Nanoantioxidant Materials: Nanoengineering Inspired by Nature
Abstract
:1. Introduction
2. Natural and Artificial Biomimetic Antioxidants
2.1. Natural Antioxidants
2.2. Artificial Biomimetic Nanoantioxidants
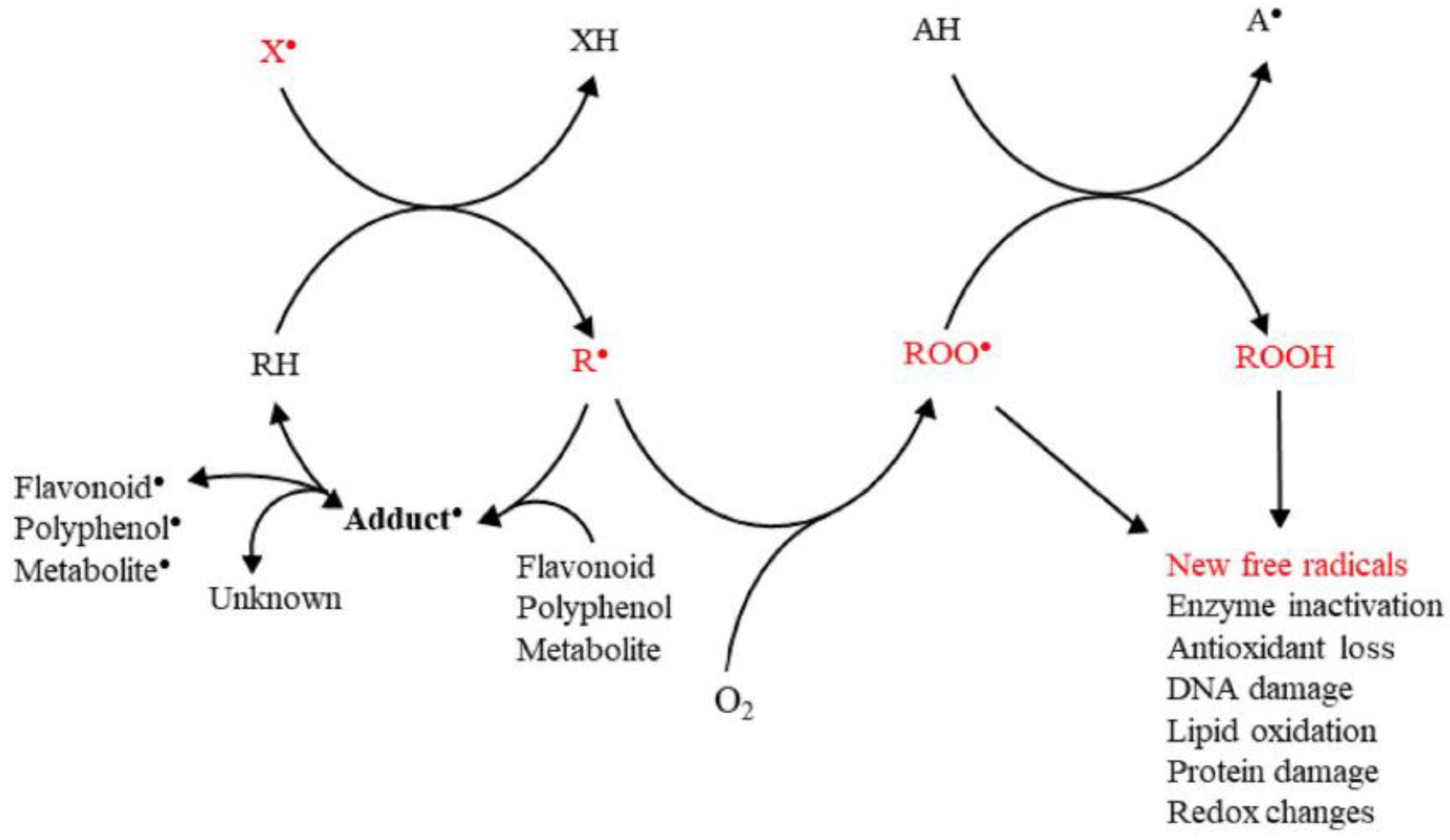
3. Oxidant Species and Counterbalancing Antioxidant Mechanisms
3.1. Free Radicals, ROS, RNS, and Other Oxidant Species
- i.
