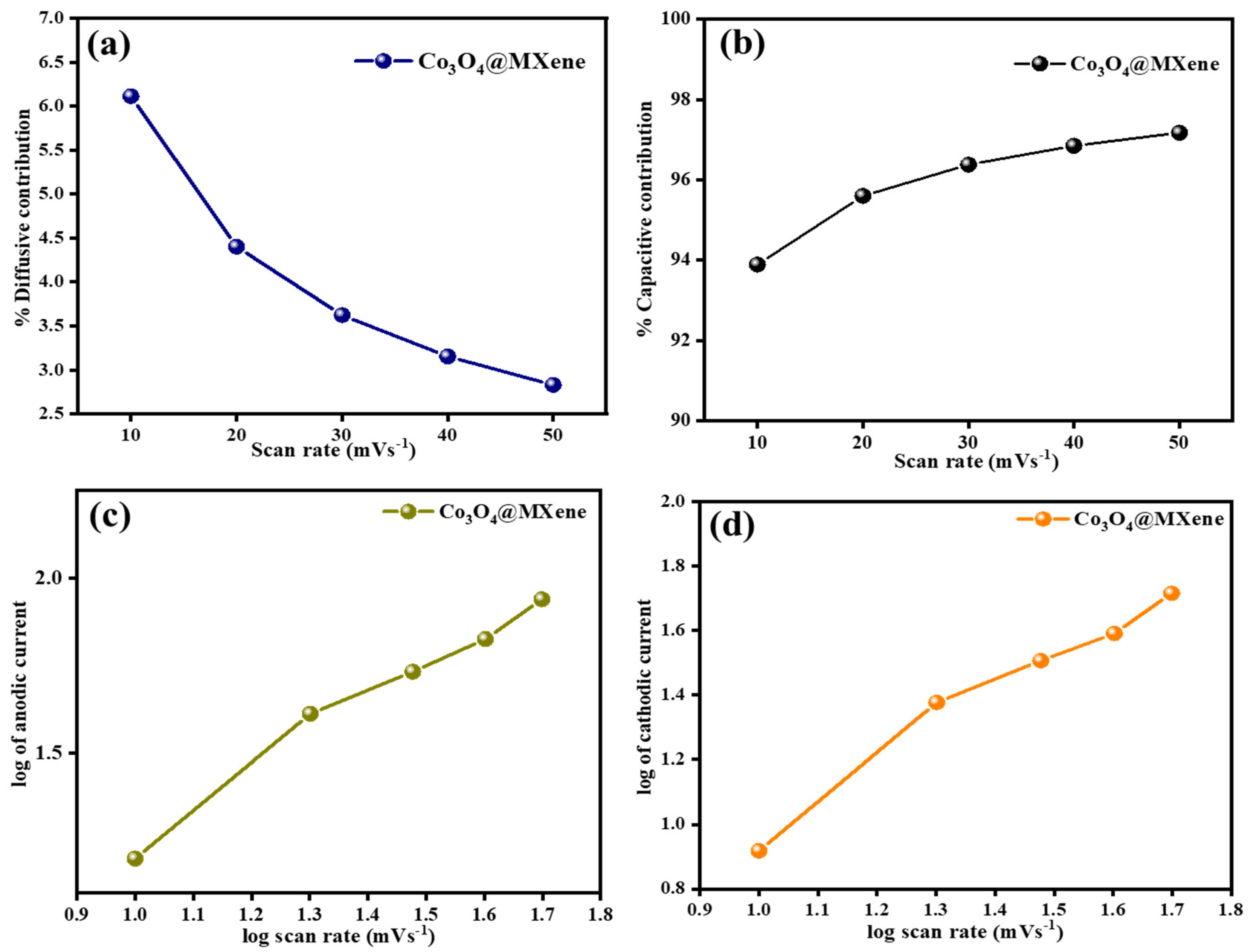
3.5. Capacitive vs. Diffusive Contribution
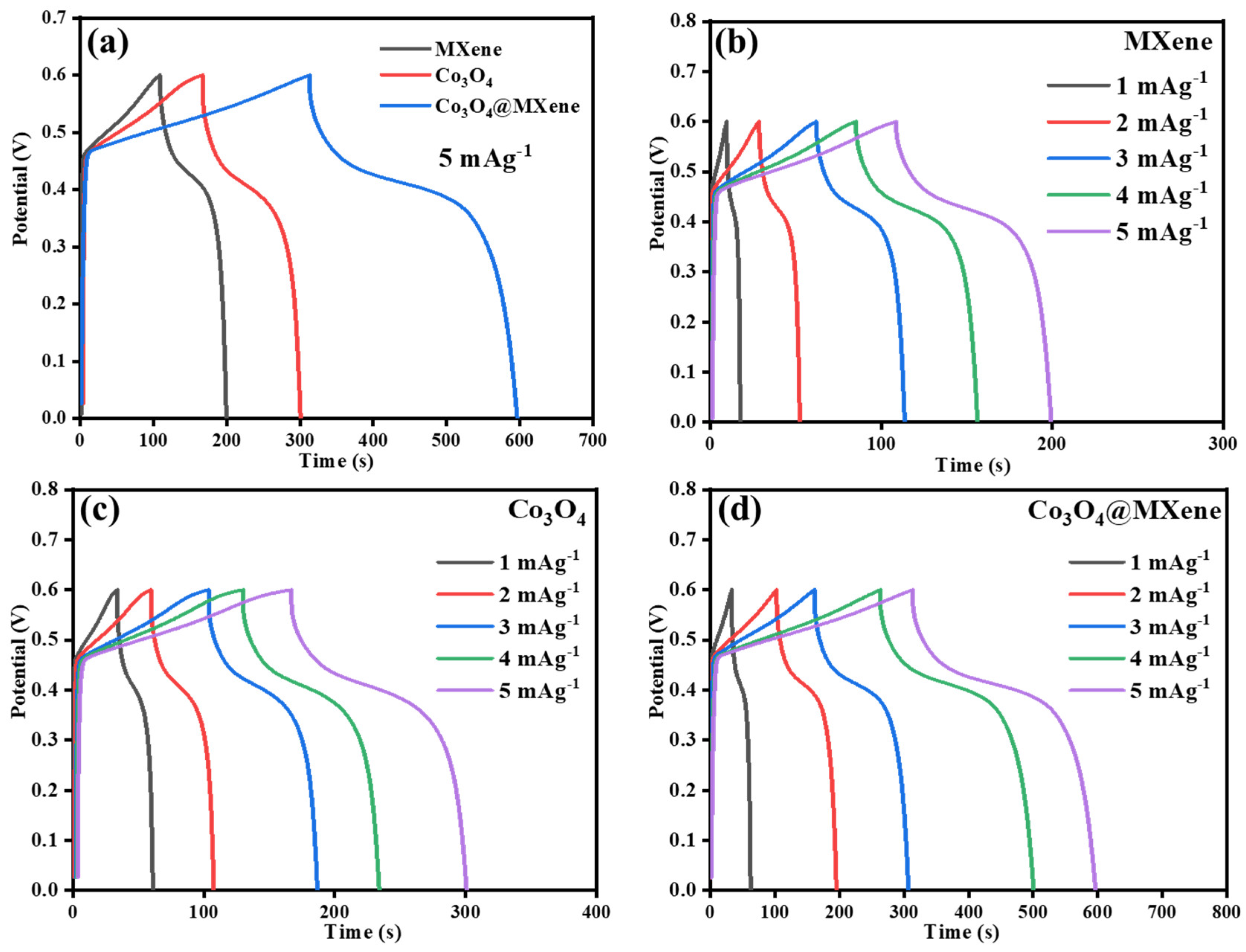
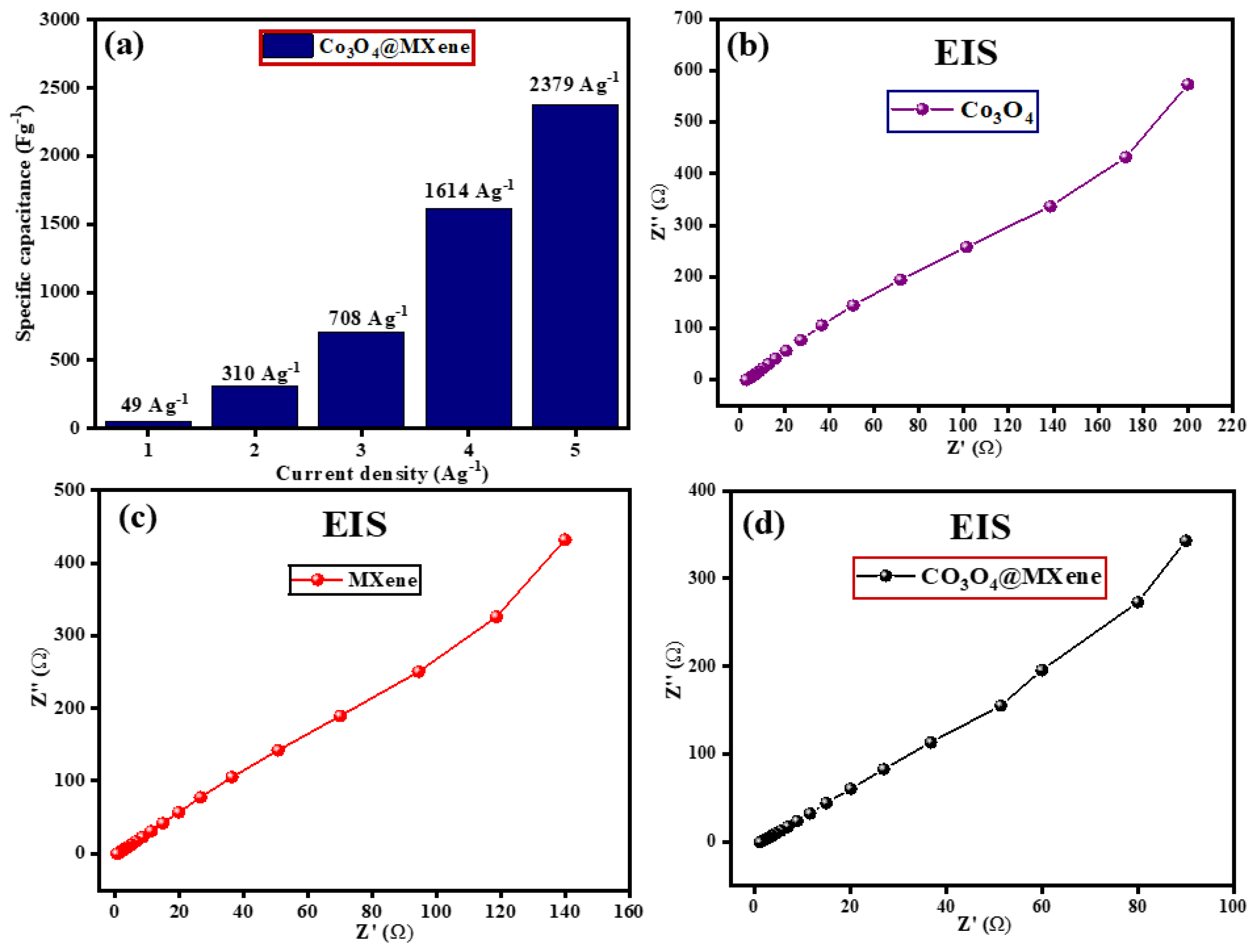
3.6. Galvanostatic Charge–Discharge
3.7. Electrochemical Impedance Spectroscopy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Conway, B.; Pell, W. Double-layer and pseudocapacitance types of electrochemical capacitors and their applications to the development of hybrid devices. J. Solid State Electrochem. 2003, 7, 637–644. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef]
- Ma, R.; Chen, Z.; Zhao, D.; Zhang, X.; Zhuo, J.; Yin, Y.; Wang, X.; Yang, G.; Yi, F. Ti3C2Tx MXene for electrode materials of supercapacitors. J. Mater. Chem. A 2021, 9, 11501–11529. [Google Scholar] [CrossRef]
- Jin, J.; Xiao, T.; Zhang, Y.-F.; Zheng, H.; Wang, H.; Wang, R.; Gong, Y.; He, B.; Liu, X.; Zhou, K. Hierarchical MXene/transition metal chalcogenide heterostructures for electrochemical energy storage and conversion. Nanoscale 2021, 13, 19740–19770. [Google Scholar] [CrossRef]
- Anand, S.; Ahmad, M.W.; Fatima, A.; Kumar, A.; Bharadwaj, A.; Yang, D.-J.; Choudhury, A. Flexible nickel disulfide nanoparticles-anchored carbon nanofiber hybrid mat as a flexible binder-free cathode for solid-state asymmetric supercapacitors. Nanotechnology 2021, 32, 495403. [Google Scholar] [CrossRef]
- Li, S.; Hao, X.; Abudula, A.; Guan, G. Nanostructured Co-based bifunctional electrocatalysts for energy conversion and storage: Current status and perspectives. J. Mater. Chem. A 2019, 7, 18674–18707. [Google Scholar] [CrossRef]
