Acoustofluidic Actuation of Living Cells
Abstract
1. Introduction
2. Suspended Single Cells in the Acoustofluidic Field
3. Acoustofluidic Cell Sorting
3.1. Automatic High-Throughput Sorting
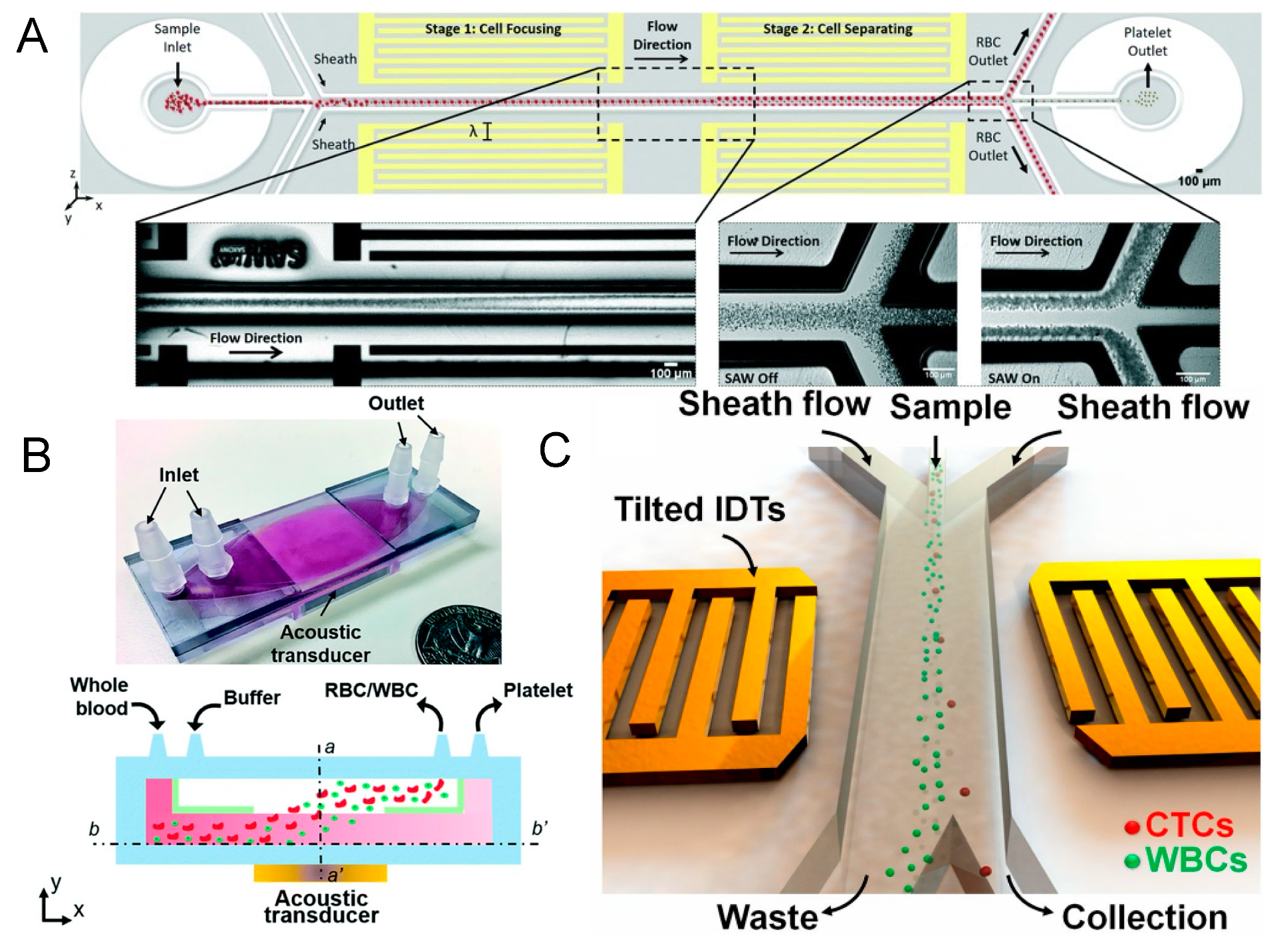
3.2. Selective High-Precision Sorting

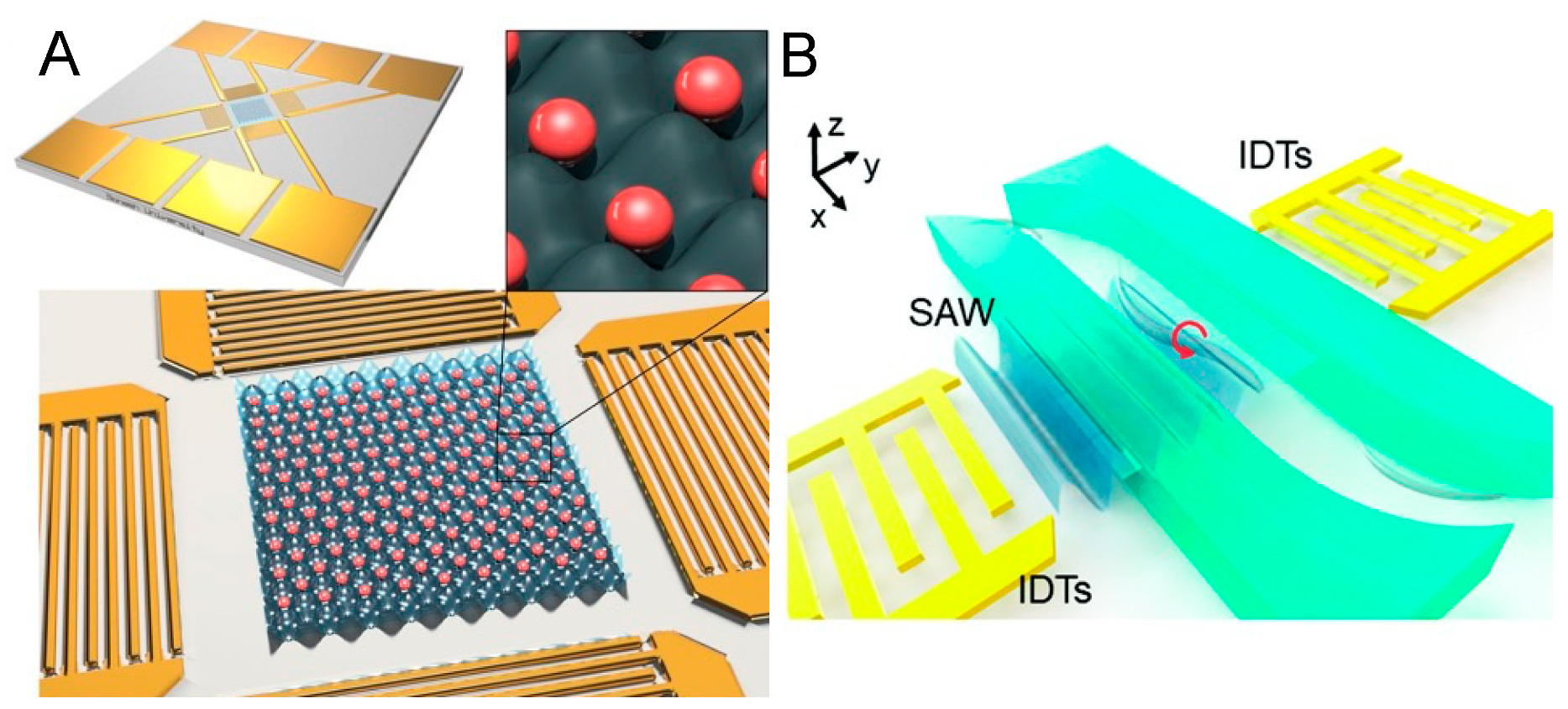
4. Acoustofluidic Patterning of Living Cells for Tissue Engineering
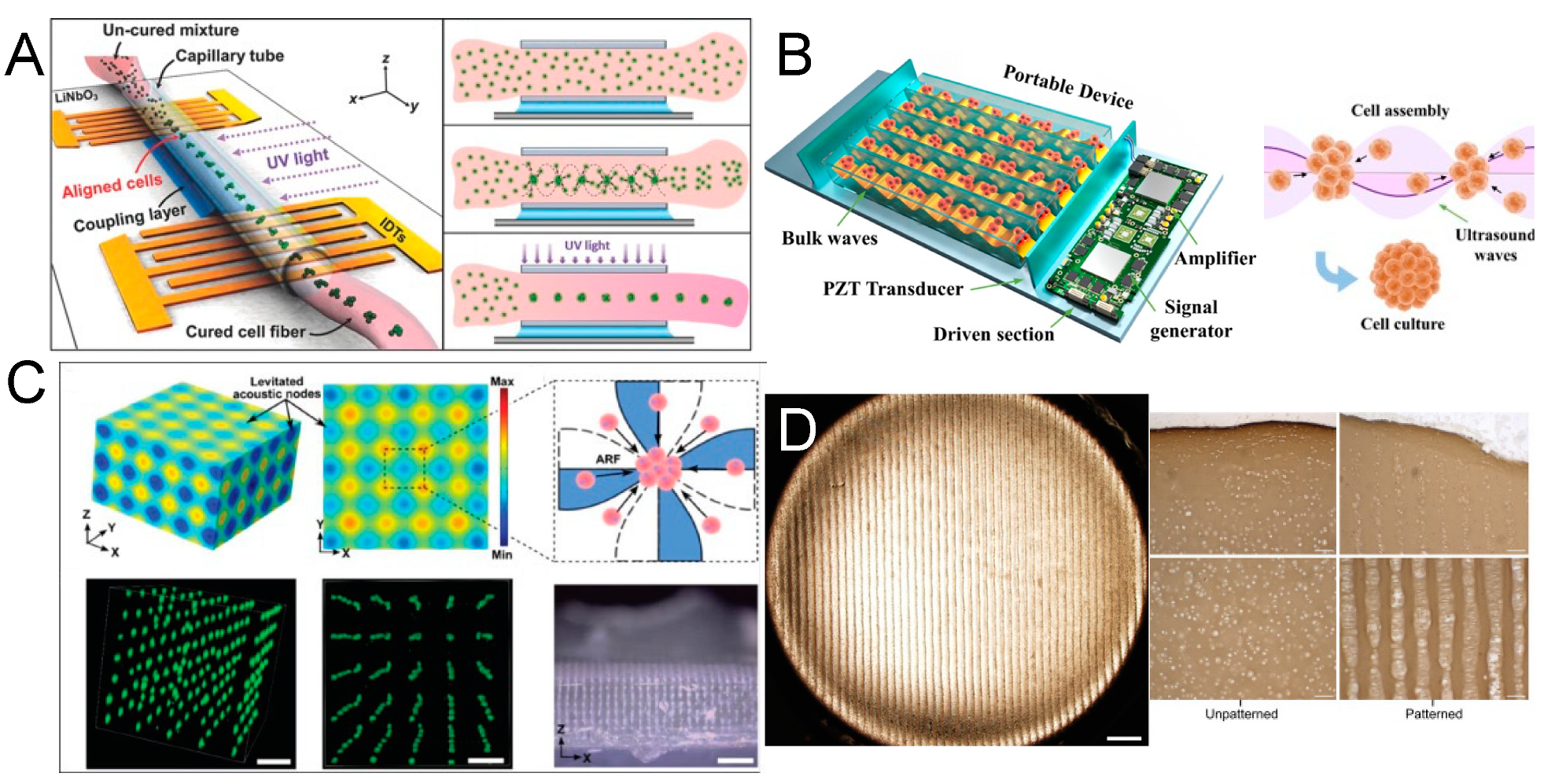
5. Acoustofluidic Microscopy
6. Acoustofluidic Biophysical Therapy

7. Acoustofluidic Droplet Printing
8. Acoustofluidic Intracellular Delivery
9. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guex, A.G.; Di Marzio, N.; Eglin, D.; Alini, M.; Serra, T. The Waves That Make the Pattern: A Review on Acoustic Manipulation in Biomedical Research. Mater. Today Bio 2021, 10, 100110. [Google Scholar] [CrossRef]
- Rufo, J.; Cai, F.; Friend, J.; Wiklund, M.; Huang, T.J. Acoustofluidics for Biomedical Applications. Nat. Rev. Methods Primers 2022, 2, 30. [Google Scholar] [CrossRef]
- Akkoyun, F.; Gucluer, S.; Ozcelik, A. Potential of the Acoustic Micromanipulation Technologies for Biomedical Research. Biomicrofluidics 2021, 15, 061301. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, Q.; Luo, Y.; Huang, Z.; Cheng, Q.; Zhang, W.; Zhou, B.; Zhou, Y.; Ma, Z. Manipulation with Sound and Vibration: A Review on the Micromanipulation System Based on Sub-MHz Acoustic Waves. Ultrason. Sonochemistry 2023, 96, 106441. [Google Scholar] [CrossRef]
- Connacher, W.; Zhang, N.; Huang, A.; Mei, J.; Zhang, S.; Gopesh, T.; Friend, J. Micro/Nano Acoustofluidics: Materials, Phenomena, Design, Devices, and Applications. Lab Chip 2018, 18, 1952–1996. [Google Scholar] [CrossRef]
- Fu, Y.Q.; Luo, J.K.; Nguyen, N.T.; Walton, A.J.; Flewitt, A.J.; Zu, X.T.; Li, Y.; McHale, G.; Matthews, A.; Iborra, E.; et al. Advances in Piezoelectric Thin Films for Acoustic Biosensors, Acoustofluidics and Lab-on-Chip Applications. Prog. Mater. Sci. 2017, 89, 31–91. [Google Scholar] [CrossRef]
- Friend, J.; Yeo, L.Y. Microscale Acoustofluidics: Microfluidics Driven via Acoustics and Ultrasonics. Rev. Mod. Phys. 2011, 83, 647–704. [Google Scholar] [CrossRef]
- Destgeer, G.; Sung, H.J. Recent Advances in Microfluidic Actuation and Micro-Object Manipulation via Surface Acoustic Waves. Lab Chip 2015, 15, 2722–2738. [Google Scholar] [CrossRef]
- Yeo, L.Y.; Friend, J.R. Surface Acoustic Wave Microfluidics. Annu. Rev. Fluid Mech. 2014, 46, 379–406. [Google Scholar] [CrossRef]
- Yeo, L.Y.; Friend, J.R. Ultrafast Microfluidics Using Surface Acoustic Waves. Biomicrofluidics 2009, 3, 12002. [Google Scholar] [CrossRef]
- Collins, D.J.; O’Rorke, R.; Neild, A.; Han, J.; Ai, Y. Acoustic Fields and Microfluidic Patterning around Embedded Micro-Structures Subject to Surface Acoustic Waves. Soft Matter 2019, 15, 8691–8705. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yazdi, S.; Lin, S.-C.S.; Ding, X.; Chiang, I.-K.; Sharp, K.; Huang, T.J. Three-Dimensional Continuous Particle Focusing in a Microfluidic Channel via Standing Surface Acoustic Waves (SSAW). Lab Chip 2011, 11, 2319–2324. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Cai, F.; Li, F.; Zhou, W.; Niu, L.; Zheng, H. Acoustic Tweezers. J. Phys. D Appl. Phys. 2019, 52, 273001. [Google Scholar] [CrossRef]
- Chen, K.; Wu, M.; Guo, F.; Li, P.; Chan, C.Y.; Mao, Z.; Li, S.; Ren, L.; Zhang, R.; Huang, T.J. Rapid Formation of Size-Controllable Multicellular Spheroids via 3D Acoustic Tweezers. Lab Chip 2016, 16, 2636–2643. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Ao, Z.; Wu, Z.; Song, S.; Mackie, K.; Guo, F. Intelligent Acoustofluidics Enabled Mini-Bioreactors for Human Brain Organoids. Lab Chip 2021, 21, 2194–2205. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Fajrial, A.K.; Yang, T.; Ding, X. Emerging On-Chip Surface Acoustic Wave Technology for Small Biomaterials Manipulation and Characterization. Biomater. Sci. 2020, 9, 1574–1582. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Lin, S.-C.S.; Kiraly, B.; Yue, H.; Li, S.; Chiang, I.-K.; Shi, J.; Benkovic, S.J.; Huang, T.J. On-Chip Manipulation of Single Microparticles, Cells, and Organisms Using Surface Acoustic Waves. Proc. Natl. Acad. Sci. USA 2012, 109, 11105–11109. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Li, P.; Lin, S.-C.S.; Stratton, Z.S.; Nama, N.; Guo, F.; Slotcavage, D.; Mao, X.; Shi, J.; Costanzo, F.; et al. Surface Acoustic Wave Microfluidics. Lab Chip 2013, 13, 3626–3649. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, X.; You, F.; Xiao, H. Review of Ultrasonic Particle Manipulation Techniques: Applications and Research Advances. Micromachines 2023, 14, 1487. [Google Scholar] [CrossRef]
- Ding, X.; Shi, J.; Lin, S.-C.S.; Yazdi, S.; Kiraly, B.; Huang, T.J. Tunable Patterning of Microparticles and Cells Using Standing Surface Acoustic Waves. Lab Chip 2012, 12, 2491–2497. [Google Scholar] [CrossRef]
- Muller, P.B.; Barnkob, R.; Jensen, M.J.H.; Bruus, H. A Numerical Study of Microparticle Acoustophoresis Driven by Acoustic Radiation Forces and Streaming-Induced Drag Forces. Lab Chip 2012, 12, 4617–4627. [Google Scholar] [CrossRef] [PubMed]
- Barnkob, R.; Augustsson, P.; Laurell, T.; Bruus, H. Acoustic Radiation- and Streaming-Induced Microparticle Velocities Determined by Microparticle Image Velocimetry in an Ultrasound Symmetry Plane. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2012, 86, 056307. [Google Scholar] [CrossRef] [PubMed]
