A Magnetic Millirobot Walks on Slippery Biological Surfaces for Targeted Cargo Delivery
Abstract
1. Introduction
2. Methods
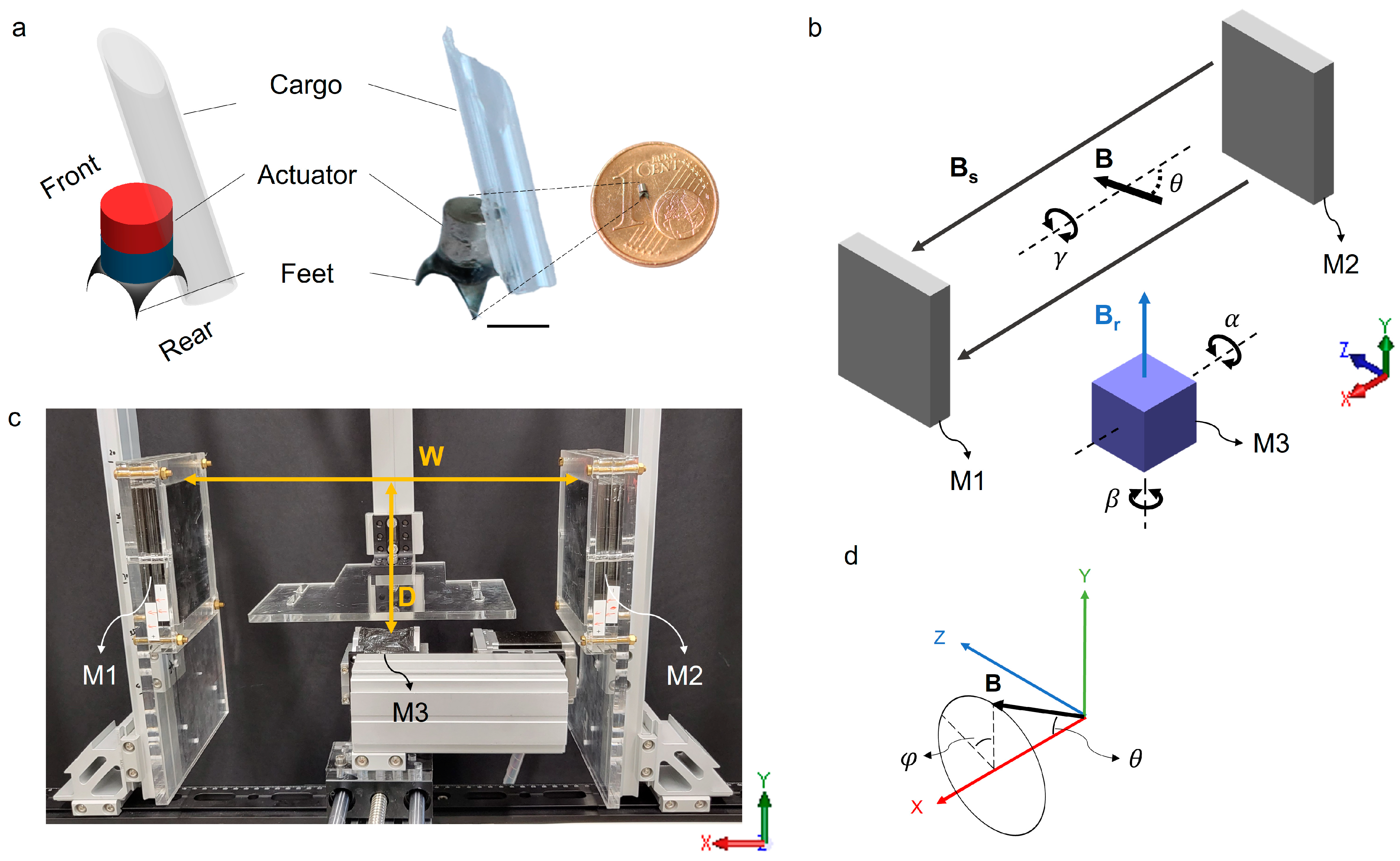
2.1. Design and Fabrication of the Millirobot
2.2. The Permanent Magnetic Actuation System
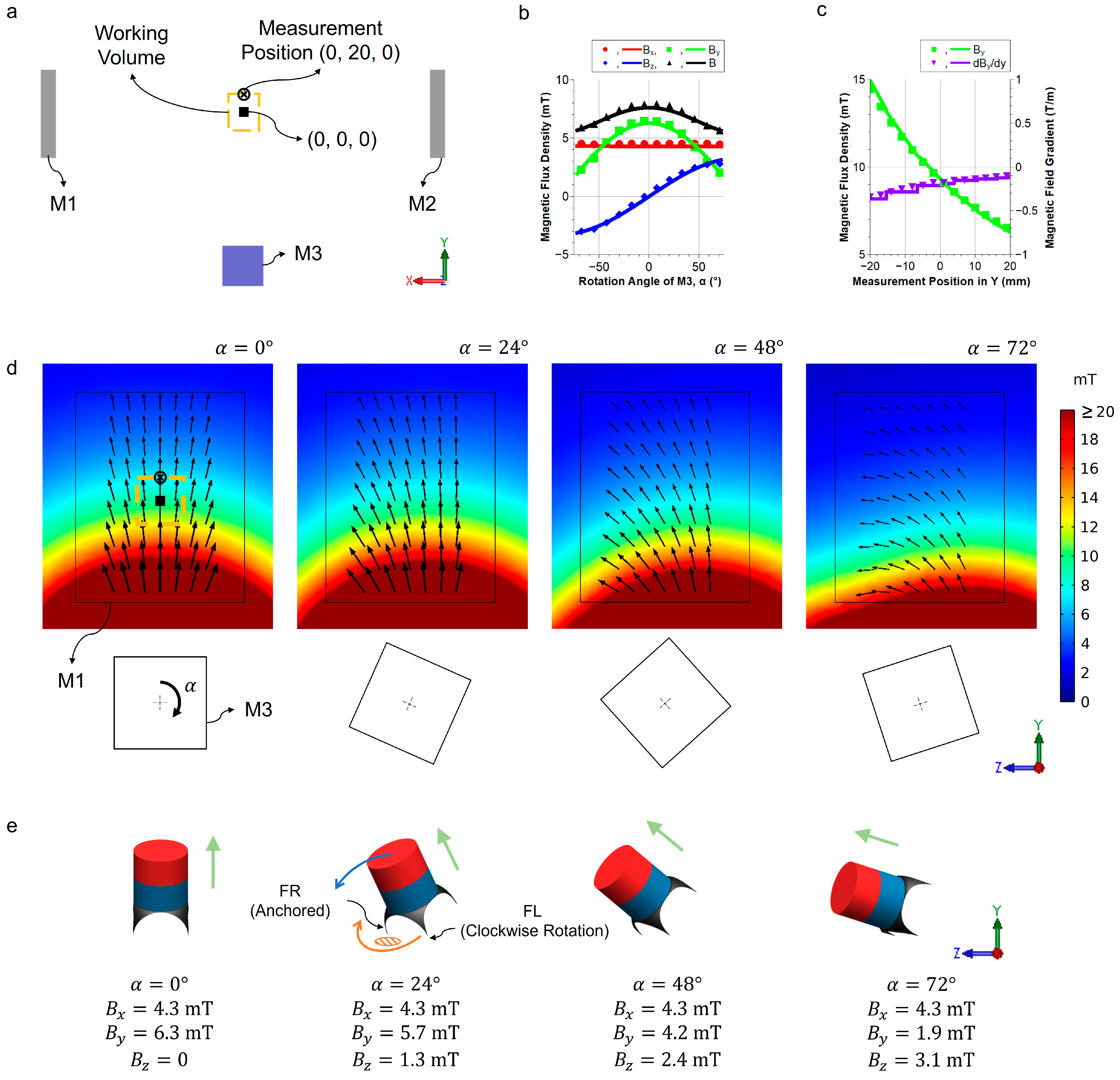
2.3. Numerical Simulation of the Actuation System
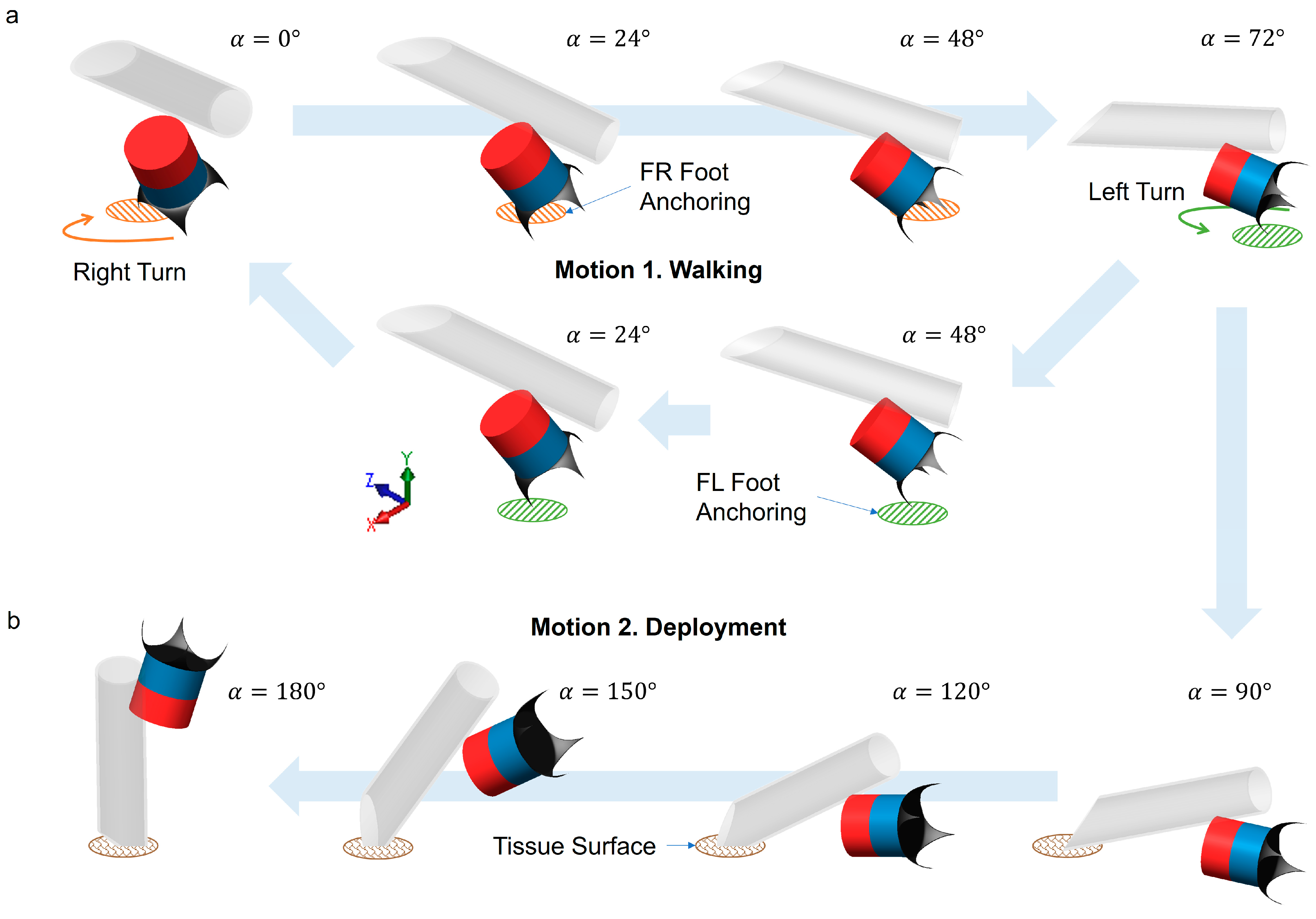
2.4. Motion Sequences of the Millirobot: Locomotion and Deployment
2.5. Testing of the Millirobot on Biological Surfaces
3. Results
3.1. Characterization of Magnetic Field for Actuation
3.2. Walking Motion
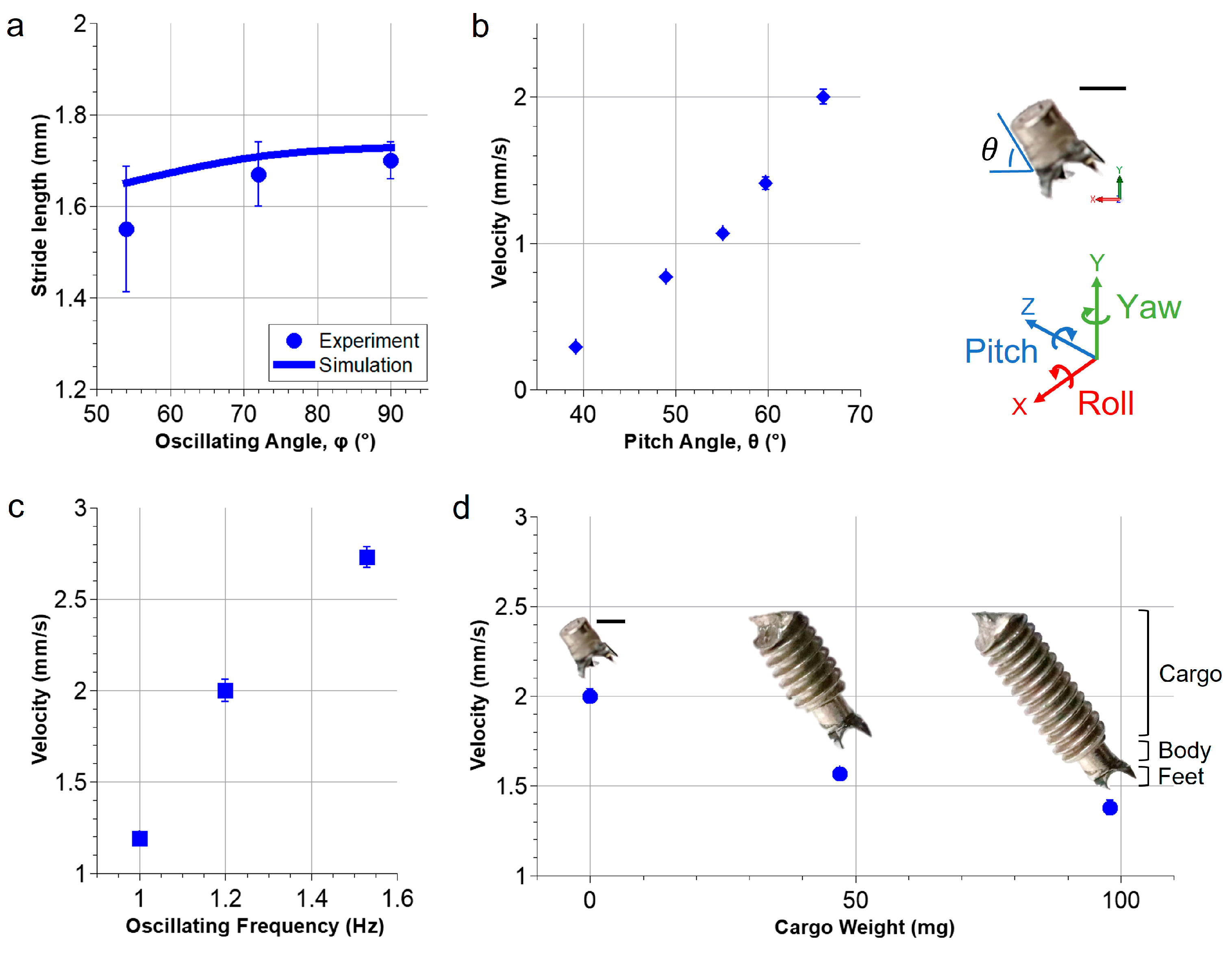
3.3. Locomotion Characterization of the Millirobot
3.4. The Control of the Millirobot
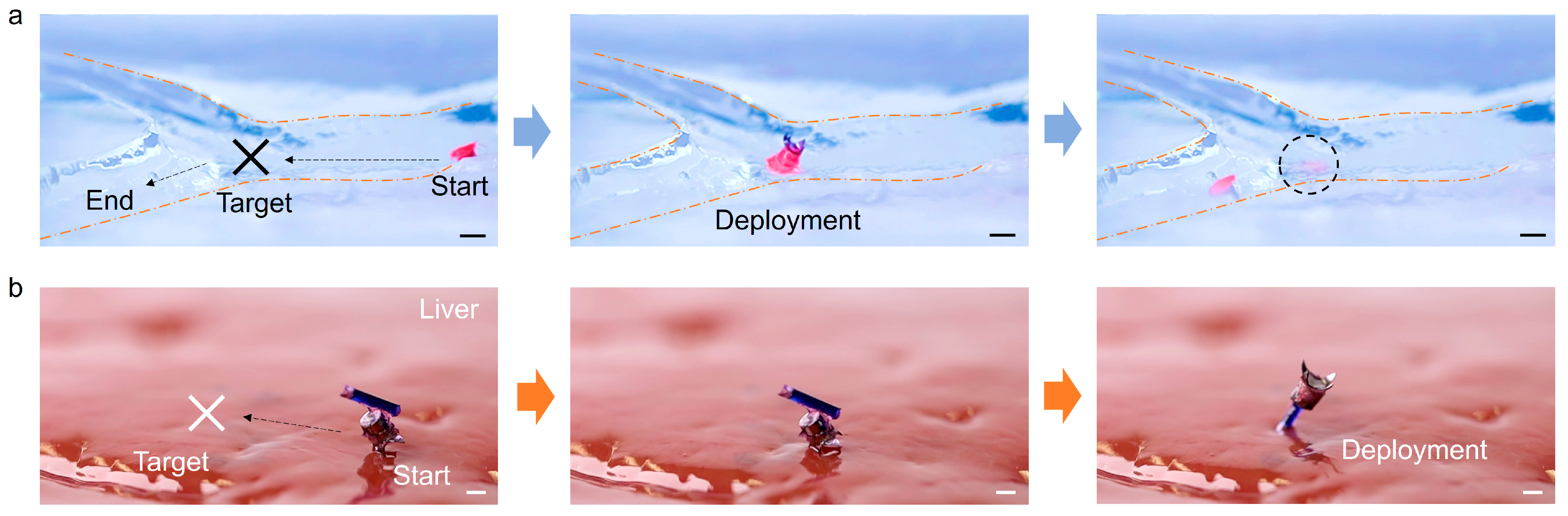
3.5. Cargo Deployment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Ávila, B.E.F.D.; Gao, W.; Zhang, L.; Wang, J. Micro/Nanorobots for Biomedicine: Delivery, Surgery, Sensing, and Detoxification. Sci. Robot. 2017, 2, 6431. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.K.; Medina-Sánchez, M.; Edmondson, R.J.; Schmidt, O.G. Engineering Microrobots for Targeted Cancer Therapies from a Medical Perspective. Nat. Commun. 2020, 11, 5618. [Google Scholar] [CrossRef]
- Wang, B.; Kostarelos, K.; Nelson, B.J.; Zhang, L. Trends in Micro-/Nanorobotics: Materials Development, Actuation, Localization, and System Integration for Biomedical Applications. Adv. Mater. 2021, 33, 2002047. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Fischer, P. Controlled Propulsion of Artificial Magnetic Nanostructured Propellers. Nano Lett. 2009, 9, 2243–2245. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Kagan, D.; Pak, O.S.; Clawson, C.; Campuzano, S.; Chuluun-Erdene, E.; Shipton, E.; Fullerton, E.E.; Zhang, L.; Lauga, E.; et al. Cargo-Towing Fuel-Free Magnetic Nanoswimmers for Targeted Drug Delivery. Small 2012, 8, 460–467. [Google Scholar] [CrossRef]
- Wu, Z.; Troll, J.; Jeong, H.H.; Wei, Q.; Stang, M.; Ziemssen, F.; Wang, Z.; Dong, M.; Schnichels, S.; Qiu, T.; et al. A Swarm of Slippery Micropropellers Penetrates the Vitreous Body of the Eye. Sci. Adv. 2018, 4, eaat4388. [Google Scholar] [CrossRef]
