Abstract
Electron transport layer (ETL) plays an undeniable role in improving the performance of n-i-p planar perovskite solar cells (PSCs). Titanium dioxide (TiO2) is known as a promising ETL material for perovskite solar cell. In this work, the effect of annealing temperature on optical, electrical, and surface morphology of the electron-beam (EB)-evaporated TiO2 ETL, and consequently on the performance of perovskite solar cell, was investigated. It was found that annealing treatment at an optimized temperature of 480 °C considerably improved the surface smoothness, density of grain boundaries, and carrier mobility of TiO2 film, which resulted in nearly 10-fold improvement in power conversion efficiency (11.16%) in comparison with the unannealed device (1.08%). The improvement in performance of the optimized PSC is attributed to the acceleration of charge carrier extraction, as well as suppression of the recombination at the ETL/Perovskite interface.
1. Introduction
Organic–inorganic hybrid perovskite solar cells (PSCs) have attracted considerable attention as one of the emerging research trends for both the academic and industrial sectors in the past decade. PSCs offer intriguing optoelectronics properties such as long carrier life time, high absorption coefficient, intrinsic low trap density, and ambipolar transport properties [1,2,3,4,5,6]. According to the National Renewable Energy Laboratory (NREL) chart, PSCs have demonstrated rapid progress in the performance enhancement from 3.8% [7] to a certified 25.7% [8]. Among the different components of PSC, the electron transport layer (ETL) plays a critical role in extracting and transporting photogenerated electron carriers and suppressing charge recombination in photovoltaic devices [9]. Therefore, interface engineering is now regarded as an efficient strategy to improve the power conversion efficiency (PCE) of perovskite solar cell devices by preventing the undesired degradation pathways [10]. This is mainly due to the fact that the photoinduced carriers not only have to transport across the interfaces in the cell but also charge extraction generally occurs at the interfaces, thereby leading to undesirable charge losses due to any possible interfacial defects and the associated specific charge distributions [10,11].
Recently, intensive efforts have been devoted to improving the electron transport layer (ETL)/perovskite layer, perovskite layer/hole transport layer, and the front and back contact interfaces [12,13,14]. Concurrently, various functional materials have been investigated to overcome the interfacial losses in PSC for performance enhancement. One of the most attractive target materials is titanium dioxide (TiO2) due to its appealing advantages, such as high transparency, favorable bandgap edge positions in relation to perovskites, environmental stability, etc. For instance, Li et al. successfully grew an array of anatase-phase TiO2 nanorods on FTO conductive glass using a hydrothermal method, enabling a 15.3% efficiency for PSC [15]. Tian et al. fabricated a composite TiO2 ETL using a mixed spray pyrolysis method, demonstrating that TiO2 nanoparticles can promote the charge extraction process at the TiO2/perovskite interfaces and improve the recombination process in perovskite layers [16]. Very recently, magnetron sputtering technology on FTO glass was also used to fabricate different thicknesses and shapes of TiO2 ETL for PSC [17,18]. Electron beam (EB) evaporation of TiO2 has emerged as a promising and cost-effective approach for large-scale production of perovskite solar cells (PSCs) [19]. The annealing process for TiO2 probably plays a key role in the crystalline perovskite phase and subsequently the charge extraction process. However, previous studies have primarily focused on PSCs utilizing indium tin oxide (ITO) substrates and organic–inorganic perovskite layers [20]. Considering the scarcity of indium, it is of greater practical significance to explore PSCs based on fluorine-doped tin oxide (FTO) substrates. Moreover, inorganic perovskite materials theoretically exhibit superior stability compared to their organic counterparts. Consequently, investigating the impact of annealing on the characteristics of perovskite solar cells prepared via EB evaporation of TiO2 on FTO substrates holds substantial importance.
In this context, we have investigated the effect of annealing temperature on the optical, electrical, and morphological properties of EB-evaporated TiO2 films to be used in PSC devices. Upon varying the annealing temperature, we demonstrated the temperature-dependent morphology and opto-electronic properties of TiO2 as a ETL for PSC. The 480 °C-heat-treated TiO2 film led to the formation of a uniform and dense surface of TiO2 film with minimum defects and high carrier mobility. Notably, the PSC with TiO2 films annealed at 480 °C showed a significant increase in the fill factor (73%), which is 3.5 times higher than that of an unannealed device (16%). For the power conversion efficiency, an approximately 10-fold improvement (11.16%) for 480 °C annealed PSC was obtained as compared to the reference device (1.08%). To unveil the internal conversion mechanism, a detailed analysis of electrical properties and charge transfer at the TiO2/perovskite interface was also performed.