- Oxygen-centered radicals
- ii.
- Nitrogen-centered radicals
- iii.
- Carbon-centered radicals
- iv.
- Sulfur-centered, phosphorous-centered, and halogen-centered radicals
3.2. Mechanisms of Antioxidant Activity
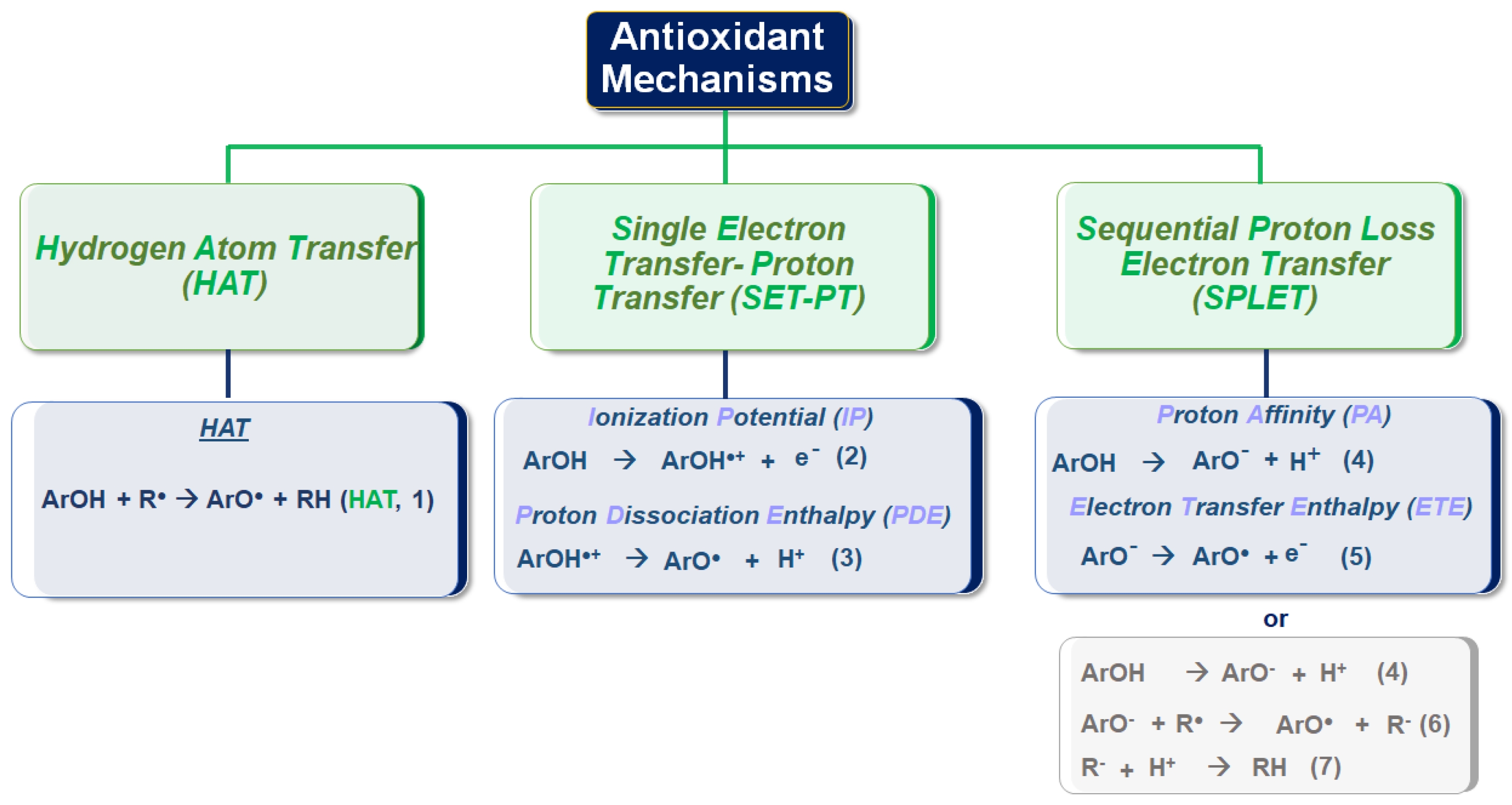
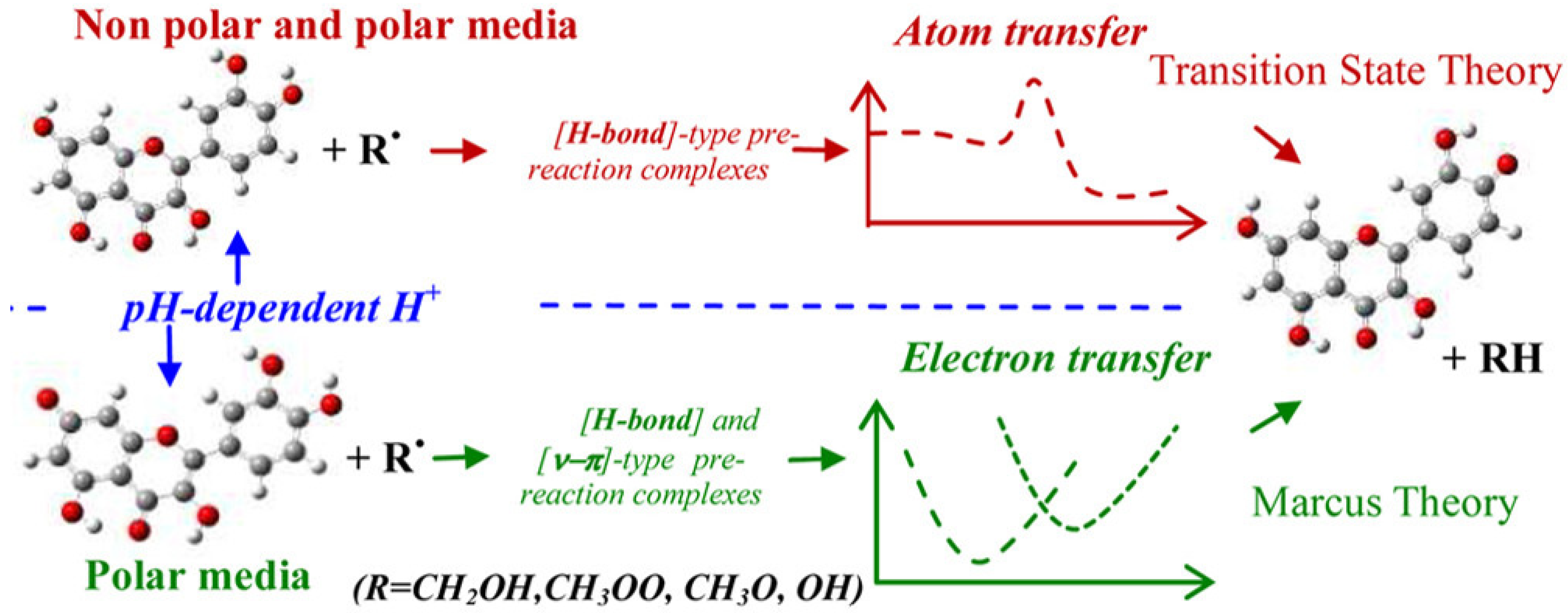
3.3. Hydrogen Atom Transfer (HAT) and Proton-Coupled Electron Transfer (PCET) Pathways
