Effects of Ni Content on Energy Density, Capacity Fade and Heat Generation in Li[NixMnyCoz]O2/Graphite Lithium-Ion Batteries
Abstract
1. Introduction
2. Experiments
3. Simulations
4. Results and Discussion
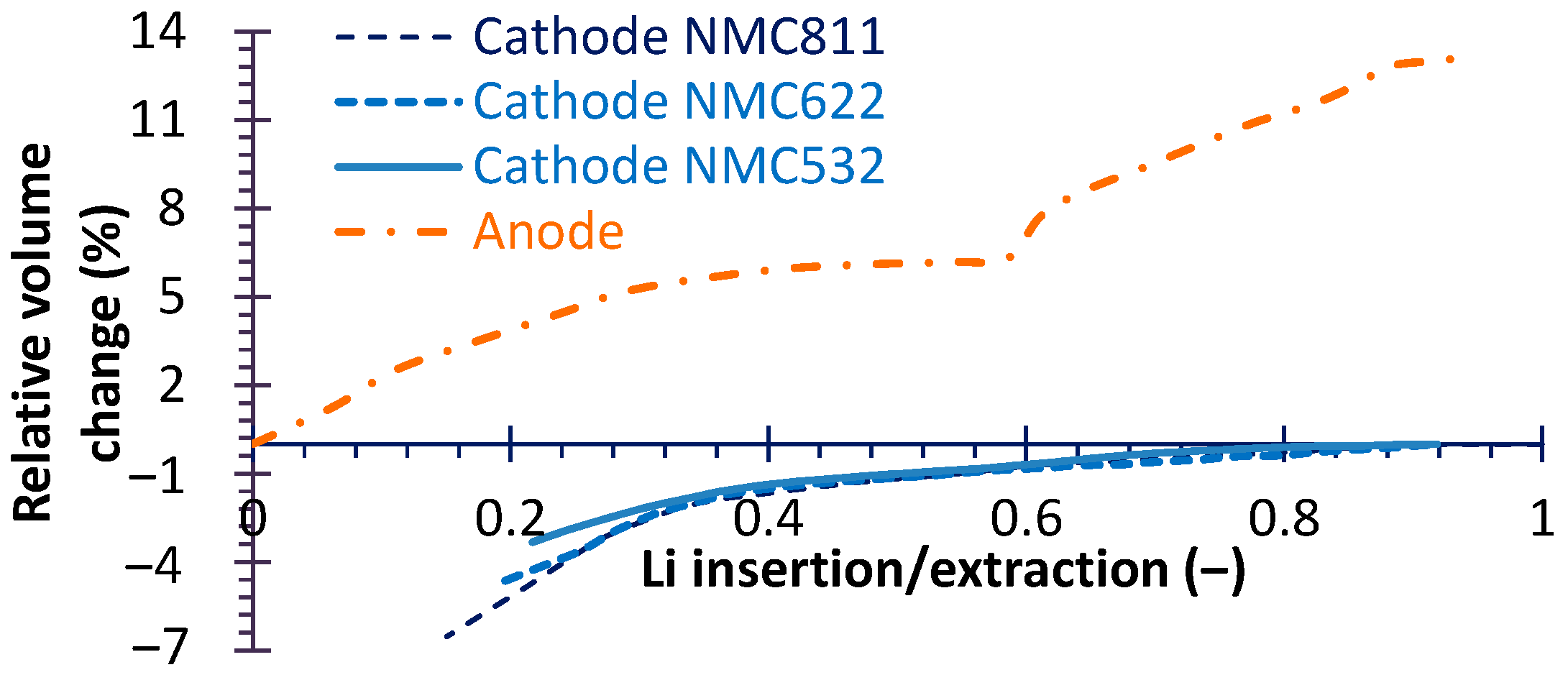
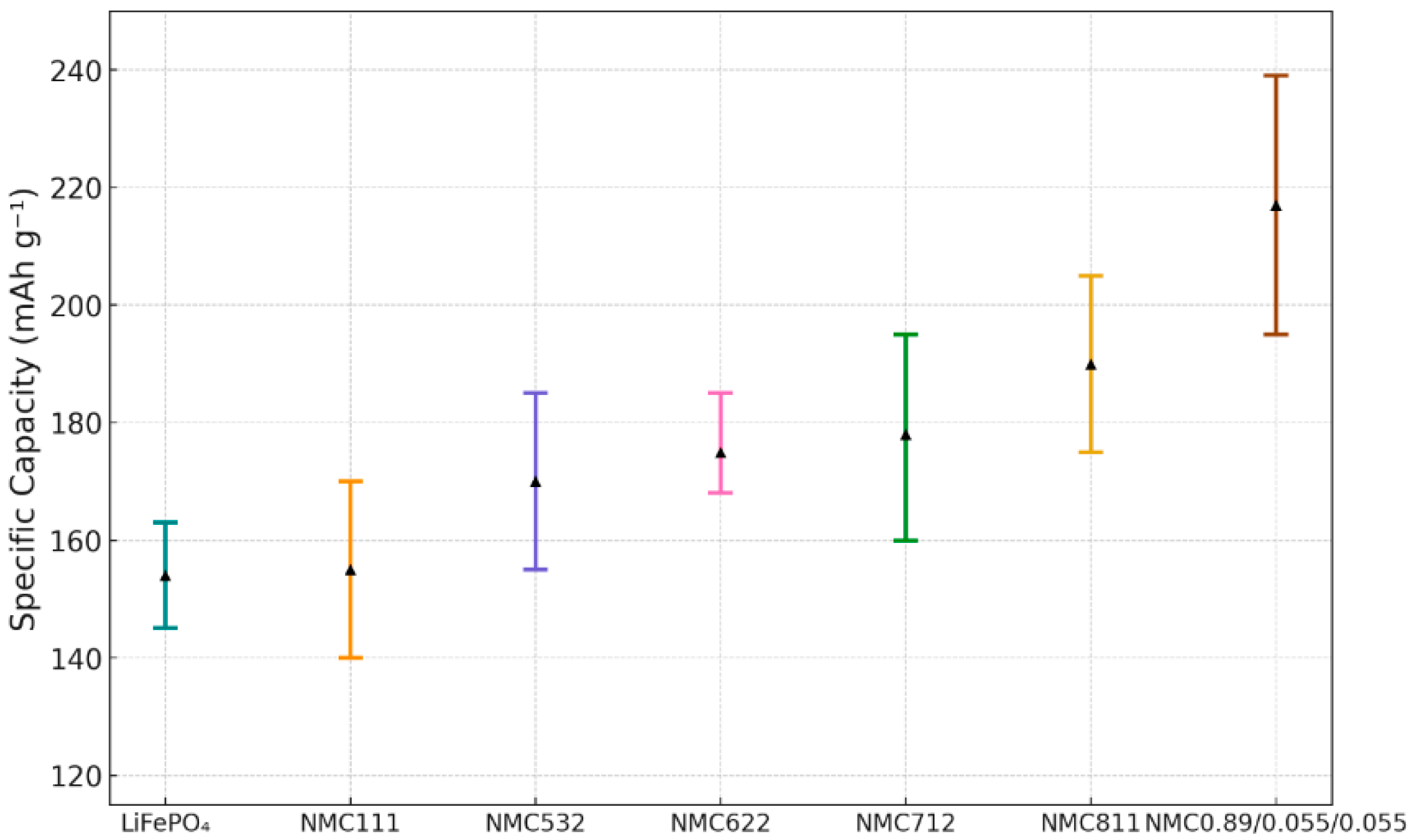
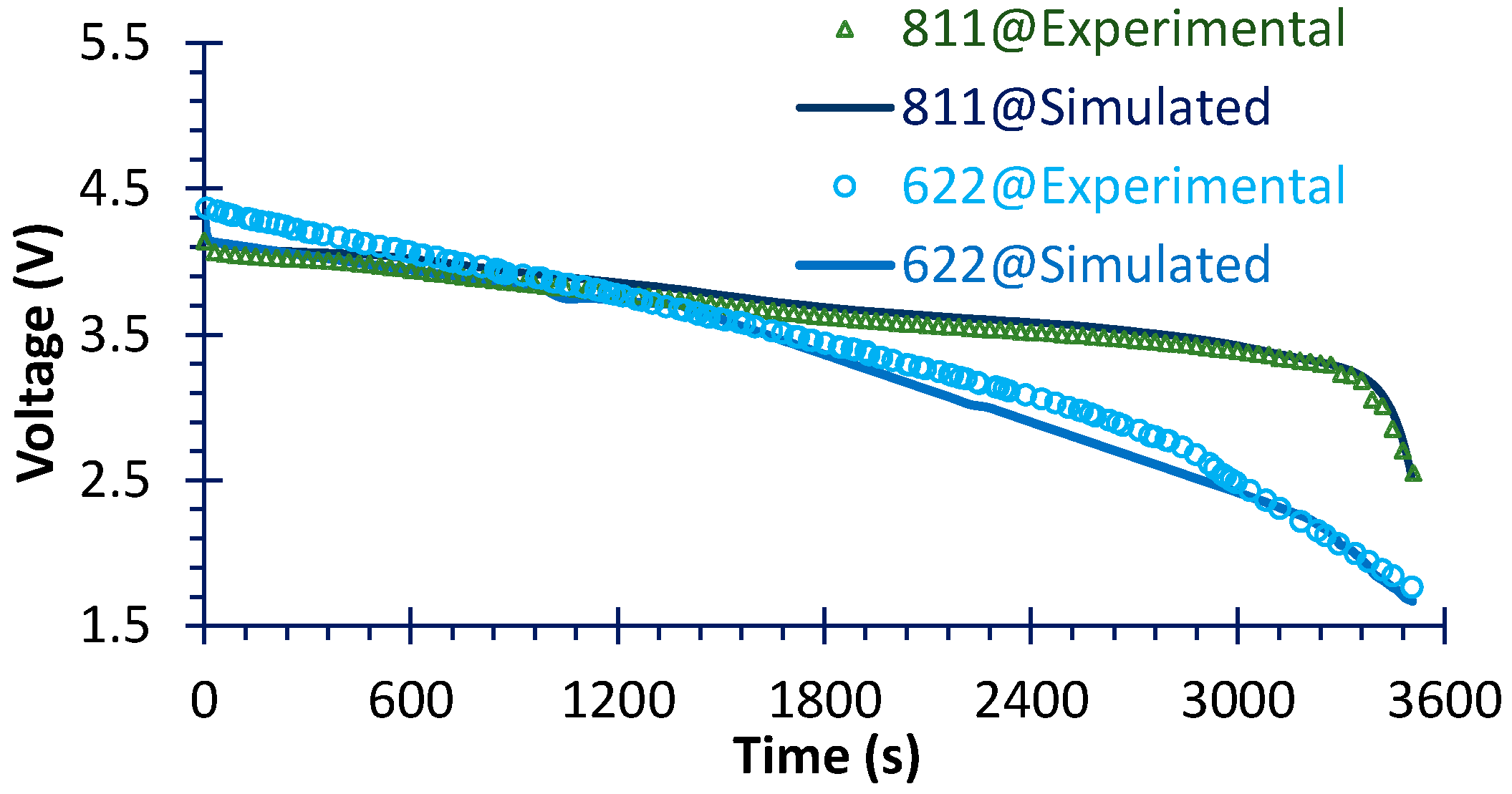
4.1. Electrical Performance
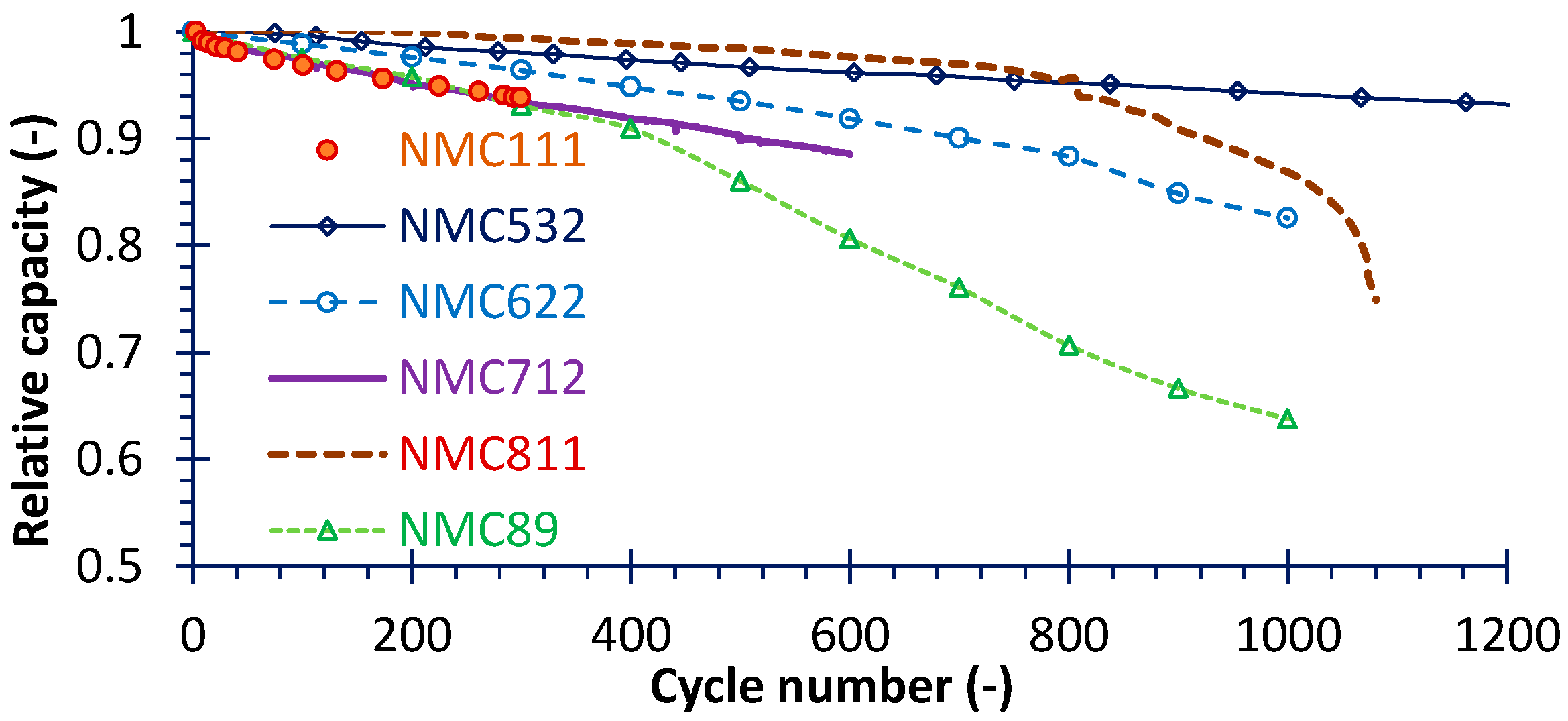
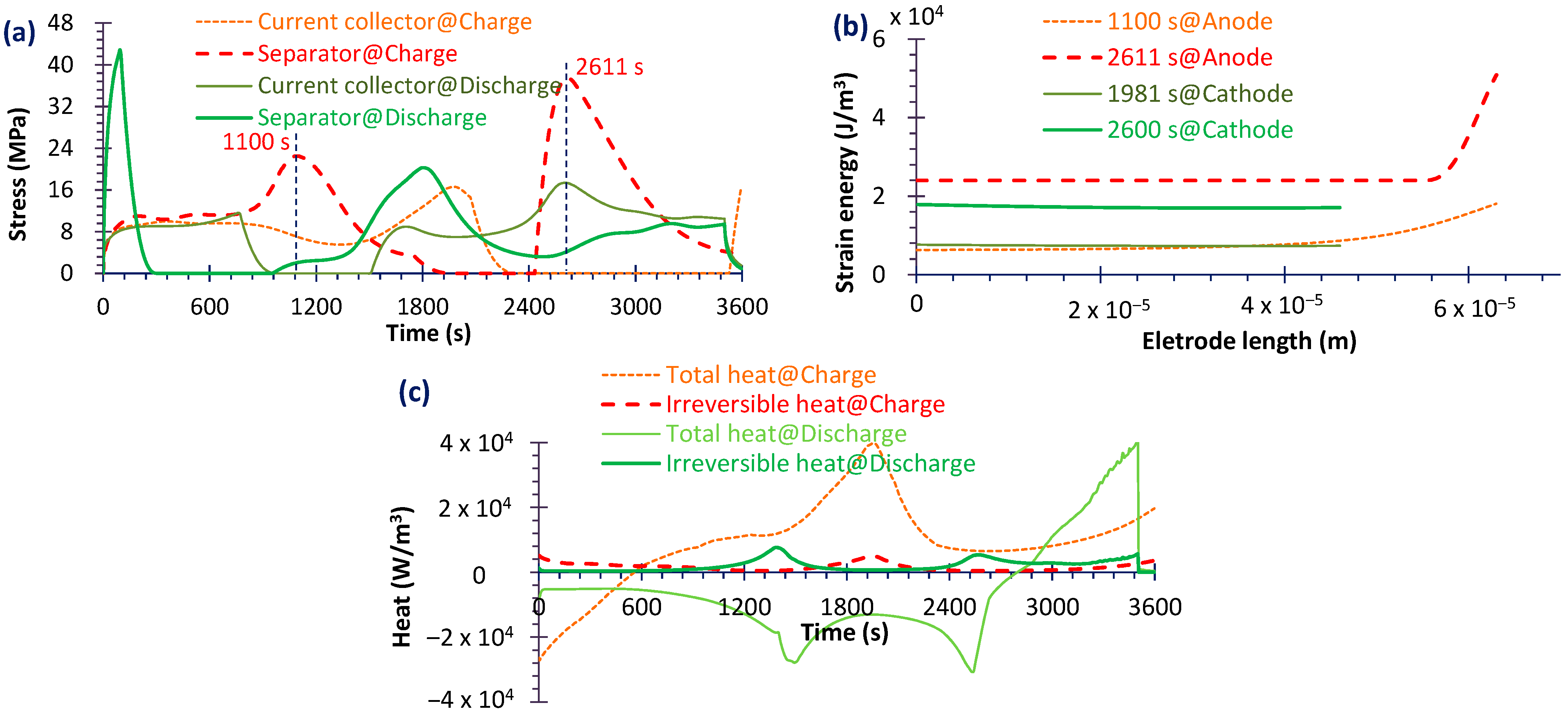
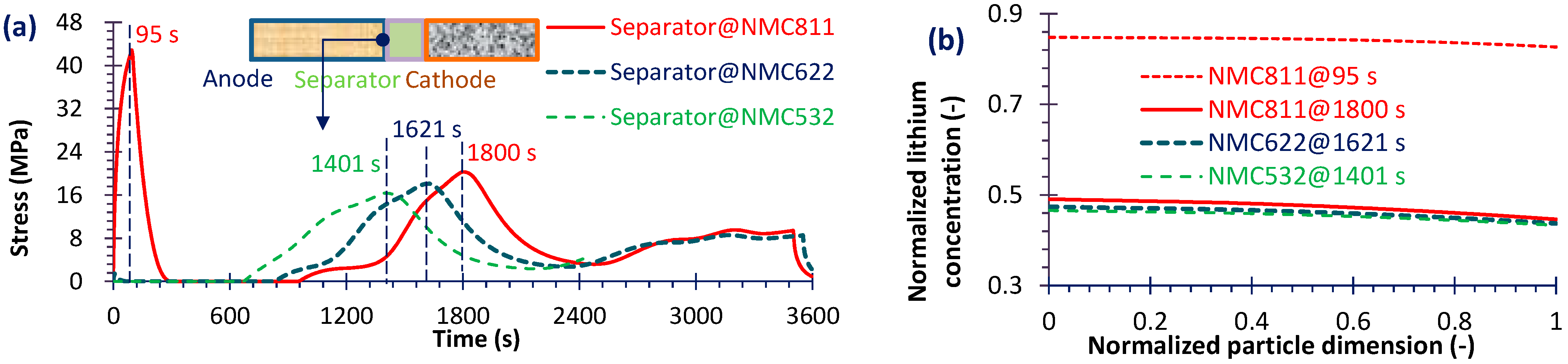
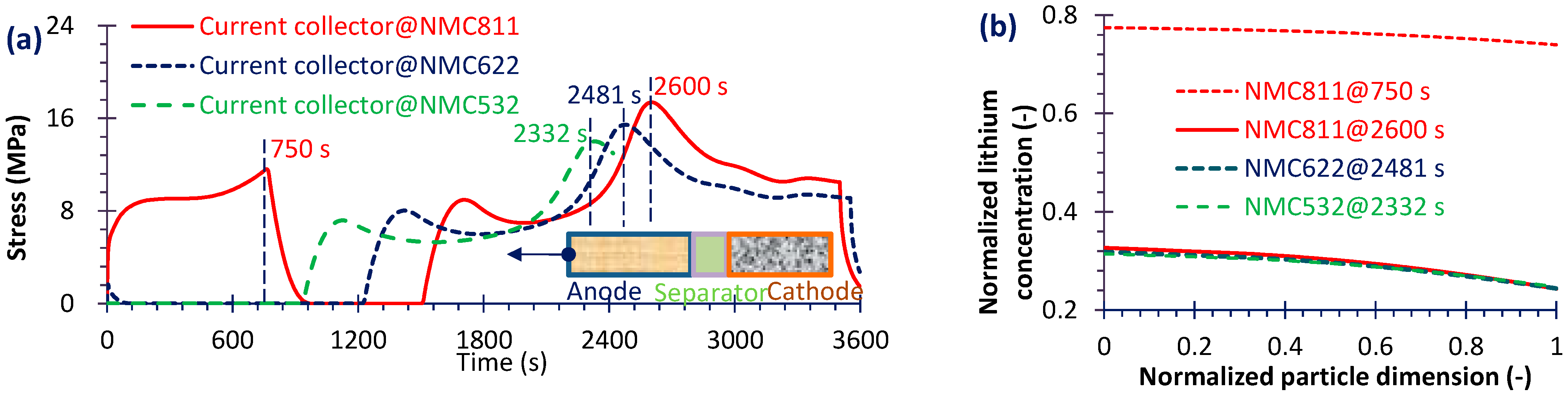
4.2. Capacity Fade
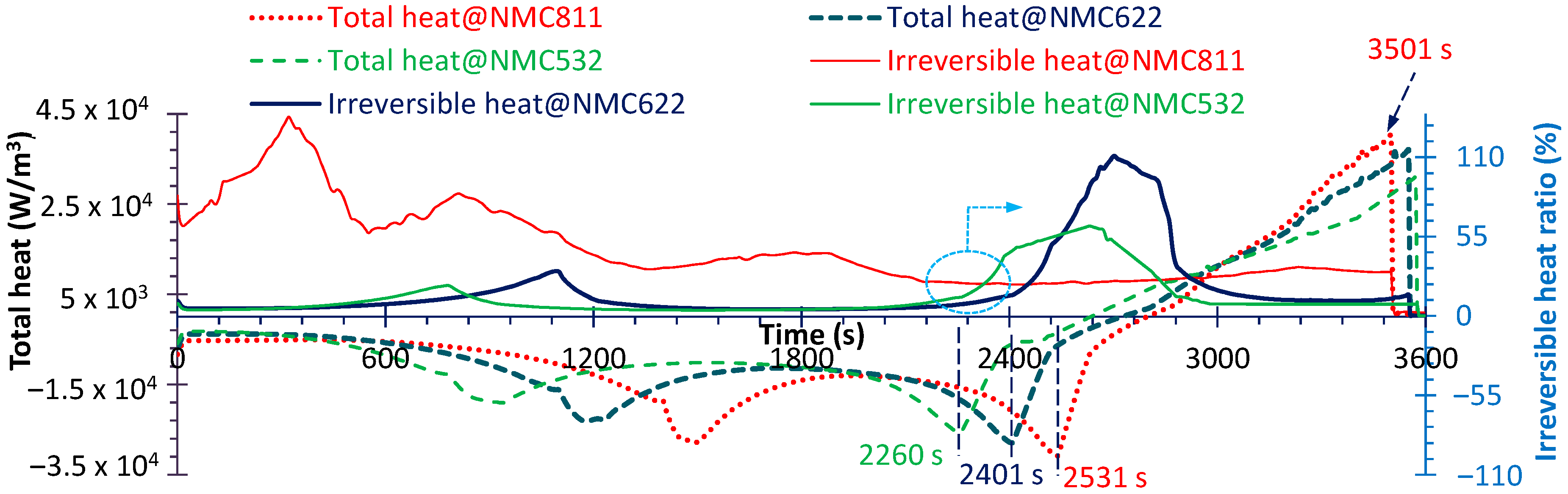