- Devendran, C.; Collins, D.J.; Neild, A. The Role of Channel Height and Actuation Method on Particle Manipulation in Surface Acoustic Wave (SAW)-Driven Microfluidic Devices. Microfluid. Nanofluidics 2022, 26, 9. [Google Scholar] [CrossRef]
- Hahn, P.; Leibacher, I.; Baasch, T.; Dual, J. Numerical Simulation of Acoustofluidic Manipulation by Radiation Forces and Acoustic Streaming for Complex Particles. Lab Chip 2015, 15, 4302–4313. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Wang, Y.; Wang, Z.; Duan, X. Microscale Acoustic Streaming for Biomedical and Bioanalytical Applications. TrAC Trends Anal. Chem. 2023, 160, 116958. [Google Scholar] [CrossRef]
- Urban, M.W. Production of Acoustic Radiation Force Using Ultrasound: Methods and Applications. Expert Rev. Med. Devices 2018, 15, 819–834. [Google Scholar] [CrossRef] [PubMed]
- Jannesar, E.A.; Hamzehpour, H. Acoustic Tweezing of Microparticles in Microchannels with Sinusoidal Cross Sections. Sci. Rep. 2021, 11, 17902. [Google Scholar] [CrossRef] [PubMed]
- Sadhal, S.S. Acoustofluidics 13: Analysis of Acoustic Streaming by Perturbation Methods. Lab Chip 2012, 12, 2292–2300. [Google Scholar] [CrossRef]
- Sadhal, S.S. Acoustofluidics 15: Streaming with Sound Waves Interacting with Solid Particles. Lab Chip 2012, 12, 2600–2611. [Google Scholar] [CrossRef]
- Gedge, M.; Hill, M. Acoustofluidics 17: Theory and Applications of Surface Acoustic Wave Devices for Particle Manipulation. Lab Chip 2012, 12, 2998–3007. [Google Scholar] [CrossRef]
- Suslick, K.S.; Didenko, Y.; Fang, M.M.; Hyeon, T.; Kolbeck, K.J.; McNamara, W.B., III; Mdleleni, M.M.; Wong, M. Acoustic Cavitation and Its Chemical Consequences. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Eng. Sci. 1999, 357, 335–353. [Google Scholar] [CrossRef]
- Mitome, H. The Mechanism of Generation of Acoustic Streaming. Electron. Commun. Jpn. (Part III Fundam. Electron. Sci.) 1998, 81, 1–8. [Google Scholar] [CrossRef]
- Wiklund, M. Acoustofluidics 12: Biocompatibility and Cell Viability in Microfluidic Acoustic Resonators. Lab Chip 2012, 12, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Mao, Z.; Bachman, H.; Li, P.; Zhang, P.; Ren, L.; Wu, M.; Huang, T.J. Acoustic Cell Separation Based on Density and Mechanical Properties. J. Biomech. Eng. 2020, 142, 031005. [Google Scholar] [CrossRef]
- Ma, Z.; Collins, D.J.; Guo, J.; Ai, Y. Mechanical Properties Based Particle Separation via Traveling Surface Acoustic Wave. Anal. Chem. 2016, 88, 11844–11851. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, K.; Hammarström, B.; Wiklund, M. Acoustic Separation of Living and Dead Cells Using High Density Medium. Lab Chip 2020, 20, 1981–1990. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Sanders, C.K.; Marrone, B.L. Separation of Escherichia Coli Bacteria from Peripheral Blood Mononuclear Cells Using Standing Surface Acoustic Waves. Anal. Chem. 2013, 85, 9126–9134. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, C.; Wang, Z.; Bachman, H.; Ouyang, Y.; Huang, P.-H.; Sadovsky, Y.; Huang, T.J. Separating Extracellular Vesicles and Lipoproteins via Acoustofluidics. Lab Chip 2019, 19, 1174–1182. [Google Scholar] [CrossRef]
- Zhou, J.; Habibi, R.; Akbaridoust, F.; Neild, A.; Nosrati, R. Paper-Based Acoustofluidics for Separating Particles and Cells. Anal. Chem. 2020, 92, 8569–8578. [Google Scholar] [CrossRef]
- Li, S.; Ren, L.; Huang, P.-H.; Yao, X.; Cuento, R.A.; McCoy, J.P.; Cameron, C.E.; Levine, S.J.; Huang, T.J. Acoustofluidic Transfer of Inflammatory Cells from Human Sputum Samples. Anal. Chem. 2016, 88, 5655–5661. [Google Scholar] [CrossRef]
- Witek, M.A.; Freed, I.M.; Soper, S.A. Cell Separations and Sorting. Anal. Chem. 2020, 92, 105–131. [Google Scholar] [CrossRef]
- Lu, N.; Tay, H.M.; Petchakup, C.; He, L.; Gong, L.; Maw, K.K.; Leong, S.Y.; Lok, W.W.; Ong, H.B.; Guo, R.; et al. Label-Free Microfluidic Cell Sorting and Detection for Rapid Blood Analysis. Lab Chip 2023, 23, 1226–1257. [Google Scholar] [CrossRef]
- Talasaz, A.H.; Powell, A.A.; Huber, D.E.; Berbee, J.G.; Roh, K.-H.; Yu, W.; Xiao, W.; Davis, M.M.; Pease, R.F.; Mindrinos, M.N.; et al. Isolating Highly Enriched Populations of Circulating Epithelial Cells and Other Rare Cells from Blood Using a Magnetic Sweeper Device. Proc. Natl. Acad. Sci. USA 2009, 106, 3970–3975. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Dubash, T.D.; Edd, J.F.; Jewett, M.K.; Garre, S.G.; Karabacak, N.M.; Rabe, D.C.; Mutlu, B.R.; Walsh, J.R.; Kapur, R.; et al. Ultrahigh-Throughput Magnetic Sorting of Large Blood Volumes for Epitope-Agnostic Isolation of Circulating Tumor Cells. Proc. Natl. Acad. Sci. USA 2020, 117, 16839–16847. [Google Scholar] [CrossRef] [PubMed]
- Miltenyi, S.; Müller, W.; Weichel, W.; Radbruch, A. High Gradient Magnetic Cell Separation with MACS. Cytometry 1990, 11, 231–238. [Google Scholar] [CrossRef]
- Shields, C.W., 4th; Reyes, C.D.; López, G.P. Microfluidic Cell Sorting: A Review of the Advances in the Separation of Cells from Debulking to Rare Cell Isolation. Lab Chip 2015, 15, 1230–1249. [Google Scholar] [CrossRef] [PubMed]
- Philpott, D.N.; Chen, K.; Atwal, R.S.; Li, D.; Christie, J.; Sargent, E.H.; Kelley, S.O. Ultrathroughput Immunomagnetic Cell Sorting Platform. Lab Chip 2022, 22, 4822–4830. [Google Scholar] [CrossRef]
- Zhang, P.; Bachman, H.; Ozcelik, A.; Huang, T.J. Acoustic Microfluidics. Annu. Rev. Anal. Chem. 2020, 13, 17–43. [Google Scholar] [CrossRef]
- Lenshof, A.; Magnusson, C.; Laurell, T. Acoustofluidics 8: Applications of Acoustophoresis in Continuous Flow Microsystems. Lab Chip 2012, 12, 1210–1223. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, Y.; Qin, X.; Nair, S.; Huang, X.; Liu, Y. Label-Free Detection of Rare Circulating Tumor Cells by Image Analysis and Machine Learning. Sci. Rep. 2020, 10, 12226. [Google Scholar] [CrossRef]
