In Situ Raman Measurement of the Growth of SiCOH Thin Film Using Hexamethyl-Disiloxane (HMDSO) Mixture Source in Semiconductor Interconnection
Abstract
1. Introduction
2. Materials and Methods
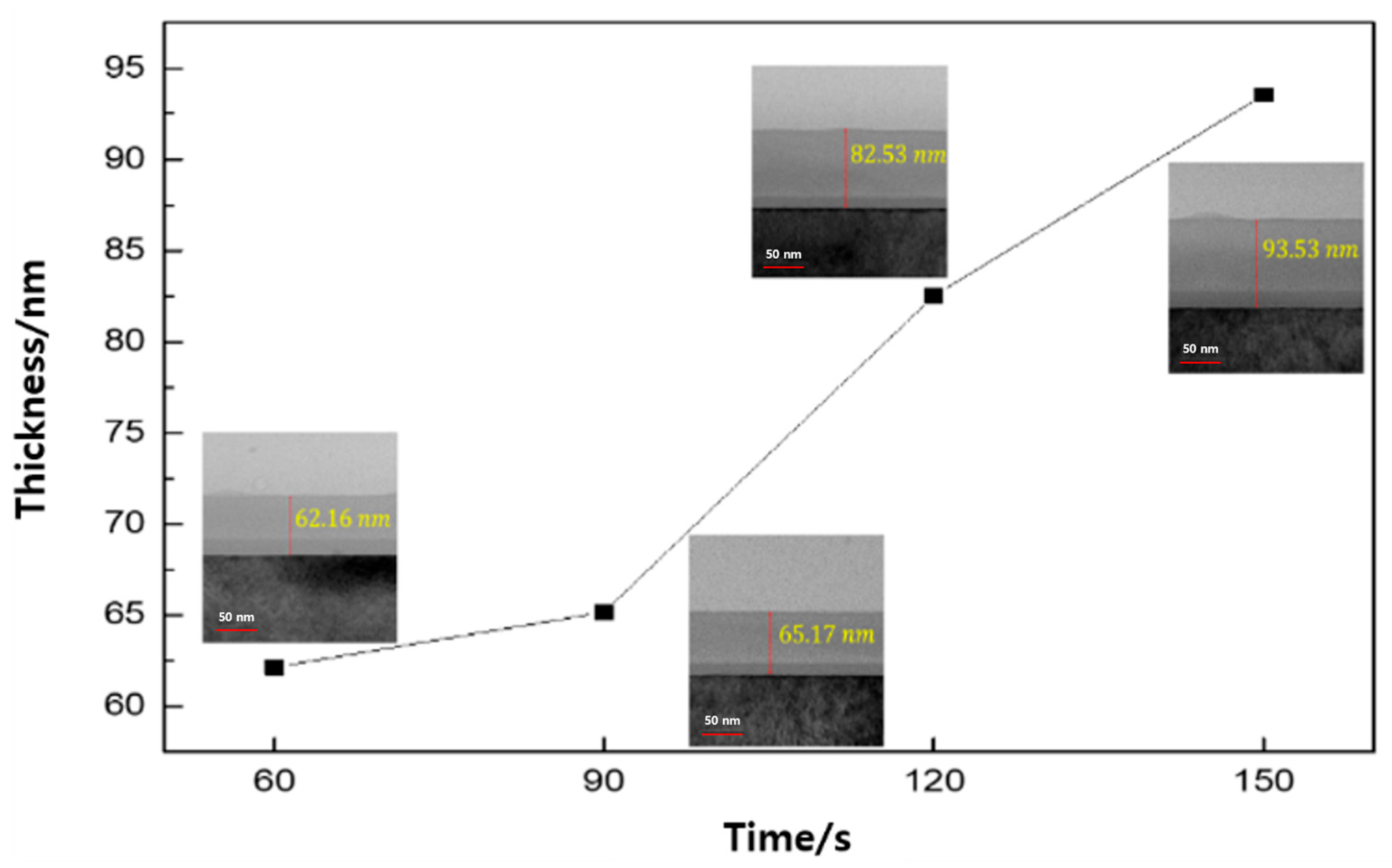
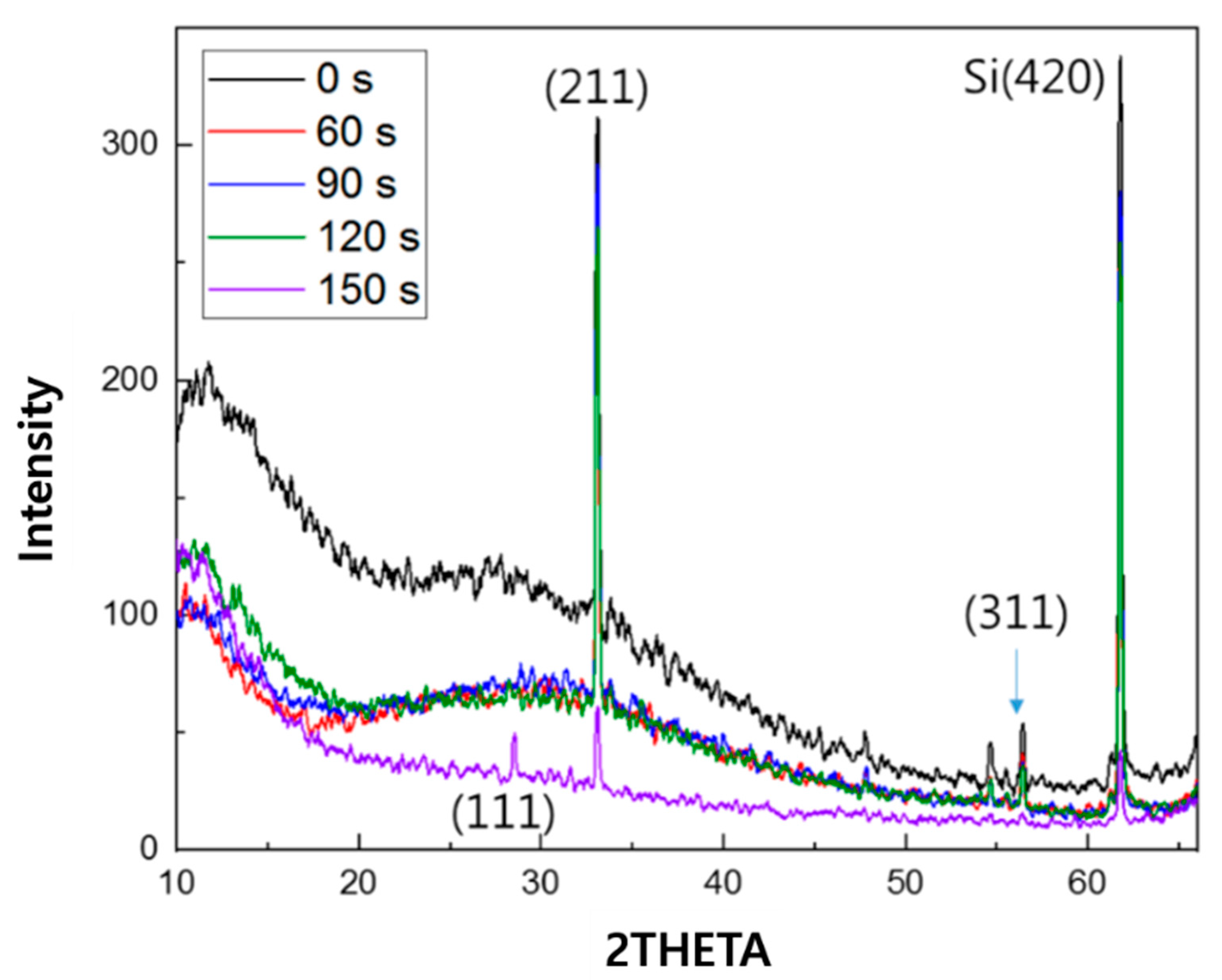
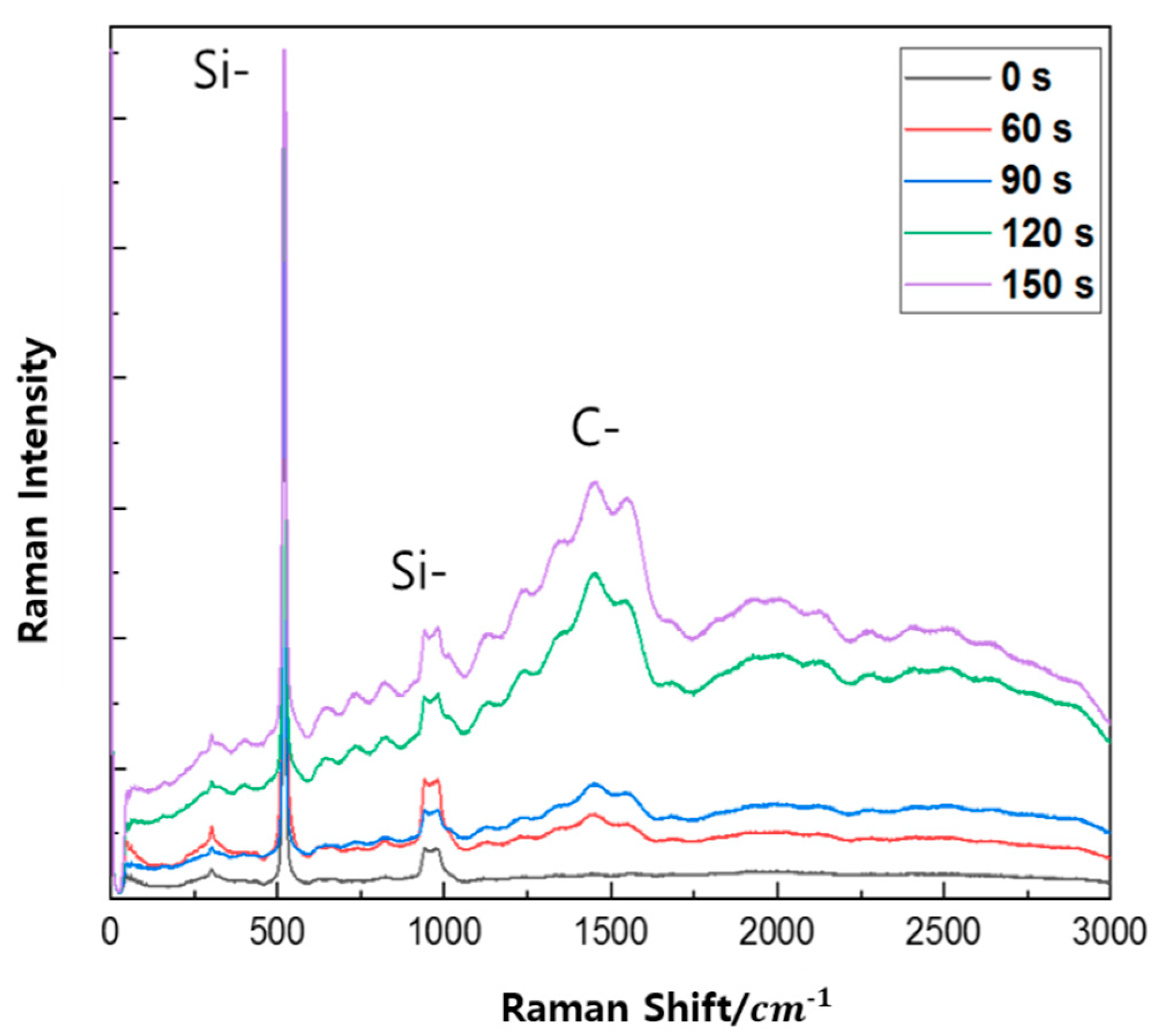
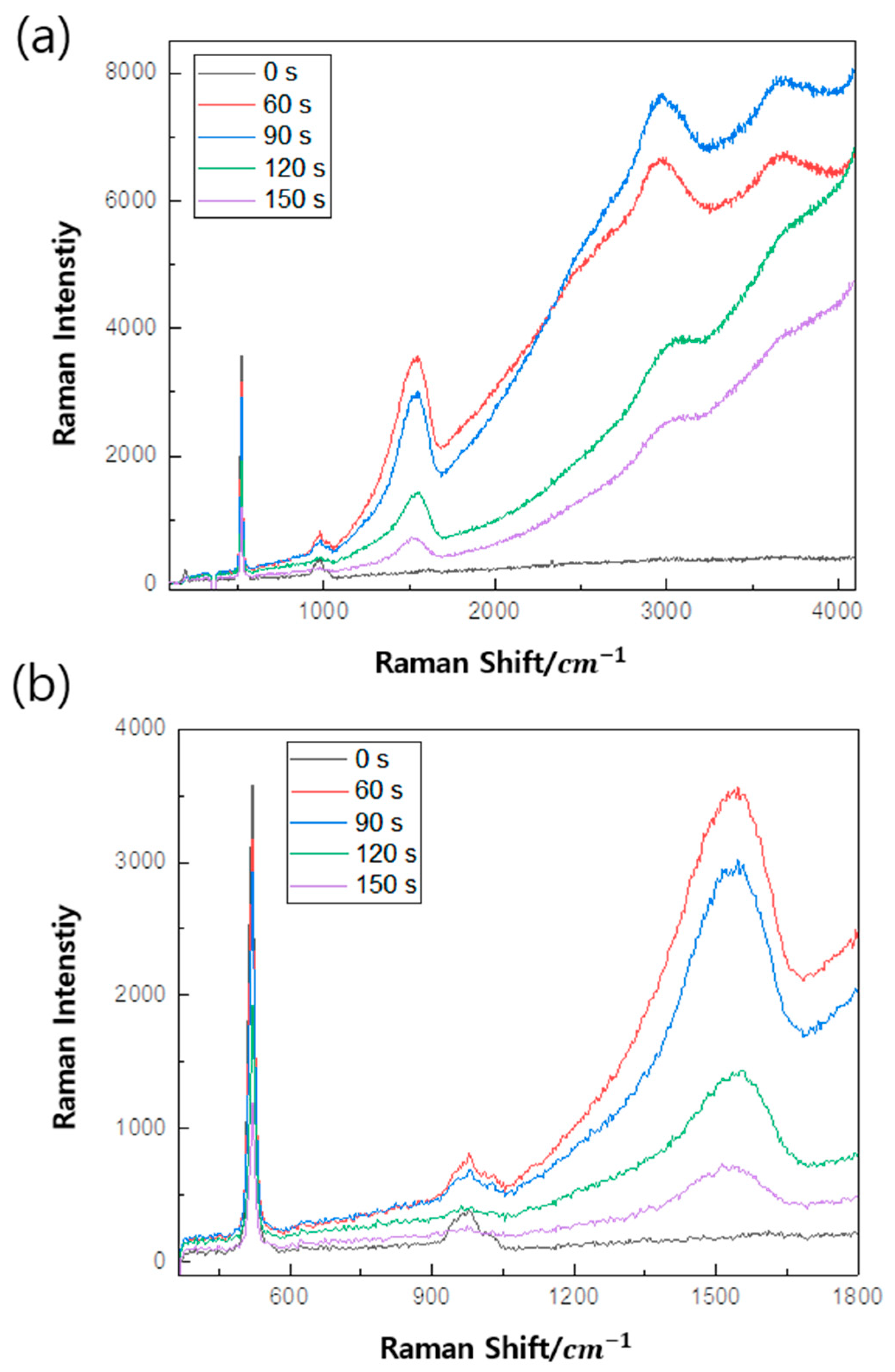
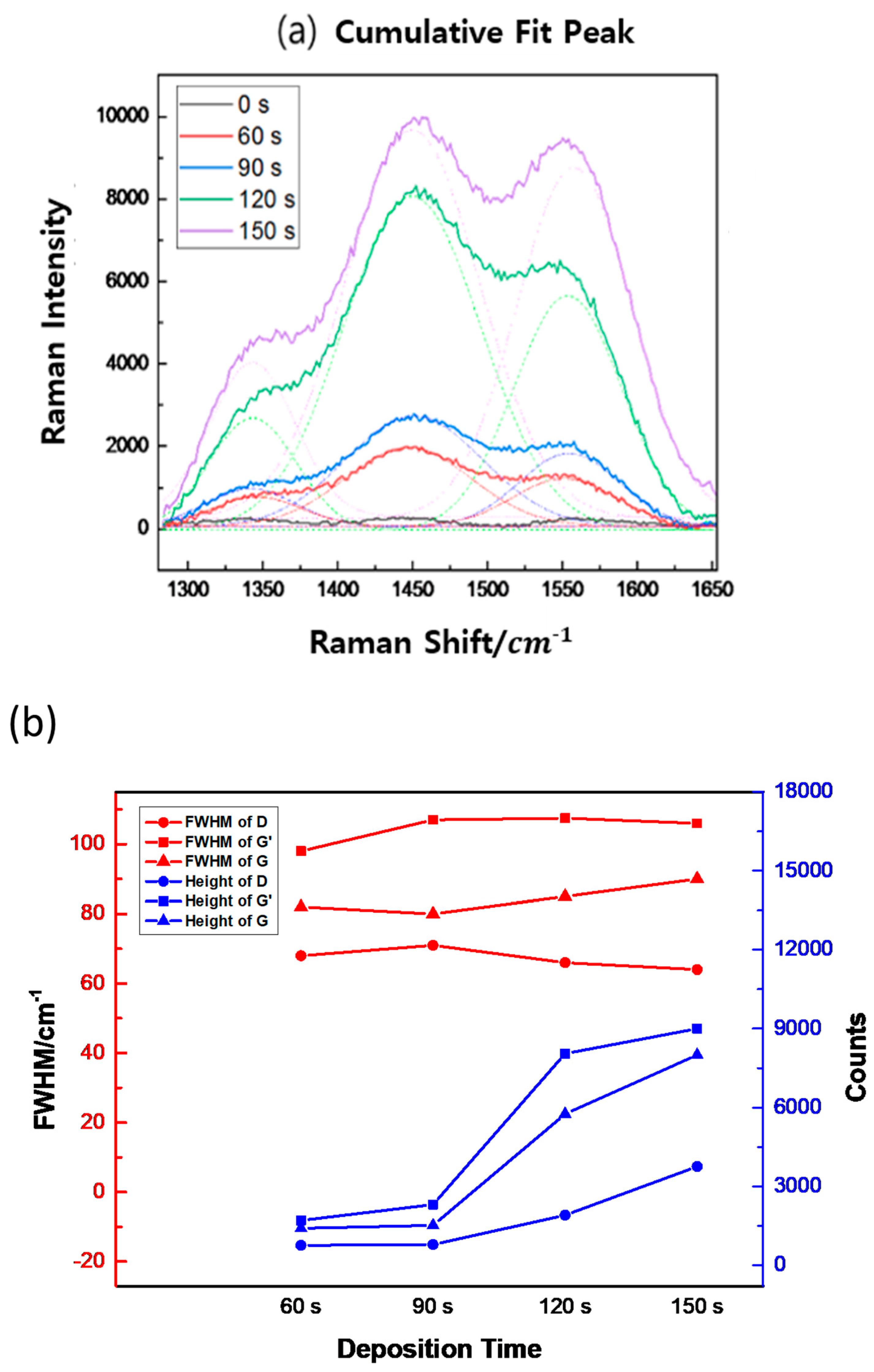
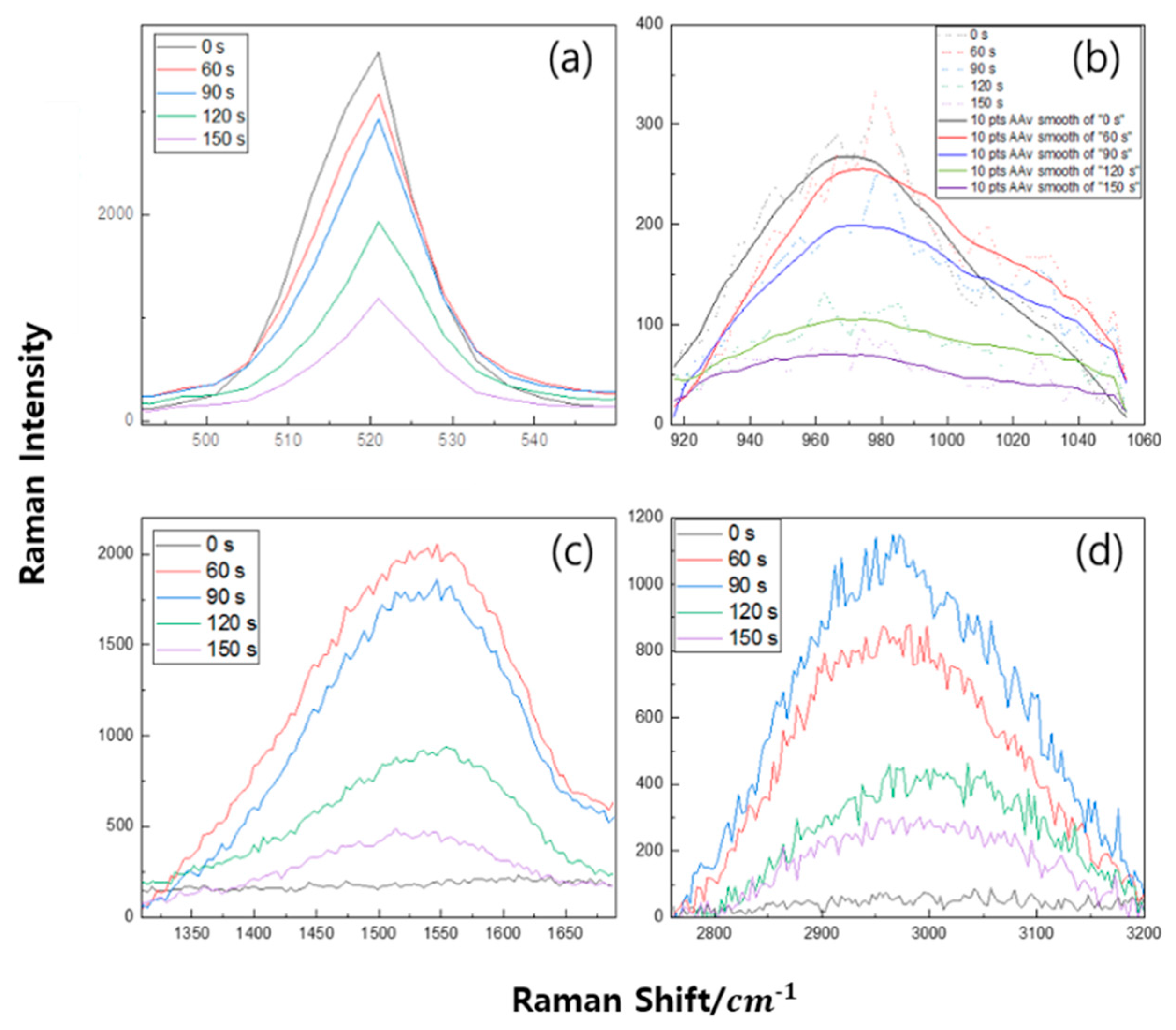
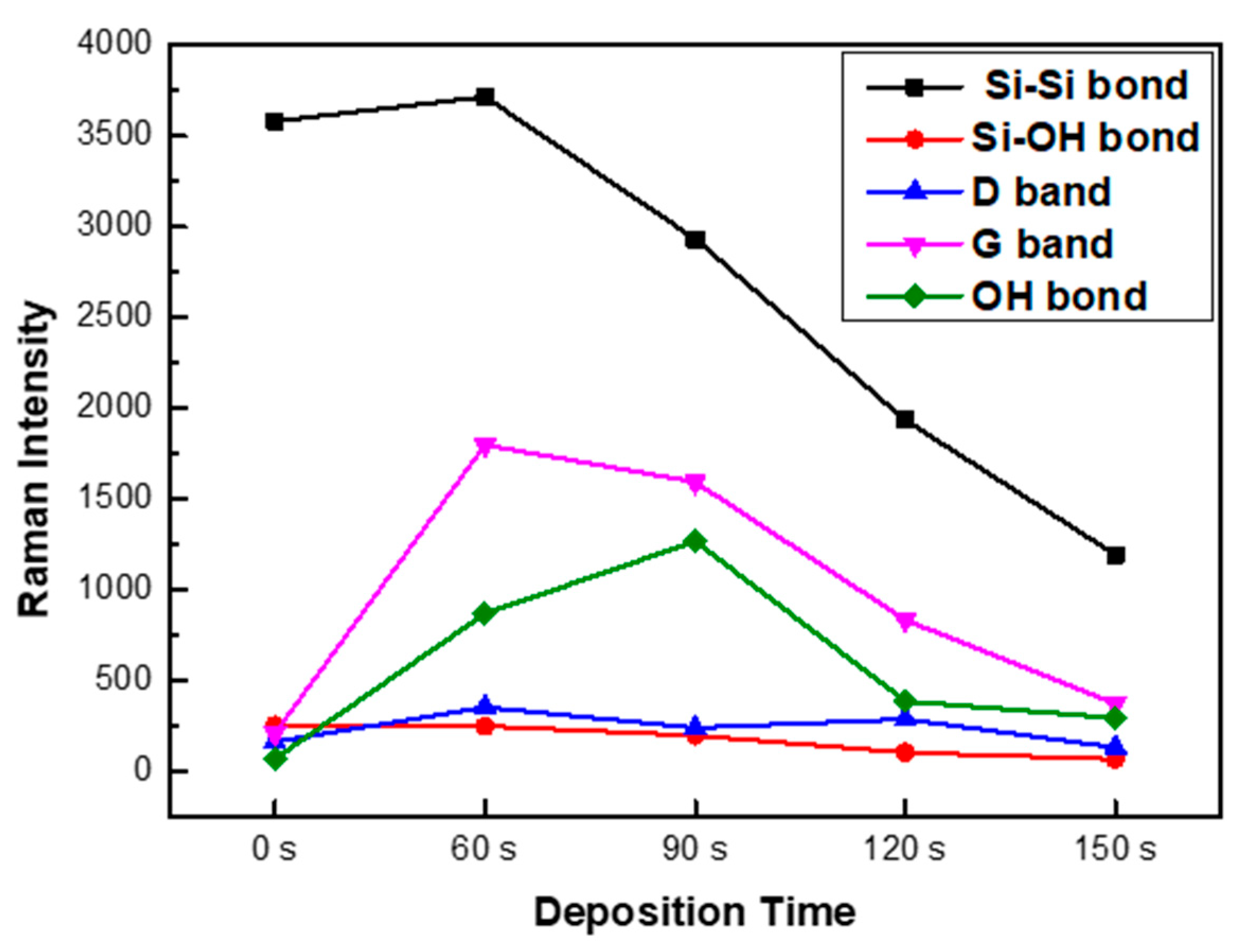
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HMDSO | Hexamethyl-disiloxane |
| CVD | Chemical vapor deposition |
| XRD | X-ray diffraction |
| LO | Longitudinal optical |
| TA | Transverse acoustic |
| DOF | Depth of Focus |
| TO | Transverse optical |
References
- Evans, A.M.; Giri, A.; Sangwan, V.K.; Xun, S.; Bartnof, M.; Torres-Castanedo, C.G.; Balch, H.B.; Rahn, M.S.; Bradshaw, N.P.; Vitaku, E. Thermally conductive ultra-low-k dielectric layers based on two-dimensional covalent organic frameworks. Nat. Mater. 2021, 20, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Nam, M.; Kim, A.; Kang, K.; Zheng, L.; Kwon, S.H.; Yoon, S.P.; Hahn, S.J.; Kim, S.-K.; Son, H. Characterization and Integration Performance of Methyl Silsesquioxane-Based Nano Porous Low-k Dielectric Films. J. Nanosci. Nanotechnol. 2016, 16, 11224–11228. [Google Scholar] [CrossRef]