4.3. Heat Generation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Coyle, E.D.; Simmons, R.A. Understanding the Global Energy Crisis; Purdue University Press: West Lafayette, IN, USA, 2014. [Google Scholar]
- Lu, Y.; Khan, Z.A.; Alvarez-Alvarado, M.S.; Zhang, Y.; Huang, Z.; Imran, M. A Critical Review of Sustainable Energy Policies for the Promotion of Renewable Energy Sources. Sustainability 2020, 12, 5078. [Google Scholar] [CrossRef]
- Sun, Y.; Ge, M.; Xie, B.; Zhang, H.; Gao, Z.; Zhao, X.; Deng, B.; Zhang, K.; Zhang, S.; Sun, C.; et al. Superior hydroxide-philic CoCo2O4@NiMn2O4 heterojunction boosting wide-voltage-window for aqueous high-energy-density supercapacitors. J. Energy Storage 2025, 133, 118060. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, G.; Tan, S.; Wang, H.; Liu, Y.; Sun, C.; Deng, B.; Zhang, K.; Gao, Z.; Huang, N. Microwave-assisted one-pot synthesis of hierarchical MnCO3/NiO heterojunction with superior energy storage performance for aqueous supercapacitor. Chem. Eng. J. 2025, 519, 165031. [Google Scholar] [CrossRef]
- Chen, W.; Liang, J.; Yang, Z.; Li, G. A review of lithium-ion battery for electric vehicle applications and beyond. Energy Procedia 2019, 158, 4363–4368. [Google Scholar] [CrossRef]