- Raza, Q.; Bibi, I.; Majid, F.; Kamal, S.; Ata, S.; Ghafoor, A.; Arshad, M.I.; Al-Mijalli, S.H.; Nazir, A.; Iqbal, M. Solar light-based photocatalytic removal of CV and RhB dyes using Bi and Al doped SrFe12O19 NPs and antibacterial properties. J. Ind. Eng. Chem. 2023, 118, 469–482. [Google Scholar] [CrossRef]
- Chen, Y.; Jing, C.; Fu, X.; Shen, M.; Li, K.; Liu, X.; Yao, H.-C.; Zhang, Y.; Yao, K.X. Synthesis of porous NiCoS nanosheets with Al leaching on ordered mesoporous carbon for high-performance supercapacitors. Chem. Eng. J. 2020, 384, 123367. [Google Scholar] [CrossRef]
- Tachikawa, T.; Ochi, T.; Kobori, Y. Crystal-face-dependent charge dynamics on a BiVO4 photocatalyst revealed by single-particle spectroelectrochemistry. Acs Catal. 2016, 6, 2250–2256. [Google Scholar] [CrossRef]
- Pan, S.; Liu, X. CdS–Graphene nanocomposite: Synthesis, adsorption kinetics and high photocatalytic performance under visible light irradiation. New J. Chem. 2012, 36, 1781–1787. [Google Scholar] [CrossRef]
- Jie, L.; Gao, X.; Cao, X.; Wu, S.; Long, X.; Ma, Q.; Su, J. A review of CdS photocatalytic nanomaterials: Morphology, synthesis methods, and applications. Mater. Sci. Semicond. Process. 2024, 176, 108288. [Google Scholar] [CrossRef]
- Zou, R.; Quan, H.; Pan, M.; Zhou, S.; Chen, D.; Luo, X. Self-assembled MXene (Ti3C2Tx)/α-Fe2O3 nanocomposite as negative electrode material for supercapacitors. Electrochim. Acta 2018, 292, 31–38. [Google Scholar] [CrossRef]
- Boota, M.; Rajesh, M.; Bécuwe, M. Multi-electron redox asymmetric supercapacitors based on quinone-coupled viologen derivatives and Ti3C2Tx MXene. Mater. Today Energy 2020, 18, 100532. [Google Scholar] [CrossRef]