- Huang, J.; Mu, J.; Cho, Y.; Winter, C.; Wang, V.; Zhang, Z.; Wang, K.; Kim, C.; Yadav, A.; Wong, K. Low-k SiOx/AlOx Nanolaminate Dielectric on Dielectric Achieved by Hybrid Pulsed Chemical Vapor Deposition. ACS Appl. Mater. Interfaces 2023, 15, 56556–56566. [Google Scholar] [CrossRef]
- Choi, J.; Yeom, H.; Chae, G.-S.; Kee, W.; Kim, K.-Y.; Lee, H.-C.; Jeong, H.-D.; Kim, J.-H. Influence of plasma parameters on low-k SiCOH film grown by plasma-enhanced chemical vapor deposition using dimethyldimethoxysilane. Vacuu 2023, 217, 112529. [Google Scholar] [CrossRef]
- Park, Y.; Liu, P.; Lee, S.; Cho, J.; Joo, E.; Kim, H.-U.; Kim, T. Diagnosing Time-Varying Harmonics in Low-k Oxide Thin Film (SiOF) Deposition by Using HDP CVD. Sensors 2023, 23, 5563. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-M.; Choi, J.K.; An, C.J.; Jin, M.L.; Kang, S.; Yun, J.; Kong, B.-S.; Jung, H.-T. Nanoporous SiCOH/CxHy dual phase films with an ultralow dielectric constant and a high Young’s modulus. J. Mater. Chem. C 2013, 1, 3414–3420. [Google Scholar] [CrossRef]
- Lee, Y.; Ban, W.; Jang, S.; Jung, D. Characterization of Flexible Low-k Dielectric SiCOH Films Prepared by Plasma-Enhanced Chemical Vapor Deposition of Tetrakis (trimethylsilyloxy) Silane Precursor. J. Nanosci. Nanotechnol. 2021, 21, 2139–2147. [Google Scholar] [CrossRef]
- Nguyen, S.; Haigh, T.; Shoudy, M.; Yao, Y.; Huang, H.; Shobha, H.; Rizzolo, M.; Canaperi, D.; Wynne, J.; Bedell, S. Flowable Chemical Vapor Deposition of Lightly Porous Low k SiCOH Dielectrics—Remote Plasma Deposition Processing, Film Analysis and Gap-Fill Application in Nano Device Fabrication. In Proceedings of the Materials Research Society (MRS) Fall Meeting, Boston, MA, USA, 27 November–2 December 2022. [Google Scholar]