- Pretini, V.; Koenen, M.H.; Kaestner, L.; Fens, M.H.A.M.; Schiffelers, R.M.; Bartels, M.; Van Wijk, R. Red Blood Cells: Chasing Interactions. Front. Physiol. 2019, 10, 945. [Google Scholar] [CrossRef] [PubMed]
- Kabat, G.C.; Kim, M.Y.; Manson, J.E.; Lessin, L.; Lin, J.; Wassertheil-Smoller, S.; Rohan, T.E. White Blood Cell Count and Total and Cause-Specific Mortality in the Women’s Health Initiative. Am. J. Epidemiol. 2017, 186, 63–72. [Google Scholar] [CrossRef]
- Snyder, E.L.; Hezzey, A.; Katz, A.J.; Bock, J. Occurrence of the Release Reaction during Preparation and Storage of Platelet Concentrates. Vox Sang. 1981, 41, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Espy, R.D.; Manicke, N.E.; Ouyang, Z.; Cooks, R.G. Rapid Analysis of Whole Blood by Paper Spray Mass Spectrometry for Point-of-Care Therapeutic Drug Monitoring. Analyst 2012, 137, 2344–2349. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, P.; Petersson, K.; Augustsson, P.; Laurell, T. Acoustic Impedance Matched Buffers Enable Separation of Bacteria from Blood Cells at High Cell Concentrations. Sci. Rep. 2018, 8, 9156. [Google Scholar] [CrossRef] [PubMed]
- Richard, C.; Fakhfouri, A.; Colditz, M.; Striggow, F.; Kronstein-Wiedemann, R.; Tonn, T.; Medina-Sánchez, M.; Schmidt, O.G.; Gemming, T.; Winkler, A. Blood Platelet Enrichment in Mass-Producible Surface Acoustic Wave (SAW) Driven Microfluidic Chips. Lab Chip 2019, 19, 4043–4051. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, K.; Yang, S.; Wang, Z.; Huang, P.-H.; Mai, J.; Li, Z.-Y.; Huang, T.J. High-Throughput Cell Focusing and Separation via Acoustofluidic Tweezers. Lab Chip 2018, 18, 3003–3010. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Lim, H.; Kim, D.; Shin, S. Separation of Platelets from Whole Blood Using Standing Surface Acoustic Waves in a Microchannel. Lab Chip 2011, 11, 3361–3364. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, C.; Augustsson, P.; Lenshof, A.; Ceder, Y.; Laurell, T.; Lilja, H. Clinical-Scale Cell-Surface-Marker Independent Acoustic Microfluidic Enrichment of Tumor Cells from Blood. Anal. Chem. 2017, 89, 11954–11961. [Google Scholar] [CrossRef]
- Wu, Z.; Jiang, H.; Zhang, L.; Yi, K.; Cui, H.; Wang, F.; Liu, W.; Zhao, X.; Zhou, F.; Guo, S. The Acoustofluidic Focusing and Separation of Rare Tumor Cells Using Transparent Lithium Niobate Transducers. Lab Chip 2019, 19, 3922–3930. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, M.; Ren, L.; Liu, J.; Whitley, P.H.; Wang, L.; Huang, T.J. High-Throughput Acoustic Separation of Platelets from Whole Blood. Lab Chip 2016, 16, 3466–3472. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Chen, C.; Wang, Z.; Huang, P.-H.; Fu, H.; Wang, L.; Wu, M.; Chen, Y.; Gao, T.; Gong, J.; et al. Plastic-Based Acoustofluidic Devices for High-Throughput, Biocompatible Platelet Separation. Lab Chip 2019, 19, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Mao, Z.; Peng, Z.; Zhou, L.; Chen, Y.; Huang, P.-H.; Truica, C.I.; Drabick, J.J.; El-Deiry, W.S.; Dao, M.; et al. Acoustic Separation of Circulating Tumor Cells. Proc. Natl. Acad. Sci. USA 2015, 112, 4970–4975. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Huang, P.-H.; Zhang, R.; Mao, Z.; Chen, C.; Kemeny, G.; Li, P.; Lee, A.V.; Gyanchandani, R.; Armstrong, A.J.; et al. Circulating Tumor Cell Phenotyping via High-Throughput Acoustic Separation. Small 2020, 16, e2004438. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ao, Z.; Cai, B.; Shu, X.; Chen, K.; Rao, L.; Luo, C.; Wang, F.-B.; Liu, W.; Bondesson, M.; et al. Size-Amplified Acoustofluidic Separation of Circulating Tumor Cells with Removable Microbeads. Nano Futures 2018, 2, 025004. [Google Scholar] [CrossRef]
- Lin, D.; Shen, L.; Luo, M.; Zhang, K.; Li, J.; Yang, Q.; Zhu, F.; Zhou, D.; Zheng, S.; Chen, Y.; et al. Circulating Tumor Cells: Biology and Clinical Significance. Signal Transduct. Target. Ther. 2021, 6, 404. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hartman, J.H.; Chen, C.; Yang, S.; Li, Q.; Tian, Z.; Huang, P.-H.; Wang, L.; Meyer, J.N.; Huang, T.J. Fluorescence-Based Sorting of Caenorhabditis Elegans via Acoustofluidics. Lab Chip 2020, 20, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, A.A.; Chen, Y.; Nama, N.; Nissly, R.H.; Ren, L.; Ozcelik, A.; Wang, L.; McCoy, J.P.; Levine, S.J.; Huang, T.J. Acoustofluidic Fluorescence Activated Cell Sorter. Anal. Chem. 2015, 87, 12051–12058. [Google Scholar] [CrossRef] [PubMed]
- Ozcelik, A.; Rich, J.; Huang, T.J. Fundamentals and Applications of Acoustics in Microfluidics. In Multidisciplinary Microfluidic and Nanofluidic Lab-on-a-Chip; Elsevier: Amsterdam, The Netherlands, 2022; pp. 297–321. [Google Scholar]
- Ren, L.; Chen, Y.; Li, P.; Mao, Z.; Huang, P.-H.; Rufo, J.; Guo, F.; Wang, L.; McCoy, J.P.; Levine, S.J.; et al. A High-Throughput Acoustic Cell Sorter. Lab Chip 2015, 15, 3870–3879. [Google Scholar] [CrossRef]
- Ren, L.; Yang, S.; Zhang, P.; Qu, Z.; Mao, Z.; Huang, P.-H.; Chen, Y.; Wu, M.; Wang, L.; Li, P.; et al. Standing Surface Acoustic Wave (SSAW)-Based Fluorescence-Activated Cell Sorter. Small 2018, 14, e1801996. [Google Scholar] [CrossRef]
- Wang, K.; Zhou, W.; Lin, Z.; Cai, F.; Li, F.; Wu, J.; Meng, L.; Niu, L.; Zheng, H. Sorting of Tumour Cells in a Microfluidic Device by Multi-Stage Surface Acoustic Waves. Sens. Actuators B Chem. 2018, 258, 1174–1183. [Google Scholar] [CrossRef]