2. Materials and Methods
2.1. Fabrication of CsPbI3-xBrx Perovskite Solar Cells
TiO2 thin films were deposited on fluorine-doped tin-oxide-coated glass (FTO/glass) by EB evaporation, as shown in step 1 of Figure 1. Before deposition, the chamber was evacuated to a base pressure of 5 × 10−3 Pa and the substrate was heated to 350 °C. TiO2 particles were used as the source for evaporation, the deposition rate and film thickness were set at 0.5 Å·s−1 and 75 nm, respectively, and oxygen was introduced during the deposition process while the pressure was kept at 3.3 × 10−2 Pa. Annealing of the TiO2 thin film was carried out in oxygen at 200 °C, 300 °C, 400 °C, 450 °C, 480 °C, and 500 °C for 2 h using a tube furnace, as shown in step 2 of Figure 1. A mixture of 380.4 mg HPbI3, 187.08 mg CsI, and 73.4 mg PbBr2 was dissolved in DMF and DMSO and filtered with a 0.2 μm syringe filter to form clear CsPbI3-xBrx solution. CsPbI3-xBrx films were casted by spin coating in a glove box at 4500 r.p.m for 30 s and dried on a hot plate at 200 °C for 5 min, as shown in step 3 of Figure 1. HTL was applied immediately after the perovskite film was cooled. The precursor solution for the HTL was prepared by dissolving 72.3 mg spiro-OMeTAD, 29 μL TBP, and 18 μL Li-TFSI solution (520 mg Li-TFSI in 1 mL acetonitrile) in 1 mL CB. The HTL was casted via spin coating at 7050 r.p.m. for 30 s in the glove box, as shown in step 4 of Figure 1. The samples were then stored in a dark desiccator for the night. A 70 nm thick Ag layer was then deposited on top of the HTL through a metal shadow mask by thermal evaporation at a base pressure of about 2 × 10−3 Pa, as shown in step 5 of Figure 1.
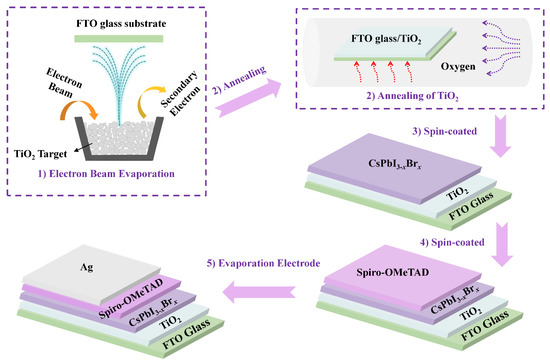
Figure 1.
Schematic illustration of the fabrication process for CsPbI3-xBrx perovskite solar cell.
2.2. Characterization
The surface morphologies of the samples were examined using a Field Emission Scanning Electron Microscope (FESEM, S-4800, Hitachi, Tokyo, Japan) and scanning probe microscope (Dimension edge, Bruker, Saarbrucken, Germany). Current density–voltage (J-V) curves were measured in dry air using a Keithley 2400 source meter under standard 1 sun AM 1.5 simulated solar irradiation system (SAN-EI 100 mw·cm−2) from Giant Force Technology Co., Ltd., New Taipei City, Taiwan. The scan rate of 50 mV·s−1 was adopted in the J-V measurement. The absorption properties of films were determined using the UV-Vis spectrophotometer (Lambda 850, Perkin Elmer, Waltham, MA, USA). Photoluminescence (PL) spectra were measured by using an Edinburgh, FLS 980 fluorescence spectrometer under excitation at 375 nm.
3. Results
3.1. Morphology Properties of TiO2 Films
In order to evaluate the surface morphology of the TiO2 film, Atomic Force Microscopy (AFM) (SPI3800N/SPA400, Rigaku, Tokyo, Japan) was employed, and Scanning Electron Microscopy (SEM) (S-4800, Hitachi, Tokyo, Japan) analyses were carried out on unannealed and annealed samples with different annealing temperatures ranging from 200 °C to 500 °C. As shown in Figure 2(A1–A7), the surface roughness of the unannealed TiO2 film was 33.91 nm. As the TiO2 film underwent annealing treatment, the roughness decreased from 32.10 nm to 26.17 nm with an increase in annealing temperature from 200 °C to 480 °C, respectively. Surprisingly, as the annealing temperature rose from 480 °C to 500 °C, the roughness remained constant and is expected to increase further for higher temperatures. Figure 2(B1–B7) represents the top-view SEM characterization of unannealed and annealed TiO2 films. It was observed that the surface of TiO2 contained a combination of large (0.325–0.75 μm) and small grains (0.025–0.3 μm), along with some pinholes and voids in the range of 0.025 μm to 0.1 μm.
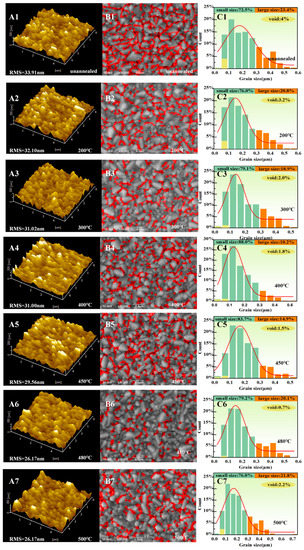
Figure 2.
(A1–A7) AFM image of TiO2 films at different annealing temperatures. (B1–B7) SEM images of the TiO2 film at different annealing temperatures. (C1–C7) Statistics on the percentage of grains and voids of TiO2 film at different annealing temperatures.
Statistical data Figure 2(C1–C7) reveals that as the annealing temperature escalated from 200 °C to 480 °C, the percentage of voids dramatically declined from 4% to 0.7%. However, as the temperature rose to 500 °C, the number of pinholes and voids significantly increased to 2%, as shown in the red indicated area in Figure 2(B1–B7). Therefore, it is assumed that 480 °C is an optimized annealing temperature resulting in the formation of TiO2 with a smooth surface with a minimum number of voids. This high-quality film is favorable for the development of crystals with regular shapes [21].
