Path Planning and Navigation of Miniature Serpentine Robot for Bronchoscopy Application
Abstract
1. Introduction
2. Mechanical Design, Control Scheme, Path Planning, and Navigation of the Miniature Serpentine Robotic Bronchoscopy
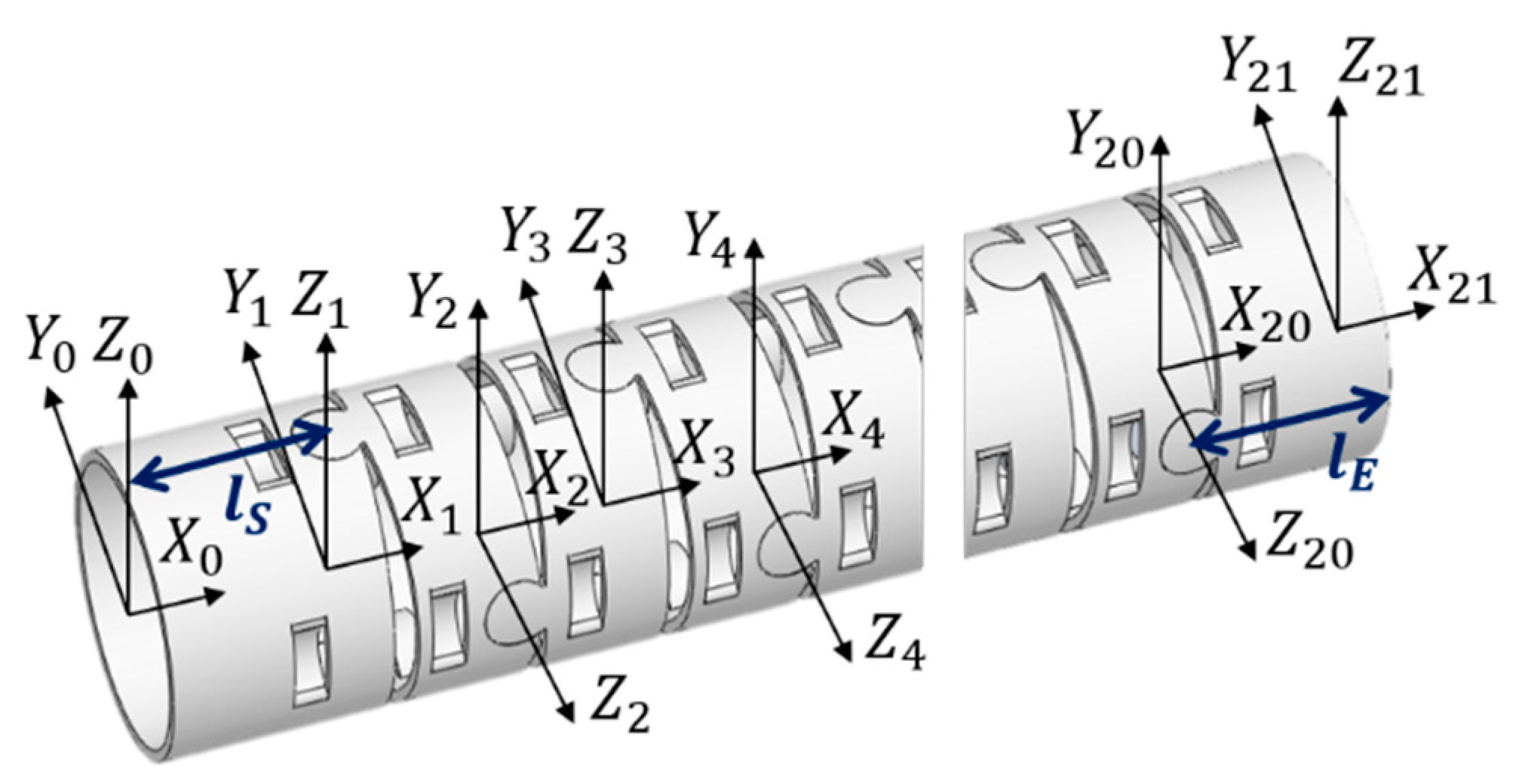
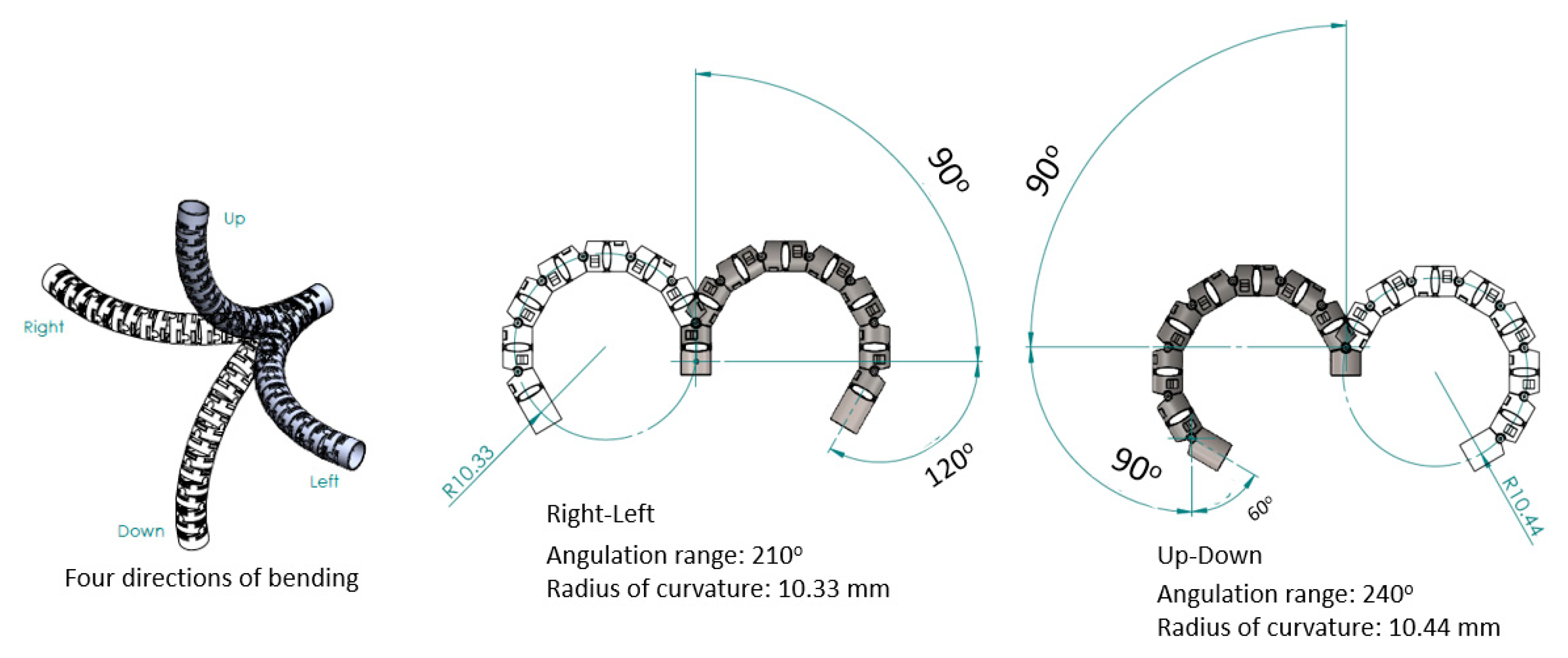
2.1. Mechanical Design
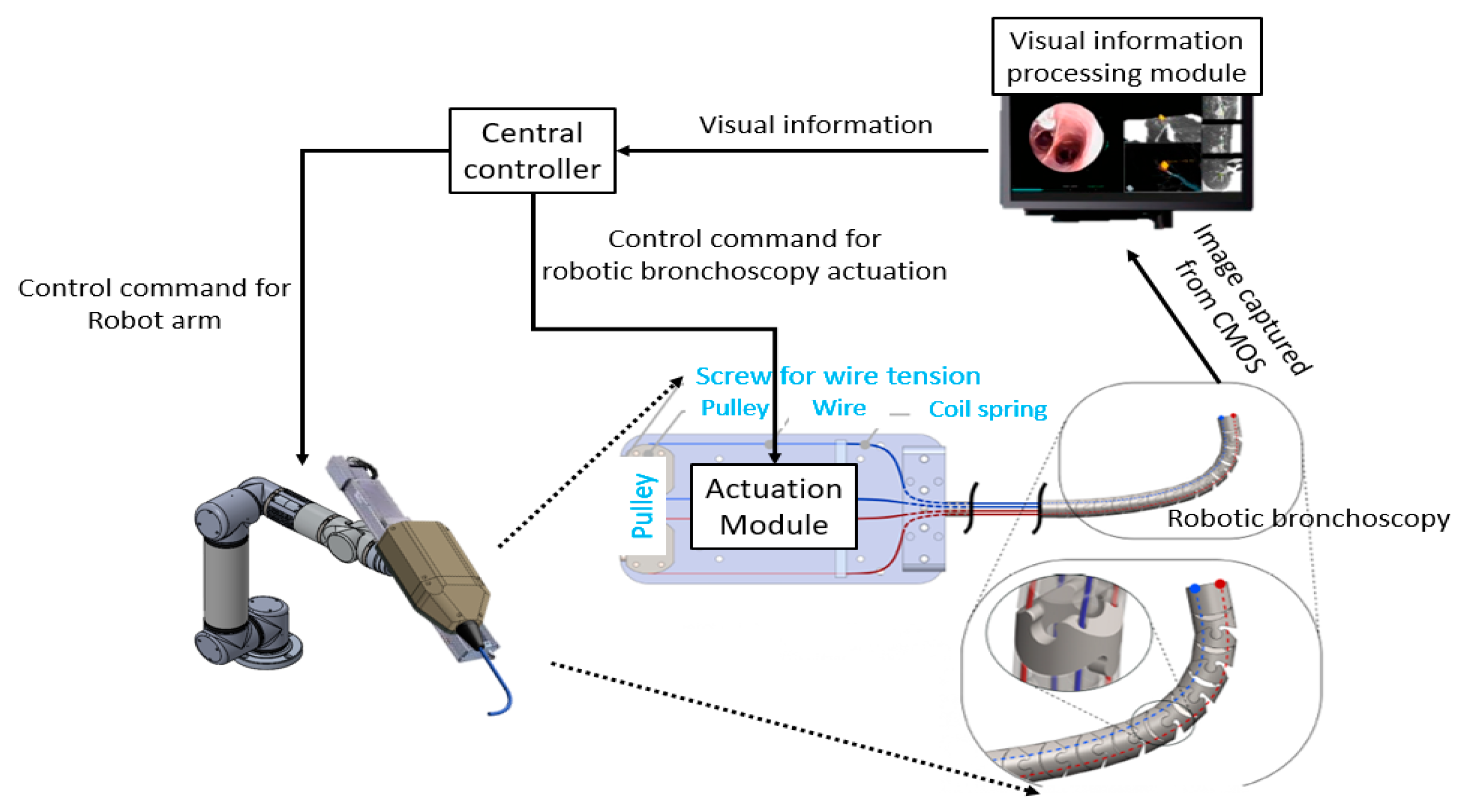
2.2. Control Scheme
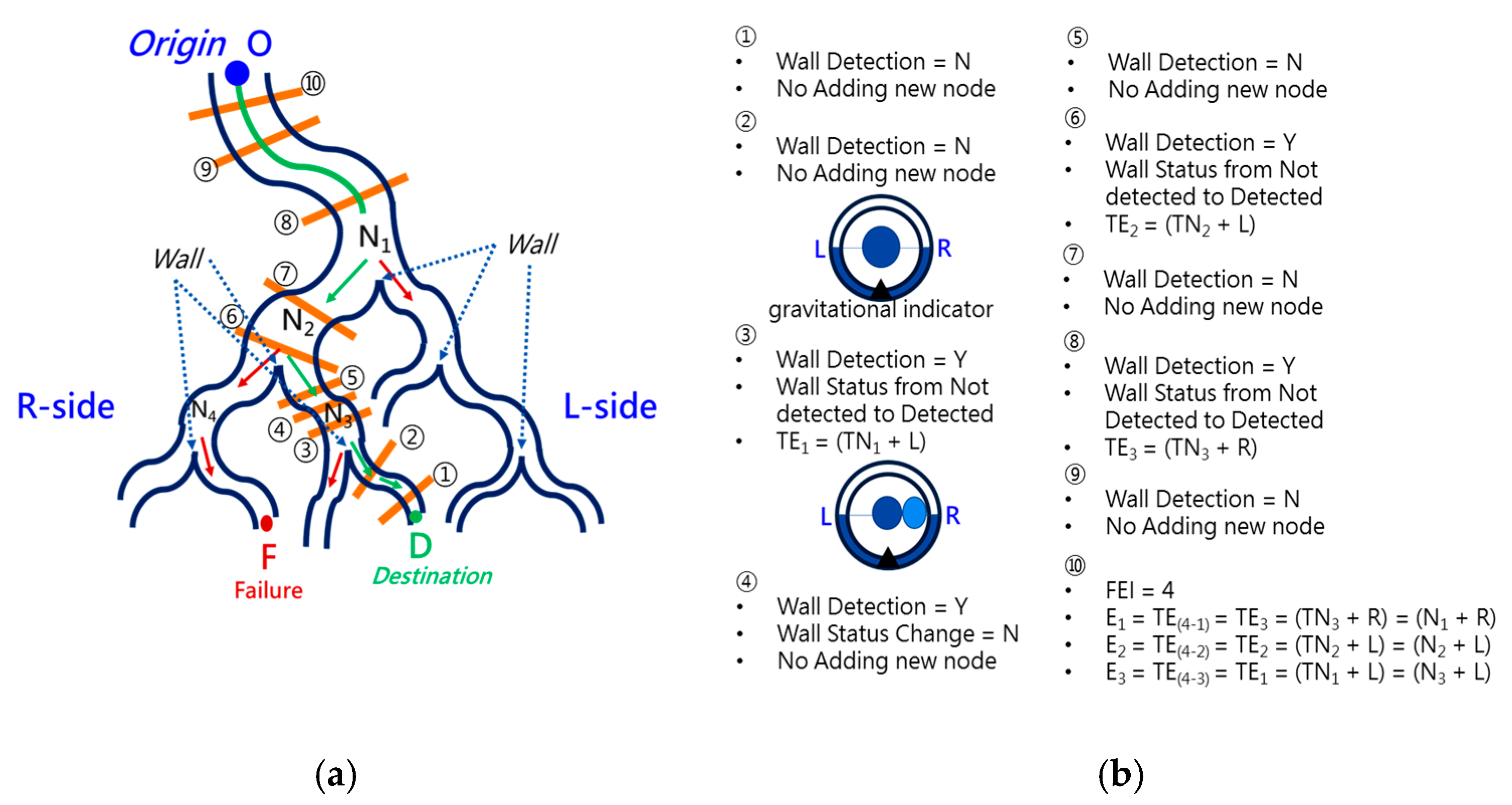
2.3. Backward Path Planning
2.4. Forward Navigation
2.5. Virtual Force for Centering
3. System Implementation and Results
3.1. Implementation
3.2. Results
4. Discussion and Conclusions
5. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMSC | Adipose-derived Mesenchymal Stem Cells |
| CMOS | Complementary Metal-Oxide-Semiconductor |
| COPD | Chronic Obstructive Pulmonary Disease |
| COVID-19 | Coronavirus Disease 2019 |
| CT | Computed Tomography |
| EMA | Electro-Magnetic Actuation |
| MRI | Magnetic Resonance Imaging |
| MSC | Mesenchymal Stem Cells |
| NOTES | Natural Orifice Transluminal Endoscopic Surgery |
References
