Innovative Fabrication of Hollow Microneedle Arrays Enabling Blood Sampling with a Self-Powered Microfluidic Patch
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
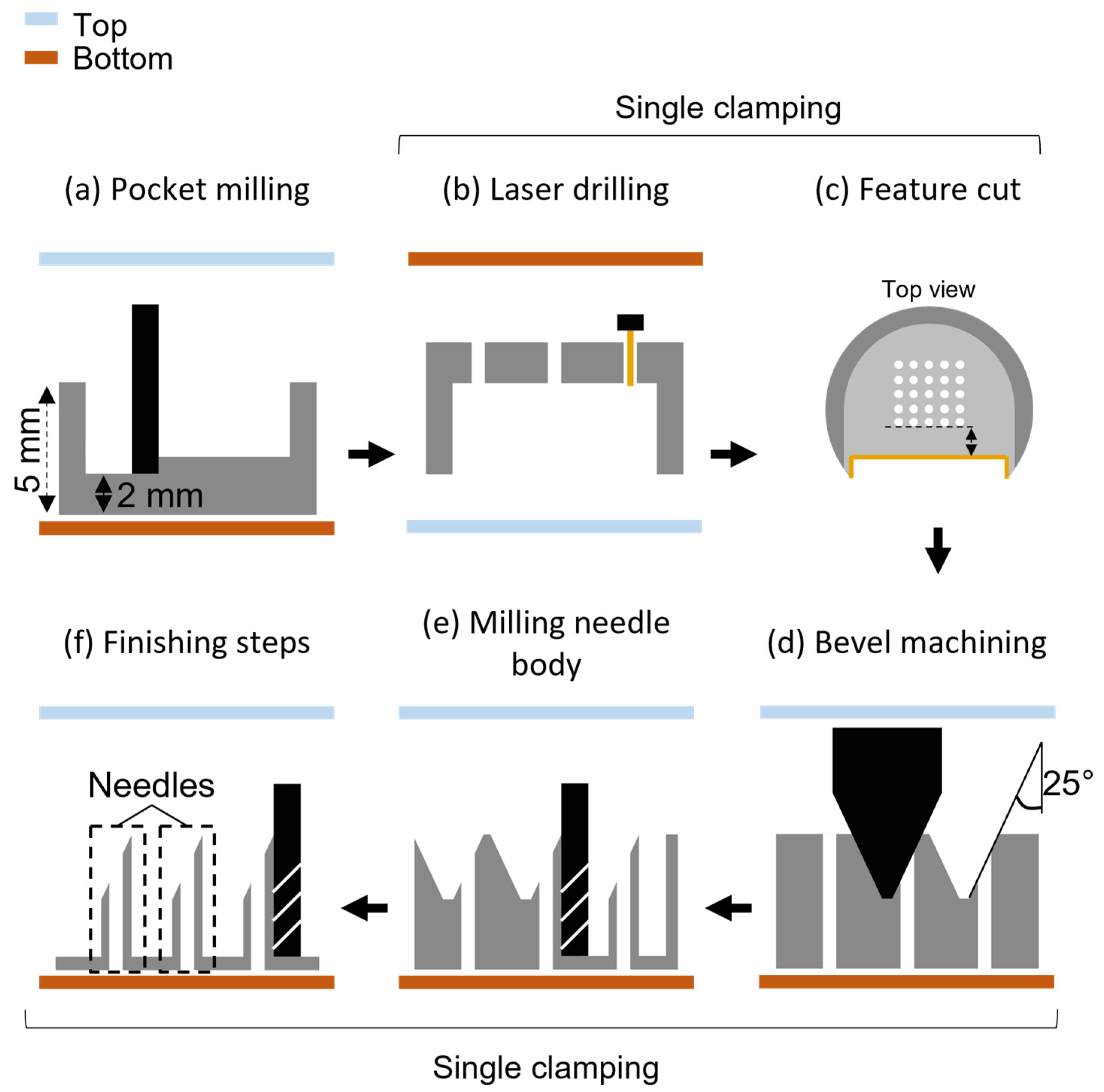
2.2. Hollow Microneedle Fabrication Process
2.3. Microscopic and Tomographic Inspections
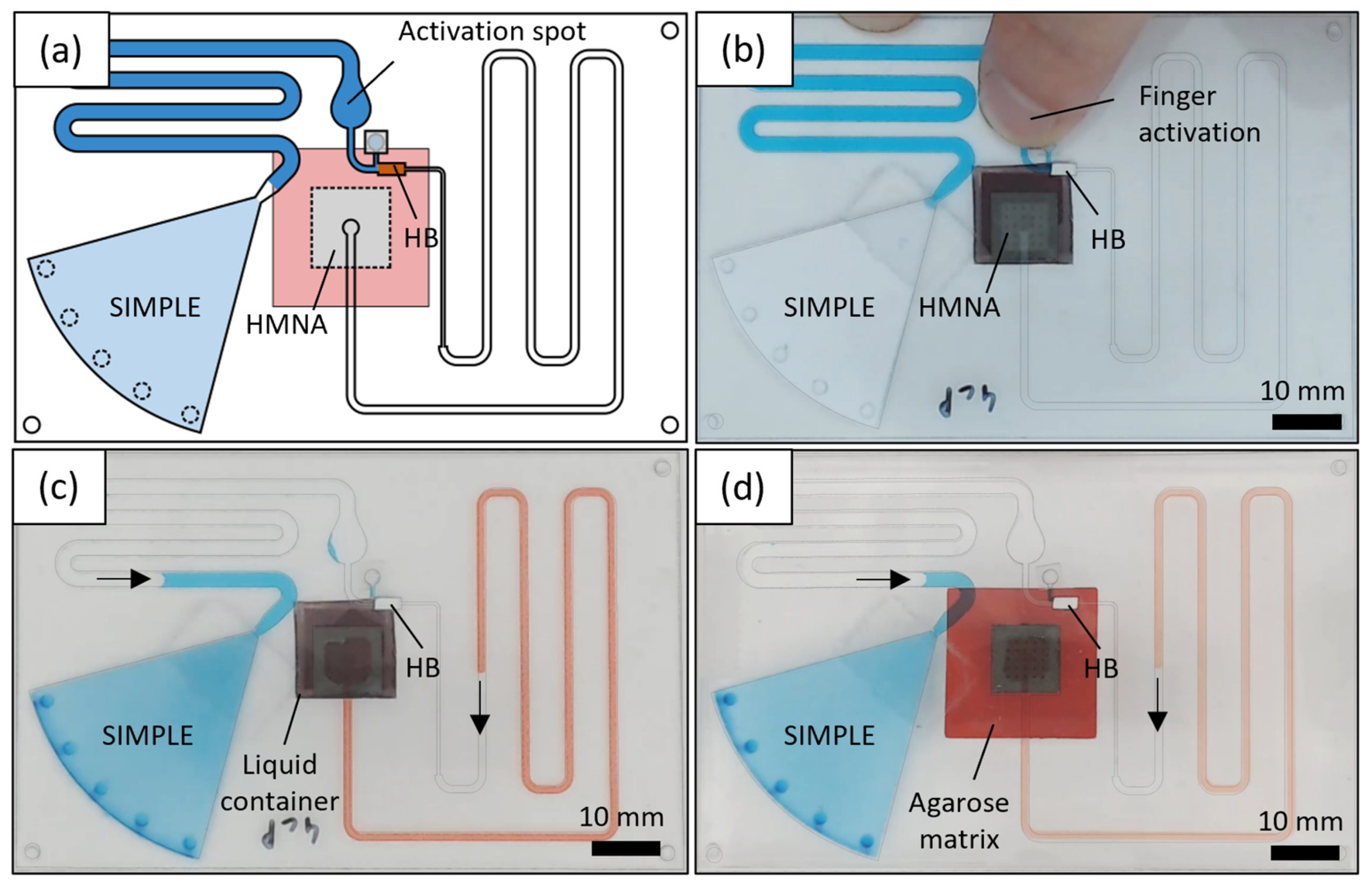
2.4. Microfluidic Chip Fabrication
2.5. Flow Characterization of the HMNAs
2.6. SIMPLE Pressure Generation
2.7. Validation of a Self-Powered Biofluid Sampling Patch with In Vitro Models
3. Results and Discussion
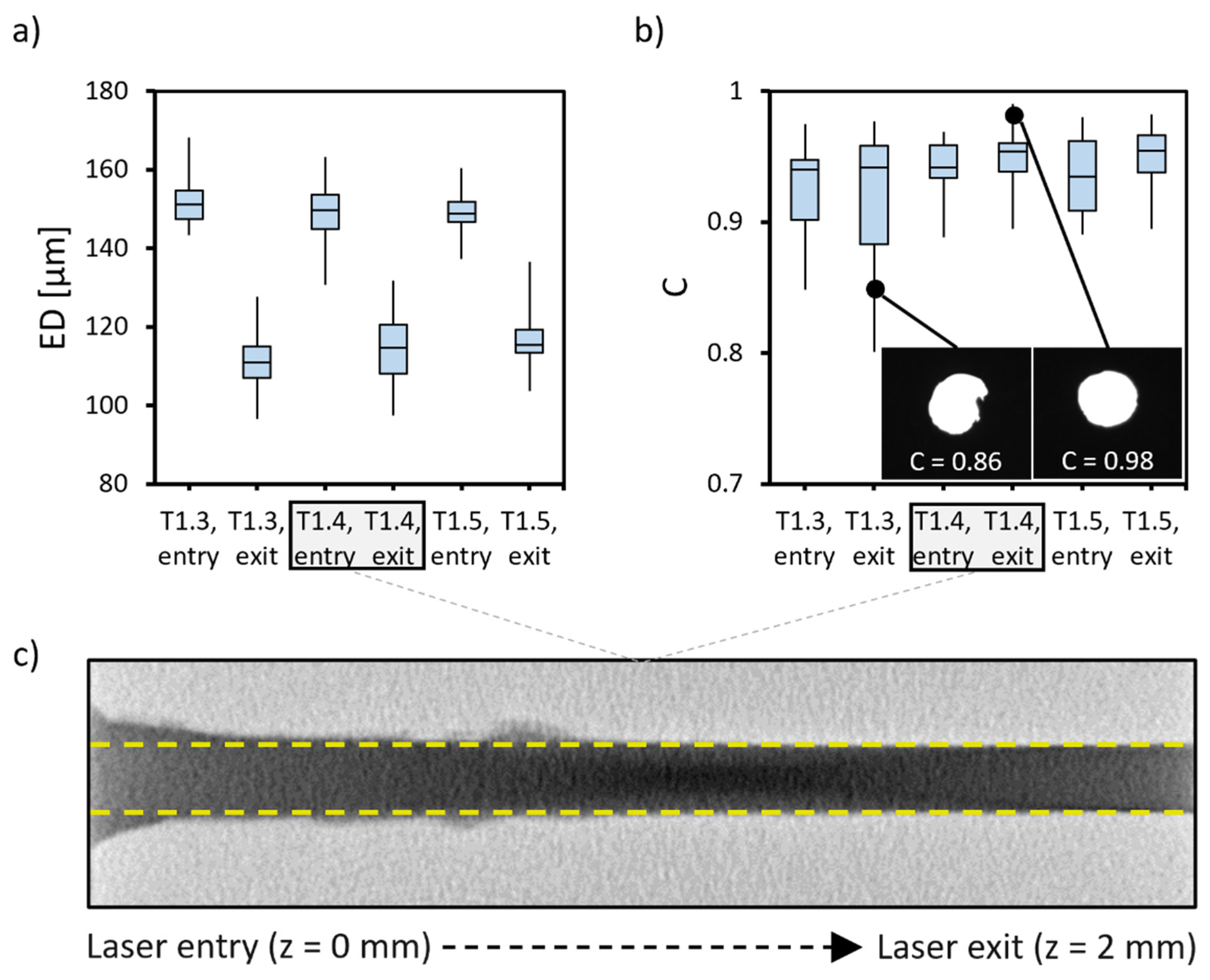
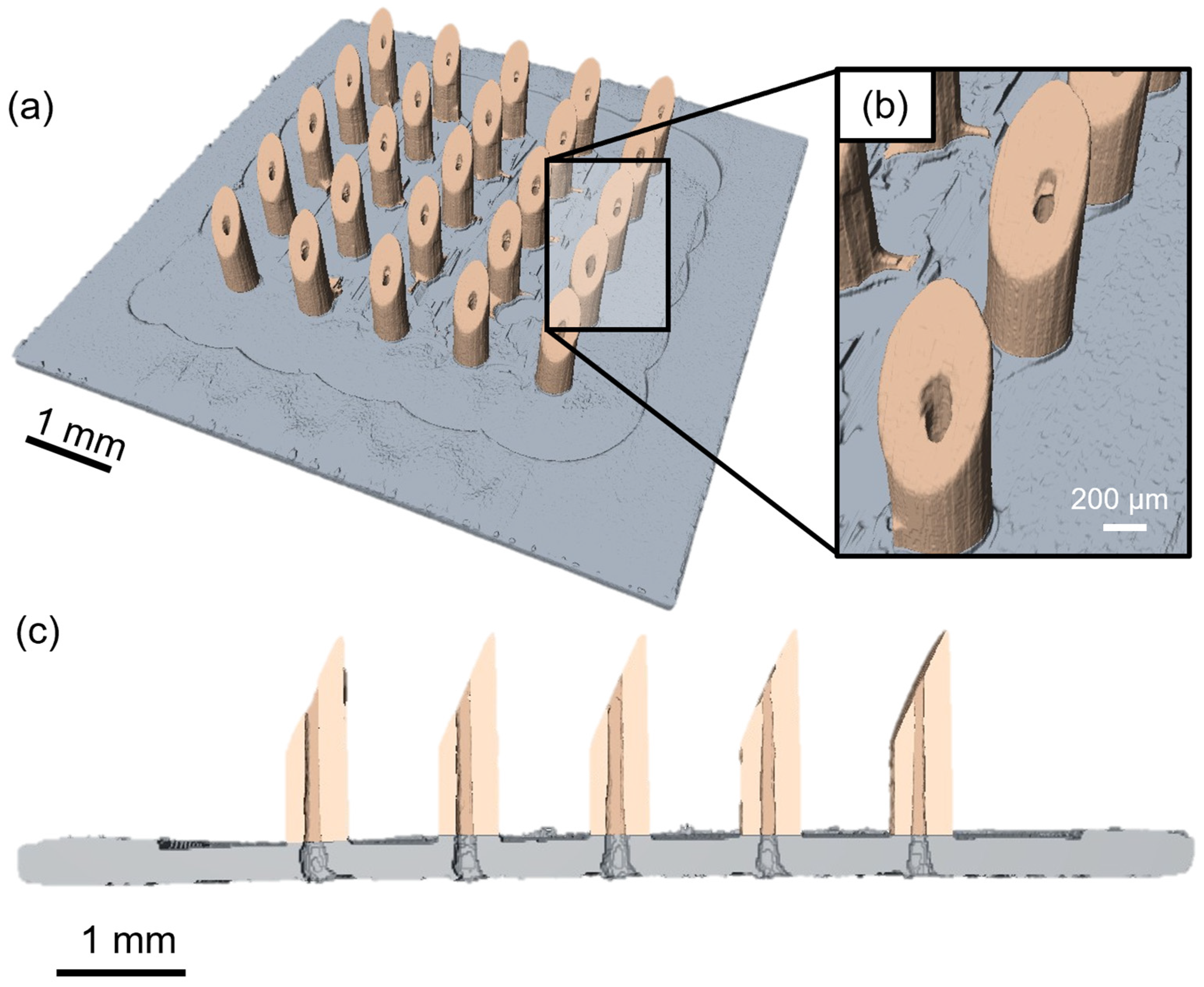
3.1. HMNA Fabrication
3.2. Flow Characterization of the HMNAs
3.3. Characterization of SIMPLE Pressure Generation
3.4. Validation of Self-Powered Biofluid Microsampling Patch with In Vitro Models
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, L.K.; Sullivan, S.; Goodnight, W.; Chang, E.Y.; Soper, D. The use of blunt needles does not reduce glove perforations during obstetrical laceration repair. Am. J. Obstet. Gynecol. 2008, 199, 639.e1–639.e4. [Google Scholar] [CrossRef] [PubMed]
