Recent Advances in Photoacoustic Imaging: Current Status and Future Perspectives
Abstract
1. Introduction
2. Principles of Photoacoustic Imaging
2.1. Basics of Photoacoustic Effect
2.2. Imaging Instrumentation
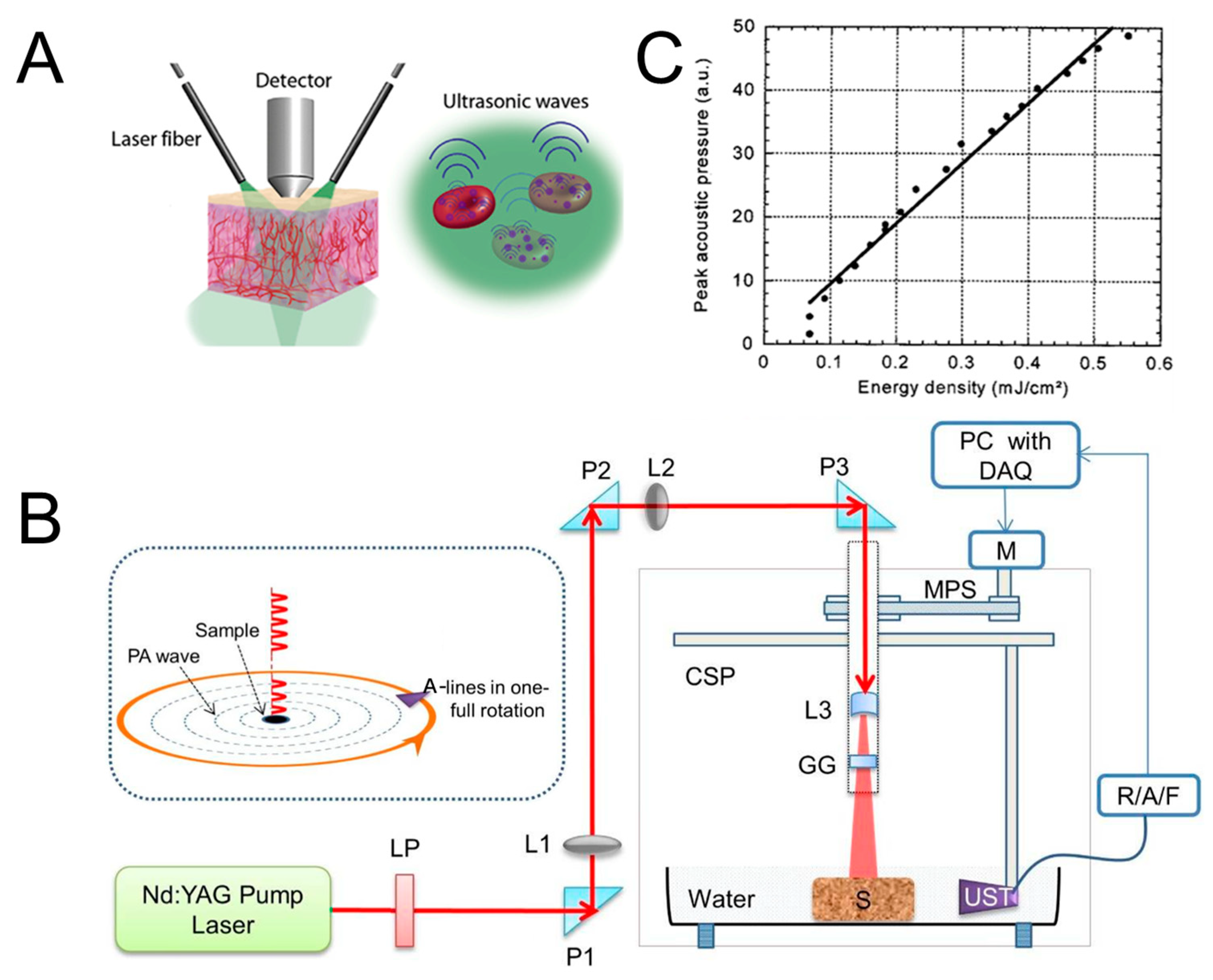
2.2.1. Light Excitation Source
2.2.2. Ultrasound Transducer
2.3. Imaging Performance
2.3.1. Imaging Algorithms
2.3.2. Contrast Agents
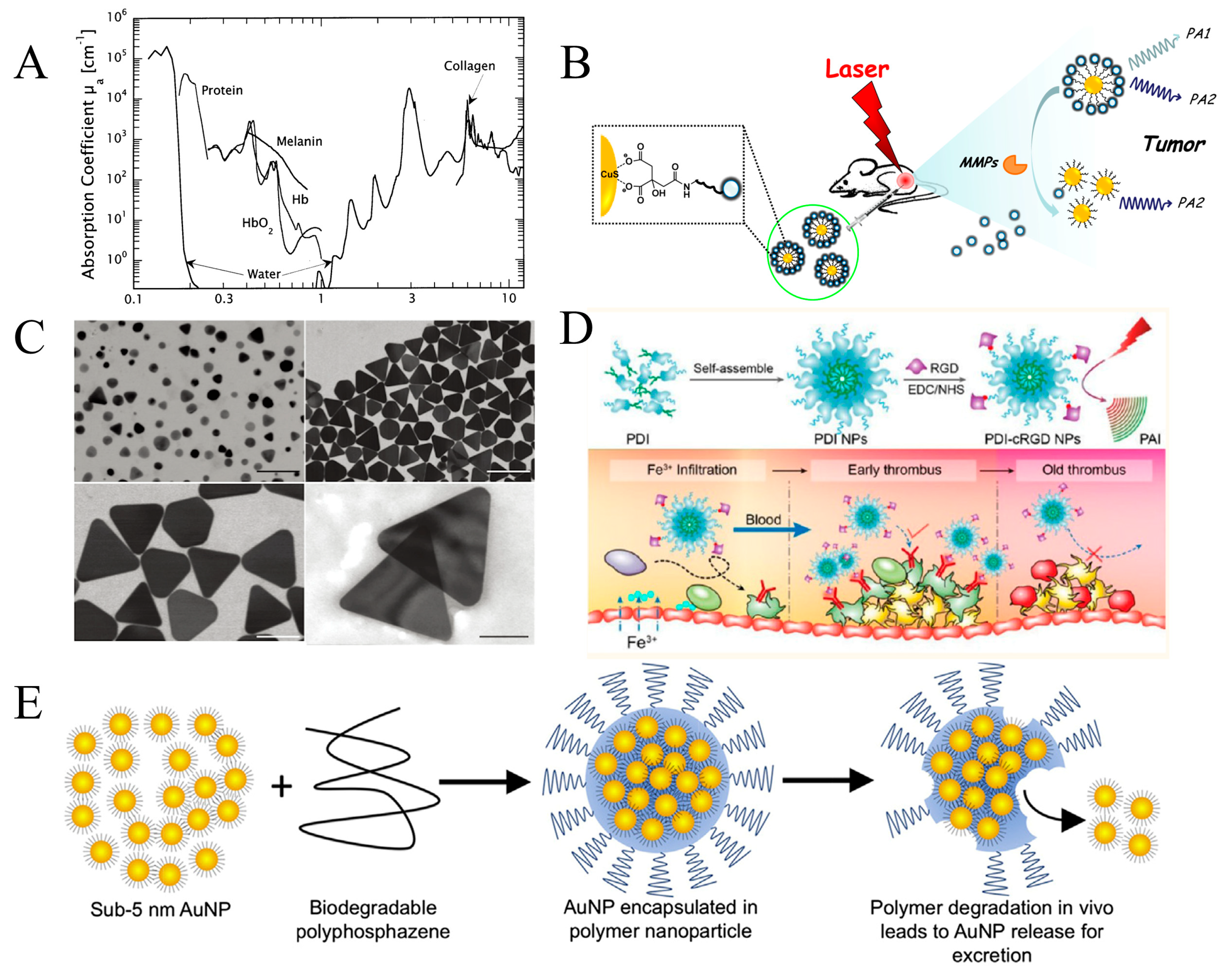
2.3.3. Imaging Resolution
3. Application
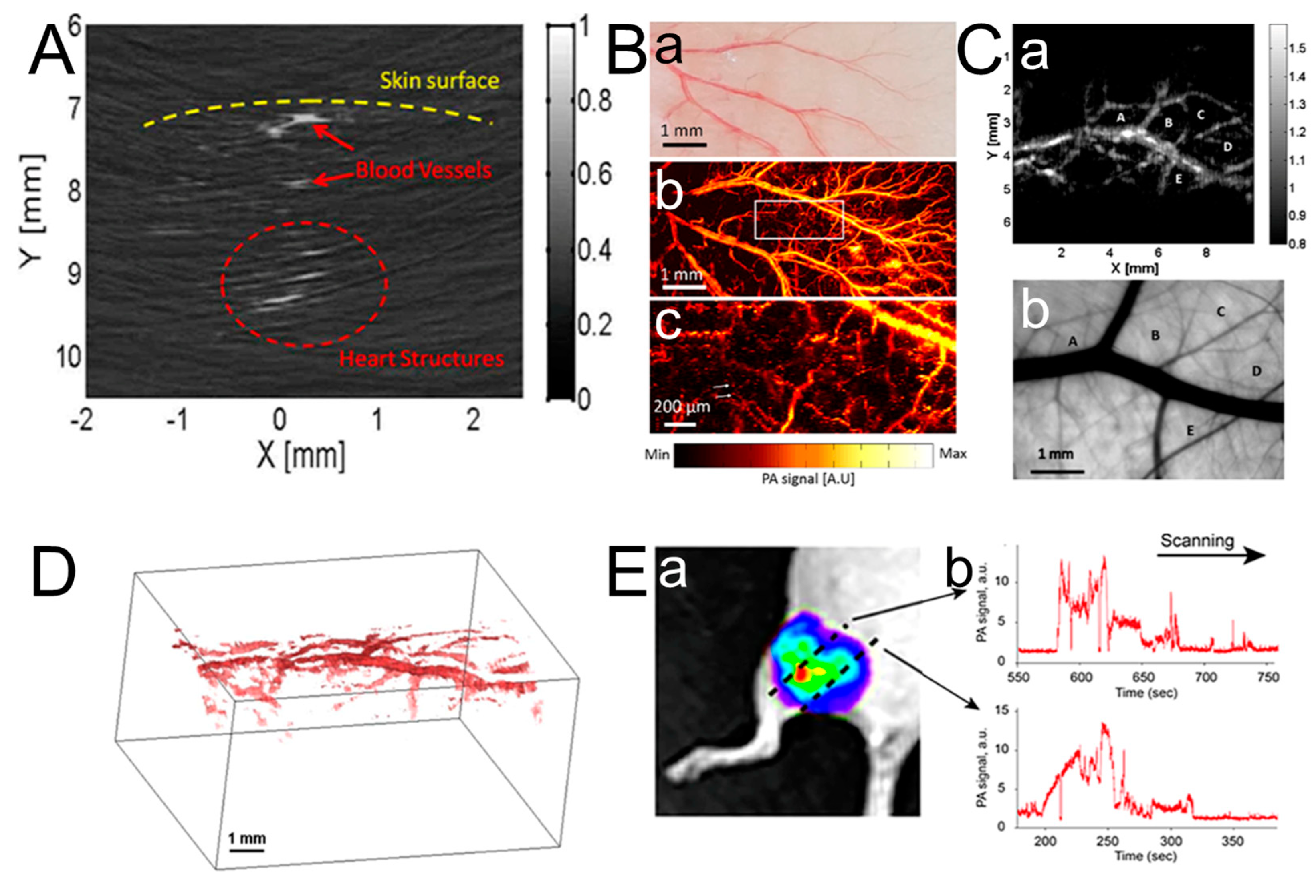
3.1. Dermatologic Imaging and Joint Imaging

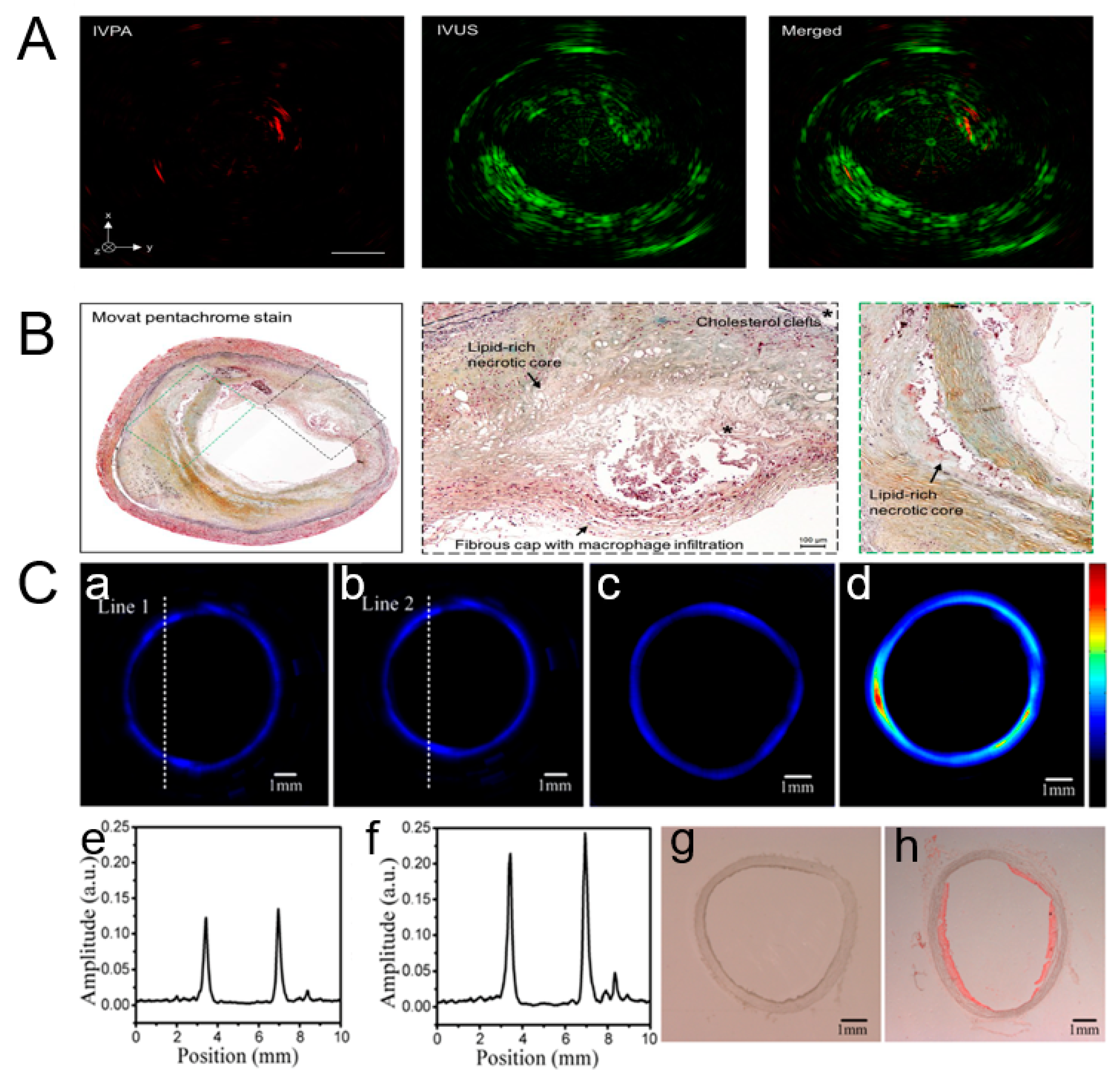
3.2. Breast Tumor Imaging and Vascular Disease Detection
3.3. Cervical Cancer

3.4. Animal Organ Imaging
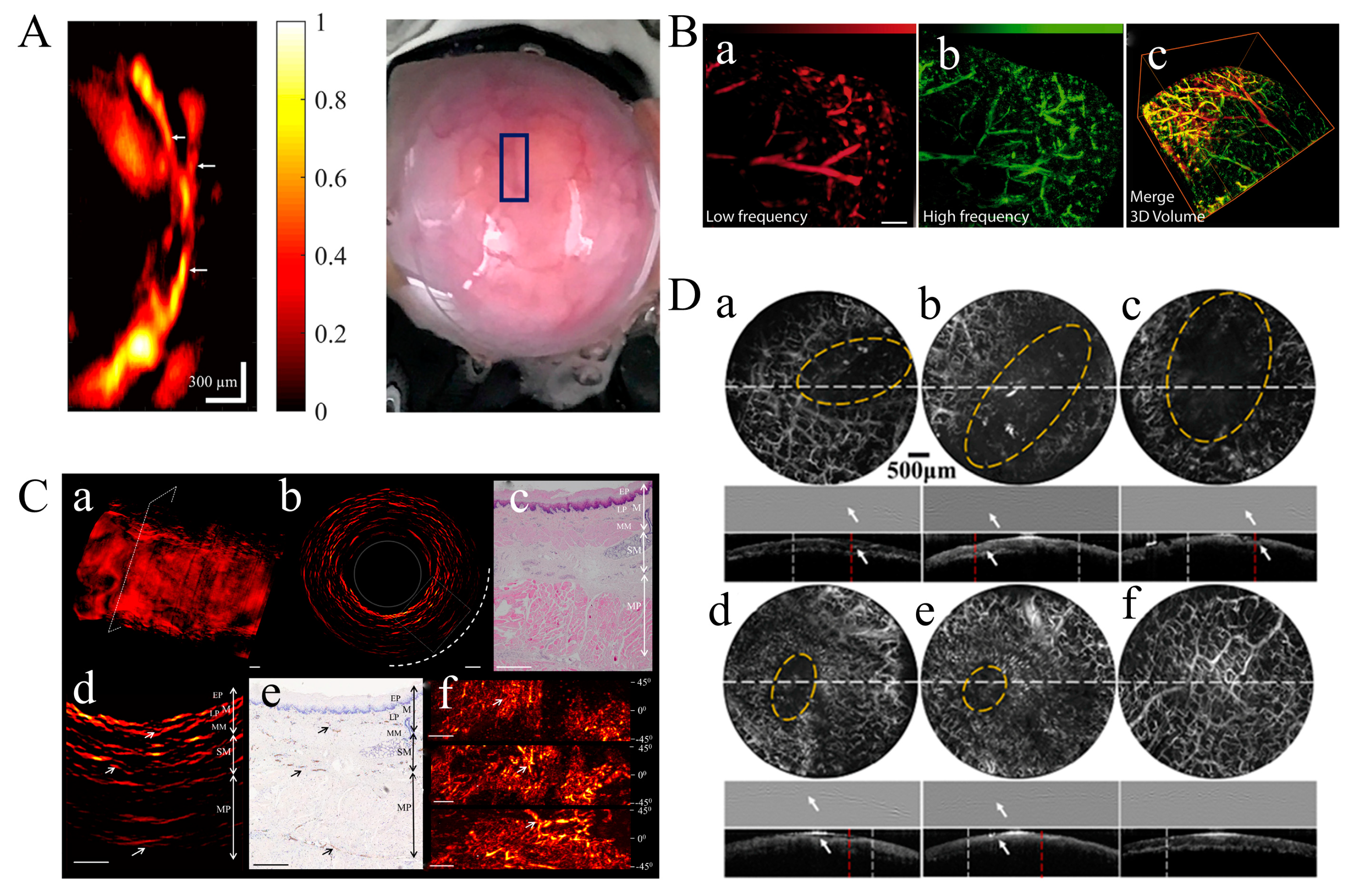
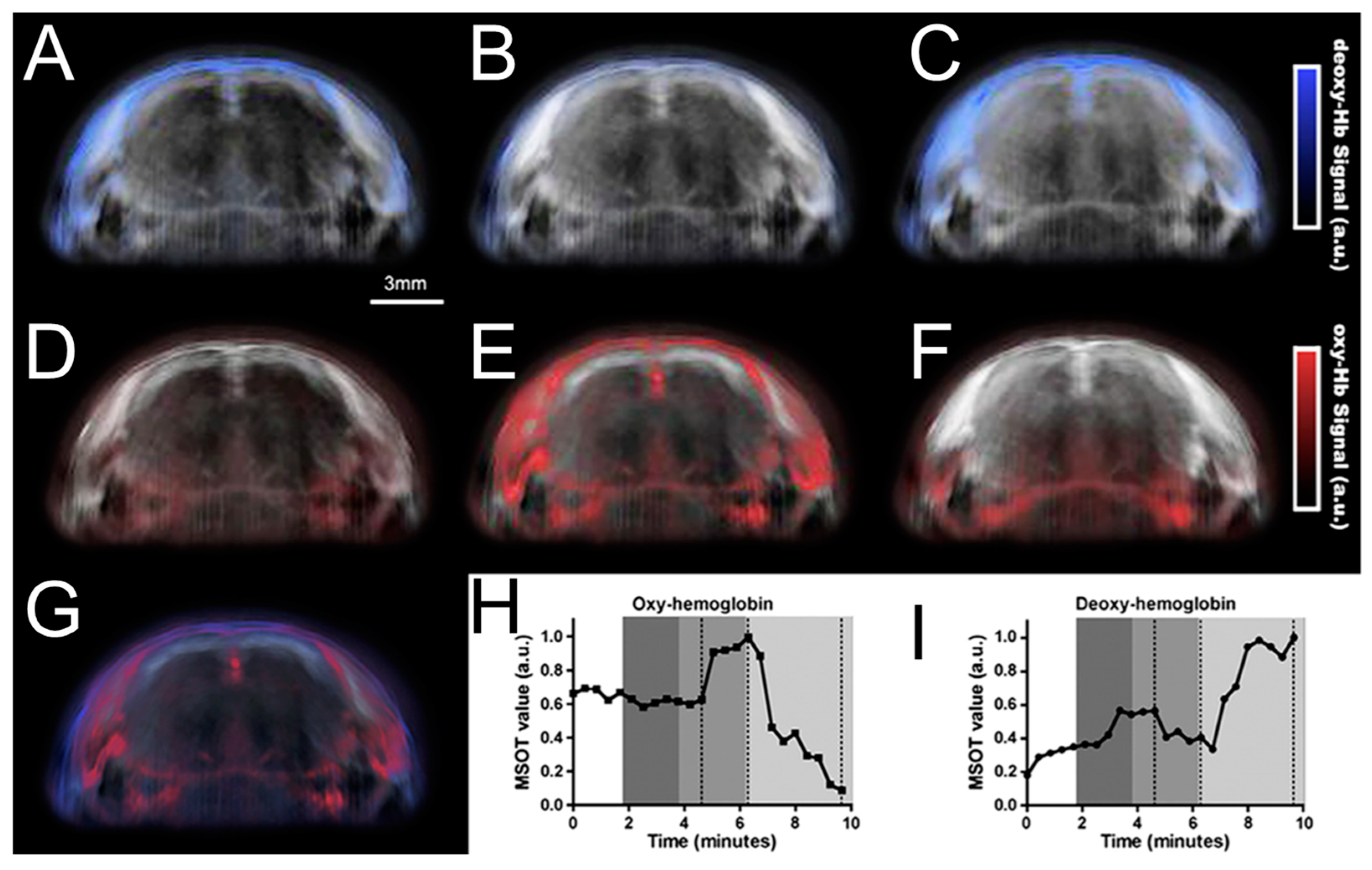
3.5. Brain Imaging
3.6. Hematologic Imaging
4. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, M.; Wang, L.V. Photoacoustic imaging in biomedicine. Rev. Sci. Instrum. 2006, 77, 041101. [Google Scholar] [CrossRef]
- Kreuzer, L.B. Ultralow Gas Concentration Infrared Absorption Spectroscopy. J. Appl. Phys. 1971, 42, 2934–2943. [Google Scholar] [CrossRef]
- Li, C.; Wang, L.V. Photoacoustic tomography and sensing in biomedicine. Phys. Med. Biol. 2009, 54, R59–R97. [Google Scholar] [CrossRef] [PubMed]
- Zackrisson, S.; van de Ven, S.M.W.Y.; Gambhir, S.S. Light In and Sound Out: Emerging Translational Strategies for Photoacoustic Imaging. Cancer Res. 2014, 74, 979–1004. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pang, Y.; Ku, G.; Xie, X.; Stoica, G.; Wang, L.V. Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain. Nat. Biotechnol. 2003, 21, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Beard, P. Biomedical photoacoustic imaging. Interface Focus 2011, 1, 602–631. [Google Scholar] [CrossRef]
- Yao, J.; Wang, L.V. Photoacoustic tomography: Fundamentals, advances and prospects. Contrast Media Mol. Imaging 2011, 6, 332–345. [Google Scholar] [CrossRef]
- Oraevsky, A.A.; Karabutov, A.A. Optoacoustic tomography. In Biomedical Photonics: Handbook; CRC Press: Boca Raton, FL, USA, 2003; pp. 31–34. [Google Scholar]
- Ntziachristos, V. Going deeper than microscopy: The optical imaging frontier in biology. Nat. Methods 2010, 7, 603–614. [Google Scholar] [CrossRef]
- Knieling, F.; Menezes, J.G.; Claussen, J.; Schwarz, M.; Neufert, C.; Fahlbusch, F.B.; Rath, T.; Thoma, O.-M.; Kramer, V.; Menchicchi, B. Raster-scanning optoacoustic mesoscopy for gastrointestinal imaging at high resolution. Gastroenterology 2018, 154, 807–809.e803. [Google Scholar] [CrossRef]
- Upputuri, P.K.; Pramanik, M. Performance characterization of low-cost, high-speed, portable pulsed laser diode photoacoustic tomography (PLD-PAT) system. Biomed. Opt. Express 2015, 6, 4118–4129. [Google Scholar] [CrossRef]
- Wang, L.V.; Zhao, X.; Sun, H.; Ku, G. Microwave-induced acoustic imaging of biological tissues. Rev. Sci. Instrum. 1999, 70, 3744–3748. [Google Scholar] [CrossRef]
- Deán-Ben, X.L.; Razansky, D. On the link between the speckle free nature of optoacoustics and visibility of structures in limited-view tomography. Photoacoustics 2016, 4, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Hai, P.; Yao, J.; Maslov, K.I.; Zhou, Y.; Wang, L.V. Near-infrared optical-resolution photoacoustic microscopy. Opt. Lett. 2014, 39, 5192–5195. [Google Scholar] [CrossRef] [PubMed]
- Sivasubramanian, K.; Pramanik, M. High frame rate photoacoustic imaging at 7000 frames per second using clinical ultrasound system. Biomed. Opt. Express 2016, 7, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Gujrati, V.; Mishra, A.; Ntziachristos, V. Molecular imaging probes for multi-spectral optoacoustic tomography. Chem. Commun. 2017, 53, 4653–4672. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.J.; Beard, P.C. High power visible light emitting diodes as pulsed excitation sources for biomedical photoacoustics. Biomed. Opt. Express 2016, 7, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shu, H.; Lu, H.; Xu, M.; Ji, X. Multispectral Depolarization Based Living-Skin Detection: A New Measurement Principle. IEEE Trans. Biomed. Eng. 2024, 71, 1937–1949. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-H.; He, P.-T.; Chen, L.; Wei, Y.; Xie, L.-P. A Measuring Method of Polarization-Maintaining Fiber Parameters based on Modulated Spectrum. Study Opt. Commun. 2021, 47, 37. [Google Scholar]