- Yang, Q.; Mei, X.; Shi, S.; Peng, K.; Qi, X.; Islam, M.Z.; Yang, X.; Si, Y.; Fu, Y. High-performance cross-linked SiCOH/polyimide composite film with an ultralow dielectric constant at high frequency. J. Appl. Polym. Sci. 2024, 141, e56025. [Google Scholar] [CrossRef]
- Park, Y.; Lim, H.; Kwon, S.; Ban, W.; Jang, S.; Jung, D. Ultralow dielectric constant SiCOH films by plasma enhanced chemical vapor deposition of decamethylcyclopentasiloxane and tetrakis (trimethylsilyloxy) silane precursors. Thin Solid Films 2021, 727, 138680. [Google Scholar] [CrossRef]
- Lopaev, D.; Zyryanov, S.; Zotovich, A.; Rakhimova, T.; Mankelevich, Y.A.; Voronina, E. Damage to porous SiCOH low-k dielectrics by O, N and F atoms at lowered temperatures. J. Phys. D Appl. Phys. 2020, 53, 175203. [Google Scholar] [CrossRef]
- Kim, M.; Hong, S.J. Investigation of Structure Modification of Underlying SiCOH Low-k Dielectrics with Subsequent Hardmask Deposition Process Conditions. Sci. Adv. Mater. 2021, 13, 2185–2193. [Google Scholar] [CrossRef]
- Poche, T.; Wirth, W.; Jang, S. Chemical structure characteristics of flexible low-k SiCOH thin films etched by inductively coupled plasma-reactive ion etching process using FTIR and XPS spectra analysis. Microelectron. Eng. 2024, 292, 112221. [Google Scholar] [CrossRef]