Figure 3 provides a schematic illustration of the reason behind the aforementioned phenomena. It is observed that prior to annealing treatment, the evaporated TiO2 film consists of tiny grains and a large number of surface defects comprising pinholes and small voids. Table 1 displays a proportion between voids and grain sizes as a function of annealing temperature. In the low-temperature mode, ranging from 200 °C to 400 °C, with increasing the annealing temperature, the number of small-sized grains outnumbered large-sized grains. At the same time, the number of voids steady declined, which is assumed to be due to the gradually growing grains. Therefore, at 400 °C, the highest proportion of small-sized grains (88.00%) to large-sized grains (10.02%) was formed. In contrast, as the annealing temperature exceeded 400 °C, enough energy was provided for large grains to absorb nearby small-sized grains and become thermodynamically stable through lowering the surface free energy [22]. Therefore, in the second temperature mode, above 450 °C, the proportion of large-sized grains to small-sized grains was reversed, and at 500 °C, the highest number of large-size grains (21.8%) to small-sized grains (76.0%) was formed [22,23]. Moreover, the number of voids experienced a decreasing trend from 200 °C (4%) to 480 °C (0.7%) and rose again as annealing temperature exceeded 480 °C. The reason behind this phenomenon is associated with the difference between the surface tensions and thermal expansion coefficients of the various grains in the film [24]. Therefore, the grains started to break up and more voids occurred as the annealing temperature rose to 500 °C.
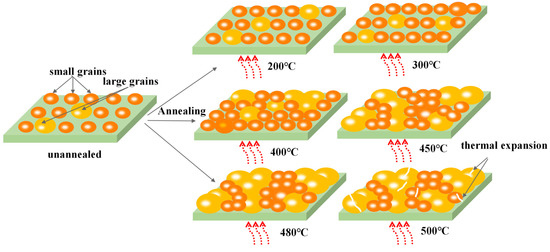
Figure 3.
The schematic diagram of TiO2 grain growth at different annealing temperatures.

Table 1.
Percentage of small grains, large grains, and voids on the surface of the SEM image of the TiO2 film.
3.2. Photovoltaic Performance of CsPbI3-xBrx Perovskite Solar Cells
To evaluate the effect of heat-treated TiO2 film as an electron transport layer on the performance of a solar cell device, TiO2 films with different annealing temperature were implemented in CsPbI3-xBrx PSCs. Figure 4a displays the current density–voltage (J-V) characteristics of PSC based on TiO2 ETLs with different annealing temperature and Table 2 shows the photovoltaic parameters of corresponding PSCs with various TiO2 ETLs. The device based on unannealed TiO2, without annealing treatment, exhibited a short circuit current density (Jsc) of 8.76 mA·cm−2, an open-circuit voltage (Voc) of 0.76 V, a fill factor (FF) of 16%, and a PCE of 1.08%. After TiO2 annealing at 200 °C, there was no change in the Voc (0.76 V); however, Jsc was increased to 12.96 mA·cm−2, FF was increased to 22%, and finally a PCE of 2.15% was achieved. This increasing trend in PSC’s photovoltaic parameters were continued as the annealing temperature was enhanced to 480 °C, where a Voc of 0.84 V, a Jsc of 18.33 mA·cm−2, a FF of 73%, and eventually a PCE of 11.16% were obtained. Surprisingly, as the annealing temperature of TiO2 was increased from 480 °C to 500 °C, the photovoltaic parameters of the PSC began to decline. Therefore, PSC with TiO2 annealed at 480 °C showed the outstanding performance among other candidates. To explore the causes behind the performance improvement of PSCs possessing TiO2 film with different annealing temperatures, the resistivity of unannealed and annealed TiO2 at various temperature ranging from 200 °C to 500 °C was measured as represented in Figure 4b. As is shown, the resistivity of unannealed TiO2 film was 1.34 Ω·m, and as the annealing temperature raised from 200 °C to 480 °C, the resistivity of TiO2 film dropped from 1.30 Ω·m to 0.10 Ω·m, respectively, and then increased to 0.24 Ω·m as the annealing temperature approached 500 °C. The series resistance of PSC is affected by several factors, including the resistivity of charge transport layers. Moreover, it is reported that the internal series resistance of the solar cell device tends to lower the fill factor substantially [25], hence limiting the overall performance of solar cell devices [26,27].
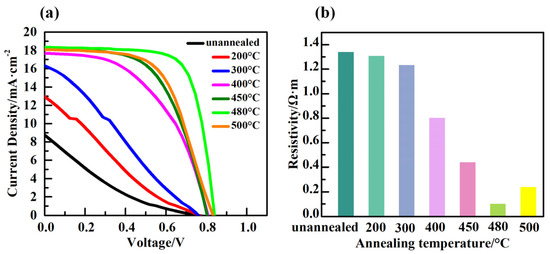
Figure 4.
(a) J-V curves of annealing at different temperatures. (b) Resistivity of TiO2 films at different annealing temperatures.

Table 2.
Photoelectric parameters of PSCs with TiO2 ETLs annealed at different temperatures.
The power lost through the solar cell can be expressed as
where Ploss is power loss, Isc is short circuit current, and Rs is internal series resistance [28]. The power loss increases with the increasing series resistance, and hence there is a drop in the fill factor as seen in Table 2. Therefore, as the resistivity of TiO2 film is increased, as a function of annealing temperature, the series resistance is enhanced, which deteriorates the performance of the device as shown in Table 2.