- Hu, W.; Lum, G.Z.; Mastrangeli, M.; Sitti, M. Small-Scale Soft-Bodied Robot with Multimodal Locomotion. Nature 2018, 554, 81–85. [Google Scholar] [CrossRef]
- Wu, Y.; Dong, X.; Kim, J.; Wang, C.; Sitti, M. Wireless Soft Millirobots for Climbing Three-Dimensional Surfaces in Confined Spaces. Sci. Adv. 2022, 8, eabn3431. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Shi, Q.; Zheng, Z.; Cui, J.; Sun, T.; Ferraro, P.; Huang, Q.; Fukuda, T. Biped Walking of Magnetic Microrobot in Oscillating Field for Indirect Manipulation of Non-Magnetic Objects. IEEE Trans. Nanotechnol. 2020, 19, 21–24. [Google Scholar] [CrossRef]
- Son, D.; Gilbert, H.; Sitti, M. Magnetically Actuated Soft Capsule Endoscope for Fine-Needle Biopsy. Soft Robot. 2020, 7, 10–21. [Google Scholar] [CrossRef]
- Esteban-Fernández de Ávila, B.; Angell, C.; Soto, F.; Lopez-Ramirez, M.A.; Báez, D.F.; Xie, S.; Wang, J.; Chen, Y. Acoustically Propelled Nanomotors for Intracellular SiRNA Delivery. ACS Nano 2016, 10, 4997–5005. [Google Scholar] [CrossRef]