- Samant, P.P.; Prausnitz, M.R. Mechanisms of sampling interstitial fluid from skin using a microneedle patch. Proc. Natl. Acad. Sci. USA 2018, 115, 4583–4588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shlomowitz, A.; Feher, M.D. Anxiety associated with self monitoring of capillary blood glucose. Br. J. Diabetes Vasc. Dis. 2014, 14, 60–63. [Google Scholar] [CrossRef] [Green Version]
- Lenicek Krleza, J.; Dorotic, A.; Grzunov, A.; Maradin, M. Capillary blood sampling: National recommendations on behalf of the Croatian society of medical biochemistry and laboratory medicine. Biochem. Med. 2015, 25, 335–358. [Google Scholar] [CrossRef]
- Morgan, P.E. Microsampling Devices for Routine Therapeutic Drug Monitoring-Are We There Yet? Ther. Drug Monit. 2021, 43, 322–334. [Google Scholar] [CrossRef]
- Dixon, R.V.; Skaria, E.; Lau, W.M.; Manning, P.; Birch-Machin, M.A.; Moghimi, S.M.; Ng, K.W. Microneedle-based devices for point-of-care infectious disease diagnostics. Acta Pharm. Sin. B 2021, 11, 2344–2361. [Google Scholar] [CrossRef]