- Carbide, T.-D.T. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 2013, 1241488, 341. [Google Scholar]
- Cheng, C.; Liang, Q.; Yan, M.; Liu, Z.; He, Q.; Wu, T.; Luo, S.; Pan, Y.; Zhao, C.; Liu, Y. Advances in preparation, mechanism and applications of graphene quantum dots/semiconductor composite photocatalysts: A review. J. Hazard. Mater. 2022, 424, 127721. [Google Scholar] [CrossRef]
- Zhong, Q.; Li, Y.; Zhang, G. Two-dimensional MXene-based and MXene-derived photocatalysts: Recent developments and perspectives. Chem. Eng. J. 2021, 409, 128099. [Google Scholar] [CrossRef]
- Boota, M.; Pasini, M.; Galeotti, F.; Porzio, W.; Zhao, M.-Q.; Halim, J.; Gogotsi, Y. Interaction of polar and nonpolar polyfluorenes with layers of two-dimensional titanium carbide (MXene): Intercalation and pseudocapacitance. Chem. Mater. 2017, 29, 2731–2738. [Google Scholar] [CrossRef]
- Tang, J.; Wu, F.; Dai, X.; Zhou, J.; Pang, H.; Duan, X.; Xiao, B.; Li, D.; Long, J. Robust MXene adding enables the stable interface of silicon anodes for high-performance Li-ion batteries. Chem. Eng. J. 2023, 452, 139139. [Google Scholar] [CrossRef]
- Fu, N.; Liu, Z.; Shen, B.; Shao, W.; Wang, T.; Zhao, H.; Wang, J.; Chen, Q.; Luo, J.; Liu, Y. Carbon and MXene Dual Confinement and Dense Structural Engineering Toward Construct High Performance Micron-SiOx Anode for Li-Ion Batteries. Adv. Funct. Mater. 2024, 34, 2410839. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, S.; Wu, Y.; Wu, F.; Tang, J. Coupling modification strategy with carbon coating and MXene conductive additive enables high-performance silicon anode for lithium-ion batteries. J. Power Sources 2025, 635, 236520. [Google Scholar] [CrossRef]