- Mutafopulos, K.; Spink, P.; Lofstrom, C.D.; Lu, P.J.; Lu, H.; Sharpe, J.C.; Franke, T.; Weitz, D.A. Traveling Surface Acoustic Wave (TSAW) Microfluidic Fluorescence Activated Cell Sorter (ΜFACS). Lab Chip 2019, 19, 2435–2443. [Google Scholar] [CrossRef]
- Li, P.; Liang, M.; Lu, X.; Chow, J.J.M.; Ramachandra, C.J.A.; Ai, Y. Sheathless Acoustic Fluorescence Activated Cell Sorting (AFACS) with High Cell Viability. Anal. Chem. 2019, 91, 15425–15435. [Google Scholar] [CrossRef]
- Li, P.; Ai, Y. Label-Free Multivariate Biophysical Phenotyping-Activated Acoustic Sorting at the Single-Cell Level. Anal. Chem. 2021, 93, 4108–4117. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, Z.; Ai, Y. Hybrid Microfluidic Sorting of Rare Cells Based on High Throughput Inertial Focusing and High Accuracy Acoustic Manipulation. RSC Adv. 2019, 9, 31186–31195. [Google Scholar] [CrossRef]
- Collins, D.J.; Neild, A.; Ai, Y. Highly Focused High-Frequency Travelling Surface Acoustic Waves (SAW) for Rapid Single-Particle Sorting. Lab Chip 2016, 16, 471–479. [Google Scholar] [CrossRef]
- Ma, Z.; Zhou, Y.; Collins, D.J.; Ai, Y. Fluorescence Activated Cell Sorting via a Focused Traveling Surface Acoustic Beam. Lab Chip 2017, 17, 3176–3185. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, A.A.; Soteriou, D.; Xu, C.K.; Goswami, R.; Herbig, M.; Guck, J.; Girardo, S. Image-Based Cell Sorting Using Focused Travelling Surface Acoustic Waves. Lab Chip 2023, 23, 372–387. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, X.; Ren, J.; Lin, F.; Wu, J. Recent Advances in Acoustofluidic Separation Technology in Biology. Microsyst. Nanoeng. 2022, 8, 94. [Google Scholar] [CrossRef]
- Xie, Y.; Bachman, H.; Huang, T.J. Acoustofluidic Methods in Cell Analysis. TrAC Trends Anal. Chem. 2019, 117, 280–290. [Google Scholar] [CrossRef]
- Wu, Z.; Pan, M.; Wang, J.; Wen, B.; Lu, L.; Ren, H. Acoustofluidics for Cell Patterning and Tissue Engineering. Eng. Regen. 2022, 3, 397–406. [Google Scholar] [CrossRef]
- Chen, B.; Wu, Z.; Wu, Y.; Chen, Y.; Zheng, L. Controllable Fusion of Multicellular Spheroids Using Acoustofluidics. Microfluid. Nanofluidics 2023, 27, 50. [Google Scholar] [CrossRef]
- Pan, H.; Mei, D.; Xu, C.; Han, S.; Wang, Y. Bisymmetric Coherent Acoustic Tweezers Based on Modulation of Surface Acoustic Waves for Dynamic and Reconfigurable Cluster Manipulation of Particles and Cells. Lab Chip 2023, 23, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Mei, D.; Xu, C.; Li, X.; Wang, Y. Acoustic Tweezers Using Bisymmetric Coherent Surface Acoustic Waves for Dynamic and Reconfigurable Manipulation of Particle Multimers. J. Colloid Interface Sci. 2023, 643, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, R.; Villegas, K.M.; Tabrizian, M. Acoustofluidics—Changing Paradigm in Tissue Engineering, Therapeutics Development, and Biosensing. Lab Chip 2023, 23, 1300–1338. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, D.V.; Reichert, P.; Zvick, J.; Labouesse, C.; Künzli, V.; Dudaryeva, O.; Bar-Nur, O.; Tibbitt, M.W.; Dual, J. Continuous Production of Acoustically Patterned Cells within Hydrogel Fibers for Musculoskeletal Tissue Engineering. Adv. Funct. Mater. 2022, 32, 2113038. [Google Scholar] [CrossRef]
- Lata, J.P.; Guo, F.; Guo, J.; Huang, P.-H.; Yang, J.; Huang, T.J. Surface Acoustic Waves Grant Superior Spatial Control of Cells Embedded in Hydrogel Fibers. Adv. Mater. 2016, 28, 8632–8638. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Li, P.; French, J.B.; Mao, Z.; Zhao, H.; Li, S.; Nama, N.; Fick, J.R.; Benkovic, S.J.; Huang, T.J. Controlling Cell-Cell Interactions Using Surface Acoustic Waves. Proc. Natl. Acad. Sci. USA 2015, 112, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Ahmed, D.; Mao, X.; Lin, S.-C.S.; Lawit, A.; Huang, T.J. Acoustic Tweezers: Patterning Cells and Microparticles Using Standing Surface Acoustic Waves (SSAW). Lab Chip 2009, 9, 2890–2895. [Google Scholar] [CrossRef]
- Li, S.; Guo, F.; Chen, Y.; Ding, X.; Li, P.; Wang, L.; Cameron, C.E.; Huang, T.J. Standing Surface Acoustic Wave Based Cell Coculture. Anal. Chem. 2014, 86, 9853–9859. [Google Scholar] [CrossRef]
- Armstrong, J.P.K.; Maynard, S.A.; Pence, I.J.; Franklin, A.C.; Drinkwater, B.W.; Stevens, M.M. Spatiotemporal Quantification of Acoustic Cell Patterning Using Voronoï Tessellation. Lab Chip 2019, 19, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Ao, Z.; Wu, Z.; Cai, H.; Hu, L.; Li, X.; Kaurich, C.; Chang, J.; Gu, M.; Cheng, L.; Lu, X.; et al. Rapid Profiling of Tumor-Immune Interaction Using Acoustically Assembled Patient-Derived Cell Clusters. Adv. Sci. 2022, 9, e2201478. [Google Scholar] [CrossRef]
- Cai, H.; Ao, Z.; Hu, L.; Moon, Y.; Wu, Z.; Lu, H.-C.; Kim, J.; Guo, F. Acoustofluidic Assembly of 3D Neurospheroids to Model Alzheimer’s Disease. Analyst 2020, 145, 6243–6253. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, S.; Luo, Z.; Zuo, Y.; Wang, F.; Zhu, J.; Chen, L.; Yang, D.; Zheng, Y.; Zheng, Y.; et al. On-Chip Hydrogel Arrays Individually Encapsulating Acoustic Formed Multicellular Aggregates for High Throughput Drug Testing. Lab Chip 2020, 20, 2228–2236. [Google Scholar] [CrossRef]
- Chen, B.; Wu, Y.; Ao, Z.; Cai, H.; Nunez, A.; Liu, Y.; Foley, J.; Nephew, K.; Lu, X.; Guo, F. High-Throughput Acoustofluidic Fabrication of Tumor Spheroids. Lab Chip 2019, 19, 1755–1763. [Google Scholar] [CrossRef]