- Romanyuk, A.V.; Zvezdin, V.N.; Samant, P.; Grenader, M.I.; Zemlyanova, M.; Prausnitz, M.R. Collection of analytes from microneedle patches. Anal. Chem. 2014, 86, 10520–10523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukerjee, E.V.; Collins, S.D.; Isseroff, R.R.; Smith, R.L. Microneedle array for transdermal biological fluid extraction and in situ analysis. Sens. Actuators A Phys. 2004, 114, 267–275. [Google Scholar] [CrossRef]
- Stoeber, B.; Liepmann, D. Arrays of hollow out-of-plane microneedles for drug delivery. J. Microelectromech. Syst. 2005, 14, 472–479. [Google Scholar] [CrossRef]
- Roxhed, N.; Gasser, T.C.; Griss, P.; Holzapfel, G.A.; Stemme, G. Penetration-enhanced ultrasharp microneedles and prediction on skin interaction for efficient transdermal drug delivery. J. Microelectromech. Syst. 2007, 16, 1429–1440. [Google Scholar] [CrossRef]
- Strambini, L.M.; Longo, A.; Diligenti, A.; Barillaro, G. A minimally invasive microchip for transdermal injection/sampling applications. Lab Chip 2012, 12, 3370–3379. [Google Scholar] [CrossRef]