- Wang, J.; Wang, L.; Su, X.; Xiao, R.; Cheng, H. Polarization Maintaining Fiber Temperature and Stress Gradient Sensitization Sensor Based on Semiconductor-Metal–Polymer Three-Layer Film Coating. ACS Appl. Mater. Interfaces 2022, 14, 20053–20061. [Google Scholar] [CrossRef]
- Tang, P.; Wang, R.K. Polarization sensitive optical coherence tomography for imaging microvascular information within living tissue without polarization-induced artifacts. Biomed. Opt. Express 2020, 11, 6379–6388. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, X.; Li, S.; Yu, G.; Shi, J.; Gui, L.; Ren, B.; Zuo, C.; Yu, B. Highly sensitive strain sensing using a polarization-maintaining photonic crystal fibre based Mach–Zehnder interferometer. J. Mod. Opt. 2022, 69, 192–199. [Google Scholar] [CrossRef]
- Chen, B.; Chu, F.; Liu, X.; Li, Y.; Rong, J.; Jiang, H. AlN-based piezoelectric micromachined ultrasonic transducer for photoacoustic imaging. Appl. Phys. Lett. 2013, 103, 031118. [Google Scholar] [CrossRef]
- Lutzweiler, C.; Razansky, D. Optoacoustic Imaging and Tomography: Reconstruction Approaches and Outstanding Challenges in Image Performance and Quantification. Sensors 2013, 13, 7345–7384. [Google Scholar] [CrossRef] [PubMed]
- Wissmeyer, G.; Pleitez, M.A.; Rosenthal, A.; Ntziachristos, V. Looking at sound: Optoacoustics with all-optical ultrasound detection. Light Sci. Appl. 2018, 7, 53. [Google Scholar] [CrossRef]
- Vaithilingam, S.; Ma, T.J.; Furukawa, Y.; Oralkan, O.; Kamaya, A.; Torashima, K.; Kupnik, M.; Wygant, I.O.; Zhuang, X.; Brooke Jeffrey, R.J.; et al. Investigating large 2D arrays for photoacoustic and acoustic imaging using CMUT technology. In Proceedings of the 2008 IEEE Ultrasonics Symposium, Beijing, China, 2–5 November 2008; pp. 1238–1241. [Google Scholar]
- Nikoozadeh, A.; Choe, J.W.; Kothapalli, S.; Moini, A.; Sanjani, S.S.; Kamaya, A.; Oralkan, Ö.; Gambhir, S.S.; Khuri-Yakub, P.T. Photoacoustic imaging using a 9F microLinear CMUT ICE catheter. In Proceedings of the 2012 IEEE International Ultrasonics Symposium, Dresden, Germany, 7–10 October 2012; pp. 24–27. [Google Scholar]
- Cheng, X.; Chen, J.; Li, C. A Miniature Capacitive Micromachined Ultrasonic Transducer Array for Minimally Invasive Photoacoustic Imaging. J. Microelectromechan. Syst. 2010, 19, 1002–1011. [Google Scholar] [CrossRef]
- Vallet, M.; Varray, F.; Kalkhoran, M.A.; Vray, D.; Boutet, J. Enhancement of photoacoustic imaging quality by using CMUT technology: Experimental study. In Proceedings of the 2014 IEEE International Ultrasonics Symposium, Chicago, IL, USA, 3–6 September 2014; pp. 1296–1299. [Google Scholar]
- Legros, M.; Meynier, C.; Dufait, R.; Ferin, G.; Tranquart, F. Piezocomposite and CMUT arrays assessment through in vitro imaging performances. In Proceedings of the 2008 IEEE Ultrasonics Symposium, Beijing, China, 2–5 November 2008; pp. 1142–1145. [Google Scholar]
- Johannes, R.; Omri, W.; Cyril, M.; Daniel, R. Optoacoustic characterization of broadband directivity patterns of capacitive micromachined ultrasonic transducers. J. Biomed. Opt. 2016, 22, 041005. [Google Scholar] [CrossRef]
- Warshavski, O.; Meynier, C.; Sénégond, N.; Chatain, P.; Johannes, R.; Johannes, R.; Daniel, R.; Daniel, R.; Felix, N.; Nguyen-Dinh, A. Experimental evaluation of cMUT and PZT transducers in receive only mode for photoacoustic imaging. In Photons Plus Ultrasound: Imaging and Sensing 2016; SPIE: San Francisco, CA, USA, 2016. [Google Scholar]
- Li, Y.; Lu, G.; Chen, J.J.; Jing, J.C.; Huo, T.; Chen, R.; Jiang, L.; Zhou, Q.; Chen, Z. PMN-PT/Epoxy 1-3 composite based ultrasonic transducer for dual-modality photoacoustic and ultrasound endoscopy. Photoacoustics 2019, 15, 100138. [Google Scholar] [CrossRef]
- Zafar, M.; Manwar, R.; Avanaki, K. Miniaturized preamplifier integration in ultrasound transducer design for enhanced photoacoustic imaging. Opt. Lett. 2024, 49, 3054–3057. [Google Scholar] [CrossRef]
- Keller, K.; Leitner, C.; Baumgartner, C.; Benini, L.; Greco, F. Fully Printed Flexible Ultrasound Transducer for Medical Applications. Adv. Mater. Technol. 2023, 8, 2300577. [Google Scholar] [CrossRef]
- Bian, J.; Wang, Y.; Liu, Z.; Shen, M.; Zhao, H.; Sun, Y.; Zhu, J. Ultra-wideband underwater acoustic transducer with a gradient impedance matching layer. Appl. Acoust. 2021, 175, 107789. [Google Scholar] [CrossRef]