- Wirth, W.; Comeaux, J.; Jang, S. Characterization of flexible low-dielectric constant carbon-doped oxide (SiCOH) thin films under repeated mechanical bending stress. J. Mater. Sci. 2022, 57, 21411–21431. [Google Scholar] [CrossRef]
- Kruchinin, V.N.; Volodin, V.A.; Rykhlitskii, S.V.; Gritsenko, V.A.; Posvirin, I.; Shi, X.; Baklanov, M.R. Atomic structure and optical properties of plasma enhanced chemical vapor deposited SiCOH low-k dielectric film. Opt. Spectrosc. 2021, 129, 645–651. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Lee, S.; Niazi, M.R.; Hwang, K.; Tang, M.-C.; Lim, D.-H.; Kang, J.-S.; Smilgies, D.-M.; Amassian, A.; Kim, D.-Y. Systematic study on the morphological development of blade-coated conjugated polymer thin films via in situ measurements. ACS Appl. Mater. Interfaces 2020, 12, 36417–36427. [Google Scholar] [CrossRef]
- Richter, L.J.; DeLongchamp, D.M.; Amassian, A. Morphology development in solution-processed functional organic blend films: An in situ viewpoint. Chem. Rev. 2017, 117, 6332–6366. [Google Scholar] [CrossRef]
- Kim, J.B.; Kim, D.S.; Kim, J.S.; Choe, J.H.; Ahn, D.W.; Jung, E.S.; Pyo, S.G. Raman scattering monitoring of thin film materials for atomic layer etching/deposition in the nano-semiconductor process integration. Chem. Phys. Rev. 2023, 4, 041311. [Google Scholar] [CrossRef]
- Shim, H.J.; Kim, J.S.; Da Ahn, W.; Choe, J.H.; Oh, D.; Kim, K.S.; Lee, S.C.; Pyo, S.G. Heterogeneous crystallinity of atomic-layer-deposited Zinc oxide thin film using resonance raman scattering analysis. Electron. Mater. Lett. 2021, 17, 362–368. [Google Scholar] [CrossRef]