- Qiu, T.; Palagi, S.; Mark, A.G.; Melde, K.; Adams, F.; Fischer, P. Active Acoustic Surfaces Enable the Propulsion of a Wireless Robot. Adv. Mater. Interfaces 2017, 4, 1700933. [Google Scholar] [CrossRef]
- Ma, Z.; Melde, K.; Athanassiadis, A.G.; Schau, M.; Richter, H.; Qiu, T.; Fischer, P. Spatial Ultrasound Modulation by Digitally Controlling Microbubble Arrays. Nat. Commun. 2020, 11, 4537. [Google Scholar] [CrossRef] [PubMed]
- Palagi, S.; Mark, A.G.; Reigh, S.Y.; Melde, K.; Qiu, T.; Zeng, H.; Parmeggiani, C.; Martella, D.; Sanchez-Castillo, A.; Kapernaum, N.; et al. Structured Light Enables Biomimetic Swimming and Versatile Locomotion of Photoresponsive Soft Microrobots. Nat. Mater. 2016, 15, 647–653. [Google Scholar] [CrossRef]
- Zhang, F.; Ye, D.; Song, S.; Meng, M.Q.-H. Design of A Novel Biopsy Capsule Robot with Anchoring Function for Intestinal Tract. In Proceedings of the 2019 IEEE International Conference on Robotics and Biomimetics (ROBIO), Dali, China, 6–8 December 2019; pp. 1471–1476. [Google Scholar]