- Zafar, M.; Manwar, R.; McGuire, L.S.; Charbel, F.T.; Avanaki, K. Ultra-widefield and high-speed spiral laser scanning OR-PAM: System development and characterization. J. Biophotonics 2023, 16, e202200383. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Roh, Y. Ultrasonic transducers for medical diagnostic imaging. Biomed. Eng. Lett. 2017, 7, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Odabaee, M.; Odabaee, M.; Pelekanos, M.; Leinenga, G.; Götz, J. Modeling ultrasound propagation through material of increasing geometrical complexity. Ultrasonics 2018, 90, 52–62. [Google Scholar] [CrossRef]
- Hübschen, G. 7—Ultrasonic techniques for materials characterization. In Materials Characterization Using Nondestructive Evaluation (NDE) Methods; Hübschen, G., Altpeter, I., Tschuncky, R., Herrmann, H.-G., Eds.; Woodhead Publishing: Sawston, UK, 2016; pp. 177–224. [Google Scholar]
- Alexander, D.; Neal, C.B.; Vasilis, N. Multispectral optoacoustic tomography at 64, 128, and 256 channels. J. Biomed. Opt. 2014, 19, 036021. [Google Scholar] [CrossRef]
- Razansky, D. Multispectral Optoacoustic Tomography—Volumetric Color Hearing in Real Time. IEEE J. Sel. Top. Quantum Electron. 2012, 18, 1234–1243. [Google Scholar] [CrossRef]
- Roger, J.Z.; Rachel, B.; Shung, K.K.; Meng-Lin, L.; George Stoica, D.V.M.; Lihong, V.W. Photoacoustic imaging of the microvasculature with a high-frequency ultrasound array transducer. J. Biomed. Opt. 2007, 12, 010501. [Google Scholar] [CrossRef]
- Wang, H.; Xing, D.; Xiang, L. Photoacoustic imaging using an ultrasonic Fresnel zone plate transducer. J. Phys. D Appl. Phys. 2008, 41, 095111. [Google Scholar] [CrossRef]
- Xu, M.; Wang, L.V. Universal back-projection algorithm for photoacoustic computed tomography. Phys. Rev. E 2005, 71, 016706. [Google Scholar] [CrossRef]
- Yang, D.; Xing, D.; Gu, H.; Tan, Y.; Zeng, L. Fast multielement phase-controlled photoacoustic imaging based on limited-field-filtered back-projection algorithm. Appl. Phys. Lett. 2005, 87, 194101. [Google Scholar] [CrossRef]
- Rosenthal, A.; Razansky, D.; Ntziachristos, V. Fast Semi-Analytical Model-Based Acoustic Inversion for Quantitative Optoacoustic Tomography. IEEE Trans. Med. Imaging 2010, 29, 1275–1285. [Google Scholar] [CrossRef]
- Su, B.; Wen, Y.; Liu, Y.; Liao, S.; Fu, J.; Quan, G.; Li, Z. A deep learning method for eliminating head motion artifacts in computed tomography. Med. Phys. 2022, 49, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Koetzier, L.R.; Mastrodicasa, D.; Szczykutowicz, T.P.; van der Werf, N.R.; Wang, A.S.; Sandfort, V.; van der Molen, A.J.; Fleischmann, D.; Willemink, M.J. Deep Learning Image Reconstruction for CT: Technical Principles and Clinical Prospects. Radiology 2023, 306, e221257. [Google Scholar] [CrossRef] [PubMed]
- Donoho, D.L. Compressed sensing. IEEE Trans. Inf. Theory 2006, 52, 1289–1306. [Google Scholar] [CrossRef]
- Candes, E.J.; Romberg, J.; Tao, T. Robust uncertainty principles: Exact signal reconstruction from highly incomplete frequency information. IEEE Trans. Inf. Theory 2006, 52, 489–509. [Google Scholar] [CrossRef]
- Xia, J.; Wang, L.V. Small-Animal Whole-Body Photoacoustic Tomography: A Review. IEEE Trans. Biomed. Eng. 2014, 61, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, L.V. Time Reversal and Its Application to Tomography with Diffracting Sources. Phys. Rev. Lett. 2004, 92, 033902. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.; Liming, N.; Robert, W.S.; Lihong, V.W.; Mark, A.A. Photoacoustic computed tomography correcting for heterogeneity and attenuation. J. Biomed. Opt. 2012, 17, 061211. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Zhang, H.; Meng, D.; Zheng, Y. InDuDoNet+: A deep unfolding dual domain network for metal artifact reduction in CT images. Med. Image Anal. 2023, 85, 102729. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Li, F. IDPCNN: Iterative denoising and projecting CNN for MRI reconstruction. J. Comput. Appl. Math. 2022, 406, 113973. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, D.; Hao, S.; Liu, J.; Quan, G.; Zhang, Y.; Ji, X.; Chen, Y. DREAM-Net: Deep Residual Error Iterative Minimization Network for Sparse-View CT Reconstruction. IEEE J. Biomed. Health Inform. 2023, 27, 480–491. [Google Scholar] [CrossRef]
- Hu, D.; Zhang, Y.; Liu, J.; Luo, S.; Chen, Y. DIOR: Deep Iterative Optimization-Based Residual-Learning for Limited-Angle CT Reconstruction. IEEE Trans. Med. Imaging 2022, 41, 1778–1790. [Google Scholar] [CrossRef] [PubMed]