- Wu, Y.; Ao, Z.; Chen, B.; Muhsen, M.; Bondesson, M.; Lu, X.; Guo, F. Acoustic Assembly of Cell Spheroids in Disposable Capillaries. Nanotechnology 2018, 29, 504006. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Chen, B.; Wu, Y.; Xia, Y.; Chen, H.; Gong, Z.; Hu, H.; Ding, Z.; Guo, S. Scaffold-Free Generation of Heterotypic Cell Spheroids Using Acoustofluidics. Lab Chip 2021, 21, 3498–3508. [Google Scholar] [CrossRef]
- Velasco, V.; Shariati, S.A.; Esfandyarpour, R. Microtechnology-Based Methods for Organoid Models. Microsyst. Nanoeng. 2020, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, S.; Win Naing, M. A Review of Manufacturing Capabilities of Cell Spheroid Generation Technologies and Future Development. Biotechnol. Bioeng. 2021, 118, 542–554. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.; Zhang, Y.; Liu, H.; Han, L. Recent Methods of Droplet Microfluidics and Their Applications in Spheroids and Organoids. Lab Chip 2023, 23, 1080–1096. [Google Scholar] [CrossRef]
- Shen, H.; Cai, S.; Wu, C.; Yang, W.; Yu, H.; Liu, L. Recent Advances in Three-Dimensional Multicellular Spheroid Culture and Future Development. Micromachines 2021, 12, 96. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Kim, E.M.; Yamamoto, M.; Park, H.; Shin, H. Engineering Multi-Cellular Spheroids for Tissue Engineering and Regenerative Medicine. Adv. Healthc. Mater. 2020, 9, e2000608. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, Y.; Qin, X.; Liu, Y. From Cell Spheroids to Vascularized Cancer Organoids: Microfluidic Tumor-on-a-Chip Models for Preclinical Drug Evaluations. Biomicrofluidics 2021, 15, 061503. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Gao, H.; Zhou, M.; Xiao, L.; Xu, T.; Zhang, X. Integrated Acoustic Chip for Culturing 3D Cell Arrays. ACS Sens. 2022, 7, 2654–2660. [Google Scholar] [CrossRef] [PubMed]
- Miao, T.; Chen, K.; Wei, X.; Huang, B.; Qian, Y.; Wang, L.; Xu, M. High-Throughput Fabrication of Cell Spheroids with 3D Acoustic Assembly Devices. Int. J. Bioprinting 2023, 9, 733. [Google Scholar] [CrossRef] [PubMed]
- Petta, D.; Basoli, V.; Pellicciotta, D.; Tognato, R.; Barcik, J.; Arrigoni, C.; Bella, E.D.; Armiento, A.R.; Candrian, C.; Richards, R.G.; et al. Sound-Induced Morphogenesis of Multicellular Systems for Rapid Orchestration of Vascular Networks. Biofabrication 2020, 13, 015004. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, J.; Zuo, Y.; Yang, D.; Zhang, J.; Cheng, Y.; Yang, Y. Versatile Biomimetic Array Assembly by Phase Modulation of Coherent Acoustic Waves. Lab Chip 2020, 20, 3515–3523. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, Y.; Islam, K.; Zhou, Y.; Omidi, S.; Berdichevsky, Y.; Liu, Y. Acoustofluidic Engineering of Functional Vessel-on-a-Chip. ACS Biomater. Sci. Eng. 2023, 9, 6273–6281. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Shin, J.; Park, H.-J.; Rhyou, C.; Kang, D.; Lee, S.-J.; Yoon, Y.-S.; Cho, S.-W.; Lee, H. High-Resolution Acoustophoretic 3D Cell Patterning to Construct Functional Collateral Cylindroids for Ischemia Therapy. Nat. Commun. 2018, 9, 5402. [Google Scholar] [CrossRef]
- Armstrong, J.P.K.; Pchelintseva, E.; Treumuth, S.; Campanella, C.; Meinert, C.; Klein, T.J.; Hutmacher, D.W.; Drinkwater, B.W.; Stevens, M.M. Tissue Engineering Cartilage with Deep Zone Cytoarchitecture by High-Resolution Acoustic Cell Patterning. Adv. Healthc. Mater. 2022, 11, e2200481. [Google Scholar] [CrossRef]
- Ren, T.; Chen, P.; Gu, L.; Ogut, M.G.; Demirci, U. Soft Ring-Shaped Cellu-Robots with Simultaneous Locomotion in Batches. Adv. Mater. 2020, 32, e1905713. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zheng, J.; Hu, Q.; Liang, L.; Yang, D.; Cheng, Y.; Li, S.-S.; Chen, L.-J.; Yang, Y. Smart Acoustic 3D Cell Construct Assembly with High-Resolution. Biofabrication 2022, 14, 045003. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Soto, F.; Ma, P.; Ahmed, R.; Yang, H.; Chen, S.; Wang, J.; Liu, C.; Akin, D.; Fu, K.; et al. Acoustic Fabrication of Living Cardiomyocyte-Based Hybrid Biorobots. ACS Nano 2022, 16, 10219–10230. [Google Scholar] [CrossRef] [PubMed]
- Melde, K.; Mark, A.G.; Qiu, T.; Fischer, P. Holograms for Acoustics. Nature 2016, 537, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Chen, C.; Rufo, J.; Shen, C.; Wang, Z.; Huang, P.-H.; Fu, H.; Zhang, P.; Cummer, S.A.; Tian, Z.; et al. Acoustofluidic Holography for Micro- to Nanoscale Particle Manipulation. ACS Nano 2020, 14, 14635–14645. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, R.; Martinez Plasencia, D.; Masuda, N.; Subramanian, S. A Volumetric Display for Visual, Tactile and Audio Presentation Using Acoustic Trapping. Nature 2019, 575, 320–323. [Google Scholar] [CrossRef]
- Ma, Z.; Holle, A.W.; Melde, K.; Qiu, T.; Poeppel, K.; Kadiri, V.M.; Fischer, P. Acoustic Holographic Cell Patterning in a Biocompatible Hydrogel. Adv. Mater. 2020, 32, e1904181. [Google Scholar] [CrossRef]
- Melde, K.; Kremer, H.; Shi, M.; Seneca, S.; Frey, C.; Platzman, I.; Degel, C.; Schmitt, D.; Schölkopf, B.; Fischer, P. Compact Holographic Sound Fields Enable Rapid One-Step Assembly of Matter in 3D. Sci. Adv. 2023, 9, eadf6182. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, J.; Harley, W.S.; Lee, P.V.S.; Collins, D.J. Programmable Acoustic Holography Using Medium-Sound-Speed Modulation. Adv. Sci. 2023, 10, e2301489. [Google Scholar] [CrossRef]
- Ghanem, M.A.; Maxwell, A.D.; Dalecki, D.; Sapozhnikov, O.A.; Bailey, M.R. Phase Holograms for the Three-Dimensional Patterning of Unconstrained Microparticles. Sci. Rep. 2023, 13, 9160. [Google Scholar] [CrossRef]
