Post Wire-Bonding Corrosion Prevention Strategies to Mitigate Chloride- and Bromide-Induced Corrosion Failures in Cu- and PCC-Based Wire-Bonded Packages
Abstract
1. Introduction
2. Materials and Methods
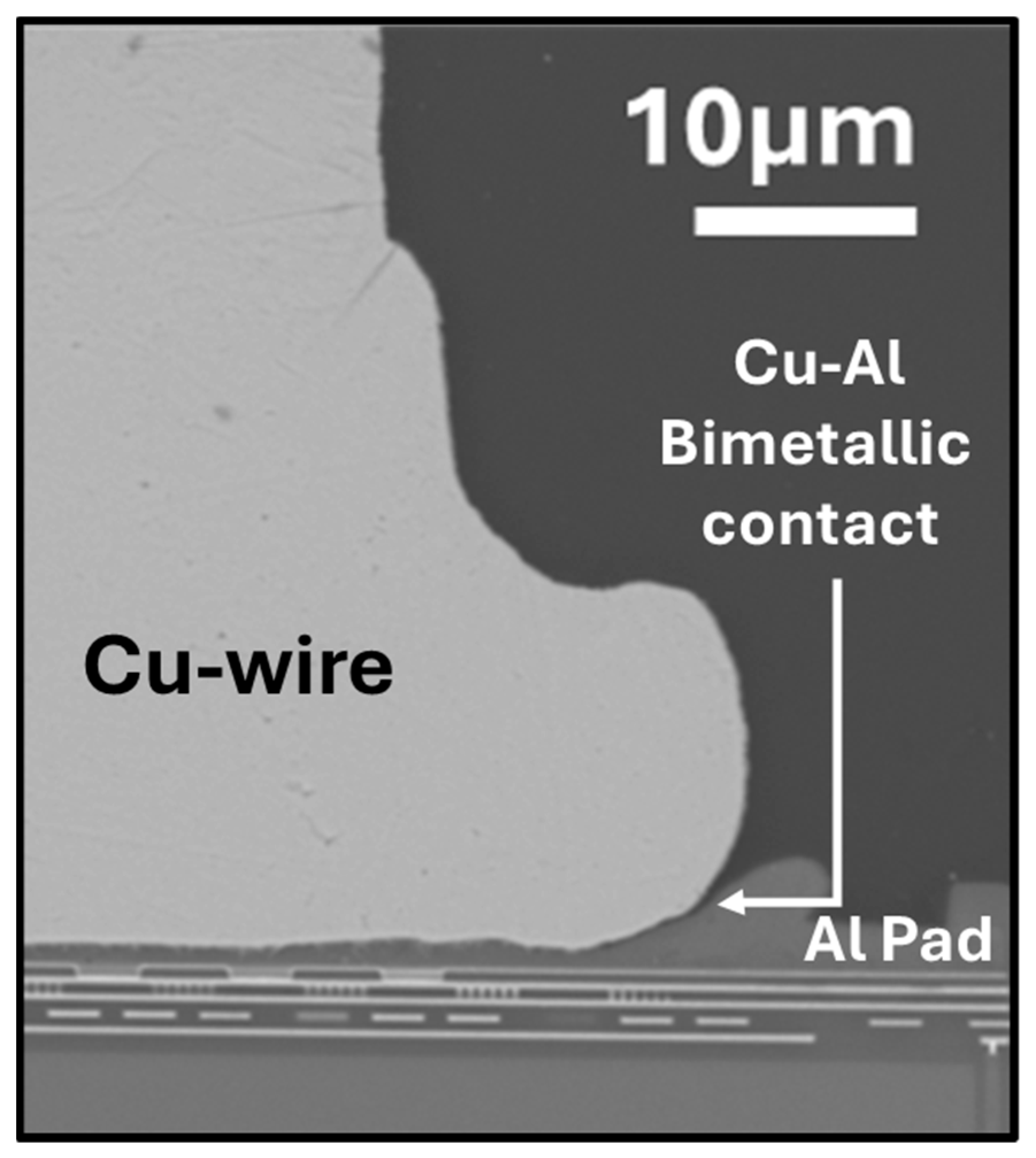
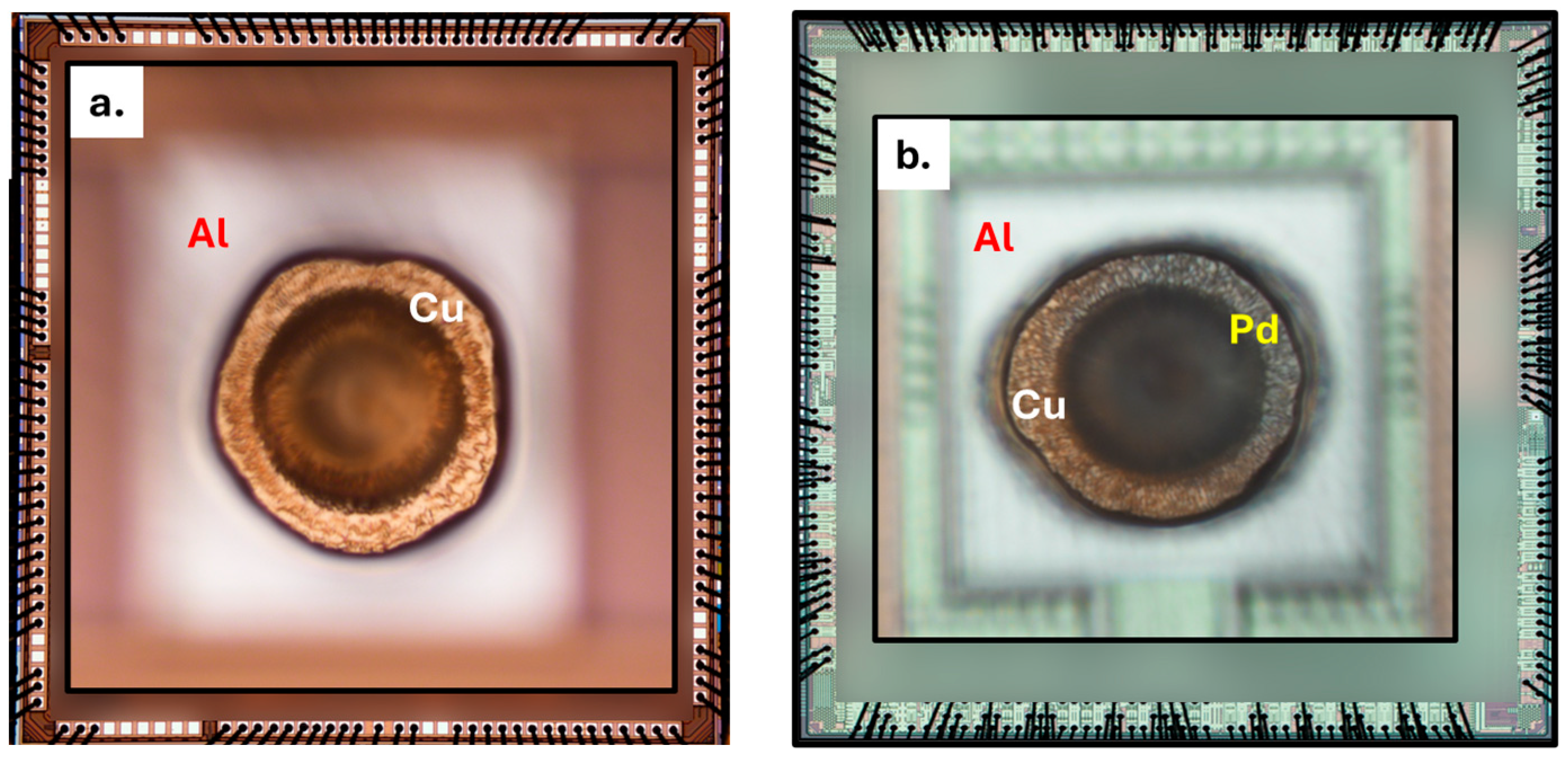
2.1. Wirebond Devices
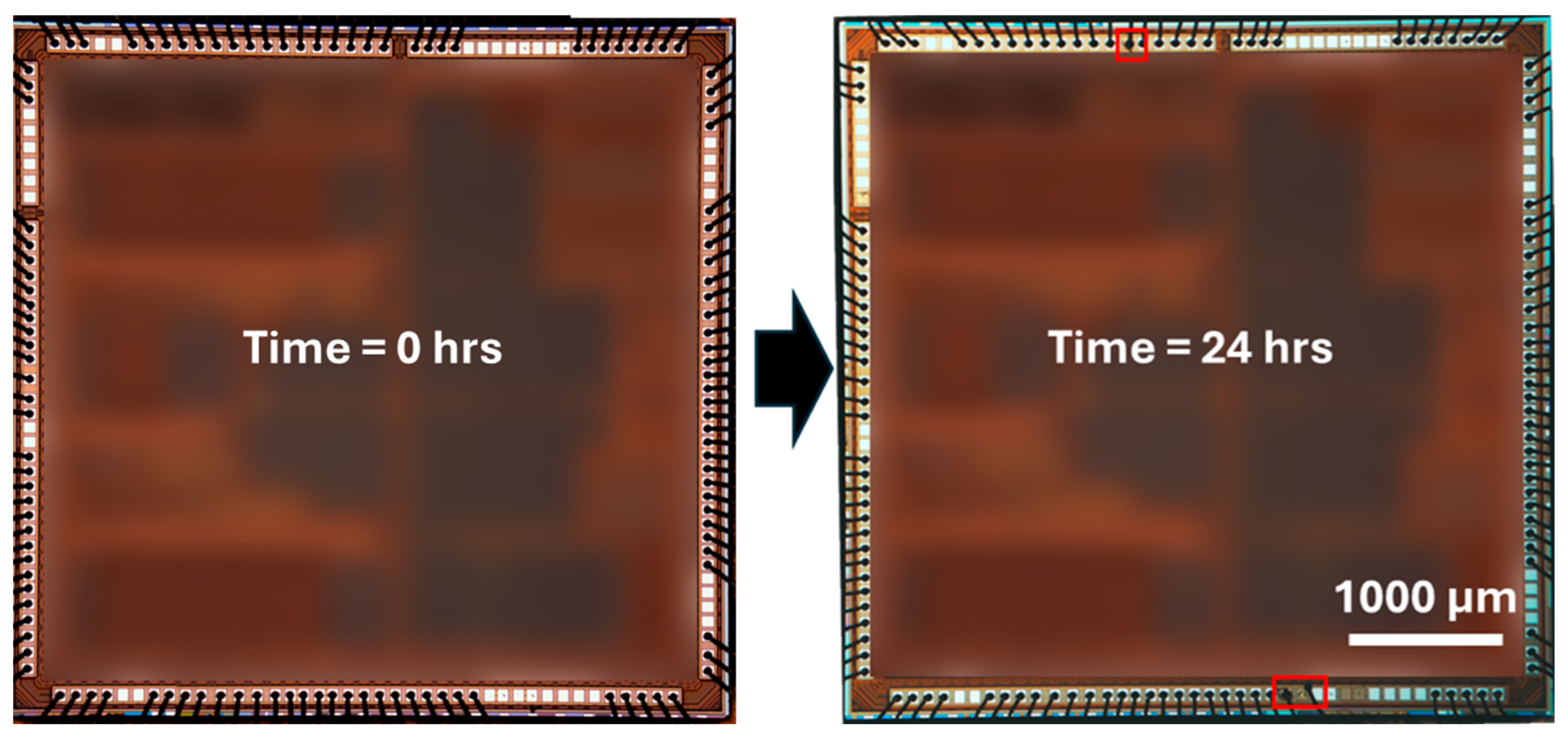
2.2. Accelerated Immersion Screening Metrology
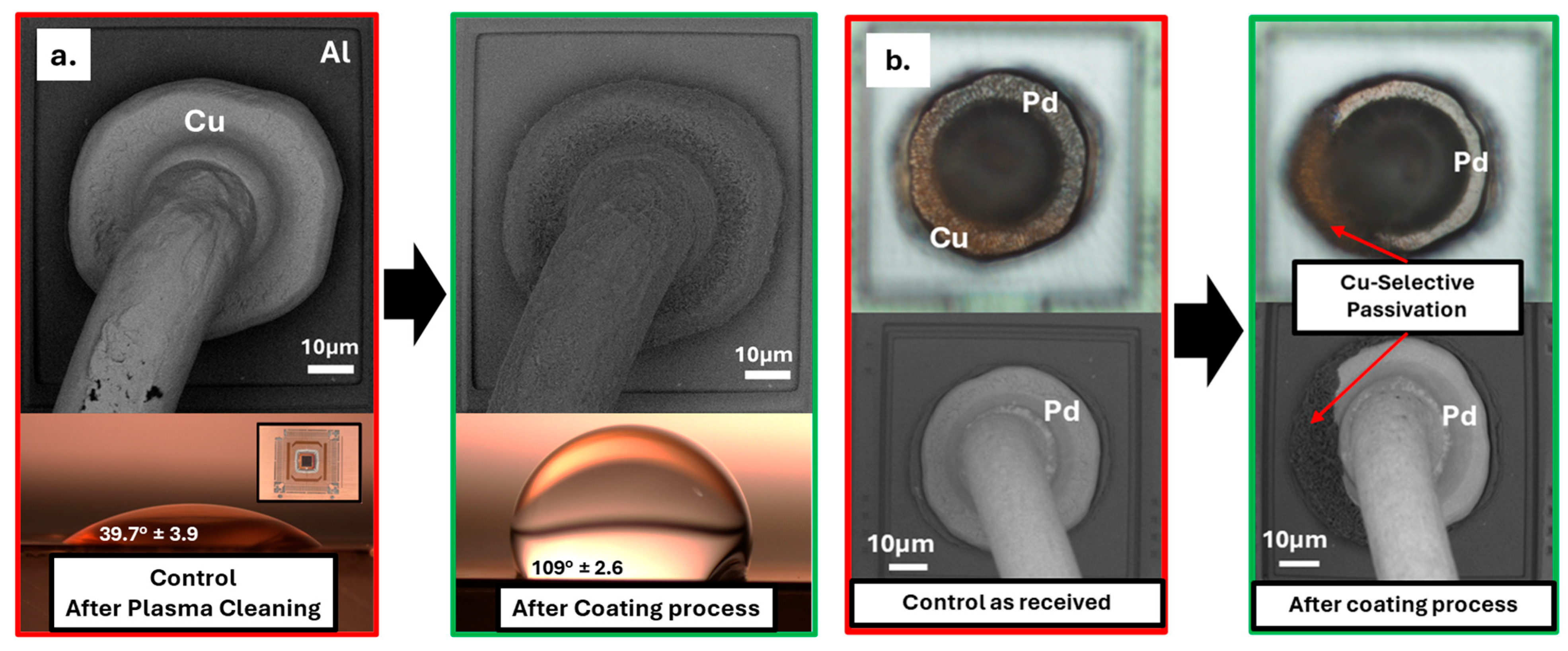
2.3. Passivation Approach and Quantification Metrologies
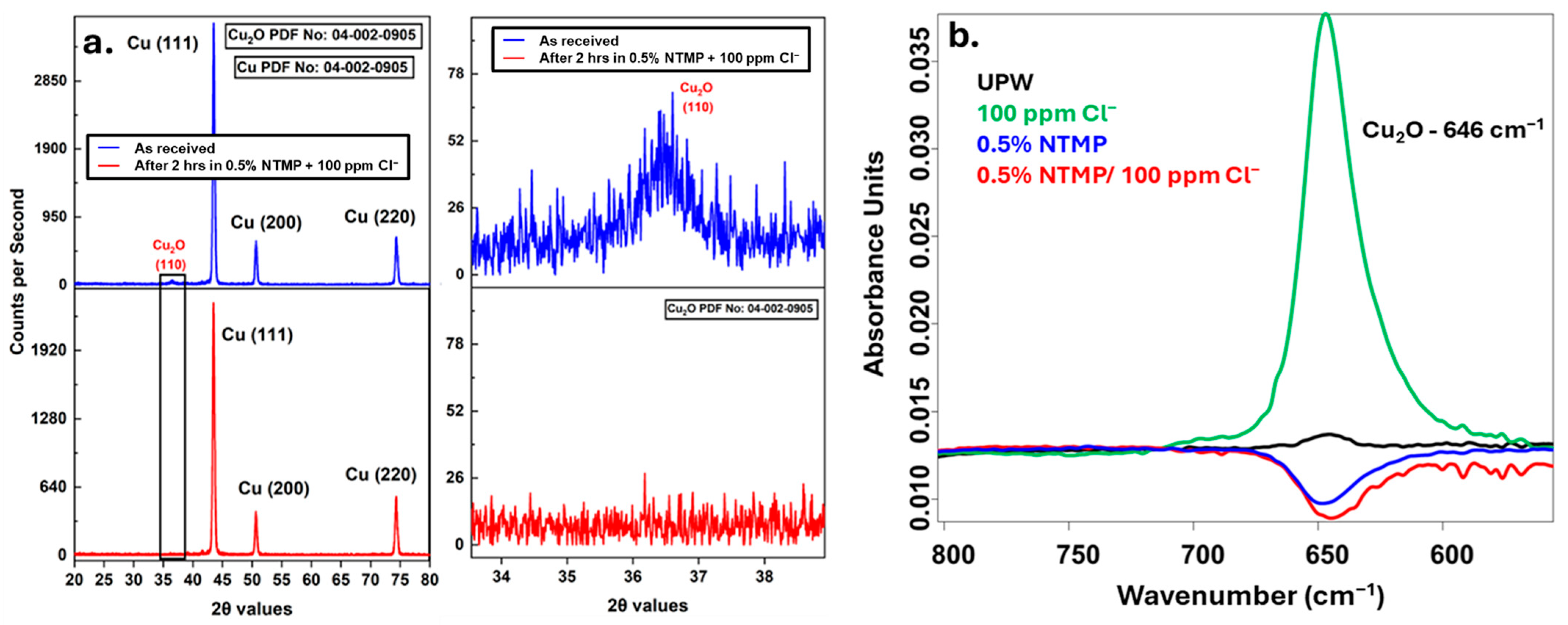
2.4. SEM, GI-XRD, and XPS Analysis
2.5. Electrochemical Analysis
3. Results and Discussion
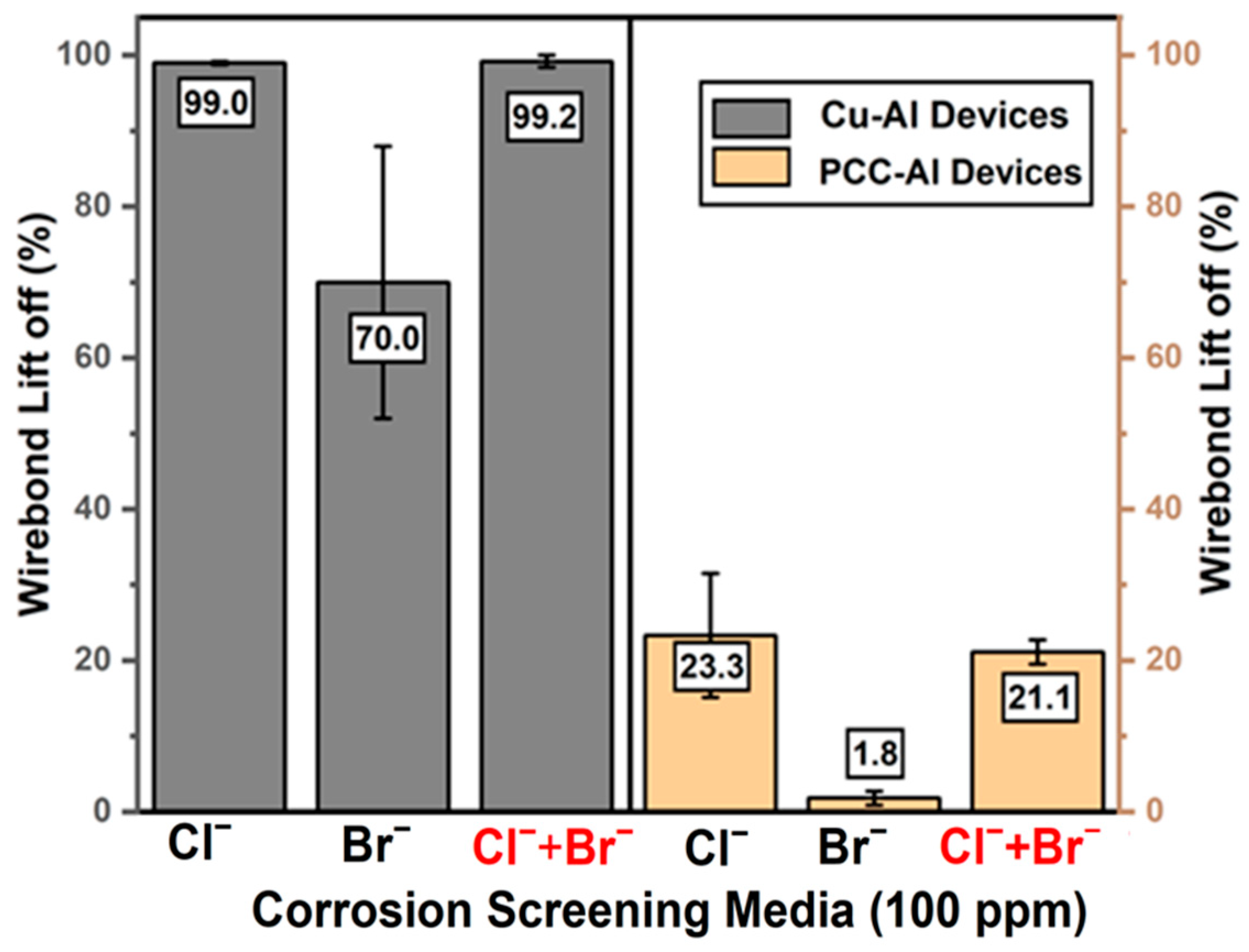
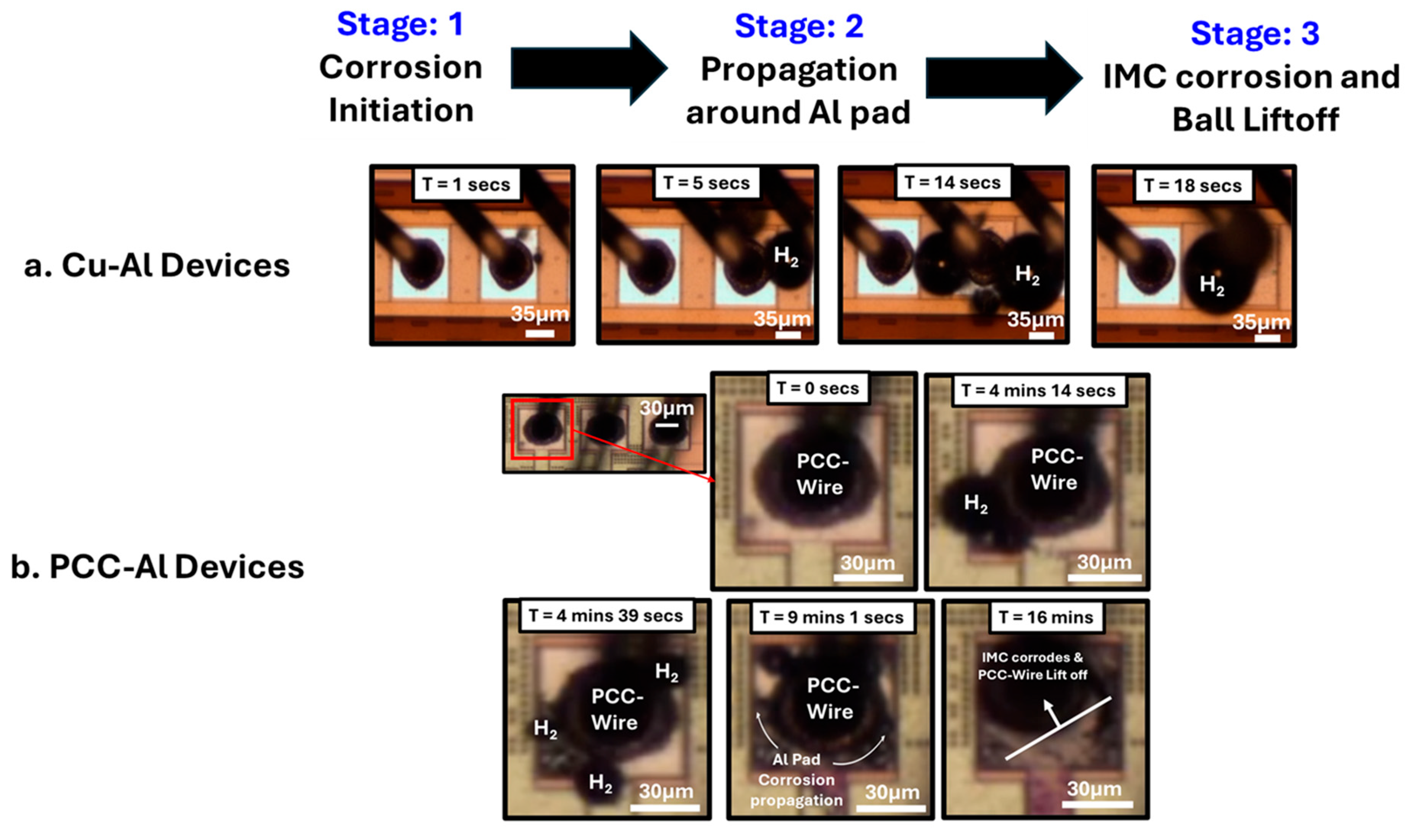
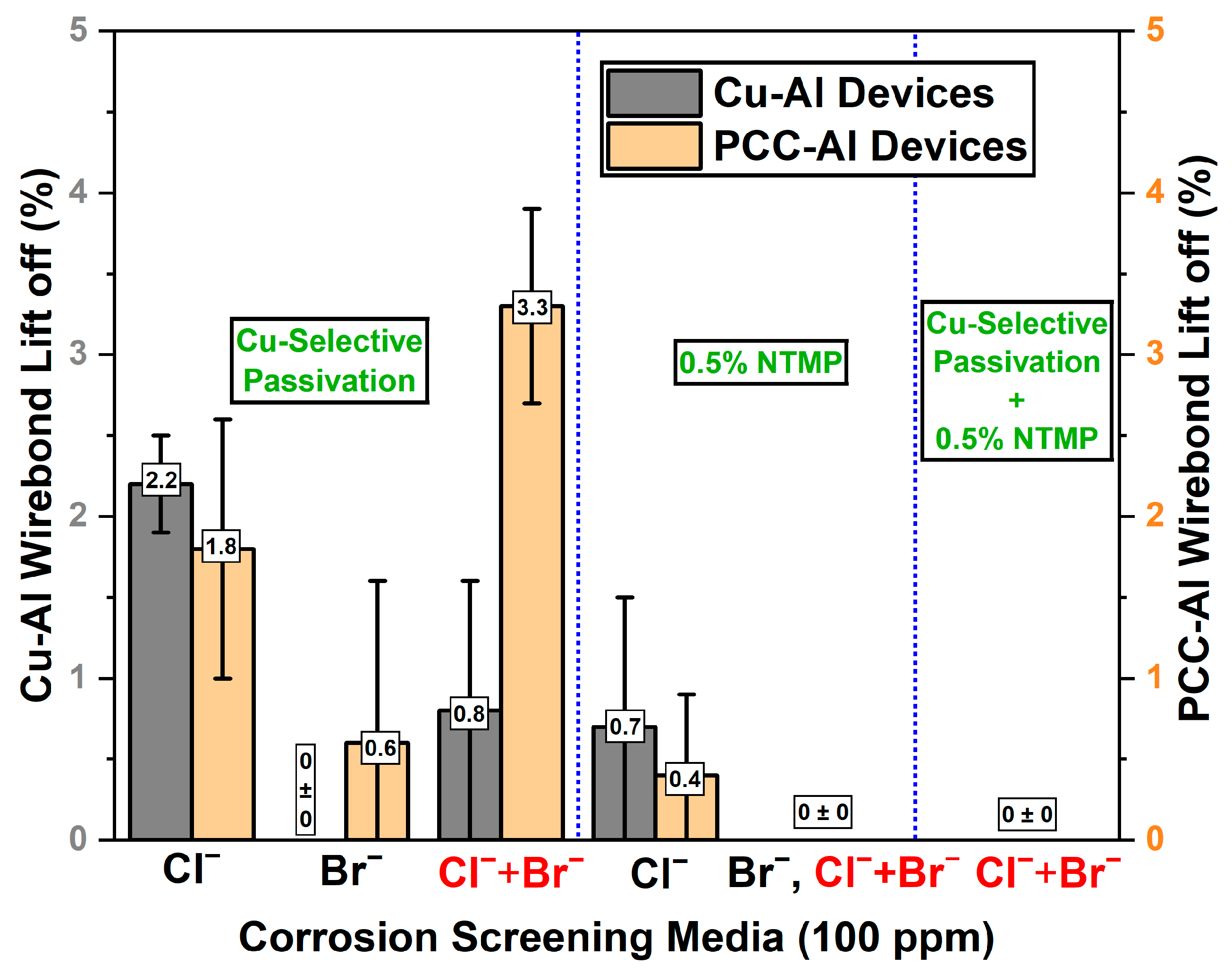
3.1. Accelerated Corrosion Screening Analysis
- Corrosion initiation is characterized by bubble formation at the Cu–Al interface.
- Propagation of Al pad corrosion, leading to dendrite and mud crack formation in the aluminum pads.
- Corrosion of the underlying IMCs ultimately results in the ball lift-off.
3.2. Optical and SEM Comparison on Passivated Wirebond Devices
3.3. Accelerated Corrosion Screening Results on Passivated Devices
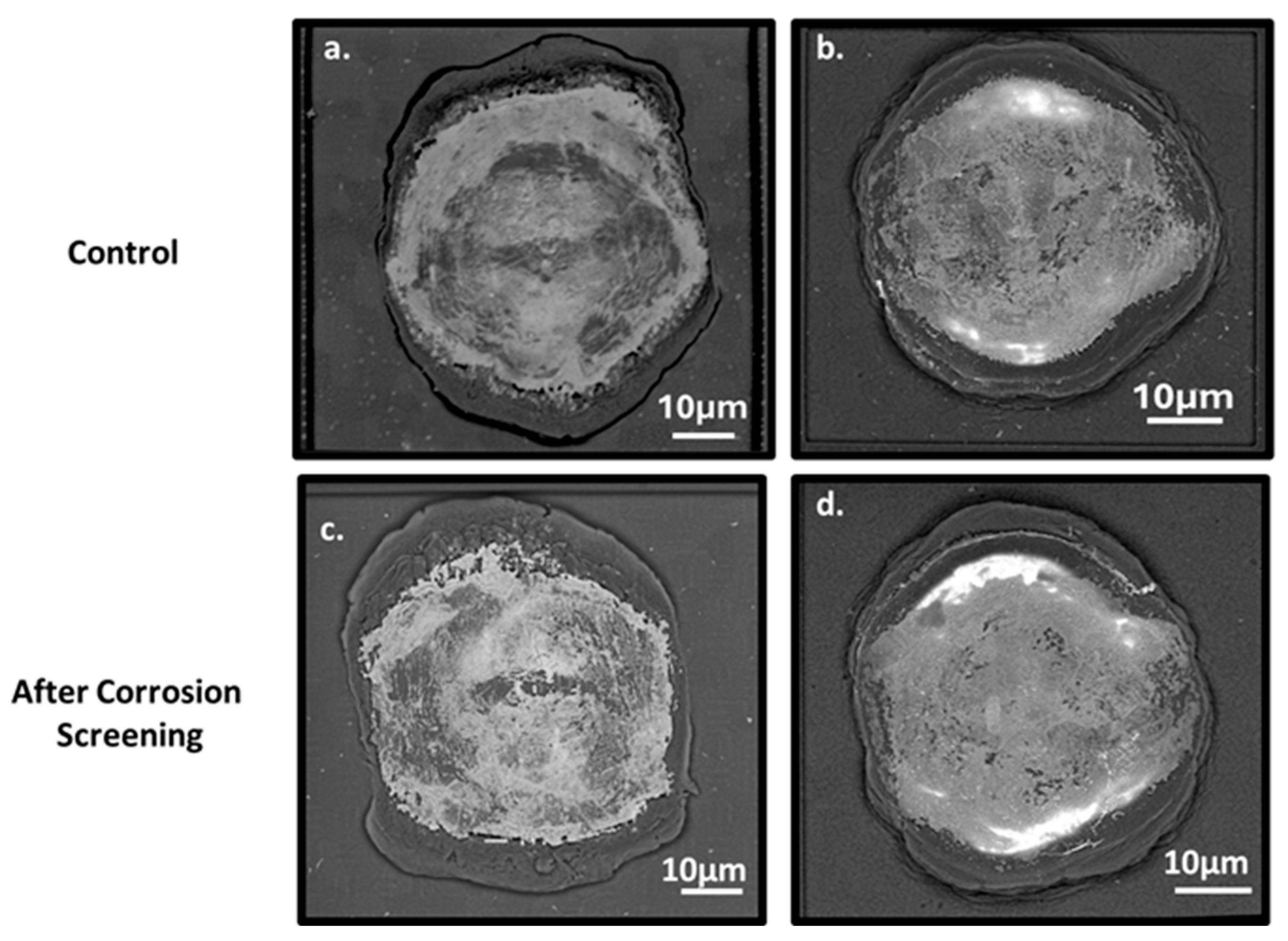
3.4. Post-Screening SEM Analysis
3.5. NTMP Interactions with Al and Cu Surface
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCC | Palladium-coated copper |
| IC | Integrated circuit |
| LQFP | Low-profile quad flat packages |
| MAPBGA | Mold array process ball grid array |
| EMC | Epoxy molding compounds |
| NTMP | Nitrilotris (methylene)phosphonic acid |
| IMC | Inter-metallic compounds |
| MX | Mixed-ion screening solution |
References