- Rejesh, N.A.; Pullagurla, H.; Pramanik, M. Deconvolution-based deblurring of reconstructed images in photoacoustic/thermoacoustic tomography. J. Opt. Soc. Am. A 2013, 30, 1994–2001. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tang, Y.; Yao, J. Photoacoustic tomography of blood oxygenation: A mini review. Photoacoustics 2018, 10, 65–73. [Google Scholar] [CrossRef]
- Zhang, H.F.; Maslov, K.; Sivaramakrishnan, M.; Stoica, G.; Wang, L.V. Imaging of hemoglobin oxygen saturation variations in single vessels in vivo using photoacoustic microscopy. Appl. Phys. Lett. 2007, 90, 053901. [Google Scholar] [CrossRef]
- Jung-Taek, O.; Meng-Lin, L.; Hao, F.Z.; Konstantin, M.; Lihong, V.W. Three-dimensional imaging of skin melanoma in vivo by dual-wavelength photoacoustic microscopy. J. Biomed. Opt. 2006, 11, 034032. [Google Scholar] [CrossRef]
- Stoffels, I.; Morscher, S.; Helfrich, I.; Hillen, U.; Leyh, J.; Burton Neal, C.; Sardella Thomas, C.P.; Claussen, J.; Poeppel Thorsten, D.; Bachmann Hagen, S.; et al. Metastatic status of sentinel lymph nodes in melanoma determined noninvasively with multispectral optoacoustic imaging. Sci. Transl. Med. 2015, 7, 317ra199. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Su, J.L.; Amirian, J.; Litovsky, S.H.; Smalling, R.; Emelianov, S. Detection of lipid in atherosclerotic vessels using ultrasound-guided spectroscopic intravascular photoacoustic imaging. Opt. Express 2010, 18, 4889–4897. [Google Scholar] [CrossRef] [PubMed]
- Sergey, A.E.; Tuenchit, K.; André, C.; Morton, H.L.; Ron, L.; Ketan, M.; Tom, M.; Alexander, A.O. Laser optoacoustic imaging system for detection of breast cancer. J. Biomed. Opt. 2009, 14, 024007. [Google Scholar] [CrossRef]
- Kumon, R.E.; Deng, C.X.; Wang, X. Frequency-Domain Analysis of Photoacoustic Imaging Data From Prostate Adenocarcinoma Tumors in a Murine Model. Ultrasound Med. Biol. 2011, 37, 834–839. [Google Scholar] [CrossRef]
- Agarwal, A.; Huang, S.W.; O’Donnell, M.; Day, K.C.; Day, M.; Kotov, N.; Ashkenazi, S. Targeted gold nanorod contrast agent for prostate cancer detection by photoacoustic imaging. J. Appl. Phys. 2007, 102, 064701. [Google Scholar] [CrossRef]
- Weber, J.; Beard, P.C.; Bohndiek, S.E. Contrast agents for molecular photoacoustic imaging. Nat. Methods 2016, 13, 639–650. [Google Scholar] [CrossRef]
- Sowers, T.; Emelianov, S. Exogenous imaging contrast and therapeutic agents for intravascular photoacoustic imaging and image-guided therapy. Phys. Med. Biol. 2018, 63, 22TR01. [Google Scholar] [CrossRef]
- Fu, Q.; Zhu, R.; Song, J.; Yang, H.; Chen, X. Photoacoustic Imaging: Contrast Agents and Their Biomedical Applications. Adv. Mater. 2019, 31, 1805875. [Google Scholar] [CrossRef]
- Ntziachristos, V.; Razansky, D. Molecular Imaging by Means of Multispectral Optoacoustic Tomography (MSOT). Chem. Rev. 2010, 110, 2783–2794. [Google Scholar] [CrossRef] [PubMed]
- Homan, K.; Mallidi, S.; Cooley, E.; Emelianov, S. Combined photoacoustic and ultrasound imaging of metal nanoparticles in vivo. In Nanoimaging; CRC Press: Boca Raton, FL, USA, 2010; Volume 3. [Google Scholar]
- Oraevsky, A.A. Gold and silver nanoparticles as contrast agents for optoacoustic tomography. In Photoacoustic Imaging and Spectroscopy; CRC Press: Boca Raton, FL, USA, 2017; pp. 373–386. [Google Scholar]
- Amy, M.W.; Konstantin, I.M.; Lihong, V.W. Noise-equivalent sensitivity of photoacoustics. J. Biomed. Opt. 2013, 18, 097003. [Google Scholar] [CrossRef]
- Kim, C.; Song, K.H.; Gao, F.; Wang, L.V. Sentinel Lymph Nodes and Lymphatic Vessels: Noninvasive Dual-Modality In Vivo Mapping by Using Indocyanine Green in Rats—Volumetric Spectroscopic Photoacoustic Imaging and Planar Fluorescence Imaging. Radiology 2010, 255, 442–450. [Google Scholar] [CrossRef]
- Wang, X.; Ku, G.; Wegiel, M.A.; Bornhop, D.J.; Stoica, G.; Wang, L.V. Noninvasive photoacoustic angiography of animal brains in vivo with near-infrared light and an optical contrast agent. Opt. Lett. 2004, 29, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Kwang Hyun, S.; Erich, W.S.; Julie, A.M.; Lihong, V.W. Noninvasive photoacoustic identification of sentinel lymph nodes containing methylene blue in vivo in a rat model. J. Biomed. Opt. 2008, 13, 054033. [Google Scholar] [CrossRef]