- Golchin, A.; Seyedjafari, E.; Ardeshirylajimi, A. Mesenchymal Stem Cell Therapy for COVID-19: Present or Future. Stem Cell Rev. Rep. 2020, 16, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Bae, K.S.; Kim, H.S.; Lee, W.-Y. Effectiveness of Mesenchymal Stem Cell Therapy for COVID-19-Induced ARDS Patients: A Case Report. Medicina 2022, 58, 1698. [Google Scholar] [CrossRef] [PubMed]
- Mallis, P.; Chatzistamatiou, T.; Dimou, Z.; Sarri, E.F.; Georgiou, E.; Salagianni, M.; Triantafyllia, V.; Andreakos, E.; Stavropoulos-Giokas, C.; Michalopoulos, E. Mesenchymal Stromal Cell Delivery as a Potential Therapeutic Strategy against COVID-19: Promising Evidence from in vitro Results. World J. Biol. Chem. 2022, 13, 47–65. [Google Scholar] [CrossRef] [PubMed]
- George, P.M.; Wells, A.U.; Jenkins, R.G. Pulmonary Fibrosis and COVID-19: The Potential Role for Antifibrotic Therapy. Lancet Respir. Med. 2020, 8, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fei, X.; Zhang, X.; Bao, W.; Han, L.; Xue, Y.; Hao, H.; Zhou, X.; Zhang, M. Adipose-derived Mesenchymal Stem Cells Suppress Ozone-mediated Airway Inflammation in a Mouse Model of Chronic Obstructive Pulmonary Disease. Mol. Immunol. 