- Jaganathan, H.; Godin, B. Biocompatibility Assessment of Si-Based Nano- and Microparticles. Adv. Drug Deliv. Rev. 2012, 64, 1800–1819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gittard, S.D.; Miller, P.R.; Boehm, R.D.; Ovsianikov, A.; Chichkov, B.N.; Heiser, J.; Gordon, J.; Monteiro-Riviere, N.A.; Narayan, R.J. Multiphoton microscopy of transdermal quantum dot delivery using two photon polymerization-fabricated polymer microneedles. Faraday Discuss. 2011, 149, 171–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceyssens, F.; Chaudhri, B.P.; Van Hoof, C.; Puers, R. Fabrication process for tall, sharp, hollow, high aspect ratio polymer microneedles on a platform. J. Micromech. Microeng. 2013, 23, 075023. [Google Scholar] [CrossRef]
- Mishra, R.; Maiti, T.K.; Bhattacharyya, T.K. Development of SU-8 hollow microneedles on a silicon substrate with microfluidic interconnects for transdermal drug delivery. J. Micromech. Microeng. 2018, 28, 105017. [Google Scholar] [CrossRef]
- Jiang, X.; Lillehoj, P.B. Microneedle-based skin patch for blood-free rapid diagnostic testing. Microsyst. Nanoeng. 2020, 6, 96. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Sharma, P.K.; Murty, U.S.; Mohan, N.H.; Thomas, R.; Dwivedy, S.K.; Banerjee, S. 3D printed hollow microneedles array using stereolithography for efficient transdermal delivery of rifampicin. Int. J. Pharm. 2021, 605, 120815. [Google Scholar] [CrossRef]
- Wu, T.; You, X.; Chen, Z. Hollow Microneedles on a Paper Fabricated by Standard Photolithography for the Screening Test of Prediabetes. Sensors 2022, 22, 4253. [Google Scholar] [CrossRef]
- Sugiyama, S.; Khumpuang, S.; Kawaguchi, G. Plain-pattern to cross-section transfer (PCT) technique for deep x-ray lithography and applications. J. Micromech. Microeng. 2004, 14, 1399–1404. [Google Scholar] [CrossRef]
- Khumpuang, S.; Kawaguchi, G.; Sugiyama, S. Quadruplets-Microneedle Array for Blood Extraction. In Technical Proceedings of NSTI-Nanotech Conference’04; Taylor & Francis Group: Boston, MA, USA, 2004; Available online: www.nsti.org (accessed on 18 September 2019).