- Sun, S.; Dong, M.; Liang, B. Distributed Real-Time Power Balancing in Renewable-Integrated Power Grids with Storage and Flexible Loads. IEEE Trans. Smart Grid 2016, 7, 2337–2349. [Google Scholar] [CrossRef]
- Kolodziejski, M.; Michalska-Pozoga, I. Battery Energy Storage Systems in Ships’ Hybrid/Electric Propulsion Systems. Energies 2023, 16, 1122. [Google Scholar] [CrossRef]
- Akbarzadeh, M.; De Smet, J.; Stuyts, J. Battery Hybrid Energy Storage Systems for Full-Electric Marine Applications. Processes 2022, 10, 2418. [Google Scholar] [CrossRef]
- Maghsoudi, R.; Ghezi, A.; Jamalpour, S.; Tamsilian, Y.; Tohidian, M.; Shahebrahimi, Y. Advantages, Limitations, and Industrial Applications of Lithium-Ion Batteries; Wiley Online Library: Hoboken, NJ, USA, 2024; pp. 793–820. [Google Scholar] [CrossRef]
- Frith, J.T.; Lacey, M.J.; Ulissi, U. A non-academic perspective on the future of lithium-based batteries. Nat. Commun. 2023, 14, 420. [Google Scholar] [CrossRef]
- CIMAC and Maritime Battery Forum. Use Cases and Application Areas for Battery Systems in Maritime Applications: A Joint White Paper. February 2024. Available online: https://www.cimac.com/cms/upload/Publication_Press/WG_Publications/Joint_battery_whitepaper_use_cases_and_application_areas_CIMAC_and_Maritime_Battery_Forum_02-09-2024.pdf (accessed on 5 July 2025).