- Li, G.; Qiu, Y.; Luo, Y.; Wang, Q.; He, S.; Zhang, X. Microstructure detection of CaO-SiO2-based mold flux at high temperature by in-situ Raman spectroscopy. J. Alloys Compd. 2025, 1022, 180116. [Google Scholar]
- Gracin, D.; Štrukil, V.; Friščić, T.; Halasz, I.; Užarević, K. Laboratory Real-Time and In Situ Monitoring of Mechanochemical Milling Reactions by Raman Spectroscopy. Angew. Chem. Int. Ed. 2014, 53, 6193–6197. [Google Scholar] [CrossRef]
- Cui, Y.; Shim, H.J.; Gao, Y.; Pyo, S.G. Real-time Raman analysis of cleaning solution: Determination of Al/Alq3 residue in cleaning mixture. J. Raman Spectrosc. 2019, 50, 571–575. [Google Scholar] [CrossRef]
- Kesari, S.; Rao, R.; Bevara, S.; Achary, S. Structural stability and anharmonicity of phonon modes of metastable Zn4V2O9: In-situ Raman spectroscopic investigation. J. Alloys Compd. 2022, 895, 162662. [Google Scholar] [CrossRef]
- Kim, B.-H.; Kim, D.; Song, S.; Park, D.; Kang, I.-S.; Jeong, D.H.; Jeon, S. Identification of metalloporphyrins with high sensitivity using graphene-enhanced resonance Raman scattering. Langmuir 2014, 30, 2960–2967. [Google Scholar] [CrossRef]
- Chang, S.-Y.; Chang, J.-Y.; Lin, S.-J.; Tsai, H.-C.; Chang, Y.-S. Interface chemistry and adhesion strength between porous sioch low-k film and sicn layers. J. Electrochem. Soc. 2007, 155, G39. [Google Scholar] [CrossRef]