- Yunus, D.E.; Sohrabi, S.; He, R.; Shi, W.; Liu, Y. Acoustic Patterning for 3D Embedded Electrically Conductive Wire in Stereolithography. J. Micromech. Microeng. 2017, 27, 045016. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Cheng, F.; Liu, G.; Li, K.; Guo, Z. Dexterous Formation of Unconventional Chladni Patterns Using Standing Bulk Acoustic Waves. Appl. Phys. Lett. 2020, 117, 184101. [Google Scholar] [CrossRef]
- Ma, Z.; Melde, K.; Athanassiadis, A.G.; Schau, M.; Richter, H.; Qiu, T.; Fischer, P. Spatial Ultrasound Modulation by Digitally Controlling Microbubble Arrays. Nat. Commun. 2020, 11, 4537. [Google Scholar] [CrossRef] [PubMed]
- Marzo, A.; Drinkwater, B.W. Holographic Acoustic Tweezers. Proc. Natl. Acad. Sci. USA 2019, 116, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Vidler, C.; Wang, J.; Chen, X.; Pan, Z.; Harley, W.S.; Lee, P.V.S.; Collins, D.J. Micro-acoustic Holograms for Detachable Microfluidic Devices. Small 2024, 2307529. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Dutcher, S.K.; Bayly, P.V.; Meacham, J.M. Robust Acoustic Trapping and Perturbation of Single-Cell Microswimmers Illuminate Three-Dimensional Swimming and Ciliary Coordination. Proc. Natl. Acad. Sci. USA 2023, 120, e2218951120. [Google Scholar] [CrossRef] [PubMed]
- Wiklund, M.; Brismar, H.; Onfelt, B. Acoustofluidics 18: Microscopy for Acoustofluidic Micro-Devices. Lab Chip 2012, 12, 3221–3234. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.J.; Morahan, B.; Garcia-Bustos, J.; Doerig, C.; Plebanski, M.; Neild, A. Two-Dimensional Single-Cell Patterning with One Cell per Well Driven by Surface Acoustic Waves. Nat. Commun. 2015, 6, 8686. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Rufo, J.; Zhong, R.; Rich, J.; Wang, Z.; Lee, L.P.; Huang, T.J. Acoustic Tweezers for High-Throughput Single-Cell Analysis. Nat. Protoc. 2023, 18, 2441–2458. [Google Scholar] [CrossRef]
- Yang, S.; Tian, Z.; Wang, Z.; Rufo, J.; Li, P.; Mai, J.; Xia, J.; Bachman, H.; Huang, P.-H.; Wu, M.; et al. Harmonic Acoustics for Dynamic and Selective Particle Manipulation. Nat. Mater. 2022, 21, 540–546. [Google Scholar] [CrossRef]
- Agnihotri, S.N.; Ugolini, G.S.; Sullivan, M.R.; Yang, Y.; De Ganzó, A.; Lim, J.W.; Konry, T. Droplet Microfluidics for Functional Temporal Analysis and Cell Recovery on Demand Using Microvalves: Application in Immunotherapies for Cancer. Lab Chip 2022, 22, 3258–3267. [Google Scholar] [CrossRef]
- Zhang, W.; Song, B.; Bai, X.; Jia, L.; Song, L.; Guo, J.; Feng, L. Versatile Acoustic Manipulation of Micro-Objects Using Mode-Switchable Oscillating Bubbles: Transportation, Trapping, Rotation, and Revolution. Lab Chip 2021, 21, 4760–4771. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, S.; Chen, C.; Hartman, J.H.; Huang, P.-H.; Wang, L.; Tian, Z.; Zhang, P.; Faulkenberry, D.; Meyer, J.N.; et al. Surface Acoustic Waves Enable Rotational Manipulation of Caenorhabditis Elegans. Lab Chip 2019, 19, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Briggs, J.P. The Zebrafish: A New Model Organism for Integrative Physiology. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282, R3–R9. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Gu, Y.; Philippe, J.; Zhang, P.; Bachman, H.; Zhang, J.; Mai, J.; Rufo, J.; Rawls, J.F.; Davis, E.E.; et al. Acoustofluidic Rotational Tweezing Enables High-Speed Contactless Morphological Phenotyping of Zebrafish Larvae. Nat. Commun. 2021, 12, 1118. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Ao, Z.; Wu, Z.; Nunez, A.; Jiang, L.; Carpenter, R.L.; Nephew, K.P.; Guo, F. Profiling Cell-Matrix Adhesion Using Digitalized Acoustic Streaming. Anal. Chem. 2020, 92, 2283–2290. [Google Scholar] [CrossRef] [PubMed]
- Greco, G.; Agostini, M.; Tonazzini, I.; Sallemi, D.; Barone, S.; Cecchini, M. Surface-Acoustic-Wave (SAW)-Driven Device for Dynamic Cell Cultures. Anal. Chem. 2018, 90, 7450–7457. [Google Scholar] [CrossRef]
- Devendran, C.; Carthew, J.; Frith, J.E.; Neild, A. Cell Adhesion, Morphology, and Metabolism Variation via Acoustic Exposure within Microfluidic Cell Handling Systems. Adv. Sci. 2019, 6, 1902326. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Nam, H.; Cha, B.; Park, J.; Sung, H.J.; Jeon, J.S. Acoustofluidic Stimulation of Functional Immune Cells in a Microreactor. Adv. Sci. 2022, 9, 2105809. [Google Scholar] [CrossRef]
- Bhadra, J.; Sridhar, N.; Fajrial, A.K.; Hammond, N.; Xue, D.; Ding, X. Acoustic Streaming Enabled Moderate Swimming Exercise Reduces Neurodegeneration in C. elegans. Sci. Adv. 2023, 9, eadf5056. [Google Scholar] [CrossRef]
- Sridhar, N.; Fajrial, A.K.; Doser, R.L.; Hoerndli, F.J.; Ding, X. Surface Acoustic Wave Microfluidics for Repetitive and Reversible Temporary Immobilization of C. elegans. Lab Chip 2022, 22, 4882–4893. [Google Scholar] [CrossRef]
- Gai, J.; Nosrati, R.; Neild, A. High DNA Integrity Sperm Selection Using Surface Acoustic Waves. Lab Chip 2020, 20, 4262–4272. [Google Scholar] [CrossRef] [PubMed]
- Gai, J.; Devendran, C.; Neild, A.; Nosrati, R. Surface Acoustic Wave-Driven Pumpless Flow for Sperm Rheotaxis Analysis. Lab Chip 2022, 22, 4409–4417. [Google Scholar] [CrossRef]
- Gai, J.; Dervisevic, E.; Devendran, C.; Cadarso, V.J.; O’Bryan, M.K.; Nosrati, R.; Neild, A. High-Frequency Ultrasound Boosts Bull and Human Sperm Motility. Adv. Sci. 2022, 9, e2104362. [Google Scholar] [CrossRef] [PubMed]
- Farooq, U.; Liu, Y.; Li, P.; Deng, Z.; Liu, X.; Zhou, W.; Yi, S.; Rong, N.; Meng, L.; Niu, L.; et al. Acoustofluidic Dynamic Interfacial Tensiometry. J. Acoust. Soc. Am. 2021, 150, 3608–3617. [Google Scholar] [CrossRef]