- Chin, C.S.; Zhang, C.; Gao, Z. Deploying battery technology for marine vessel electrification. IEEE Potentials 2021, 40, 24–33. [Google Scholar] [CrossRef]
- Kaur, D.; Singh, M.; Singh, S. Lithium–sulfur batteries for marine applications. In Lithium-Sulfur Batteries: Materials, Challenges and Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 549–577. [Google Scholar] [CrossRef]
- Bugryniec, P.J.; Khanna, S.; Wootton, M.; Williams, D.; Brown, S.F. Assessment of the risks posed by thermal runaway within marine Li-ion battery energy storage systems—Considering past incidents, current guidelines and future mitigation measures. J. Energy Storage 2025, 128, 117070. [Google Scholar] [CrossRef]
- Yin, R.; Du, M.; Shi, F.; Cao, Z.; Wu, W.; Shi, H.; Zheng, Q. Risk analysis for marine transport and power applications of lithium ion batteries: A review. Process Saf. Environ. Prot. 2024, 181, 266–293. [Google Scholar] [CrossRef]
- MI News Network. USCG Warns of Fire Risks from Lithium-Ion Battery Installations on Ships. Marine Insight, 15 July 2025. Available online: https://www.marineinsight.com/shipping-news/uscg-warns-of-fire-risks-from-lithium-ion-battery-installations-on-ships/ (accessed on 5 July 2025).
- Koech, A.K.; Mwandila, G.; Mulolani, F.; Mwaanga, P. Lithium-ion battery fundamentals and exploration of cathode materials: A review. S. Afr. J. Chem. Eng. 2024, 50, 321–339. [Google Scholar] [CrossRef]
- Ohzuku, T.; Brodd, R.J. An overview of positive-electrode materials for advanced lithium-ion batteries. J. Power Sources 2007, 174, 449–456. [Google Scholar] [CrossRef]
- Sivakumar, S.; Somasundaram, P. An investigation on influence of battery materials for efficient lithium-ion battery pack design. AIP Conf. Proc. 2022, 2446, 080001. [Google Scholar] [CrossRef]
- Fergus, J.W. Recent developments in cathode materials for lithium ion batteries. J. Power Sources 2010, 195, 939–954. [Google Scholar] [CrossRef]
- Lee, S.; Li, W.; Dolocan, A.; Celio, H.; Park, H.; Warner, J.H.; Manthiram, A. In-Depth Analysis of the Degradation Mechanisms of High-Nickel, Low/No-Cobalt Layered Oxide Cathodes for Lithium-Ion Batteries. Adv. Energy Mater. 2021, 11, 2100858. [Google Scholar] [CrossRef]
- Li, W.; Lee, S.; Manthiram, A. High-Nickel NMA: A Cobalt-Free Alternative to NMC and NCA Cathodes for Lithium-Ion Batteries. Adv. Mater. 2020, 32, 2002718. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Lu, L.; Zheng, Y.; Feng, X.; Li, Z.; Li, J.; Ouyang, M. A review on the key issues of the lithium ion battery degradation among the whole life cycle. eTransportation 2019, 1, 100005. [Google Scholar] [CrossRef]
- Kondrakov, A.O.; Schmidt, A.; Xu, J.; Geßwein, H.; Mönig, R.; Hartmann, P.; Sommer, H.; Brezesinski, T.; Janek, O. Anisotropic lattice strain and mechanical degradation of High- and Low-Nickel NCM cathode materials for Li-Ion batteries. J. Phys. Chem. C 2017, 121, 3286–3294. [Google Scholar] [CrossRef]
- Jung, R.; Metzger, M.; Maglia, F.; Stinner, C.; Gasteiger, H.A. Oxygen release and its effect on the cycling stability of LiNixMnyCozO2 (NMC) cathode materials for Li-ion batteries. J. Electrochem. Soc. 2017, 164, A1361–A1377. [Google Scholar] [CrossRef]
- Xu, G.; Liu, X.; Daali, A.; Amine, R.; Chen, Z.; Amine, K. Challenges and Strategies to Advance High-Energy Nickel-Rich Layered Lithium Transition Metal Oxide Cathodes for Harsh Operation. Adv. Funct. Mater. 2020, 30, 2004748. [Google Scholar] [CrossRef]
- Deng, B.L.; Li, W.B.; Cai, W.Y.; Liu, L.R.; Liao, C.; Xiao, M.W.; Li, M. Capacity fade of high-energy Li[Ni0.8Mn0.1Co0.1]O2/Graphite lithium-ion battery as affected by cell mechanical constraint and subsequent stresses. J. Clean. Prod. 2024, 448, 141722. [Google Scholar] [CrossRef]
- Doyle, M.; Fuller, T.F.; Newman, J. Modeling of galvanostatic charge and discharge of the lithium/polymer/insertion cell. J. Electrochem. Soc. 1993, 140, 1526–1533. [Google Scholar] [CrossRef]
- Chen, Z.Q.; Danilov, D.L.; Zhang, Q.; Jiang, M.; Zhou, J.; Eichel, R.-A.; Notten, P.H.L. Modeling NCA/C6-Si Battery Ageing. Electrochim. Acta 2022, 430, 141077. [Google Scholar] [CrossRef]
- Zuo, D.X.; Li, P.C. Aging characteristics of lithium-ion battery under fast charging based on electrochemical-thermal-mechanical coupling model. J. Electrochem. 2024, 30, 2402061. [Google Scholar] [CrossRef]