- Li, M.; Oh, J.; Xie, X.; Ku, G.; Wang, W.; Li, C.; Lungu, G.; Stoica, G.; Wang, L.V. Simultaneous Molecular and Hypoxia Imaging of Brain Tumors In Vivo Using Spectroscopic Photoacoustic Tomography. Proc. IEEE 2008, 96, 481–489. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, L.; Nie, L.; Sun, X.; Cheng, L.; Wu, C.; Niu, G.; Chen, X.; Liu, Z. Visualization of protease activity in vivo using an activatable photo-acoustic imaging probe based on CuS nanoparticles. Theranostics 2014, 4, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.V.; Hu, S. Photoacoustic Tomography: In Vivo Imaging from Organelles to Organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef]
- Kim, J.-W.; Galanzha, E.I.; Shashkov, E.V.; Moon, H.-M.; Zharov, V.P. Golden carbon nanotubes as multimodal photoacoustic and photothermal high-contrast molecular agents. Nat. Nanotechnol. 2009, 4, 688–694. [Google Scholar] [CrossRef]
- Homan, K.A.; Souza, M.; Truby, R.; Luke, G.P.; Green, C.; Vreeland, E.; Emelianov, S. Silver Nanoplate Contrast Agents for In Vivo Molecular Photoacoustic Imaging. ACS Nano 2012, 6, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Yang, Z.; Hu, X.; Wu, J.; Shou, K.; Ma, H.; Jian, C.; Zhao, Y.; Qi, B.; Hu, X.; et al. Organic Semiconducting Nanoparticles as Efficient Photoacoustic Agents for Lightening Early Thrombus and Monitoring Thrombolysis in Living Mice. ACS Nano 2017, 11, 3298–3310. [Google Scholar] [CrossRef]
- Cheheltani, R.; Ezzibdeh, R.M.; Chhour, P.; Pulaparthi, K.; Kim, J.; Jurcova, M.; Hsu, J.C.; Blundell, C.; Litt, H.I.; Ferrari, V.A.; et al. Tunable, biodegradable gold nanoparticles as contrast agents for computed tomography and photoacoustic imaging. Biomaterials 2016, 102, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.J.; Mallidi, S.; Tam, J.M.; Tam, J.O.; Murthy, A.; Johnston, K.P.; Sokolov, K.V.; Emelianov, S.Y. Utility of biodegradable plasmonic nanoclusters in photoacoustic imaging. Opt. Lett. 2010, 35, 3751–3753. [Google Scholar] [CrossRef]
- Huang, P.; Lin, J.; Li, W.; Rong, P.; Wang, Z.; Wang, S.; Wang, X.; Sun, X.; Aronova, M.; Niu, G. Biodegradable gold nanovesicles with an ultrastrong plasmonic coupling effect for photoacoustic imaging and photothermal therapy. Angew. Chem. 2013, 125, 14208–14214. [Google Scholar] [CrossRef]
- Tam, J.M.; Tam, J.O.; Murthy, A.; Ingram, D.R.; Ma, L.L.; Travis, K.; Johnston, K.P.; Sokolov, K.V. Controlled Assembly of Biodegradable Plasmonic Nanoclusters for Near-Infrared Imaging and Therapeutic Applications. ACS Nano 2010, 4, 2178–2184. [Google Scholar] [CrossRef]
- Huang, X.; Tang, S.; Mu, X.; Dai, Y.; Chen, G.; Zhou, Z.; Ruan, F.; Yang, Z.; Zheng, N. Freestanding palladium nanosheets with plasmonic and catalytic properties. Nat. Nanotechnol. 2011, 6, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Vu, L.; Ramos, J.; Potta, T.; Rege, K. Generation of a focused poly (amino ether) library: Polymer-mediated transgene delivery and gold-nanorod based theranostic systems. Theranostics 2012, 2, 1160–1173. [Google Scholar] [CrossRef]
- Nie, L.; Chen, M.; Sun, X.; Rong, P.; Zheng, N.; Chen, X. Palladium nanosheets as highly stable and effective contrast agents for in vivo photoacoustic molecular imaging. Nanoscale 2014, 6, 1271–1276. [Google Scholar] [CrossRef]
- Vogel, A.; Venugopalan, V. Mechanisms of Pulsed Laser Ablation of Biological Tissues. Chem. Rev. 2003, 103, 577–644. [Google Scholar] [CrossRef]
- Telenkov, S.A.; Mandelis, A. Fourier-domain methodology for depth-selective photothermoacoustic imaging of tissue chromophores. Eur. Phys. J. Spec. Top. 2008, 153, 443–448. [Google Scholar] [CrossRef]
- Upputuri, P.K.; Pramanik, M. Pulsed laser diode based optoacoustic imaging of biological tissues. Biomed. Phys. Eng. Express 2015, 1, 045010. [Google Scholar] [CrossRef]
- Szabo, T. Diagnostic Ultrasound Imaging—Inside Out; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Valluru, K.S.; Chinni, B.K.; Rao, N.A.; Bhatt, S.; Dogra, V.S. Basics and clinical applications of photoacoustic imaging. Ultrasound Clin. 2009, 4, 403–429. [Google Scholar] [CrossRef]
- Deán-Ben, X.L.; Razansky, D.; Ntziachristos, V. The effects of acoustic attenuation in optoacoustic signals. Phys. Med. Biol. 2011, 56, 6129–6148. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, M.; Soliman, D.; Omar, M.; Buehler, A.; Ovsepian, S.V.; Aguirre, J.; Ntziachristos, V. Optoacoustic Dermoscopy of the Human Skin: Tuning Excitation Energy for Optimal Detection Bandwidth With Fast and Deep Imaging in vivo. IEEE Trans. Med. Imaging 2017, 36, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.V.; Yao, J. A practical guide to photoacoustic tomography in the life sciences. Nat. Methods 2016, 13, 627–638. [Google Scholar] [CrossRef]