- Qin, I.; Yauw, O.; Schulze, G.; Shah, A.; Chylak, B.; Wong, N. Advances in Wire Bonding Technology for 3D Die Stacking and Fan Out Wafer Level Package. In Proceedings of the 2017 IEEE 67th Electronic Components and Technology Conference (ECTC), Orlando, FL, USA, 30 May–2 June 2017. [Google Scholar] [CrossRef]
- Zhou, H.; Chang, A.; Fan, J.; Cao, J.; An, B.; Xia, J.; Yao, J.; Cui, X.; Zhang, Y. Copper Wire Bonding: A Review. Micromachines 2023, 14, 1612. [Google Scholar] [CrossRef]
- Gan, C.L.; Hashim, U. Reliability Assessment and Activation Energy Study of Au and Pd-Coated Cu Wires Post High Temperature Aging in Nanoscale Semiconductor Packaging. ASME. J. Electron. Packag. 2013, 135, 021010. [Google Scholar] [CrossRef]
- An, B.; Zhou, H.; Cao, J.; Ming, P.; Persic, J.; Yao, J.; Chang, A. A Review of Silver Wire Bonding Techniques. Micromachines 2023, 14, 2129. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.W. Overview of wire bonding using copper wire or insulated wire. Microelectron. Reliab. 2011, 51, 4–12. [Google Scholar] [CrossRef]
- Liu, C.P.; Chang, S.J.; Liu, Y.F.; Chen, W.S. Cu-Al interfacial formation and kinetic growth behavior during HTS reliability test. J. Mater. Process. Technol. 2019, 267, 90–102. [Google Scholar] [CrossRef]
- Yoo, Y.-R.; Kim, G.; Jeon, S.-M.; Park, H.-J.; Seo, W.-W.; Moon, J.-T.; Kim, Y.-S. Influence of HCl Concentration on Corrosion Behavior between Au or Cu Bonding Wires and the Bond Pad for Semiconductor Packaging. Materials 2023, 16, 7275. [Google Scholar] [CrossRef]
- Uno, T. Bond reliability under humid environment for coated copper wire and bare copper wire. Microelectron. Reliab. 2011, 51, 148–156. [Google Scholar] [CrossRef]
- Liu, C.; Chang, S.; Liu, Y.; Su, J. Corrosion-induced degradation and its mechanism study of Cu–Al interface for Cu-wire bonding under HAST conditions. J. Alloys Compd. 2020, 825, 154046. [Google Scholar] [CrossRef]