SET-PT Single Electron Transfer-Proton Transfer
Sequential Proton-Loss Electron Transfer (SPLET)
4. Evaluation of the Antioxidant Activity
4.1. Evaluation Based on Electron Paramagnetic (EPR) Resonance Spectroscopy
4.2. Evaluating Antioxidant Activity via Fluorescence Spectroscopy
4.2.1. Radical Trapping Antioxidant Parameter (TRAP) Assay
4.2.2. Oxygen Radical Absorbance Capacity (ORAC) Assay
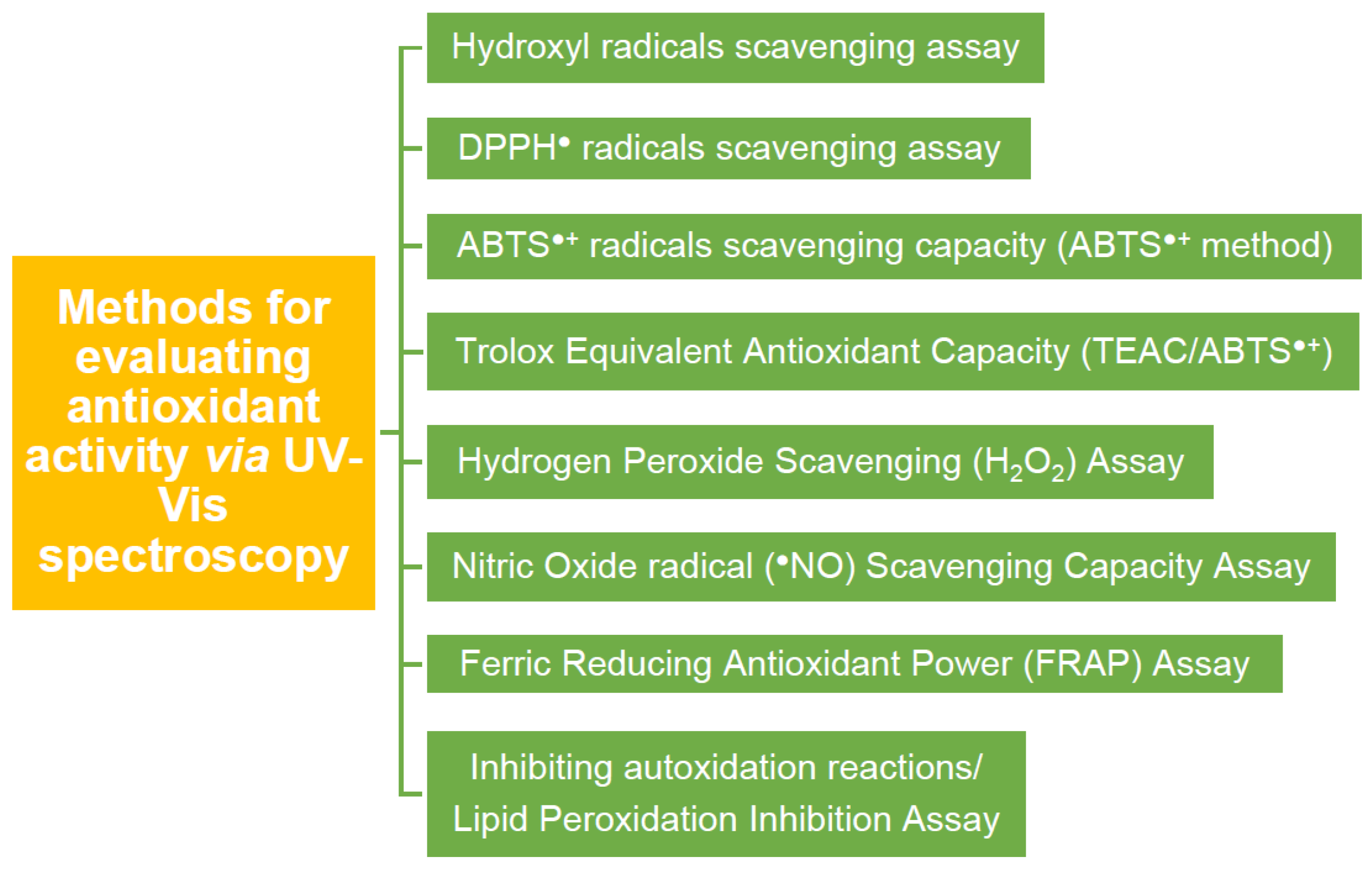
4.3. Evaluating Antioxidant Activity via UV–Vis Spectroscopy
4.3.1. Evaluating ●OH Radicals Scavenging Capacity via Assay
4.3.2. Evaluating DPPH● Radicals Scavenging Capacity
4.3.3. ABTS●+ Radicals Scavenging Capacity (ABTS●+ Method) or Trolox Equivalent Antioxidant Capacity (TEAC/ABTS●+)
4.3.4. Hydrogen Peroxide Scavenging (H2O2) Assay