- Zeng, L.; Liu, G.; Yang, D.; Ji, X. Portable optical-resolution photoacoustic microscopy with a pulsed laser diode excitation. Appl. Phys. Lett. 2013, 102, 053704. [Google Scholar] [CrossRef]
- Huikeshoven, M.; Koster, P.H.L.; de Borgie, C.A.J.M.; Beek, J.F.; van Gemert, M.J.C.; van der Horst, C.M.A.M. Redarkening of Port-Wine Stains 10 Years after Pulsed-Dye–Laser Treatment. N. Engl. J. Med. 2007, 356, 1235–1240. [Google Scholar] [CrossRef]
- Gao, K.; Huang, Z.; Yuan, K.H.; Zhang, B.; Hu, Z.Q. Side-by-side comparison of photodynamic therapy and pulsed-dye laser treatment of port-wine stain birthmarks. Br. J. Dermatol. 2013, 168, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Shiyong, Z.; Ying, G.; Ping, X.; Jin, G.; Tingmei, S.; Tianshi, W.; Naiyan, H.; Li, Z.; Haixia, Q.; Yu, X.; et al. Imaging port wine stains by fiber optical coherence tomography. J. Biomed. Opt. 2010, 15, 036020. [Google Scholar] [CrossRef]
- Liu, G.; Jia, W.; Nelson, J.S.; Chen, Z. In vivo, high-resolution, three-dimensional imaging of port wine stain microvasculature in human skin. Lasers Surg. Med. 2013, 45, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Jasaitiene, D.; Valiukeviciene, S.; Linkeviciute, G.; Raisutis, R.; Jasiuniene, E.; Kazys, R. Principles of high-frequency ultrasonography for investigation of skin pathology. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, M.; Omar, M.; Buehler, A.; Aguirre, J.; Ntziachristos, V. Implications of Ultrasound Frequency in Optoacoustic Mesoscopy of the Skin. IEEE Trans. Med. Imaging 2015, 34, 672–677. [Google Scholar] [CrossRef]
- Xu, D.; Yang, S.; Wang, Y.; Gu, Y.; Xing, D. Noninvasive and high-resolving photoacoustic dermoscopy of human skin. Biomed. Opt. Express 2016, 7, 2095–2102. [Google Scholar] [CrossRef] [PubMed]
- Haroon, Z.; Aedán, B.; Hrebesh, M.S.; Martin, J.L. Linear-array-based photoacoustic imaging of human microcirculation with a range of high frequency transducer probes. J. Biomed. Opt. 2014, 20, 051021. [Google Scholar] [CrossRef]
- Erfanzadeh, M.; Kumavor, P.D.; Zhu, Q. Laser scanning laser diode photoacoustic microscopy system. Photoacoustics 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Nandy, S.; Salehi, H.S.; Kumavor, P.D.; Zhu, Q. A low-cost photoacoustic microscopy system with a laser diode excitation. Biomed. Opt. Express 2014, 5, 3053–3058. [Google Scholar] [CrossRef]
- Mohsen, E.; Hassan, S.S.; Patrick, K.; Quing, Z. Improvement and evaluation of a low-cost laser diode photoacoustic microscopy system for ovarian tissue imaging. In Proceedings of the SPIE Photonics West, Photons Plus Ultrasound: Imaging and Sensing 2016, Francisco, CA, USA, 14–17 February 2016. [Google Scholar]
- Zeng, L.; Piao, Z.; Huang, S.; Jia, W.; Chen, Z. Label-free optical-resolution photoacoustic microscopy of superficial microvasculature using a compact visible laser diode excitation. Opt. Express 2015, 23, 31026–31033. [Google Scholar] [CrossRef]
- Daoudi, K.; van den Berg, P.J.; Rabot, O.; Kohl, A.; Tisserand, S.; Brands, P.; Steenbergen, W. Handheld probe integrating laser diode and ultrasound transducer array for ultrasound/photoacoustic dual modality imaging. Opt. Express 2014, 22, 26365–26374. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, J.; Schwarz, M.; Soliman, D.; Buehler, A.; Omar, M.; Ntziachristos, V. Broadband mesoscopic optoacoustic tomography reveals skin layers. Opt. Lett. 2014, 39, 6297–6300. [Google Scholar] [CrossRef] [PubMed]
- Jason, Z.; Don, H.; Bryan, C.; Tom, M.; Kenneth, K.; Dornbluth, N.C.; McCorvey, B.M.; Pamela, O.; Sergey, E.; Vyacheslav, N.; et al. Clinical feasibility study of combined optoacoustic and ultrasonic imaging modality providing coregistered functional and anatomical maps of breast tumors. Proc. SPIE 2013, 8581, 858103. [Google Scholar]
- Zalev, J.; Clingman, B.; Herzog, D.; Miller, T.; Ulissey, M.; Stavros, A.T.; Oraevsky, A.; Lavin, P.; Kist, K.; Dornbluth, N.C.; et al. Opto-acoustic image fusion technology for diagnostic breast imaging in a feasibility study. Proc. SPIE 2015, 9419, 941909. [Google Scholar]