2022, 151, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Nejaddehbashi, F.; Radan, M.; Bayati, V.; Dianat, M.; Ali Mard, S.; Mansouri, Z. Adipose-derived Mesenchymal Stem Cells in Emphysema: Comparison of Inflammatory Markers Changes in Response to Intratracheal and Systemic Delivery Method. Tissue Cell 2023, 80, 102011. [Google Scholar] [CrossRef] [PubMed]
- Go, G.; Jeong, S.G.; Yoo, A.; Han, J.; Kang, B.; Kim, S.J.; Nguyen, T.; Jin, Z.; Kim, C.S.; Seo, Y.; et al. Human Adipose–derived Mesenchymal Stem Cell–based Medical Microrobot System for Knee Cartilage Regeneration in vivo. Sci. Robot. 2020, 5, eaay6626. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.C.; Pastis, N.J.; Mahajan, A.K.; Khandhar, S.J.; Simoff, M.J.; Machuzak, M.S.; Cicenia, J.; Gildea, T.R.; Silvestri, G.A. Robotic Bronchoscopy for Peripheral Pulmonary Lesions: A Multicenter Pilot and Feasibility Study (BENEFIT). Chest 2021, 159, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Donno, A.D.; Zorn, L.; Zanne, P.; Nageotte, F.; Mathelin, M.D. Introducing STRAS: A New Flexible Robotic System for Minimally Invasive Surgery. In Proceedings of the IEEE International Conference on Robotics and Automation, Karlsruhe, Germany, 6–10 May 2013; pp. 1213–1220. [Google Scholar]
- Webster, R.J.; Okamura, A.M.; Cowan, N.J. Toward Active Cannulas: Miniature Snake-like Surgical Robots. In Intelligent Robots and Systems; IEEE: New York, NY, USA, 2006; pp. 2857–2863. [Google Scholar]
- Simaan, N.; Xu, K.; Kapoor, A.; Wei, W.; Kazanzides, P.; Flint, P.; Taylor, R. Design and Integration of a Telerobotic System for Minimally Invasive Surgery of the Throat. Int. J. Robot. Res.-Spec. Issue Med. Robot. 2009, 28, 1134–1153. [Google Scholar] [CrossRef] [PubMed]
- Yeshmukhametov, A.; Koganezawa, K.; Yamamoto, Y. Design and Kinematics of Cable-Driven Continuum Robot Arm with Universal Joint Backbone. In Proceedings of the IEEE International Conference on Robotics and Biomimetics (ROBIO), Kuala Lumpur, Malaysia, 12–15 December 2018; pp. 2444–2449. [Google Scholar]