- Das, D.; Manna, S.; Puravankara, S. Electrolytes, Additives and Binders for NMC Cathodes in Li-Ion Batteries—A Review. Batteries 2023, 9, 193. [Google Scholar] [CrossRef]
- Liu, J.H.; Lin, W.C.; Wang, Z.; Wang, Y.; Chen, T.W.; Zheng, J.X. Elastic Mechanics Study of Layered Li(NixMnyCoz)O2. PRX Energy 2024, 3, 013012. [Google Scholar] [CrossRef]
- Syed, M.A.; Salehabadi, M.; Obrovac, M.N. High Energy Density Large Particle LiFePO4. Chem. Mater. 2024, 36, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Commandeur, D.; Sabado, C.; Ashton, T.E.; Darr, J.A. Combinatorial Performance Mapping of Near-NMC111 Li-ion Cathodes. J. Mater. 2022, 8, 437–445. [Google Scholar] [CrossRef]
- An, S.J.; Li, J.; Mohanty, D.; Daniel, C.; Polzin, B.J.; Croy, J.R.; Trask, S.E.; Wood, D.L., III. Correlation of electrolyte volume and electrochemical performance in lithium-ion pouch cells with graphite anodes and NMC532 cathodes. J. Electrochem. Soc. 2017, 164, A1195–A1203. [Google Scholar] [CrossRef]
- Yerkinbekova, Y.; Kumarov, A.; Tatykayev, B.; Mentbayeva, A.; Repo, E.; Laakso, E. Ni-rich cathode materials with concentration gradients for high-energy and safe lithium-ion batteries: A comprehensive review. J. Power Sources 2025, 626, 235686. [Google Scholar] [CrossRef]
- E, X.S.; Lu, T.F.; Wu, S.M.; Liang, Y.; Liu, X.; Wen, Y.; Jiang, W.W.; Wei, Z.; Cui, Y.; Zhang, Z.H. Electronic structure and lithium ion diffusion in Cr, Mo, W doped LiNiO2 for Li-ion batteries cathode material: First-principles study. Comput. Condens. Matter 2025, 44, e01081. [Google Scholar] [CrossRef]
- Kalnaus, S.; Livingston, K.; Hawley, W.B.; Wang, H.; Li, J.L. Design and processing for high performance Li ion battery electrodes with double-layer structure. J. Energy Storage 2021, 44, 103582. [Google Scholar] [CrossRef]
- Wihba, R.; Doğan, E.; Moeez, I.; Bhatti, A.H.U.; Akbar, M.; Chung, K.Y.; Altin, E.; Ateş, M.N.; Altundağ, S.; Stoyanova, R.; et al. Evaluation of the Effect of Precursor NMC622@TiO2 Core–Shell Powders Using a Prelithiated Anode from Fig Seeds: Spotlight on Li-ion Full-Cell Performance. ACS Appl. Mater. Interfaces 2024, 16, 70442–70459. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zheng, J.; Lu, J.; Li, Y.; Yan, P.; Zhang, Y. Realizing superior cycling stability of Ni-Rich layered cathode by combination of grain boundary engineering and surface coating. Nano Energy 2019, 62, 30–37. [Google Scholar] [CrossRef]
- Soloy, A.; Flahaut, D.; Allouche, J.; Foix, D.; Vallverdu, G.S.; Suard, E.; Dumont, E.; Gal, L.; Weill, F.; Croguennec, L. Effect of Particle Size on LiNi0.6Mn0.2Co0.2O2 Layered Oxide Performance in Li-Ion Batteries. ACS Appl. Energy Mater. 2022, 5, 5617–5632. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kwak, E.; Oh, K.-Y. Degradation pathways dependency of a lithium iron phosphate battery on temperature and compressive force. Int. J. Energy Res. 2021, 45, 6888–6906. [Google Scholar] [CrossRef]
- Li, J.; Li, H.Y.; Stone, W.; Glazier, S.; Dahn, J.R. Development of Electrolytes for Single Crystal NMC532/Artificial Graphite Cells with Long Lifetime. J. Electrochem. Soc. 2018, 165, A626–A635. [Google Scholar] [CrossRef]
- Fu, R.J.; Xiao, M.; Choe, S.-Y. Modeling, validation and analysis of mechanical stress generation and dimension changes of a pouch type high power Li-ion battery. J. Power Sources 2013, 224, 211–224. [Google Scholar] [CrossRef]
- Berckmans, G.; Sutter, L.D.; Marinaro, M.; Smekens, J.; Jaguemont, J.; Wohlfahrt-Mehrens, M.; van Mierlo, J.; Omar, N. Analysis of the effect of applying external mechanical pressure on next generation silicon alloy lithium-ion cells. Electrochim. Acta 2019, 306, 387–395. [Google Scholar] [CrossRef]
- Sutter, L.D.; Berckmans, G.; Marinaro, M.; Wohlfahrt-Mehrens, M.; Berecibar, M.; Mierlo, J.V. Mechanical behavior of Silicon-Graphite pouch cells under external compressive load: Implications and opportunities for battery pack design. J. Power Sources 2020, 451, 227774. [Google Scholar] [CrossRef]
- Hamed, S.; Obrezkov, F.; Huotari, S.; Colalongo, M.; Mousavihashemi, S.; Kallio, T. Optimized NMC622 electrodes with a high content of the active material: A comprehensive study. J. Power Sources 2024, 608, 234549. [Google Scholar] [CrossRef]
- Ouyang, D.X.; Weng, J.W.; Hu, J.Y.; Chen, M.Y.; Huang, Q.; Wang, J. Experimental investigation of thermal failure propagation in typical lithiumion battery modules. Thermochim. Acta 2019, 676, 205–213. [Google Scholar] [CrossRef]