Figure 5a schematically illustrates the mechanism behind the movement of carriers in EB-evaporated TiO2 film, hence affecting the performance of PSC. Essentially, TiO2 film consists of grain, grain boundaries, and numerous defects which play a role as barriers hindering the movement of photogenerated electrons. These crystal defects scatter the conduction of electrons and impede the flow of current leading to performance degradation of PSC [29].
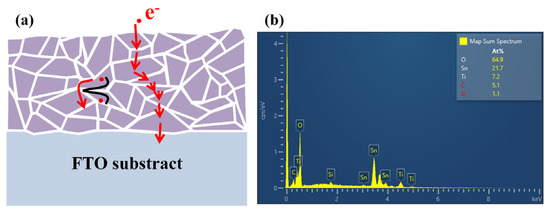
Figure 5.
(a) Schematic diagram of the internal grain boundaries of TiO2. (b) EDS pattern of TiO2 thin film.
Elemental composition analysis of the TiO2 film was performed using Energy-Dispersive X-ray Spectroscopy (EDS) (S-4800, Hitachi, Tokyo, Japan). Figure 5b shows the EDS spectra of the surface of TiO2 film annealed at 480 °C (for more details, please see Figure A1 in Appendix A). It is revealed that the surface of TiO2 film annealed at 480 °C contains less imperfections with the minimum ratio of titanium to oxygen 1:2. This minimum amount of imperfections provides less interfacial obstacles in the way of electron transition in crystal structure of the ETL TiO2 film [30].
The statistical analysis of the photovoltaic device involved the use of 20 devices. Among the different annealing temperatures, the TiO2 ETLs film annealed at 480 °C exhibited favorable characteristics, such as good surface coverage and smooth surface without gaps. These attributes contributed to the reduction in defect state density and the accumulation of interface charges, resulting in a decreased hysteresis effect (for more details, please see Table A1 and Figure A2 in Appendix A). To evaluate the long-term stability of the devices, they were stored and tested in ambient air humidity conditions at room temperature within the range of (20 ± 5) °C and RH = 20 ± 5%, without encapsulation. The findings reveal that the PSCs fabricated with TiO2 ETLs annealed at 480 °C exhibited notable advantages in terms of reliability, reproducibility, and overall performance (for more details, please see Figure A3 in Appendix A).
3.3. Opto-Physical Properties
Optical transparency of ETL is a critical factor determining the performance of n-i-p-structured PSC since it allows the incoming photons to pass though it and reach to the perovskite layer. Figure 6a shows the transmission spectra of TiO2 film with different annealing temperature in the wavelength range of 380 nm to 780 nm. Results show that all annealed samples exhibited an excellent optical transmittance with an average transmittance of above 85% in visible range of wavelength. The small change in optical transparency of TiO2 samples with constant thickness (75 nm) is assumed to be due to the difference in refractive index of samples, which is mostly associated with the grain size of TiO2 films [31]. We determined the optical band gap (Eg) of TiO2 films through the first derivative of transmissivity (T) in relation to energy (E) [32]. Figure 6b shows the effect of annealing temperature on the optical bandgap energy of the TiO2 film. It is evident that there is a slight difference in the optical bandgap of TiO2 film as a function of different annealing conditions. This result demonstrates that there is a negligible effect of annealing temperature on the optical characteristic of TiO2 film. Different annealing conditions easily cause changes in the structure and composition of TiO2 films, which in turn can affect their electronic structure and the energy required to excite an electron. For example, annealing at high temperatures can induce crystallization and grain growth, leading to changes in the crystal structure and defects within the film. Consequently, the annealing-treated TiO2 films result in a shift in the optical bandgap towards higher or lower energies. In general, the bandgap of a material decreases as the grain size increases, which are attributed to, the presence of grain boundaries in a polycrystalline material induce the defect states in the bandgap, which can act as trapping sites for electrons and holes. These defect states can then lead to a broadening of the bandgap, resulting in a decrease in the optical bandgap of the material. The decrease in bandgap with increasing grain size can be described by the quantum confinement effect, where the electronic states in the material are confined by the grain boundaries, leading to a change in the electronic band structure. The degree of quantum confinement is related to the grain size, with smaller grains resulting in a greater degree of confinement and a larger increase in bandgap. However, it is important to note that the relationship between grain size and bandgap can also be influenced by other factors, such as strain and doping. In some cases, an increase in grain size can lead to a decrease in strain, which can in turn lead to a decrease in the bandgap. Additionally, doping can introduce new electronic states into the bandgap, which can affect the bandgap energy and modify the relationship between grain size and bandgap.
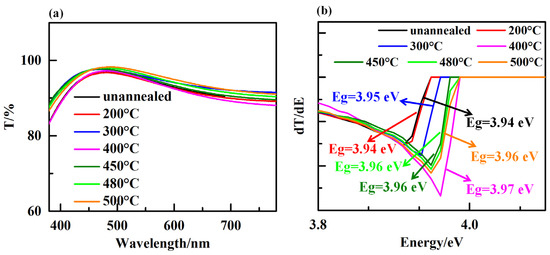
Figure 6.
(a) Transmittance spectra images of the TiO2 film. (b) Plots of the derivative of the transmittance with respect to energy.