- Van Soestbergen, M.; Mavinkurve, A.; Zaal, J.J.M.; O’Halloran, G.M.; Rongen, R.T.H.; Farrugia, M.L. Crevice Corrosion of Ball Bond Intermetallics of Cu and Ag Wire. In Proceedings of the 2016 IEEE 66th Electronic Components and Technology Conference (ECTC), Las Vegas, NV, USA, 31 May–3 June 2016; pp. 774–781. [Google Scholar] [CrossRef]
- Qin, W.; Anderson, H.; Anderson, T.; Chang, G.; Barrientos, D. Corrosion Mechanisms of Cu Wire Bonding on Al Pads. In Proceedings of the 2018 IEEE 68th Electronic Components and Technology Conference (ECTC), San Diego, CA, USA, 29 May–1 June 2018. [Google Scholar] [CrossRef]
- Belfort, Y.; Caignard, J.M.; Keller, S.; Guerveno, J.-P. Failures on DC–DC Modules Following a Change of Wire Bonding Material from Gold to Copper. Microelectron. Reliab. 2015, 55, 2003–2006. [Google Scholar] [CrossRef]
- Gan, C.L.; Ng, E.K.; Chan, B.L.; Hashim, U.; Classe, F.C. Technical Barriers and Development of Cu Wirebonding in Nanoelectronics Device Packaging. J. Nanomater. 2012, 173025. [Google Scholar] [CrossRef]
- Osenbach, J.; Wang, B.Q.; Emerich, S.; DeLucca, J.; Meng, D. Corrosion of the Cu/Al Interface in Cu-Wire-Bonded Integrated Circuits. In Proceedings of the 2013 IEEE 63rd Electronic Components and Technology Conference (ECTC), Las Vegas, NV, USA, 28–31 May 2013; IEEE: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Mathew, V.; Chopin, S. Corrosion Reliability of Copper Wirebond (CuWB) Packages-Impact of Voltage and Corrosive Ions from Packaging Materials. IMAPSource Proc. 2015, 2015, 286–291. [Google Scholar] [CrossRef]