- Wu, Z.; Cai, H.; Ao, Z.; Nunez, A.; Liu, H.; Bondesson, M.; Guo, S.; Guo, F. A Digital Acoustofluidic Pump Powered by Localized Fluid-Substrate Interactions. Anal. Chem. 2019, 91, 7097–7103. [Google Scholar] [CrossRef]
- Ning, J.; Lei, Y.; Hu, H.; Gai, C. A Comprehensive Review of Surface Acoustic Wave-Enabled Acoustic Droplet Ejection Technology and Its Applications. Micromachines 2023, 14, 1543. [Google Scholar] [CrossRef]
- Darmawan, M.; Byun, D. Focused Surface Acoustic Wave Induced Jet Formation on Superhydrophobic Surfaces. Microfluid. Nanofluidics 2015, 18, 1107–1114. [Google Scholar] [CrossRef]
- Sankaranarayanan, S.K.R.S.; Bhethanabotla, V.R. Design of Efficient Focused Surface Acoustic Wave Devices for Potential Microfluidic Applications. J. Appl. Phys. 2008, 103, 064518. [Google Scholar] [CrossRef]
- Tan, M.K.; Friend, J.R.; Yeo, L.Y. Interfacial Jetting Phenomena Induced by Focused Surface Vibrations. Phys. Rev. Lett. 2009, 103, 024501. [Google Scholar] [CrossRef]
- Castro, J.O.; Ramesan, S.; Rezk, A.R.; Yeo, L.Y. Continuous Tuneable Droplet Ejection via Pulsed Surface Acoustic Wave Jetting. Soft Matter 2018, 14, 5721–5727. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Sui, C.; Wu, Y.; Ao, Z.; Guo, S.-S.; Guo, F. A Digital Acoustofluidic Device for On-Demand and Oil-Free Droplet Generation. Nanotechnology 2019, 30, 084001. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wu, Z.; Gong, Z.; Xia, Y.; Li, J.; Du, L.; Zhang, Y.; Gao, X.; Fan, Z.; Hu, H.; et al. Acoustic Bioprinting of Patient-Derived Organoids for Predicting Cancer Therapy Responses. Adv. Healthc. Mater. 2022, 11, e2102784. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Du, L.; Li, J.; Wu, Z.; Gong, Z.; Xia, Y.; Fan, Z.; Qian, Q.; Ding, Z.; Hu, H.; et al. Modeling Cancer Metastasis Using Acoustically Bio-Printed Patient-Derived 3D Tumor Microtissues. J. Mater. Chem. B Mater. Biol. Med. 2022, 10, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Chen, H.; Li, J.; Hu, H.; Qian, Q.; He, R.-X.; Ding, Z.; Guo, S.-S. Acoustic Droplet-Assisted Superhydrophilic-Superhydrophobic Microarray Platform for High-Throughput Screening of Patient-Derived Tumor Spheroids. ACS Appl. Mater. Interfaces 2021, 13, 23489–23501. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Huang, L.-X.; Chen, H.; Li, J.; Chen, K.-K.; Hu, H.; Wang, F.-B.; Ding, Z.; Guo, S.-S. Acoustic Droplet Vitrification Method for High-Efficiency Preservation of Rare Cells. ACS Appl. Mater. Interfaces 2021, 13, 12950–12959. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Chen, K.; Cai, B.; Rao, L.; Wang, Z.; Sun, Y.; Yu, M.; Liu, W.; Guo, S.; Zhao, X.-Z. An Acoustic Droplet-Induced Enzyme Responsive Platform for the Capture and on-Demand Release of Single Circulating Tumor Cells. ACS Appl. Mater. Interfaces 2019, 11, 41118–41126. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Li, J.; Huang, L.-X.; Hua, B.; Guo, S.-S. In Situ Microreaction Platform Based on Acoustic Droplet Manipulation for Ultra-High-Precision Multiplex Bioassay. Anal. Chem. 2022, 94, 6347–6354. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Jiang, E.; Wei, X.; Xia, Y.; Wu, Z.; Gong, Z.; Shang, Z.; Guo, S. The Acoustic Droplet Printing of Functional Tumor Microenvironments. Lab Chip 2021, 21, 1604–1612. [Google Scholar] [CrossRef]
- Gong, Z.; Huang, L.; Tang, X.; Chen, K.; Wu, Z.; Zhang, L.; Sun, Y.; Xia, Y.; Chen, H.; Wei, Y.; et al. Acoustic Droplet Printing Tumor Organoids for Modeling Bladder Tumor Immune Microenvironment within a Week. Adv. Healthc. Mater. 2021, 10, e2101312. [Google Scholar] [CrossRef]
- Morshedi Rad, D.; Alsadat Rad, M.; Razavi Bazaz, S.; Kashaninejad, N.; Jin, D.; Ebrahimi Warkiani, M. A Comprehensive Review on Intracellular Delivery. Adv. Mater. 2021, 33, e2005363. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-E.; Khoo, H.; Hur, S.C. Recent Advances in Microscale Electroporation. Chem. Rev. 2022, 122, 11247–11286. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.P.; Langer, R.; Jensen, K.F. Intracellular Delivery by Membrane Disruption: Mechanisms, Strategies, and Concepts. Chem. Rev. 2018, 118, 7409–7531. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Stewart, M.; Sharei, A.; Weaver, J.C.; Langer, R.S.; Jensen, K.F. High-Throughput Nuclear Delivery and Rapid Expression of DNA via Mechanical and Electrical Cell-Membrane Disruption. Nat. Biomed. Eng. 2017, 1, 0039. [Google Scholar] [CrossRef] [PubMed]
- Ramesan, S.; Rezk, A.R.; Dekiwadia, C.; Cortez-Jugo, C.; Yeo, L.Y. Acoustically-Mediated Intracellular Delivery. Nanoscale 2018, 10, 13165–13178. [Google Scholar] [CrossRef] [PubMed]
- Ramesan, S.; Rezk, A.R.; Yeo, L.Y. High Frequency Acoustic Permeabilisation of Drugs through Tissue for Localised Mucosal Delivery. Lab Chip 2018, 18, 3272–3284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, Y.; Zhang, H.; Tang, Z.; Liu, W.; Lu, Y.; Wang, Z.; Yang, H.; Pang, W.; Zhang, H.; et al. Hypersonic Poration: A New Versatile Cell Poration Method to Enhance Cellular Uptake Using a Piezoelectric Nano-Electromechanical Device. Small 2017, 13, 1602962. [Google Scholar] [CrossRef] [PubMed]
- Ambattu, L.A.; Yeo, L.Y. Sonomechanobiology: Vibrational Stimulation of Cells and Its Therapeutic Implications. Biophys. Rev. 2023, 4, 021301. [Google Scholar] [CrossRef] [PubMed]
- Rich, J.; Tian, Z.; Huang, T.J. Sonoporation: Past, Present, and Future. Adv. Mater. Technol. 2022, 7, 2100885. [Google Scholar] [CrossRef]
- Wu, P.; Bai, L.; Lin, W. On the Definition of Cavitation Intensity. Ultrason. Sonochem. 2020, 67, 105141. [Google Scholar] [CrossRef]