- Yasuhara, S.; Sasaki, T.; Shimayama, T.; Tajima, K.; Yano, H.; Kadomura, S.; Yoshimaru, M.; Matsunaga, N.; Samukawa, S. Super-low-k SiOCH film (k = 1.9) with extremely high water resistance and thermal stability formed by neutral-beam-enhanced CVD. J. Phys. D: Appl. Phys. 2010, 43, 065203. [Google Scholar] [CrossRef]
- Myers, J.N.; Zhang, X.; Bielefeld, J.D.; Chen, Z. Plasma treatment effects on molecular structures at dense and porous low-K SiCOH film surfaces and buried interfaces. J. Phys. Chem. C 2015, 119, 22514–22525. [Google Scholar] [CrossRef]
- Yamin, H.; Tan, H.; Wang, D.; Lam, J.; Mai, Z. Experiments and results of Raman and FTIR complementary vibrational spectroscopy for IC reliability failure analysis. In Proceedings of the 21th International Symposium on the Physical and Failure Analysis of Integrated Circuits (IPFA), Singapore, 30 June–4 July 2014; pp. 291–294. [Google Scholar]
- Bokobza, L.; Bruneel, J.-L.; Couzi, M. Raman spectroscopy as a tool for the analysis of carbon-based materials (highly oriented pyrolitic graphite, multilayer graphene and multiwall carbon nanotubes) and of some of their elastomeric composites. Vib. Spectrosc. 2014, 74, 57–63. [Google Scholar] [CrossRef]
- Jeong, J.; Jang, K.; Lee, H.S.; Chung, G.-S.; Kim, G.-Y. Raman scattering studies of polycrystalline 3C-SiC deposited on SiO2 and AlN thin films. Phys. B: Condens. Matter 2009, 404, 7–10. [Google Scholar] [CrossRef]