- Mathew, V.; Chopin, S.; Higgins, L.; Zhang, Y. Copper Wirebond Compatibility with Organic and Inorganic Ions Present in Mold Compounds. J. Microelectron. Electron. Packag. 2014, 11, 70–74. [Google Scholar] [CrossRef]
- Ross, N.; Asokan, M.; Ashok Kumar, G.I.; Caperton, J.; Alptekin, J.; Salunke, A.S.; Chyan, O.M. Mechanistic Study of Copper Wire-Bonding Failures on Packaging Devices in Acidic Chloride Environments. Microelectron. Reliab. 2020, 113, 113917. [Google Scholar] [CrossRef]
- Mathew, V.; Chopin, S.; Li, G.; Xu, S. Post wirebonding coating for prevention of corrosion of wire bonded packages by chlorine containing foreign particles. In Proceedings of the 2022 IEEE 72nd Electronic Components and Technology Conference (ECTC), San Diego, CA, USA, 31 May–3 June 2022; pp. 495–501. [Google Scholar] [CrossRef]
- Qin, I.; Xu, H.; Clauberg, H.; Cathcart, R.; Acoff, V.L.; Chylak, B.; Huynh, C. Wire Bonding of Cu and Pd Coated Cu Wire: Bondability, Reliability, and IMC Formation. In Proceedings of the 2011 IEEE 61st Electronic Components and Technology Conference (ECTC), Lake Buena Vista, FL, USA, 31 May–3 June 2011. [Google Scholar] [CrossRef]
- Lim, A.B.Y.; Neo, W.J.; Yauw, O.; Chylak, B.; Gan, C.L.; Chen, Z. Evaluation of the Corrosion Performance of Cu–Al Intermetallic Compounds and the Effect of Pd Addition. Microelectron. Reliab. 2016, 56, 155–161. [Google Scholar] [CrossRef]
- Qin, W.; Anderson, T.; Chang, G. Mechanism to Improve the Reliability of Copper Wire Bonding with Palladium-Coating of the Wire. Microelectron. Reliab. 2019, 99, 239–244. [Google Scholar] [CrossRef]
- Kumaravel, D.K.; Jesu Durai, K.A.; Nair, S.M.; Tran, K.T.A.; Chyan, O.; Poliah, R.; Chan, M.Z.; Mathew, V. Innovative Cu-Selective Passivation Coatings for Enhanced Reliability in Cu Interconnects for IC Packaging. In Proceedings of the 2024 IEEE 26th Electronics Packaging Technology Conference (EPTC), Singapore, 3–6 December 2024; pp. 534–540. [Google Scholar] [CrossRef]