- Evens, T.; van Hileghem, L.; Dosso, F.D.; Lammertyn, J.; Malek, O.; Castagne, S.; Seveno, D.; van Bael, A. Producing Hollow Polymer Microneedles Using Laser Ablated Molds in an Injection Molding Process. J. Micro Nano-Manuf. 2021, 9, 030902. [Google Scholar] [CrossRef]
- Aksit, A.; Rastogi, S.; Nadal, M.L.; Parker, A.M.; Lalwani, A.K.; West, A.C.; Kysar, J.W. Drug delivery device for the inner ear: Ultra-sharp fully metallic microneedles. Drug Deliv. Transl. Res. 2021, 11, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.P.; Martanto, W.; Allen, M.G.; Prausnitz, M.R. Hollow metal microneedles for insulin delivery to diabetic rats. IEEE Trans. Biomed. Eng. 2005, 52, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Vinayakumar, K.B.; Kulkarni, P.G.; Nayak, M.M.; Dinesh, N.S.; Hegde, G.M.; Ramachandra, S.G.; Rajanna, K. A hollow stainless steel microneedle array to deliver insulin to a diabetic rat. J. Micromech. Microeng. 2016, 26, 065013. [Google Scholar] [CrossRef]
- Rajabi, M.; Roxhed, N.; Shafagh, R.Z.; Haraldson, T.; Fischer, A.C.; Van Der Wijngaart, W.; Stemme, G.; Niklaus, F. Flexible and stretchable microneedle patches with integrated rigid stainless steel microneedles for transdermal biointerfacing. PLoS ONE 2016, 11, e0166330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhri, B.P.; Ceyssens, F.; De Moor, P.; Van Hoof, C.; Puers, R. A high aspect ratio SU-8 fabrication technique for hollow microneedles for transdermal drug delivery and blood extraction. J. Micromech. Microeng. 2010, 20, 064006. [Google Scholar] [CrossRef]
- Liu, G.S.; Kong, Y.; Wang, Y.; Luo, Y.; Fan, X.; Xie, X.; Yang, B.R.; Wu, M.X. Microneedles for transdermal diagnostics: Recent advances and new horizons. Biomaterials 2020, 232, 119740. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Yao, J.; Yang, C.; Ding, G. A minimally invasive micro sampler for quantitative sampling with an ultrahigh-aspect-ratio microneedle and a PDMS actuator. Biomed. Microdevices 2016, 18, 59. [Google Scholar] [CrossRef]
- Le Thanh, H.; Le The, H.; Nguyen, V.; Tran-Minh, N.; Wang, K.; Karlsen, F. Optimal design of polymer-based microneedle for improved collection of whole blood from human fingers. Micro Nano Lett. 2014, 9, 644–649. [Google Scholar] [CrossRef]
- Le Thanh, H.; Ta, B.Q.; Le The, H.; Nguyen, V.; Wang, K.; Karlsen, F. Low-Cost Fabrication of Hollow Microneedle Arrays Using CNC Machining and UV Lithography. J. Microelectromech. Syst. 2015, 24, 1583–1593. [Google Scholar] [CrossRef]
- Miller, P.R.; Gittard, S.D.; Edwards, T.L.; Lopez, D.A.M.; Xiao, X.; Wheeler, D.R.; Monteiro-Riviere, N.A.; Brozik, S.M.; Polsky, R.; Narayan, R.J. Integrated carbon fiber electrodes within hollow polymer microneedles for transdermal electrochemical sensing. Biomicrofluidics 2011, 5, 013415. [Google Scholar] [CrossRef] [Green Version]
- Miller, P.R.; Xiao, X.; Brener, I.; Burckel, D.B.; Narayan, R.; Polsky, R. Microneedle-Based Transdermal Sensor for On-Chip Potentiometric Determination of K+. Adv. Healthc. Mater. 2014, 3, 876–881. [Google Scholar] [CrossRef]