4.3.5. Nitric Oxide Radical (●NO) Scavenging Assay
4.3.6. Ferric Reducing Antioxidant Power (FRAP) Assay
4.3.7. Inhibiting Autoxidation Reactions/Lipid Peroxidation Inhibition Assay
4.4. Expressing Antioxidant Capacity
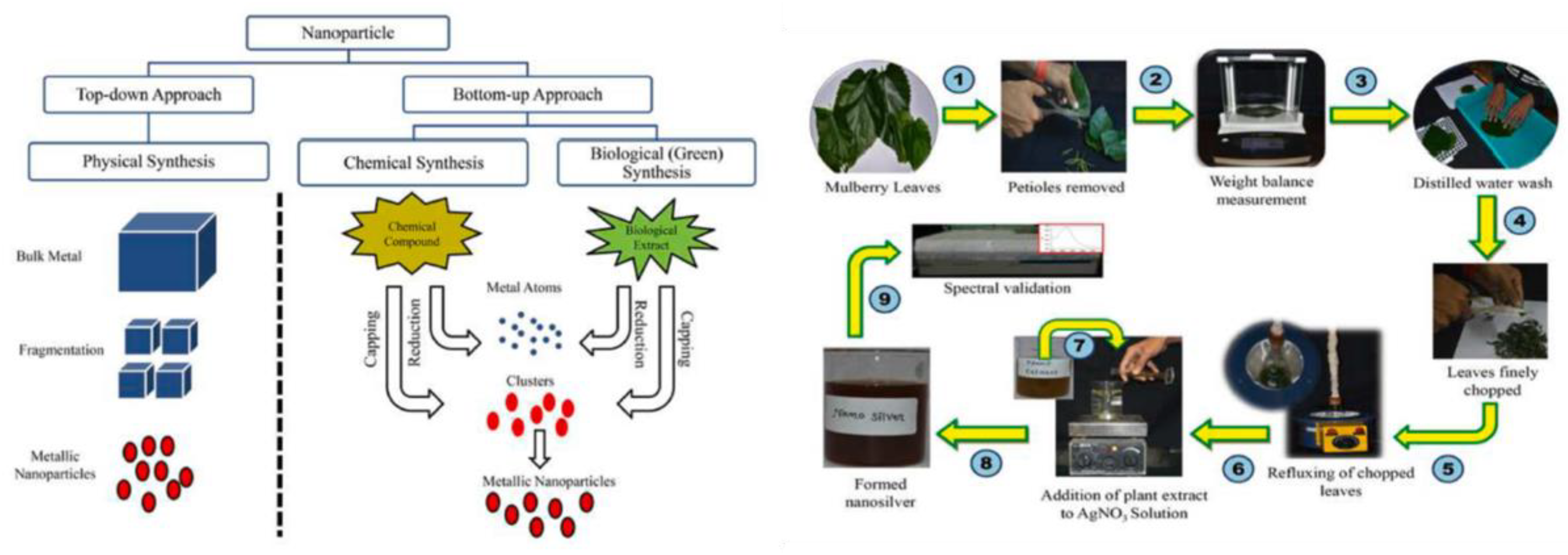
5. Optimizing Antioxidant Systems by Biomimetic Nanoengineering
5.1. Biological Nanoengineering