- Cao, X.; Li, Z.; Yang, F.; Xie, J.; Shi, X.; Yuan, P.; Ding, X.; Lu, X. Ultralow charge voltage triggering exceptional post-charging antibacterial capability of Co3O4/MnOOH nanoneedles for skin infection treatment. Adv. Sci. 2023, 10, 2207594. [Google Scholar] [CrossRef]
- Babu, S.K.; Gunasekaran, B.; Sridharan, M.; Vijayakumar, T. Decorating MnO2 nanosheets on MOF-derived Co3O4 as a battery-type electrode for hybrid supercapacitors. RSC Adv. 2022, 12, 28818–28830. [Google Scholar] [CrossRef] [PubMed]
- Leong, W.X.R.; Al-Dhahebi, A.M.; Ahmad, M.R.; Saheed, M.S.M. Ti3C2Tx MXene-polymeric strain sensor with huge gauge factor for body movement detection. Micromachines 2022, 13, 1302. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Numan, A.; Khalid, M.; Bello, I.; Panza, E.; Cinti, S. Facile and affordable design of MXene-Co3O4-based nanocomposites for detection of hydrogen peroxide in cancer cells: Toward portable tool for cancer management. Small 2023, 19, 2208209. [Google Scholar] [CrossRef] [PubMed]
- Meyyappan, M. Nanostructured materials for supercapacitors. J. Vac. Sci. Technol. A 2013, 31, 050803. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. In MXenes; Jenny Stanford Publishing: Singapore, 2023; pp. 15–29. [Google Scholar]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.-Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. In MXenes; Jenny Stanford Publishing: Singapore, 2023; pp. 379–399. [Google Scholar]
- Mozafari, M.; Soroush, M. Surface functionalization of MXenes. Mater. Adv. 2021, 2, 7277–7307. [Google Scholar] [CrossRef]