- Lee, K.; Lee, H.C.; Lee, D.S.; Jung, H. Drawing lithography: Three-dimensional fabrication of an ultrahigh-aspect-ratio microneedle. Adv. Mater. 2010, 22, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Li, C.G.; Lee, C.Y.; Lee, K.; Jung, H. An optimized hollow microneedle for minimally invasive blood extraction. Biomed. Microdevices 2013, 15, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Li, C.G.; Joung, H.A.; Noh, H.; Song, M.B.; Kim, M.G.; Jung, H. One-touch-activated blood multidiagnostic system using a minimally invasive hollow microneedle integrated with a paper-based sensor. Lab Chip 2015, 15, 3286–3292. [Google Scholar] [CrossRef]
- Niinomi, M. Recent research and development in metallic materials for biomedical, dental and healthcare products applications. Mater. Sci. Forum 2007, 539–543, 193–200. [Google Scholar] [CrossRef]
- Yuen, P.K.; Goral, V.N. Low-cost rapid prototyping of flexible microfluidic devices using a desktop digital craft cutter. Lab Chip 2010, 10, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Dal Dosso, F.; Decrop, D.; Pérez-Ruiz, E.; Daems, D.; Agten, H.; Al-Ghezi, O.; Bollen, O.; Breukers, J.; De Rop, F.; Katsafadou, M.; et al. Creasensor: SIMPLE technology for creatinine detection in plasma. Anal. Chim. Acta 2018, 1000, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Dal Dosso, F.; Kokalj, T.; Belotserkovsky, J.; Spasic, D.; Lammertyn, J. Self-powered infusion microfluidic pump for ex vivo drug delivery. Biomed. Microdevices 2018, 20, 44. [Google Scholar] [CrossRef]
- Dal Dosso, F.; Bondarenko, Y.; Kokalj, T.; Lammertyn, J. SIMPLE analytical model for smart microfluidic chip design. Sens. Actuators A Phys. 2019, 287, 131–137. [Google Scholar] [CrossRef]
- Ordutowski, H.; Dal Dosso, F.; De Wispelaere, W.; Van Tricht, C.; Vermeire, S.; Geukens, N.; Gils, A.; Spasic, D.; Lammertyn, J. Next generation point-of-care test for therapeutic drug monitoring of adalimumab in patients diagnosed with autoimmune diseases. Biosens. Bioelectron. 2022, 208, 114189. [Google Scholar] [CrossRef]
- Dal Dosso, F.; Tripodi, L.; Spasic, D.; Kokalj, T.; Lammertyn, J. Innovative Hydrophobic Valve Allows Complex Liquid Manipulations in a Self-Powered Channel-Based Microfluidic Device. ACS Sens. 2019, 4, 694–703. [Google Scholar] [CrossRef]
- Cheng, J.; Perrie, W.; Edwardson, S.P.; Fearon, E.; Dearden, G.; Watkins, K.G. Effects of laser operating parameters on metals micromachining with ultrafast lasers. Appl. Surf. Sci. 2009, 256, 1514–1520. [Google Scholar] [CrossRef]
- Radmanesh, M.; Kiani, A. ND:YAG Laser Pulses Ablation Threshold of Stainless Steel 304. Mater. Sci. Appl. 2015, 06, 634–645. [Google Scholar] [CrossRef] [Green Version]
- Blicharz, T.M.; Gong, P.; Bunner, B.M.; Chu, L.L.; Leonard, K.M.; Wakefield, J.A.; Williams, R.E.; Dadgar, M.; Tagliabue, C.A.; El Khaja, R.; et al. Microneedle-based device for the one-step painless collection of capillary blood samples. Nat. Biomed. Eng. 2018, 2, 151–157. [Google Scholar] [CrossRef]
- Kou, Z.; Wan, Y.; Cai, Y.; Liang, X.; Liu, Z. Burr Controlling in Micro Milling with Supporting Material Method. Procedia Manuf. 2015, 1, 501–511. [Google Scholar] [CrossRef] [Green Version]
- Kokalj, T.; Park, Y.; Vencelj, M.; Jenko, M.; Lee, L.P. Self-powered Imbibing Microfluidic Pump by Liquid Encapsulation: SIMPLE. Lab Chip 2014, 14, 4329–4333. [Google Scholar] [CrossRef] [PubMed]