To evaluate the carrier dynamics at the interface between the absorber layer (CsPbI3-xBrx films) and the ELT layer (TiO2 film) with different annealing temperatures, photoluminescence (PL) spectra analysis was carried out in the wavelength range of 550 nm to 850 nm. Figure 7 shows the steady-state PL spectra of CsPbI3-xBrx perovskite film coated with TiO2 with different annealing temperatures. As is observed, the PL luminescence intensity is maximized for TiO2 film without annealing treatment; however, as the annealing temperature is enhanced, the intensity of the PL peak is quenched and reaches its minimum value at 480 °C, indicating more effective electron extraction. The PL intensity is raised again when the annealing temperature reaches 500 °C. As is known, defects such as voids, grain boundaries, and vacancies that form at the interface between the TiO2 ETL and perovskite film can contribute to recombination loss [33]. Thus, TiO2 film annealed at 480 °C significantly minimizes the number of these trap states and consequently enhances the acceleration of the carrier’s extraction from the perovskite layer. As the annealing temperature approaches 500 °C, this tendency is broken and the PL intensity is raised again due to an increase in the number of recombination sites, such as defects and voids, as shown in Table 1.
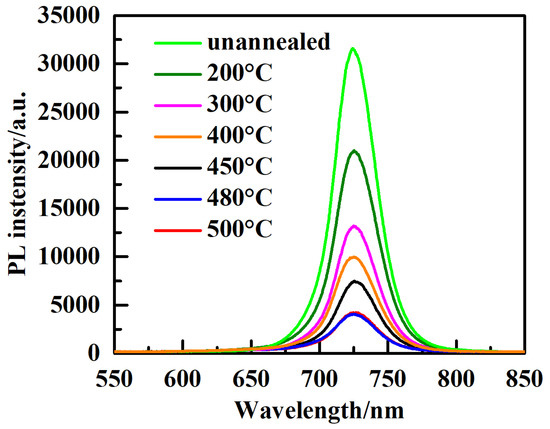
Figure 7.
PL spectra of perovskite films prepared with TiO2 ETL annealed at different temperatures.
The space-charge-limited-current (SCLC) test was conducted using the structure shown in Figure 8a, and the results are presented in Figure 8b, providing valuable insights into the recombination behavior at the interface between the ETL and the perovskite material. This analysis offers an additional perspective that aids in understanding the observed trend in the open-circuit voltage (Voc). SCLC is a useful technique for investigating carrier mobility and recombination properties in materials. In SCLC, when trap centers capture photogenerated free carriers, the density of free carriers decreases, resulting in a small current with a shallow slope at low bias voltage. However, as the voltage increases, all traps become filled. Beyond the inflection point, the current increases rapidly with a steeper slope, and this inflection point is known as the trap-filled limit voltage (VTFL). The value of VTFL can be estimated by calculating the intersection point of the two slopes. Additionally, VTFL can be determined by the trap state density (Nt). The values of Nt for the TiO2 films before and after annealing at 200 °C, 300 °C, 400 °C, 450 °C, 480 °C, and 500 °C were calculated using the formula , resulting in 8.86 × 1019 cm−3, 7.67 × 1019 cm−3, 6.38 × 1019 cm−3, 6.27 × 1019 cm−3, 4.10 × 1019 cm−3, 3.13 × 1019 cm−3, and 4.10 × 1019 cm−3, respectively. These results indicate that the increase in Jsc is primarily attributed to the reduction in trap state density and the decrease in resistivity, as mentioned in this study. Moreover, the decrease in defect state density suggests that the TiO2 film annealed at 480 °C exhibits better surface quality, with fewer ETL/perovskite interface recombination sites and defect centers, thereby minimizing the probability of interface recombination.
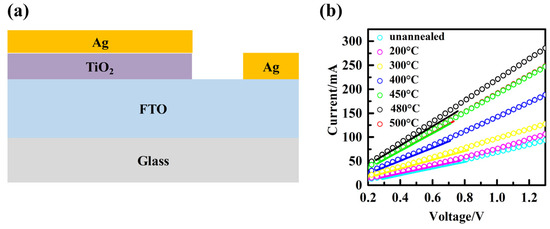
Figure 8.
(a) Schematic illustration of devices with FTO/TiO2/Ag. (b) Current–voltage characteristics of devices with FTO/TiO2/Ag.
4. Conclusions
In summary, we systematically investigated the effect of annealing temperature on electrical and morphological characteristics of EB-evaporated TiO2 thin film as ETL to improve the performance of n-i-p planar PSC. It was found that as the annealing temperature increased from 200 °C to 480 °C, the electrical and morphological properties of TiO2 film were improved, which resulted in higher performance of PSC. However, above 480 °C, the quality of TiO2 film was deteriorated, which led to lower performance of PSC. It was revealed that the TiO2 film annealed at 480 °C contained the lowest amount of surface defects and trap states, which resulted in minimum recombination loss of photogenerated carries. Transmission tests showed an average optical transmittance of above 85% in the visible range of wavelength for the TiO2 film annealed at 480 °C, and it was determined that the annealing temperature did not have any influence on the band gap of the TiO2 film. It was concluded that annealing treatment at 480 °C remarkably improved the quality of TiO2 ETL, resulting in a 3.5-fold improvement in FF and 10-fold increase in device PCE compared to an unannealed device. Our findings provide a straightforward method for improving the FF of perovskite solar cells at a low cost and on a large scale, establishing the groundwork for the commercialization of perovskite solar cells.