- Xu, X.; Yang, L.; Zheng, W.; Zhang, H.; Wu, F.; Tian, Z.; Zhang, P.; Sun, Z. MXenes with applications in supercapacitors and secondary batteries: A comprehensive review. Mater. Rep. Energy 2022, 2, 100080. [Google Scholar] [CrossRef]
- Al Boukhari, J.; Khalaf, A.; Awad, R. Structural analysis and dielectric investigations of pure and rare earth elements (Y and Gd) doped NiO nanoparticles. J. Alloys Compd. 2020, 820, 153381. [Google Scholar] [CrossRef]
- Tsukamoto, T.; Aoki, T.; Kanesaka, H.; Taniguchi, T.; Takayama, T.; Motegi, H.; Takayama, R.; Tanaka, S.; Komiyama, K.; Yoneda, M. Three-dimensional numerical simulation of full-scale proton exchange membrane fuel cells at high current densities. J. Power Sources 2021, 488, 229412. [Google Scholar] [CrossRef]
- Ahmed, I.B.; Diaby, M.; Nafati, H.; Bardaoui, A.; Santos, D.; Chtourou, R.; Assaker, I.B. Supercapacitive performance of 3D-cobalt oxide (Co3O4) nanowires grown onto anodized stainless-steel substrate: Effect of anodization time. Solid State Sci. 2024, 152, 107537. [Google Scholar] [CrossRef]
- Shinde, N.M.; Pumera, M. High Performance MXene/MnCo2O4 Supercapacitor Device for Powering Small Robotics. ACS Appl. Electron. Mater. 2024, 6, 7339–7345. [Google Scholar] [CrossRef]
- Pholauyphon, W.; Charoen-Amornkitt, P.; Suzuki, T.; Tsushima, S. Perspectives on accurately analyzing cyclic voltammograms for surface-and diffusion-controlled contributions. Electrochem. Commun. 2024, 159, 107654. [Google Scholar] [CrossRef]