- Ho, E.; Hedstrom, G.; Septimiu, M. Robotic Bronchoscopy in Diagnosing Lung Cancer—The Evidence, Tips and Tricks: A Clinical Practice Review. Ann. Transl. Med. 2023. [Google Scholar] [CrossRef]
- Duan, X.; Xie, D.; Zhang, R.; Li, X.; Sun, J.; Qian, C.; Song, X.; Li, C. A Novel Robotic Bronchoscope System for Navigation and Biopsy of Pulmonary Lesions. Cyborg Bionic Syst. 2023, 4, 0013. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Wu, H.Y.; Huang, S.; Chang, J.Y. Kinematic Analysis and Mechanism Design of Surgical Tendon-driven Serpentine Manipulators. In Proceedings of the International Conference on Advanced Mechanism and Machine Technology (CSMMT), Bengaluru, India, 9–10 July 2021. Paper Number 058. [Google Scholar]
- Wu, H.Y.; Huang, S.; Wu, C.Y.; Kuan, C.P.; Wang, A.P. The Design and Control Scheme of Miniature Serpentine Robot for In-Body Visual Servo Applications. In Proceedings of the World Congress on Micro and Nano Manufacturing (WCMNM 2022), Leuven, Belgium, 19–22 September 2022. [Google Scholar]