- Li, Z.; Merz, K.M. Systematic Evaluation of Ion Diffusion and Water Exchange. J. Chem. Theory Comput. 2022, 18, 3017–3026. [Google Scholar] [CrossRef]
- Lantz, L.; Pecht, M.G. Pecht Ion transport in encapsulants used in microcircuit packaging. IEEE Trans. Compon. Packag. Technol. 2003, 26, 199–205. [Google Scholar] [CrossRef]
- Van Soestbergen, M.; Ernst, L.J.; Zhang, G.Q.; Rongen, R.T.H. Transport of Corrosive Constituents in Epoxy Moulding Compounds. In Proceedings of the 2007 International Conference on Thermal, Mechanical and Multi-Physics Simulation Experiments in Microelectronics and Micro-Systems EuroSime 2007, London, UK, 16–18 April 2007; pp. 1–5. [Google Scholar] [CrossRef]
- Paniagua, S.A.; Giordano, A.J.; Smith, O.L.; Barlow, S.; Li, H.; Armstrong, N.R.; Pemberton, J.E.; Brédas, J.; Ginger, D.; Marder, S.R. Phosphonic Acids for Interfacial Engineering of Transparent Conductive Oxides. Chem. Rev. 2016, 116, 7117. [Google Scholar] [CrossRef]
- Karlsson, P.M.; Anderson, M.W.; Palmqvist, A.E.C. Adsorption of sodium dodecyl sulfate and sodium dodecyl phosphate at the surface of aluminium oxide studied with AFM. Corros. Sci. 2009, 52, 1103. [Google Scholar] [CrossRef]
- Zhao, R.; Hauert, R.; Crockett, R.; Cancellieri, C.; Gaan, S.; Schmutz, P.; Heuberger, M.; Jeurgens, L.P.H. Aqueous phosphonic acid treatments of aluminium: Effect of oxide dissolution and re-oxidation on adsorbate formation. Appl. Surf. Sci. 2024, 667, 160363. [Google Scholar] [CrossRef]