- Sharma, S.; Chand, P. Supercapacitor and electrochemical techniques: A brief review. Results Chem. 2023, 5, 100885. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Hassan, W.H.; Trivedi, T.; Kumar, A.; Reddy, B.S.R.; Thakur, R.; Shomurotova, S.; Aljanabi, H.Y.A.; Sahib, A.A.A.; Alkahtani, H.M. Interface engineering through embedding Co2O3 nanoparticles onto MXenes layered structure for boosting supercapacitor performance. Electrochim. Acta 2025, 525, 146121. [Google Scholar] [CrossRef]
- Moya, A. A physically consistent electric circuit to interpret the galvanostatic charge-discharge curves of electric double layer capacitors. J. Power Sources 2025, 656, 238050. [Google Scholar] [CrossRef]
- Lazanas, A.C.; Prodromidis, M.I. Electrochemical impedance spectroscopy—A tutorial. ACS Meas. Sci. Au 2023, 3, 162–193. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raza, Q.; Lim, S. Interfacial Engineering of Co3O4@MXene for Superior Charge Storage: A Route to High-Capacitance Supercapacitors. Micromachines 2025, 16, 1313. https://doi.org/10.3390/mi16121313
Raza Q, Lim S. Interfacial Engineering of Co3O4@MXene for Superior Charge Storage: A Route to High-Capacitance Supercapacitors. Micromachines. 2025; 16(12):1313. https://doi.org/10.3390/mi16121313
Chicago/Turabian StyleRaza, Qasim, and Sooman Lim. 2025. "Interfacial Engineering of Co3O4@MXene for Superior Charge Storage: A Route to High-Capacitance Supercapacitors" Micromachines 16, no. 12: 1313. https://doi.org/10.3390/mi16121313
APA StyleRaza, Q., & Lim, S. (2025). Interfacial Engineering of Co3O4@MXene for Superior Charge Storage: A Route to High-Capacitance Supercapacitors. Micromachines, 16(12), 1313. https://doi.org/10.3390/mi16121313