- Monarch Platform. Available online: https://www.jnjmedtech.com/en-US/product-family/monarch (accessed on 12 March 2023).
- Intuitive Da Vinci SP. Available online: https://www.intuitive.com/en-us/products-and-services/da-vinci/systems/sp (accessed on 12 March 2023).
- Marinho, M.M.; Ishida, H.; Harada, K.; Deie, K.; Mitsuishi, M. Virtual Fixture Assistance for Suturing in Robot-Aided Pediatric Endoscopic Surgery. IEEE Robot. Autom. Lett. 2019, 5, 524–531. [Google Scholar] [CrossRef]

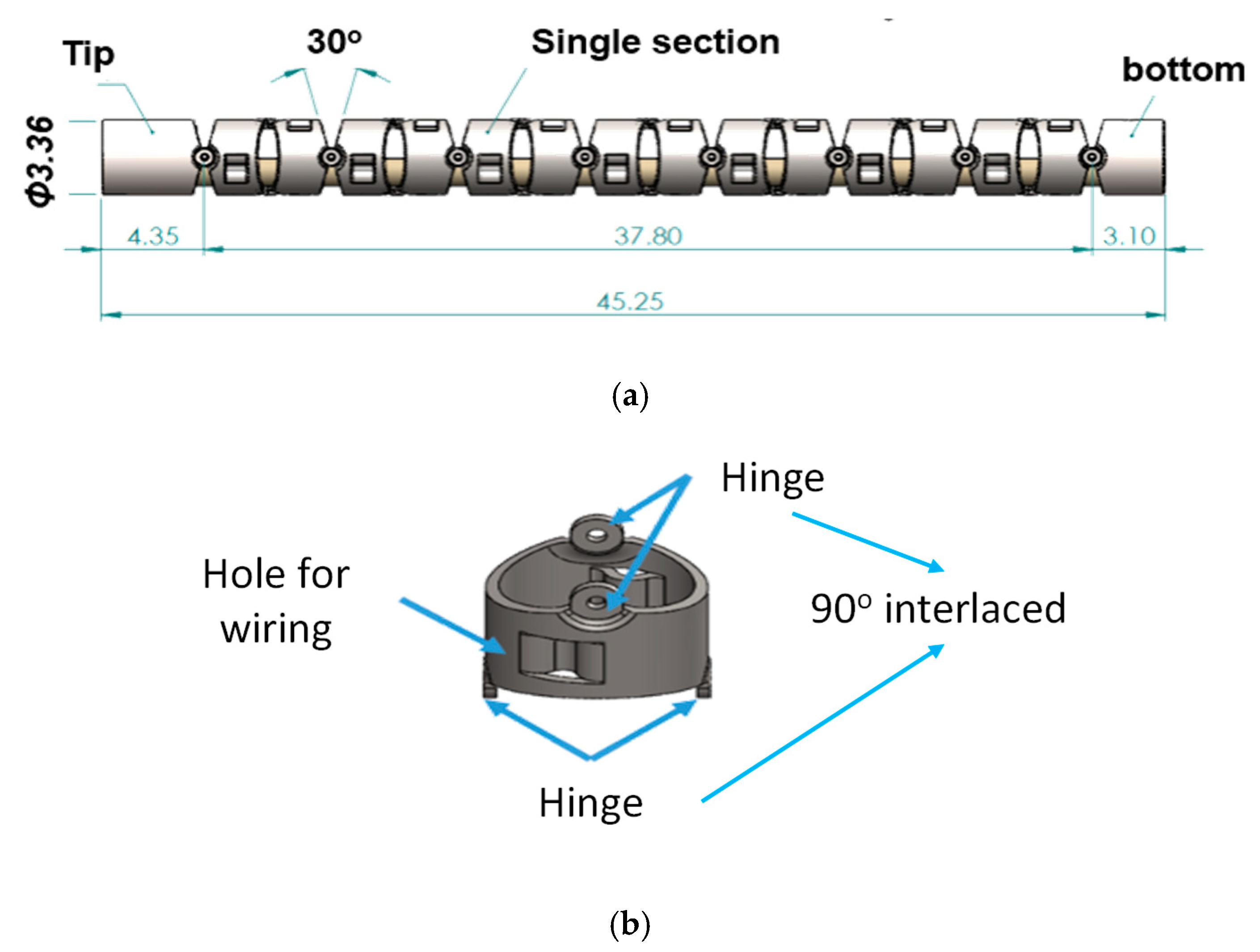












| Item | Description | Guidelines/Requirements |
|---|---|---|
| 1. | Bending section at the front portion of the robotic bronchoscopy |
|
| 2. | Outer diameter of the bending section |
|
| 3. | Real-time and in-situ visual information |
|
| Joint | θ | d | a | α |
|---|---|---|---|---|
| 1 | 0 | 0 | lS | 0° |
| 2 | θ1 | 0 | g + h | 90° |
| 3 | θ2 | 0 | g + h | −90° |
| 4 | θ3 | 0 | g + h | 90° |
| 14 | θ13 | 0 | g + h | 90° |
| 15 | θ14 | 0 | lE | −90° |
| Event | TEvent | TNode | Node | Decision | FEI = 4 |
|---|---|---|---|---|---|
| Event1 | TEvent(4-1) = TEvent3 | TNode3 | Node1 | R | |