- Xia, D.; Pan, C.; Qin, Z.; Fan, B.; Song, S.; Jin, W.; Hu, W. Covalent surface modification of LY12 aluminum alloy surface by self-assembly dodecyl phosphate film towards corrosion protection. Prog. Org. Coat. 2020, 143, 105638. [Google Scholar] [CrossRef]
- Alptekin, J.; Antony Jesu Durai, K.; Kumaravel, D.K.; Estridge, L.; Chyan, O.; Ibrahim, R.; Li, G.; Mathew, V. A Cu–Cu Wire-Bonding Enabled by a Cu-Selective Passivation Coating to Enhance Packaging Reliability. IEEE Trans. Compon. Packag. Manufact. Technol. 2023, 13, 1923. [Google Scholar] [CrossRef]
- Chyan, O.; Ashokan, M. Selective Surface Finishing for Corrosion Inhibition via Chemical Vapor Deposition. Google Patents US20210071308A1, 11 March 2021. Available online: https://patents.google.com/patent/US20210071308A1/en (accessed on 4 March 2025).
- Fairley, N.; Fernandez, V.; Richard-Plouet, M.; Guillot-Deudon, C.; Walton, J.; Smith, E.; Flahaut, D.; Greiner, M.; Biesinger, M.; Tougaard, S.; et al. Systematic and collaborative approach to problem solving using X-ray photoelectron spectroscopy. Appl. Surf. Sci. Adv. 2021, 5, 100112. [Google Scholar] [CrossRef]
- O’Halloran, G.M.; Van Ijzerloo, A.; Rongen, R.; Zachariasse, F. Planar Analysis of Copper-Aluminium Intermetallics. In Proceedings of the 39th International Symposium for Testing and Failure Analysis, San Jose, CA, USA, 3–7 November 2013. [Google Scholar] [CrossRef]
- Mavinkurve, A.; Goumans, L.; O’Halloran, G.M.; Rongen, R.T.H.; Farrugia, M.-L. Copper wire interconnect reliability evaluation using in-situ High Temperature Storage Life (HTSL) tests. Microelectron. Reliab. 2014, 54, 1661–1665. [Google Scholar] [CrossRef]
- Hoque, E.; DeRose, J.A.; Hoffmann, P.; Mathieu, H.J.; Bhushan, B.; Cichomski, M. Phosphonate self-assembled monolayers on aluminum surfaces. J. Chem. Phys. 2006, 124, 174710. [Google Scholar] [CrossRef] [PubMed]