- Rehan, M.; Yeo, A.G.; Yousuf, M.U.; Avci, E. Anchoring Mechanism for Capsule Endoscope: Mechanical Design, Fabrication and Experimental Evaluation. Micromachines 2022, 13, 2045. [Google Scholar] [CrossRef]
- Abbott, J.J.; Peyer, K.E.; Lagomarsino, M.C.; Zhang, L.; Dong, L.; Kaliakatsos, I.K.; Nelson, B.J. How Should Microrobots Swim? Int. J. Robot. Res. 2009, 28, 1434–1447. [Google Scholar] [CrossRef]
- Qiu, T.; Jeong, M.; Goyal, R.; Kadiri, V.M.; Sachs, J.; Fischer, P. Magnetic Micro-/Nanopropellers for Biomedicine. In Field-Driven Micro and Nanorobots for Biology and Medicine; Sun, Y., Wang, X., Yu, J., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 389–411. ISBN 978-3-030-80197-7. [Google Scholar]
- Leibinger, A.; Forte, A.E.; Tan, Z.; Oldfield, M.J.; Beyrau, F.; Dini, D.; Rodriguez y Baena, F. Soft Tissue Phantoms for Realistic Needle Insertion: A Comparative Study. Ann. Biomed. Eng. 2016, 44, 2442–2452. [Google Scholar] [CrossRef]
- McDowell, M.A.; Fryar, C.D.; Ogden, C.L. Anthropometric Reference Data for Children and Adults: United States, 1988–1994. Vital Health Stat. 11 2009, 1–68. Available online: https://pubmed.ncbi.nlm.nih.gov/19642512/ (accessed on 12 July 2023).