- Hui, J.; Cao, Y.; Zhang, Y.; Kole, A.; Wang, P.; Yu, G.; Eakins, G.; Sturek, M.; Chen, W.; Cheng, J.-X. Real-time intravascular photoacoustic-ultrasound imaging of lipid-laden plaque in human coronary artery at 16 frames per second. Sci. Rep. 2017, 7, 1417. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Xiong, K.; Yang, S.; Xing, D. Intravascular confocal photoacoustic endoscope with dual-element ultrasonic transducer. Opt. Express 2015, 23, 9130–9136. [Google Scholar] [CrossRef]
- Peng, K.; He, L.; Wang, B.; Xiao, J. Detection of cervical cancer based on photoacoustic imaging—The in-vitro results. Biomed. Opt. Express 2015, 6, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Thekkek, N.; Richards-Kortum, R. Optical imaging for cervical cancer detection: Solutions for a continuing global problem. Nat. Rev. Cancer 2008, 8, 725–731. [Google Scholar] [CrossRef]
- Triratanachat, S.; Niruthisard, S.; Trivijitsilp, P.; Tresukosol, D.; Jarurak, N. Angiogenesis in cervical intraepithelial neoplasia and early-staged uterine cervical squamous cell carcinoma: Clinical significance. Int. J. Gynecol. Cancer 2006, 16, 575. [Google Scholar] [CrossRef]
- Tang, S.; Chen, J.; Samant, P.; Stratton, K.; Xiang, L. Transurethral Photoacoustic Endoscopy for Prostate Cancer: A Simulation Study. IEEE Trans. Med. Imaging 2016, 35, 1780–1787. [Google Scholar] [CrossRef]
- Dima, A.; Ntziachristos, V. In-vivo handheld optoacoustic tomography of the human thyroid. Photoacoustics 2016, 4, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.J.; Kwak, J.Y.; Kim, M.J.; Son, E.J.; Kim, E.-K. Can Vascularity at Power Doppler US Help Predict Thyroid Malignancy? Radiology 2010, 255, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Levi, J.; Kothapalli, S.-R.; Bohndiek, S.; Yoon, J.-K.; Dragulescu-Andrasi, A.; Nielsen, C.; Tisma, A.; Bodapati, S.; Gowrishankar, G.; Yan, X.; et al. Molecular Photoacoustic Imaging of Follicular Thyroid Carcinoma. Clin. Cancer Res. 2013, 19, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Razansky, D.; Harlaar, N.J.; Hillebrands, J.L.; Taruttis, A.; Herzog, E.; Zeebregts, C.J.; van Dam, G.M.; Ntziachristos, V. Multispectral Optoacoustic Tomography of Matrix Metalloproteinase Activity in Vulnerable Human Carotid Plaques. Mol. Imaging Biol. 2012, 14, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002, 29, 15–18. [Google Scholar] [CrossRef]
- McDonald, D.M.; Choyke, P.L. Imaging of angiogenesis: From microscope to clinic. Nat. Med. 2003, 9, 713–725. [Google Scholar] [CrossRef]
- Bohndiek, S.E.; Sasportas, L.S.; Machtaler, S.; Jokerst, J.V.; Hori, S.; Gambhir, S.S. Photoacoustic Tomography Detects Early Vessel Regression and Normalization During Ovarian Tumor Response to the Antiangiogenic Therapy Trebananib. J. Nucl. Med. 2015, 56, 1942. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Stylogiannis, A.; Afshari, P.; Wiedemann, T.; Steiger, K.; Buehler, A.; Zakian, C.; Ntziachristos, V. Capsule optoacoustic endoscopy for esophageal imaging. J. Biophotonics 2019, 12, e201800439. [Google Scholar] [CrossRef]
- He, H.; Buehler, A.; Bozhko, D.; Jian, X.; Cui, Y.; Ntziachristos, V. Importance of Ultrawide Bandwidth for Optoacoustic Esophagus Imaging. IEEE Trans. Med. Imaging 2018, 37, 1162–1167. [Google Scholar] [CrossRef]
- Qin, W.; Qi, W.; Jin, T.; Guo, H.; Xi, L. In vivo oral imaging with integrated portable photoacoustic microscopy and optical coherence tomography. Appl. Phys. Lett. 2017, 111, 263704. [Google Scholar] [CrossRef]
- Jin, T.; Guo, H.; Jiang, H.; Ke, B.; Xi, L. Portable optical resolution photoacoustic microscopy (pORPAM) for human oral imaging. Opt. Lett. 2017, 42, 4434–4437. [Google Scholar] [CrossRef] [PubMed]
- Burton, N.C.; Patel, M.; Morscher, S.; Driessen, W.H.P.; Claussen, J.; Beziere, N.; Jetzfellner, T.; Taruttis, A.; Razansky, D.; Bednar, B.; et al. Multispectral Opto-acoustic Tomography (MSOT) of the Brain and Glioblastoma Characterization. NeuroImage 2013, 65, 522–528. [Google Scholar] [CrossRef] [PubMed]
- McGuire, L.S.; Zafar, M.; Manwar, R.; Charbel, F.T.; Avanaki, K. A Bilateral Craniectomy Technique for In Vivo Photoacoustic Brain Imaging. Appl. Sci. 2023, 13, 12951. [Google Scholar] [CrossRef]