- Vloemans, D.; Van Hileghem, L.; Verbist, W.; Thomas, D.; Dal Dosso, F.; Lammertyn, J. Precise sample metering method by coordinated burst action of hydrophobic burst valves applied to dried blood spot collection. Lab Chip 2021, 21, 4445–4454. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, K.; Nakanishi, N.; Uetsuji, Y.; Nakamachi, E. Development of blood extraction system for health monitoring system. Biomed. Microdevices 2005, 7, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Ramasubramanian, M.K.; Barham, O.M.; Swaminathan, V. Mechanics of a mosquito bite with applications to microneedle design. Bioinspir. Biomim. 2008, 3, 046001. [Google Scholar] [CrossRef] [Green Version]
- Boron, W.; Boulpaep, E. Medical Physiology; Elsevier Health Sciences: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Lima, R.; Ishikawa, T.; Imai, Y.; Yamaguchi, T. Blood flow behavior in microchannels: Past, current and future trends. In Single and Two-Phase Flows on Chemical and Biomedical Engineering; Bentham Science Publishers: Sharjah, UAE, 2012; pp. 513–547. ISBN 9781608053476. [Google Scholar] [CrossRef] [Green Version]
- Prentner, S.; Allen, D.M.; Larcombe, L.; Marson, S.; Jenkins, K.; Saumer, M. Effects of channel surface finish on blood flow in microfluidic devices. Microsyst. Technol. 2010, 16, 1091–1096. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Das, D.B.; Rielly, C.D. Microneedle Assisted Micro-Particle Delivery from Gene Guns: Experiments Using Skin-Mimicking Agarose Gel. J. Pharm. Sci. 2014, 103, 613–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, J.H.; Ordutowski, H.; Van Tricht, C.; Verbruggen, R.; Barcenas Gallardo, A.; Bulcaen, M.; Ciwinska, M.; Gutierrez Cisneros, C.; Devriese, C.; Guluzade, S.; et al. Point-of-care therapeutic drug monitoring of adalimumab by integrating a FO-SPR biosensor in a self-powered microfluidic cartridge. Biosens. Bioelectron. 2022, 206, 114125. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Hileghem, L.; Kushwaha, S.; Piovesan, A.; Verboven, P.; Nicolaï, B.; Reynaerts, D.; Dal Dosso, F.; Lammertyn, J. Innovative Fabrication of Hollow Microneedle Arrays Enabling Blood Sampling with a Self-Powered Microfluidic Patch. Micromachines 2023, 14, 615. https://doi.org/10.3390/mi14030615
Van Hileghem L, Kushwaha S, Piovesan A, Verboven P, Nicolaï B, Reynaerts D, Dal Dosso F, Lammertyn J. Innovative Fabrication of Hollow Microneedle Arrays Enabling Blood Sampling with a Self-Powered Microfluidic Patch. Micromachines. 2023; 14(3):615. https://doi.org/10.3390/mi14030615
Chicago/Turabian StyleVan Hileghem, Lorenz, Shashwat Kushwaha, Agnese Piovesan, Pieter Verboven, Bart Nicolaï, Dominiek Reynaerts, Francesco Dal Dosso, and Jeroen Lammertyn. 2023. "Innovative Fabrication of Hollow Microneedle Arrays Enabling Blood Sampling with a Self-Powered Microfluidic Patch" Micromachines 14, no. 3: 615. https://doi.org/10.3390/mi14030615
APA StyleVan Hileghem, L., Kushwaha, S., Piovesan, A., Verboven, P., Nicolaï, B., Reynaerts, D., Dal Dosso, F., & Lammertyn, J. (2023). Innovative Fabrication of Hollow Microneedle Arrays Enabling Blood Sampling with a Self-Powered Microfluidic Patch. Micromachines, 14(3), 615. https://doi.org/10.3390/mi14030615