- Peyer, K.E.; Zhang, L.; Nelson, B.J. Bio-Inspired Magnetic Swimming Microrobots for Biomedical Applications. Nanoscale 2013, 5, 1259–1272. [Google Scholar] [CrossRef]
- Pore-Size Dependence and Slow Relaxation of Hydrogel Friction on Smooth Surfaces|PNAS. Available online: https://www.pnas.org/doi/10.1073/pnas.1922364117 (accessed on 22 June 2023).
- Bi, C.; Guix, M.; Johnson, B.V.; Jing, W.; Cappelleri, D.J. Design of Microscale Magnetic Tumbling Robots for Locomotion in Multiple Environments and Complex Terrains. Micromachines 2018, 9, 68. [Google Scholar] [CrossRef]
- Krachler, A.-M.; Orth, K. Adhesins During Infection. In Encyclopedia of Microbiology, 4th ed.; Schmidt, T.M., Ed.; Academic Press: Oxford, UK, 2019; pp. 28–37. ISBN 978-0-12-811737-8. [Google Scholar]
- Miranda, I.; Souza, A.; Sousa, P.; Ribeiro, J.; Castanheira, E.M.S.; Lima, R.; Minas, G. Properties and Applications of PDMS for Biomedical Engineering: A Review. J. Funct. Biomater. 