5.2. Nanoantioxidant Hybrids via Non-Covalent Modification
5.3. Nano(en)zymes
| Nanoantioxidant | Target | Evaluation Methods/Antioxidant Efficiency * | Ref. | |
|---|---|---|---|---|
| Non-covalent surface modification | ||||
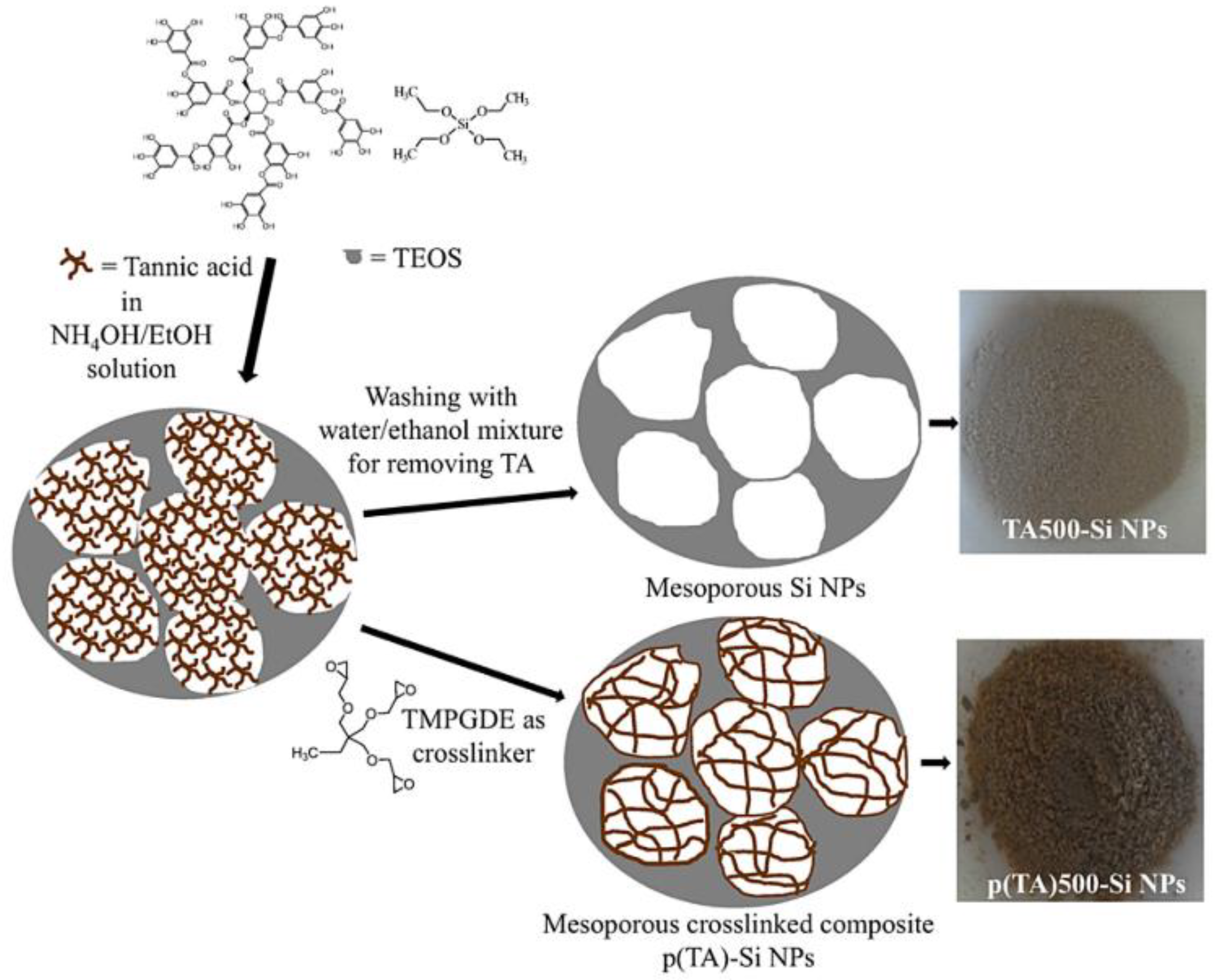
| 1 | Mesoporous poly-(Tannic Acid) (p(TA)-Si NPs) | ABTS●+ | Total phenol content(TPC): GA equivalencyp(TA)1000eSi NPs = 14 ± 