- Zemp, R.J.; Song, L.; Bitton, R.; Shung, K.K.; Wang, L.V. Realtime Photoacoustic Microscopy of Murine Cardiovascular Dynamics. Opt. Express 2008, 16, 18551–18556. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, C.; Park, K.; Lim, G.; Kim, C. Fast optical-resolution photoacoustic microscopy using a 2-axis water-proofing MEMS scanner. Sci. Rep. 2015, 5, 7932. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.; McGuire, L.S.; Ranjbaran, S.M.; Matchynski, J.I.; Manwar, R.; Conti, A.C.; Perrine, S.A.; Avanaki, K. Spiral laser scanning photoacoustic microscopy for functional brain imaging in rats. Neurophotonics 2024, 11, 015007. [Google Scholar] [CrossRef]
- Liang, S.; Konstantin, I.M.; Rachel, B.; Shung, K.K.; Lihong, V.W. Fast 3-D dark-field reflection-mode photoacoustic microscopy in vivo with a 30-MHz ultrasound linear array. J. Biomed. Opt. 2008, 13, 054028. [Google Scholar] [CrossRef]
- Nedosekin, D.A.; Juratli, M.A.; Sarimollaoglu, M.; Moore, C.L.; Rusch, N.J.; Smeltzer, M.S.; Zharov, V.P.; Galanzha, E.I. Photoacoustic and photothermal detection of circulating tumor cells, bacteria and nanoparticles in cerebrospinal fluid in vivo and ex vivo. J. Biophotonics 2013, 6, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Galanzha, E.I.; Zaharoff, D.A.; Griffin, R.J.; Zharov, V.P. Nanotheranostics of Circulating Tumor Cells, Infections and Other Pathological Features in Vivo. Mol. Pharm. 2013, 10, 813–830. [Google Scholar] [CrossRef]
- Zharov, V.P.; Galanzha, E.I.; Tuchin, V.V. In vivo photothermal flow cytometry: Imaging and detection of individual cells in blood and lymph flow. J. Cell. Biochem. 2006, 97, 916–932. [Google Scholar] [CrossRef]
- Galanzha, E.I.; Shashkov, E.V.; Kelly, T.; Kim, J.-W.; Yang, L.; Zharov, V.P. In vivo magnetic enrichment and multiplex photoacoustic detection of circulating tumour cells. Nat. Nanotechnol. 2009, 4, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Jansen, K.; van der Steen, A.F.W.; van Beusekom, H.M.M.; Oosterhuis, J.W.; van Soest, G. Intravascular photoacoustic imaging of human coronary atherosclerosis. Opt. Lett. 2011, 36, 597–599. [Google Scholar] [CrossRef] [PubMed]
- Manohar, S.; Razansky, D. Photoacoustics: A historical review. Adv. Opt. Photon. 2016, 8, 586–617. [Google Scholar] [CrossRef]


| Modality | Spatial Resolution (L: Lateral Resolution, A: Axial Resolution) | Penetration Depth | Validation |
|---|---|---|---|
| PAE | L: 421 μm, A: 69 μm | 1.5 mm | Esophagus wall imaging of a pig |
| PAM | L: 70 μm, A: 25 μm | 3 mm | Subcutaneous vasculature in rats |
| PAT | L: 140 μm, A: 40 μm | 15 mm (40 Mhz) | Subcutaneous vasculature in human forearm |
| PAT | L: 30 μm, A: 7 μm | 5 mm | Drosophila fly and drosophila ex vivo |
| OR-PAM | L: 6.2 μm, A: 27 μm | 3.2 mm | Fresh chicken breast tissue |
| AR-PAE | L: 18 μm, A: 4 μm | 5 mm | Phantom |
| PAM | L: 100 μm, A: 25 μm | 3 mm | Microvessels in a rat |
| Research | Characteristics | Reference | |
|---|---|---|---|
| Application | Dermatologic imaging and joint imaging | Advantages of PAI for imaging skin diseases. | [100,101,102,103,104,105,106,107,108,109,110,111,112,113] |
| Breast tumor imaging and vascular disease detection | Advantages of high contrast and high spatial resolution of PA imaging for breast cancer diagnosis. | [114,115,116,117] | |
| Cervical cancer | Optimization of PAI for cervical cancer diagnosis. | [67,71,118,119,120,121,122,123,124,125] | |
| Animal organ imaging | Effective application of PAI on animal organs. | [10,108,126,127,128,129,130,131,132] | |
| Brain imaging | PAI is used in brain imaging to assist the research of image analysis. | [133,134,135,136,137,138] | |
| Hematologic imaging | Detection of circulating tumor cells by PAI. | [135,136,138,139,140,141,142] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Teng, X.; Yu, S.; Yang, W.; Kong, T.; Liu, T. Recent Advances in Photoacoustic Imaging: Current Status and Future Perspectives. Micromachines 2024, 15, 1007. https://doi.org/10.3390/mi15081007
Liu H, Teng X, Yu S, Yang W, Kong T, Liu T. Recent Advances in Photoacoustic Imaging: Current Status and Future Perspectives. Micromachines. 2024; 15(8):1007. https://doi.org/10.3390/mi15081007
Chicago/Turabian StyleLiu, Huibin, Xiangyu Teng, Shuxuan Yu, Wenguang Yang, Tiantian Kong, and Tangying Liu. 2024. "Recent Advances in Photoacoustic Imaging: Current Status and Future Perspectives" Micromachines 15, no. 8: 1007. https://doi.org/10.3390/mi15081007
APA StyleLiu, H., Teng, X., Yu, S., Yang, W., Kong, T., & Liu, T. (2024). Recent Advances in Photoacoustic Imaging: Current Status and Future Perspectives. Micromachines, 15(8), 1007. https://doi.org/10.3390/mi15081007