2021, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Sitti, M.; Ceylan, H.; Hu, W.; Giltinan, J.; Turan, M.; Yim, S.; Diller, E. Biomedical Applications of Untethered Mobile Milli/Microrobots. Proc. IEEE 2015, 103, 205–224. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, M.; Tan, X.; Fischer, F.; Qiu, T. A Magnetic Millirobot Walks on Slippery Biological Surfaces for Targeted Cargo Delivery. Micromachines 2023, 14, 1439. https://doi.org/10.3390/mi14071439
Jeong M, Tan X, Fischer F, Qiu T. A Magnetic Millirobot Walks on Slippery Biological Surfaces for Targeted Cargo Delivery. Micromachines. 2023; 14(7):1439. https://doi.org/10.3390/mi14071439
Chicago/Turabian StyleJeong, Moonkwang, Xiangzhou Tan, Felix Fischer, and Tian Qiu. 2023. "A Magnetic Millirobot Walks on Slippery Biological Surfaces for Targeted Cargo Delivery" Micromachines 14, no. 7: 1439. https://doi.org/10.3390/mi14071439
APA StyleJeong, M., Tan, X., Fischer, F., & Qiu, T. (2023). A Magnetic Millirobot Walks on Slippery Biological Surfaces for Targeted Cargo Delivery. Micromachines, 14(7), 1439. https://doi.org/10.3390/mi14071439