- Kozlica, D.K.; Milošev, I. Corrosion inhibition of copper and aluminium by 2-mercaptobenzimidazole and octylphosphonic acid—Surface pre-treatment and method of film preparation. Electrochim. Acta 2022, 431, 141154. [Google Scholar] [CrossRef]
- Pan, C.; Wang, X.; Behnamian, Y.; Wu, Z.; Qin, Z.; Xia, D.-H.; Hu, W. Monododecyl Phosphate Film on LY12 Aluminum Alloy: PH-Controlled Self-Assembly and Corrosion Resistance. J. Electrochem. Soc. 2020, 167, 161510. [Google Scholar] [CrossRef]
- Attavar, S.; Diwekar, M.; Linford, M.R.; Davis, M.A.; Blair, S. Passivation of aluminum with alkyl phosphonic acids for biochip applications. Appl. Surf. Sci. 2010, 256, 7146–7150. [Google Scholar] [CrossRef]
- Hoque, E.; DeRose, J.A.; Kulik, G.; Hoffmann, P.; Mathieu, H.J.; Bhushan, B. Alkylphosphonate Modified Aluminum Oxide Surfaces. J. Phys. Chem. B 2006, 110, 10855–10861. [Google Scholar] [CrossRef]
- Scandurra, A.; Currò, G.; Frisina, F.; Pignataro, S. Corrosion Inhibition of Al Metal in Microelectronic Devices Assembled in Plastic Packages. J. Electrochem. Soc. 2001, 148, B289. [Google Scholar] [CrossRef]
- Wan, X.; Lieberman, I.; Asyuda, A.; Resch, S.; Seim, H.; Kirsch, P.; Zharnikov, M. Thermal Stability of Phosphonic Acid Self-Assembled Monolayers on Alumina Substrates. J. Phys. Chem. C 2020, 124, 2531. [Google Scholar] [CrossRef]
- Smecca, E.; Motta, A.; Fragalà, M.E.; Aleeva, Y.; Condorelli, G.G. Spectroscopic and Theoretical Study of the Grafting Modes of Phosphonic Acids on ZnO Nanorods. J. Phys. Chem. C 2013, 117, 5364–5372. [Google Scholar] [CrossRef]

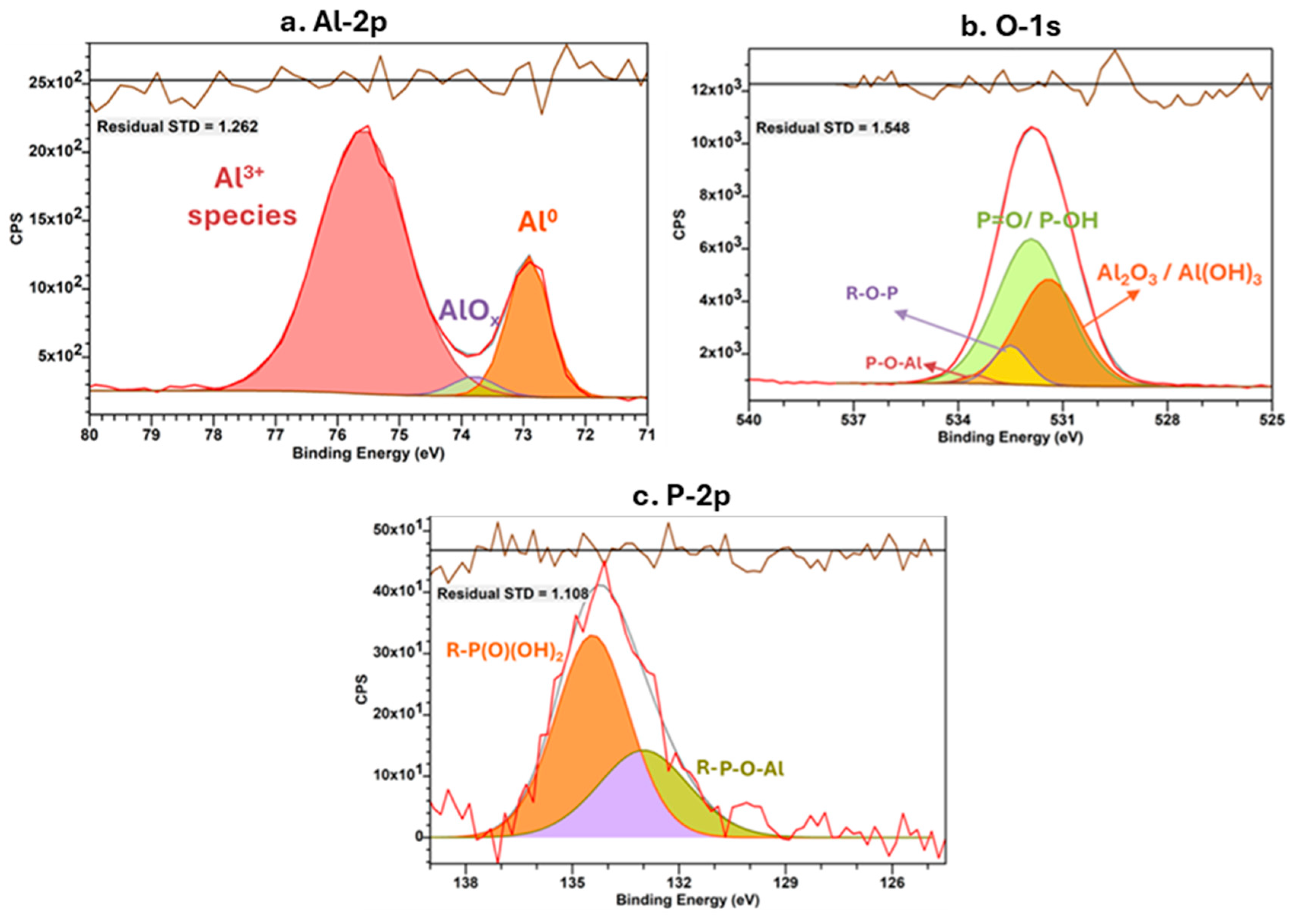
| Element | Components | Binding Energy | Atom% | References |