- Röhrl, J.; Hundhausen, M.; Emtsev, K.; Seyller, T.; Graupner, R.; Ley, L. Raman spectra of epitaxial graphene on SiC (0001). Appl. Phys. Lett. 2008, 92, 201918. [Google Scholar] [CrossRef]
- Cançado, L.G.; Monken, V.P.; Campos, J.L.E.; Santos, J.C.; Backes, C.; Chacham, H.; Neves, B.R.; Jorio, A. Science and metrology of defects in graphene using Raman spectroscopy. Carbon 2024, 220, 118801. [Google Scholar] [CrossRef]
- Wasyluk, J.; Perova, T.S.; Kukushkin, S.A.; Osipov, A.V.; Feoktistov, N.A.; Grudinkin, S.A. Raman investigation of different polytypes in SiC thin films grown by solid-gas phase epitaxy on Si (111) and 6H-SiC substrates. Mater. Sci. Forum 2010, 645, 359–362. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095. [Google Scholar] [CrossRef]
- Shim, H.J.; Kim, J.S.; Ahn, D.W.; Choe, J.H.; Jung, E.; Oh, D.; Kim, K.S.; Lee, S.C.; Pyo, S.G. Raman Shift of Surface Reaction and Plasma Induced Surface Damage by TNF3/BNF3 Reactive Ion Etching Process. Electron. Mater. Lett. 2022, 18, 321–329. [Google Scholar] [CrossRef]
- Yao, L.; Ao, J.; Jeng, M.-J.; Bi, J.; Gao, S.; Sun, G.; He, Q.; Zhou, Z.; Zhang, Y.; Sun, Y. Reactive mechanism of Cu2ZnSnSe4 thin films prepared by reactive annealing of the cu/Zn metal layer in a SnSex + se atmosphere. Crystals 2018, 9, 10. [Google Scholar] [CrossRef]
- Ye, C.; Ning, Z.; Wang, T.; Yu, X.; Xin, Y. Effect of Si–OH group on characteristics of SiCOH films prepared by decamethylcyclopentasiloxane electron cyclotron resonance plasma. Thin Solid Films 2006, 496, 221–226. [Google Scholar] [CrossRef]