Author Contributions
Conceptualization, T.X. and D.C.; methodology, T.X.; validation, D.C. and T.L.; formal analysis, T.X. and F.Z.; investigation, T.X. and D.C.; data curation, D.C.; writing—original draft preparation, T.X. and D.C.; writing—review and editing, T.X., X.C., X.W., Z.T., F.Z., J.H., K.G. and A.V.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (grant numbers 51802184, 62004120, 61904100); the Shaanxi Province Innovation Capability Support Plan-Youth Science and Technology Nova Project (2023KJXX-141); and the Scientific Research Startup Foundation of Shaanxi University of Science and Technology (grant numbers 2017BJ-03, 126022255).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
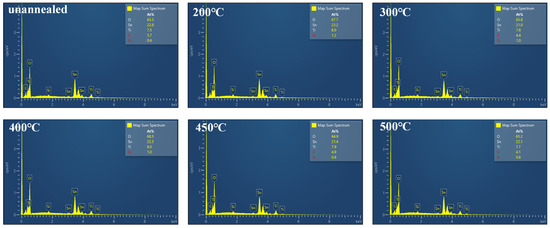
Figure A1.
EDS pattern of TiO2 thin film.

Table A1.
Performance parameters of PSC devices with TiO2 ETL at different annealing temperatures.
Table A1.
Performance parameters of PSC devices with TiO2 ETL at different annealing temperatures.
| Temperature/°C | Scan Direction | Voc/V | Jsc/mA·cm−2 | FF/% | PCE/% | HI |
|---|---|---|---|---|---|---|
| unannealed | Forward | 0.48 | 5.28 | 17 | 0.42 | 0.61 |
| Reverse | 0.76 | 8.76 | 16 | 1.08 | ||
| Average | 0.61 ± 0.15 | 5.07 ± 1.49 | 17 ± 6.47 | 0.48 ± 0.18 | ||
| 200 | Forward | 0.74 | 10.75 | 24 | 1.90 | 0.12 |
| Reverse | 0.76 | 12.96 | 22 | 2.15 | ||
| Average | 0.69 ± 0.04 | 10.98 ± 2.90 | 24 ± 3.69 | 1.73 ± 0.21 | ||
| 300 | Forward | 0.60 | 16.65 | 29 | 2.89 | 0.13 |
| Reverse | 0.76 | 16.33 | 27 | 3.32 | ||
| Average | 0.78 ± 0.07 | 12.74 ± 2.18 | 26 ± 8.56 | 2.59 ± 0.40 | ||
| 400 | Forward | 0.74 | 16.40 | 51 | 6.24 | 0.12 |
| Reverse | 0.80 | 17.66 | 50 | 7.08 | ||
| Average | 0.70 ± 0.04 | 16.92 ± 1.97 | 39 ± 6.64 | 4.73 ± 1.23 | ||
| 450 | Forward | 0.76 | 18.20 | 55 | 7.57 | 0.10 |
| Reverse | 0.80 | 18.10 | 58 | 8.43 | ||
| Average | 0.78 ± 0.03 | 15.60 ± 1.74 | 42 ± 13.24 | 5.39 ± 2.15 | ||
| 480 | Forward | 0.83 | 18.08 | 70 | 10.49 | 0.06 |
| Reverse | 0.84 | 18.33 | 73 | 11.16 | ||
| Average | 0.80 ± 0.05 | 18.37 ± 0.30 | 56 ± 5.30 | 8.23 ± 1.23 | ||
| 500 | Forward | 0.81 | 17.10 | 55 | 7.59 | 0.16 |
| Reverse | 0.83 | 18.07 | 59 | 9.00 | ||
| Average | 0.79 ± 0.03 | 16.20 ± 2.31 | 54.9 ± 8.02 | 7.15 ± 1.73 |
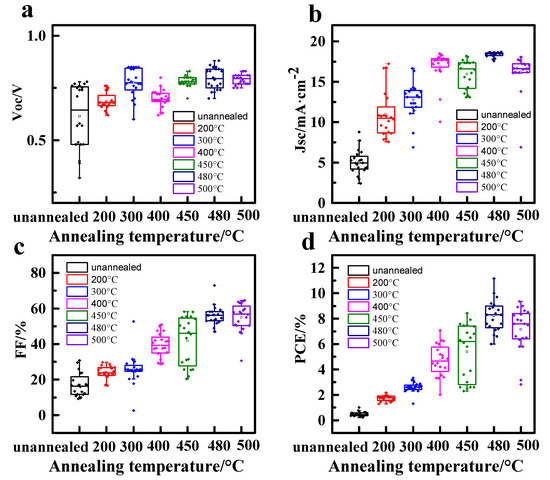
Figure A2.
Box plots of PSCs devices with different annealing temperatures of TiO2 ETL (a) Voc, (b) Jsc, (c) FF, and (d) PCE.
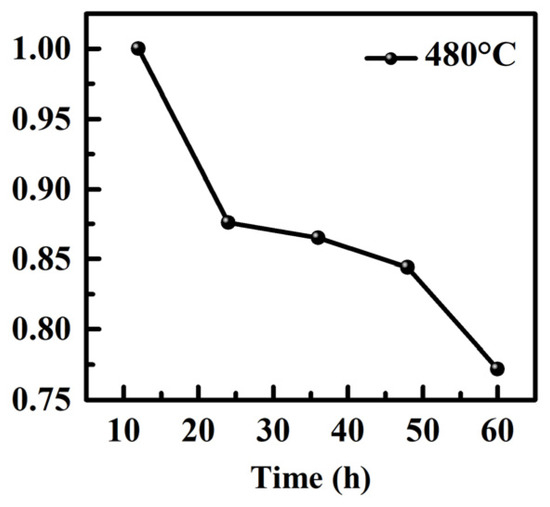
Figure A3.