| Event2 | TEvent(4-2) = TEvent2 | TNode2 | Node2 | L | |
| Event3 | TEvent(4-3) = TEvent2 | TNode1 | Node3 | L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuan, C.-P.; Huang, S.; Wu, H.-Y.; Wang, A.-P.; Wu, C.-Y. Path Planning and Navigation of Miniature Serpentine Robot for Bronchoscopy Application. Micromachines 2023, 14, 969. https://doi.org/10.3390/mi14050969
Kuan C-P, Huang S, Wu H-Y, Wang A-P, Wu C-Y. Path Planning and Navigation of Miniature Serpentine Robot for Bronchoscopy Application. Micromachines. 2023; 14(5):969. https://doi.org/10.3390/mi14050969
Chicago/Turabian StyleKuan, Cheng-Peng, Shu Huang, Hao-Yan Wu, An-Peng Wang, and Chien-Yu Wu. 2023. "Path Planning and Navigation of Miniature Serpentine Robot for Bronchoscopy Application" Micromachines 14, no. 5: 969. https://doi.org/10.3390/mi14050969
APA StyleKuan, C.-P., Huang, S., Wu, H.-Y., Wang, A.-P., & Wu, C.-Y. (2023). Path Planning and Navigation of Miniature Serpentine Robot for Bronchoscopy Application. Micromachines, 14(5), 969. https://doi.org/10.3390/mi14050969