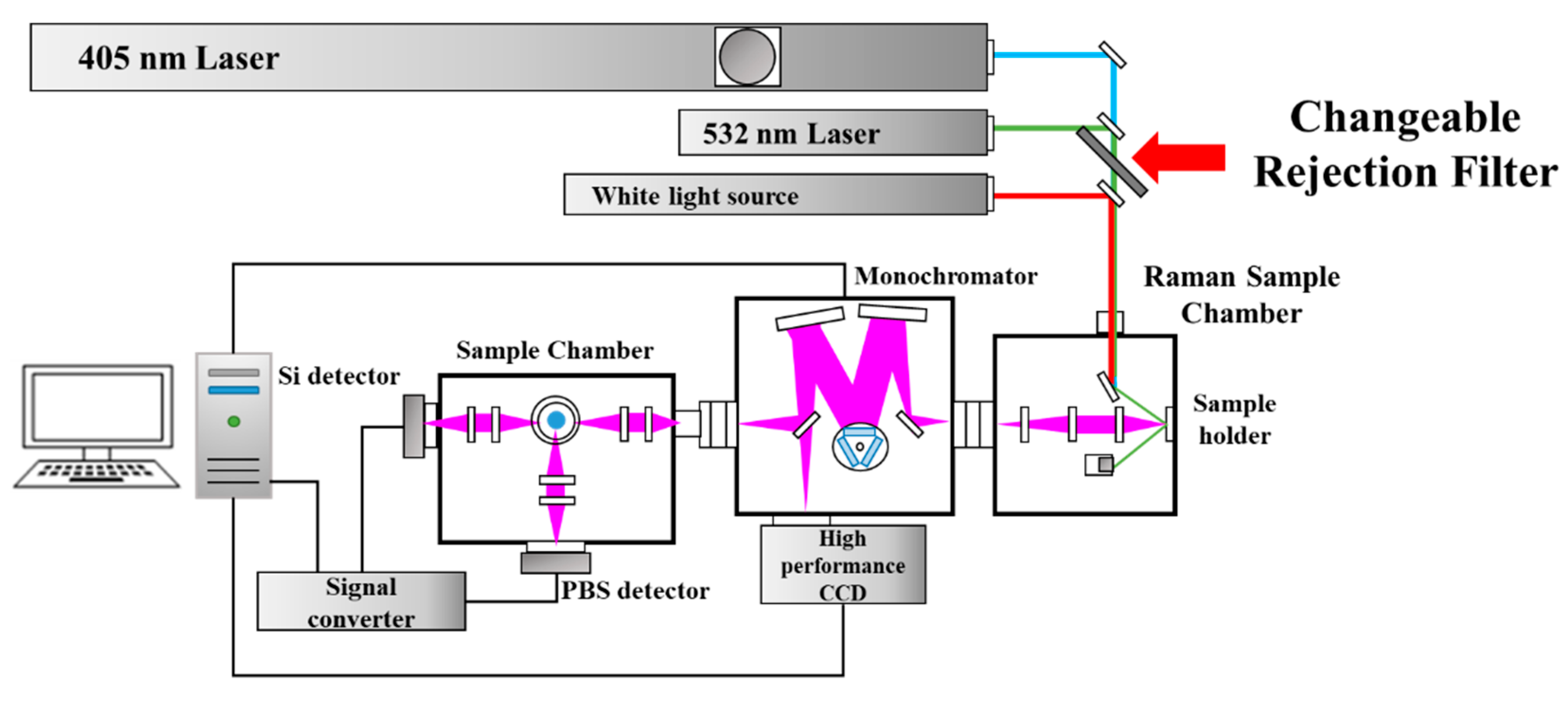
| 405 nm | 532 nm | |
|---|---|---|
| Light Source | 405 nm laser, 30 mW | 532 nm DPSS laser, 100 mW |
| Raman Filter Set | Grade 4: 200 cm−1 (150 cm−1 typ.) | Grade 3: 70 cm−1 (50 cm−1 typ.) |
| CCD | -ICX674 Camera (Sony, Tokyo, Japan) -1932 × 1452 pixels | -ICX674 Camera -1932 × 1452 pixels |
| Grating | 2400 lpmm@532 nm grating | 2400 lpmm@532 nm grating |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.R.; Choi, T.M.; Pyo, S.G. In Situ Raman Measurement of the Growth of SiCOH Thin Film Using Hexamethyl-Disiloxane (HMDSO) Mixture Source in Semiconductor Interconnection. Micromachines 2025, 16, 1202. https://doi.org/10.3390/mi16111202
Lee HR, Choi TM, Pyo SG. In Situ Raman Measurement of the Growth of SiCOH Thin Film Using Hexamethyl-Disiloxane (HMDSO) Mixture Source in Semiconductor Interconnection. Micromachines. 2025; 16(11):1202. https://doi.org/10.3390/mi16111202
Chicago/Turabian StyleLee, Hwa Rim, Tae Min Choi, and Sung Gyu Pyo. 2025. "In Situ Raman Measurement of the Growth of SiCOH Thin Film Using Hexamethyl-Disiloxane (HMDSO) Mixture Source in Semiconductor Interconnection" Micromachines 16, no. 11: 1202. https://doi.org/10.3390/mi16111202
APA StyleLee, H. R., Choi, T. M., & Pyo, S. G. (2025). In Situ Raman Measurement of the Growth of SiCOH Thin Film Using Hexamethyl-Disiloxane (HMDSO) Mixture Source in Semiconductor Interconnection. Micromachines, 16(11), 1202. https://doi.org/10.3390/mi16111202