- Gao, S.; Feng, X.N.; Lu, L.G.; Kamyab, N.; Du, J.Y.; Coman, P.; White, R.E.; Ouyang, M.G. An experimental and analytical study of thermal runaway propagation in a large format lithium ion battery module with NCM pouch-cells in parallel. Int. J. Heat Mass Transf. 2019, 135, 93–103. [Google Scholar] [CrossRef]
- Lyu, P.Z.; Liu, X.J.; Qu, J.; Zhao, J.T.; Huo, Y.T.; Qu, Z.G.; Rao, Z.H. Recent advances of thermal safety of lithium ion battery for energy storage. Energy Storage Mater. 2020, 31, 195–220. [Google Scholar] [CrossRef]



| Equipment | Brand and Model | Range | Accuracy | Error |
|---|---|---|---|---|
| Thermostat | ESPEC SEWTH-A-150LHS (ESPEC Corp., Fukuchiyama, Japan) | −40–85 °C | ±0.3 °C ±2.5% RH | ±1.0% |
| Charge/Discharge System | RUINENG-HRCDS-100V (Ruineng Pumps (Fujian) Co., Ltd., Quanzhou, China) | 0–100 V 0–200 A | ±0.05% F.S ±0.02% V/A | ±0.1% |
| Data Collector | KEYSIGHT-34970A (Keysight Technologies (formerly Agilent), Loveland, CO, USA) | DC/AC | 0.0035% | / |
| No. | Procedure | Step Details | Notes |
|---|---|---|---|
| 1 | Parameter calibration | Check voltage/current range, accuracy (±0.05% F.S. for voltage and ±0.02% V/A for current) and system connection. Calibrate temp sensor (±1 °C max error) | Record and store verification data |
| 2 | Set ambient temp: 25/45 °C (811/712) | Maintain temp within thermostat for 30 min, keep fluctuation ≤ ±0.5 °C | / |
| 3 | Charge to 100% SOC under 1C or 1/3C (811/712) | Apply CC-CV charge: cutoff voltage at 4.25 V, stop at 0.05C current | Record and store charge curve with timestamp |
| 4 | Discharge 0% SOC under 1C or 1/3C (811/712) | CC discharge to cutoff voltage | Record and store V and T every 5 min |
| 5 | Set temp to thermal equilibrium | Set chamber temp to 25 °C/45 °C, hold 30 min after reaching T0 to ensure battery T_core = T_ambient | Equilibrium condition: ΔT_batt ≤ ±0.3 °C/10 min |
| 6 | Initiate 30 min idle period | Rest battery (no load) | Record OCV every 10 min |
| 7 | Charge to 100% SOC under 1C or 1/3C (811/712) | Same as Step 3, record full capacity after charge | Compute fade rate vs. initial capacity |
| 8 | Discharge 0% SOC under 1C or 1/3C (811/712) | Same as Step 4, record discharged capacity after discharge | Compute fade rate vs. initial capacity |
| 9 | Repeat steps 7–8/each 100 cycles | Every 100 cycles, record total capacity fade (%) | Stop test if swelling/leakage occurs |
| 10 | Repeat steps 2–9 | If capacity < 80% stop test and change cell | Recalibrate parameters |
| Cathode | Manufacturer | Voltage (V) | Fading Rate (%/Cycle) | Temperature (°C)/Current Rate (C) | Calculated Duration (Cycle) | Ref. |
|---|---|---|---|---|---|---|
| NMC111 | Self-synthesized | 4.4 | 0.0204 | 25/1 | 1–300 | [25] |
| NMC532 | LiFun Technology (Zhuzhou, China) | 4.4 | 0.00522 | 40/0.33 | 1–1667 | [43] |
| NMC622 | Self-synthesized | 4.2 | 0.0175 | 25/1 | 1–1000 | [39] |
| NMC712 | Farasis Energy (Ganzhou, China) | 4.25 | 0.0191 | 45/0.33 | 1–600 | This study |
| NMC811 | Farasis Energy (Ganzhou, China) | 4.25 | 0.0234 | 25/1 | 6–1080 | This study |
| NMC89 | Self-synthesized | 4.2 | 0.0362 | 25/1 | 1–1000 | [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Tan, S.; Sun, C.; Zhang, K.; Deng, B.; Liao, C. Effects of Ni Content on Energy Density, Capacity Fade and Heat Generation in Li[NixMnyCoz]O2/Graphite Lithium-Ion Batteries. Micromachines 2025, 16, 1075. https://doi.org/10.3390/mi16101075
Zhang G, Tan S, Sun C, Zhang K, Deng B, Liao C. Effects of Ni Content on Energy Density, Capacity Fade and Heat Generation in Li[NixMnyCoz]O2/Graphite Lithium-Ion Batteries. Micromachines. 2025; 16(10):1075. https://doi.org/10.3390/mi16101075
Chicago/Turabian StyleZhang, Gaoyong, Shuhuang Tan, Chengqi Sun, Kun Zhang, Banglin Deng, and Cheng Liao. 2025. "Effects of Ni Content on Energy Density, Capacity Fade and Heat Generation in Li[NixMnyCoz]O2/Graphite Lithium-Ion Batteries" Micromachines 16, no. 10: 1075. https://doi.org/10.3390/mi16101075
APA StyleZhang, G., Tan, S., Sun, C., Zhang, K., Deng, B., & Liao, C. (2025). Effects of Ni Content on Energy Density, Capacity Fade and Heat Generation in Li[NixMnyCoz]O2/Graphite Lithium-Ion Batteries. Micromachines, 16(10), 1075. https://doi.org/10.3390/mi16101075