|---|---|---|---|---|
| Al-2p | Al (0) metal | 72.9 | 19.6 | [35,36,37,38,39] |
| AlOx | 73.8 | 2.9 | ||
| Al3+ species | 75.6 | 77.5 | ||
| O-1s | Al2O3/Al (OH)3 | 531.4 | 36.6 | [36] |
| P = O/P-OH | 531.9 | 54.61 | ||
| P-O-R | 532.5 | 7.61 | ||
| P-O-Al | 533.5 | 1.23 | ||
| P-2p | R-P(O)(OH)2 | 134.4 | 65.3 | [36,41,42] |
| R-P-O-Al | 133.0 | 34.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumaravel, D.K.; Sridharan Nair, S.; Tran, K.T.A.; Ahluwalia, P.; Antony Jesu Durai, K.; Chyan, O. Post Wire-Bonding Corrosion Prevention Strategies to Mitigate Chloride- and Bromide-Induced Corrosion Failures in Cu- and PCC-Based Wire-Bonded Packages. Micromachines 2025, 16, 1155. https://doi.org/10.3390/mi16101155
Kumaravel DK, Sridharan Nair S, Tran KTA, Ahluwalia P, Antony Jesu Durai K, Chyan O. Post Wire-Bonding Corrosion Prevention Strategies to Mitigate Chloride- and Bromide-Induced Corrosion Failures in Cu- and PCC-Based Wire-Bonded Packages. Micromachines. 2025; 16(10):1155. https://doi.org/10.3390/mi16101155
Chicago/Turabian StyleKumaravel, Dinesh Kumar, Shinoj Sridharan Nair, Khanh Tuyet Anh Tran, Pavan Ahluwalia, Kevin Antony Jesu Durai, and Oliver Chyan. 2025. "Post Wire-Bonding Corrosion Prevention Strategies to Mitigate Chloride- and Bromide-Induced Corrosion Failures in Cu- and PCC-Based Wire-Bonded Packages" Micromachines 16, no. 10: 1155. https://doi.org/10.3390/mi16101155
APA StyleKumaravel, D. K., Sridharan Nair, S., Tran, K. T. A., Ahluwalia, P., Antony Jesu Durai, K., & Chyan, O. (2025). Post Wire-Bonding Corrosion Prevention Strategies to Mitigate Chloride- and Bromide-Induced Corrosion Failures in Cu- and PCC-Based Wire-Bonded Packages. Micromachines, 16(10), 1155. https://doi.org/10.3390/mi16101155