- Meng, L.; Liu, X.; Wang, Y.; Zhang, W.; Zhou, W.; Cai, F.; Li, F.; Wu, J.; Xu, L.; Niu, L.; et al. Sonoporation of Cells by a Parallel Stable Cavitation Microbubble Array. Adv. Sci. 2019, 6, 1900557. [Google Scholar] [CrossRef] [PubMed]
- Helfield, B.; Chen, X.; Watkins, S.C.; Villanueva, F.S. Biophysical Insight into Mechanisms of Sonoporation. Proc. Natl. Acad. Sci. USA 2016, 113, 9983–9988. [Google Scholar] [CrossRef]
- Salari, A.; Appak-Baskoy, S.; Coe, I.R.; Abousawan, J.; Antonescu, C.N.; Tsai, S.S.H.; Kolios, M.C. Dosage-Controlled Intracellular Delivery Mediated by Acoustofluidics for Lab on a Chip Applications. Lab Chip 2021, 21, 1788–1797. [Google Scholar] [CrossRef]
- Ramesan, S.; Rezk, A.R.; Cevaal, P.M.; Cortez-Jugo, C.; Symons, J.; Yeo, L.Y. Acoustofection: High-Frequency Vibrational Membrane Permeabilization for Intracellular SiRNA Delivery into Nonadherent Cells. ACS Appl. Bio Mater. 2021, 4, 2781–2789. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Sun, M.; Yang, Y.; Xu, H.; Liu, J.; He, S.; Wang, Y.; Xu, L.; Pang, W.; Duan, X. Controllable Cell Deformation Using Acoustic Streaming for Membrane Permeability Modulation. Adv. Sci. 2021, 8, 2002489. [Google Scholar] [CrossRef] [PubMed]
- Belling, J.N.; Heidenreich, L.K.; Tian, Z.; Mendoza, A.M.; Chiou, T.-T.; Gong, Y.; Chen, N.Y.; Young, T.D.; Wattanatorn, N.; Park, J.H.; et al. Acoustofluidic Sonoporation for Gene Delivery to Human Hematopoietic Stem and Progenitor Cells. Proc. Natl. Acad. Sci. USA 2020, 117, 10976–10982. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, J.; Li, R.; Wang, Y.; Zhang, M.; Li, J.; Yin, S.; Liu, G.; Zhang, L.; Li, B.; et al. Stretchable Electronic Facial Masks for Sonophoresis. ACS Nano 2022, 16, 5961–5974. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Cai, H.; Wu, Z.; Li, X.; Tian, C.; Ao, Z.; Niu, V.C.; Xiao, X.; Jiang, L.; Khodoun, M.; et al. Acoustic Metamaterials-Driven Transdermal Drug Delivery for Rapid and on-Demand Management of Acute Disease. Nat. Commun. 2023, 14, 869. [Google Scholar] [CrossRef] [PubMed]
- Ozcelik, A.; Rufo, J.; Guo, F.; Gu, Y.; Li, P.; Lata, J.; Huang, T.J. Acoustic Tweezers for the Life Sciences. Nat. Methods 2018, 15, 1021–1028. [Google Scholar] [CrossRef]
- Rufo, J.; Zhang, P.; Zhong, R.; Lee, L.P.; Huang, T.J. A Sound Approach to Advancing Healthcare Systems: The Future of Biomedical Acoustics. Nat. Commun. 2022, 13, 3459. [Google Scholar] [CrossRef]
- Kang, S.; Wu, X.; Qi, H.; Yang, K.; Feng, R.; Guo, W.; Sun, C.; Duan, X.; Wang, Y. Effect of Hydrogel Matrix and Fluid Shear Stress on the Behavioral Regulation of Mesenchymal Stem Cells. Small Struct. 2024, 2300432. [Google Scholar] [CrossRef]
- Durrer, J.; Agrawal, P.; Ozgul, A.; Neuhauss, S.C.F.; Nama, N.; Ahmed, D. A Robot-Assisted Acoustofluidic End Effector. Nat. Commun. 2022, 13, 6370. [Google Scholar] [CrossRef]
- Zhang, Z.; Shi, Z.; Ahmed, D. SonoTransformers: Transformable Acoustically Activated Wireless Microscale Machines. Proc. Natl. Acad. Sci. USA 2024, 121, e2314661121. [Google Scholar] [CrossRef] [PubMed]
- Richard, C.; Vargas-Ordaz, E.J.; Zhang, Y.; Li, J.; Cadarso, V.J.; Neild, A. Acousto-Optofluidic 3D Single Cell Imaging of Macrophage Phagocytosis of Pseudomonas aeruginosa. Lab Chip 2024, 24, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Harley, W.S.; Kolesnik, K.; Xu, M.; Heath, D.E.; Collins, D.J. 3D Acoustofluidics via Sub-wavelength Micro-resonators. Adv. Funct. Mater. 2023, 33, 2211422. [Google Scholar] [CrossRef]
- Maramizonouz, S.; Tao, X.; Rahmati, M.; Jia, C.; Tao, R.; Torun, H.; Zheng, T.; Jin, H.; Dong, S.; Luo, J.; et al. Flexible and Bendable Acoustofluidics for Particle and Cell Patterning. Int. J. Mech. Sci. 2021, 202–203, 106536. [Google Scholar] [CrossRef]
- Yang, Z.; Jin, S.; Zhang, C.; Ren, J.; Jing, W.; Wei, X. Microfluidics-Assisted Synthesis of Hydrogel Microparticles with Acoustic-Magnetic Control. Chem. Eng. Sci. 2023, 281, 119082. [Google Scholar] [CrossRef]
- Chen, Z.; Pei, Z.; Zhao, X.; Zhang, J.; Wei, J.; Hao, N. Acoustic Microreactors for Chemical Engineering. Chem. Eng. J. 2022, 433, 133258. [Google Scholar] [CrossRef]
- Yiannacou, K.; Sharma, V.; Sariola, V. Programmable Droplet Microfluidics Based on Machine Learning and Acoustic Manipulation. Langmuir 2022, 38, 11557–11564. [Google Scholar] [CrossRef]
- Mohanty, S.; Paul, A.; Matos, P.M.; Zhang, J.; Sikorski, J.; Misra, S. CeFlowBot: A Biomimetic Flow-Driven Microrobot That Navigates under Magneto-Acoustic Fields. Small 2022, 18, e2105829. [Google Scholar] [CrossRef]
- Li, X.; Ji, Z.; Zhou, J.; Guo, Y.; He, Y.; Zhang, J.; Fu, Y. Machine Learning as a New Strategy for Designing Surface Acoustic Wave Resonators. Sens. Actuators A Phys. 2024, 369, 115158. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, Z.; Qiu, Y.; Hao, N. Acoustic Microfluidics for Colloidal Materials and Interface Engineering. Mater. Adv. 2023, 4, 988–994. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Gai, J.; Zhao, Y.; Liu, Y.; Liu, Y. Acoustofluidic Actuation of Living Cells. Micromachines 2024, 15, 466. https://doi.org/10.3390/mi15040466
Wu Y, Gai J, Zhao Y, Liu Y, Liu Y. Acoustofluidic Actuation of Living Cells. Micromachines. 2024; 15(4):466. https://doi.org/10.3390/mi15040466
Chicago/Turabian StyleWu, Yue, Junyang Gai, Yuwen Zhao, Yi Liu, and Yaling Liu. 2024. "Acoustofluidic Actuation of Living Cells" Micromachines 15, no. 4: 466. https://doi.org/10.3390/mi15040466
APA StyleWu, Y., Gai, J., Zhao, Y., Liu, Y., & Liu, Y. (2024). Acoustofluidic Actuation of Living Cells. Micromachines, 15(4), 466. https://doi.org/10.3390/mi15040466