Stability testing of PSC devices annealed at 480 °C for TiO2 ETL.
References
- Sun, S.; Salim, T.; Mathews, N.; Duchamp, M.; Boothroyd, C.; Xing, G.; Sum, T.C.; Lam, Y.M. The origin of high efficiency in low-temperature solution-processable bilayer organometal halide hybrid solar cells. Energy Environ. Sci. 2014, 7, 399–407. [Google Scholar] [CrossRef]
- Ogomi, Y.; Morita, A.; Tsukamoto, S.; Saitho, T.; Fujikawa, N.; Shen, Q.; Toyoda, T.; Yoshino, K.; Pandey, S.S.; Ma, T.; et al. CH3NH3SnxPb(1-x)I3 Perovskite Solar Cells Covering up to 1060 nm. J. Phys. Chem. Lett. 2014, 5, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Correa-Baena, J.P.; Abate, A.; Saliba, M.; Tress, W.; Jacobsson, T.J.; Gratzel, M.; Hagfeldt, A. The rapid evolution of highly efficient perovskite solar cells. Energy Environ. Sci. 2017, 10, 710–727. [Google Scholar] [CrossRef]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-Hole Diffusion Lengths Exceeding 1 Micrometer in an Organometal Trihalide Perovskite Absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef]
- Xing, G.; Mathews, N.; Sun, S.; Lim, S.S.; Lam, Y.M.; Graetzel, M.; Mhaisalkar, S.; Sum, T.C. Long-Range Balanced Electron- and Hole-Transport Lengths in Organic-Inorganic CH3NH3PbI3. Science 2013, 342, 344–347. [Google Scholar] [CrossRef]
- Wehrenfennig, C.; Eperon, G.E.; Johnston, M.B.; Snaith, H.J.; Herz, L.M. High Charge Carrier Mobilities and Lifetimes in Organolead Trihalide Perovskites. Adv. Mater. 2014, 26, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- NREL. Best Research-Cell Efficiency Chart. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 1 January 2023).
- Zhou, H.; Chen, Q.; Li, G.; Luo, S.; Song, T.-B.; Duan, H.-S.; Hong, Z.; You, J.; Liu, Y.; Yang, Y. Interface engineering of highly efficient perovskite solar cells. Science 2014, 345, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Meng, X.; Yang, S. Interface Engineering for Highly Efficient and Stable Planar p-i-n Perovskite Solar Cells. Adv. Energy Mater. 2018, 8, 1701883. [Google Scholar] [CrossRef]
- Yang, Z.; Babu, B.H.; Wu, S.; Liu, T.; Fang, S.; Xiong, Z.; Han, L.; Chen, W. Review on Practical Interface Engineering of Perovskite Solar Cells: From Efficiency to Stability. Sol. RRL 2019, 4, 1900257. [Google Scholar] [CrossRef]
- Choi, I.Y.; Baek, S.-D.; Takaloo, A.V.; Lee, S.Y.; Hajibabaei, A.; Kim, K.S.; Myoung, J.-M. Highly Efficient Pure-Blue Perovskite Light-Emitting Diode Leveraging CsPbBrxCl3-x/Cs4PbBrxCl6-x Nanocomposite Emissive Layer with Shallow Valence Band. Adv. Opt. Mater. 2022, 10, 2102502. [Google Scholar] [CrossRef]
- Xiao, W.; Pu, W.H.; Wang, J.W.; Wang, X.; Sun, L.; Cui, J.D.; Wang, L.G. Theoretical investigation of the structural and electronic properties of Al-decorated TiO2/perovskite interfaces. Appl. Surf. Sci. 2019, 492, 369–373. [Google Scholar] [CrossRef]
- Han, F.; Hao, G.; Wan, Z.; Luo, J.; Xia, J.; Jia, C. Bifunctional electron transporting layer/perovskite interface linker for highly efficient perovskite solar cells. Electrochim. Acta 2019, 296, 75–81. [Google Scholar] [CrossRef]
- Li, T.; Rui, Y.; Zhang, X.; Shi, J.; Wang, X.; Wang, Y.; Yang, J.; Zhang, Q. Anatase TiO2 nanorod arrays as high-performance electron transport layers for perovskite solar cells. J. Alloys Compd. 2020, 849, 156629. [Google Scholar] [CrossRef]
- Tian, J.; Li, H.; Wang, H.; Zheng, B.; Xue, Y.; Liu, X. TiO2 composite electron transport layers for planar perovskite solar cells by mixed spray pyrolysis with precursor solution incorporating TiO2 nanoparticles. Chin. Phys. B 2018, 27, 018810. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, T.-H.; Wei, Q.-Y.; Yu, S.-J.; Gao, H.; Guo, P.-C.; Li, J.-K.; Wang, Y.-X. Preparation of TiO2 electron transport layer by magnetron sputtering and its effect on the properties of perovskite solar cells. Energy Rep. 2022, 8, 3166–3175. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Wang, Y.; Wang, Q.; Liu, Z.; Geng, R.; Wang, H.; Jiang, W.; Ding, W. Improving the performance of perovskite solar cells via TiO2 electron transport layer prepared by direct current pulsed magnetron sputtering. J. Alloys Compd. 2022, 929, 167278. [Google Scholar] [CrossRef]
- Hossain, M.F.; Naka, S.; Okada, H. Annealing effect of E-beam evaporated TiO2 films and their performance in perovskite solar cells. J. Photochem. Photobiol. A Chem. 2018, 360, 109–116. [Google Scholar] [CrossRef]
- Chen, C.-M.; Hsu, Y.-C.; Cherng, S.-J. Effects of annealing conditions on the properties of TiO2/ITO-based photoanode and the photovoltaic performance of dye-sensitized solar cells. J. Alloys Compd. 2011, 509, 872–877. [Google Scholar] [CrossRef]
- Islam, S.; Bakhtiar, H.; Bidin, N.; Salim, A.A.; Riaz, S.; Krishnan, G.; Naseem, S. Crack-free high surface area silica-titania nanocomposite coating as opto-chemical sensor device. Sens. Actuators A Phys. 2018, 270, 153–161. [Google Scholar] [CrossRef]
- Fang, Z.; Guo, Y.; Fu, B.; Wei, L.; Chen, J.; Pang, L.; Wang, Z. Effect of Shear Bands Induced by Asymmetric Rolling on Microstructure and Texture Evolution of Non-Oriented 3.3% Si Steel. Materials 2020, 13, 4696. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xu, Y.; He, Y.; Jiao, H.; Yue, S.; Li, J. A quasi in-situ EBSD study of the nucleation and growth of Goss grains during primary and secondary recrystallization of a strip-cast Fe-6.5 wt% Si alloy. J. Alloys Compd. 2021, 861, 158550. [Google Scholar] [CrossRef]
- Saidaminov, M.I.; Kim, J.; Jain, A.; Quintero-Bermudez, R.; Tan, H.; Long, G.; Tan, F.; Johnston, A.; Zhao, Y.; Voznyy, O.; et al. Suppression of atomic vacancies via incorporation of isovalent small ions to increase the stability of halide perovskite solar cells in ambient air. Nat. Energy 2018, 3, 648–654. [Google Scholar] [CrossRef]
- Shyma, A.P.; Sellappan, R. Computational Probing of Tin-Based Lead-Free Perovskite Solar Cells: Effects of Absorber Parameters and Various Electron Transport Layer Materials on Device Performance. Materials 2022, 15, 7859. [Google Scholar] [CrossRef]
- Takaloo, A.V.; Lee, H.J.; Park, T.H.; Dongale, T.D.; Kim, Y.U.; Choi, D.H.; Kim, T.G. Haze-enhanced ZnO/Ag/ZnO nanomesh electrode for flexible, high-efficiency indoor organic photovoltaics. J. Power Sources 2021, 515, 230589. [Google Scholar] [CrossRef]
- Park, J.W.; Takaloo, A.V.; Kim, S.H.; Son, K.R.; Kang, D.Y.; Kang, S.K.; Lee, C.B.; Choi, H.; Shim, J.W.; Kim, T.G. Surface-modified ultra-thin indium zinc oxide films with tunable work function for efficient hole transport in flexible indoor organic photovoltaics. J. Power Sources 2021, 489, 229507. [Google Scholar] [CrossRef]
- Baig, H.; Kanda, H.; Asiri, A.M.; Nazeeruddin, M.K.; Mallick, T. Increasing efficiency of perovskite solar cells using low concentrating photovoltaic systems. Sustain. Energy Fuels 2020, 4, 4301–4302. [Google Scholar] [CrossRef]
- Pu, W.; Xiao, W.; Wang, J.; Li, X.-W.; Wang, L. Stress and Defect Effects on Electron Transport Properties at SnO2/Perovskite Interfaces: A First-Principles Insight. ACS Omega 2022, 7, 16187–16196. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, W.; Guo, H.; Zhao, Y.; Shan, X.; Jin, K.; Tian, H.; Zhao, Q.; Yu, D.; Lu, X.; et al. Interfacial Oxygen Vacancies as a Potential Cause of Hysteresis in Perovskite Solar Cells. Chem. Mater. 2016, 28, 802–812. [Google Scholar] [CrossRef]
- Wang, J.-L.; Chen, C.-K.; Li, X.; Jiang, M.-Y.; Hu, X.-J. Influences of grain size and microstructure on optical properties of microcrystalline diamond films. Chin. Phys. B 2020, 29, 018103. [Google Scholar] [CrossRef]
- Touam, T.; Atoui, M.; Hadjoub, I.; Chelouche, A.; Boudine, B.; Fischer, A.; Boudrioua, A.; Doghmane, A. Effects of dip-coating speed and annealing temperature on structural, morphological and optical properties of sol-gel nano-structured TiO2 thin films. Eur. Phys. J. Appl. Phys. 2014, 67, 30302. [Google Scholar] [CrossRef]
- He, Q.; Zhang, H.; Han, S.; Xing, Y.; Li, Y.; Zhang, X.; Wang, R. Improvement of nanopore structure SnO2 electron-transport layer for carbon-based CsPbIBr2 perovskite solar cells. Mater. Sci. Semicond. Process. 2022, 148, 106787. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).