0.3 μg/mL TEAC: p(TA)1000eSi NPs = 68± 6 mM Trolox equivalent g−1 | [27] |
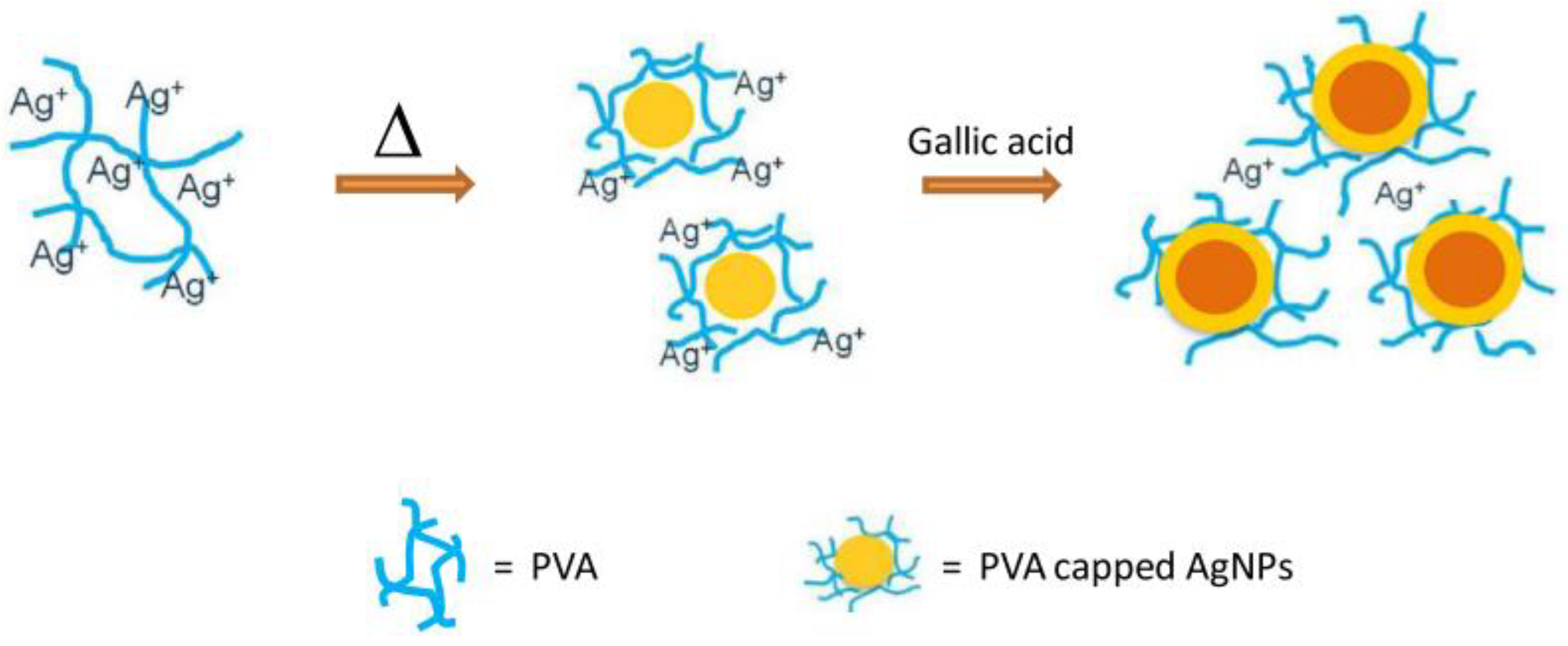
| 2 | PVA-Ag NPs, Poly(vinyl alcohol) | ABTS●+ | ABTS●+:TAC values of gingerSupplemental ginger capsule 3 = 3.199 ± 0.025 mg gallic acid/g sample | [103] |
| 3 | Fe2O3/C | DPPH (●N) | ●N (DPPH method): RSC Fe2O3/C NPs = 89%, for 10 mg of Fe2O3/C in the solution | [106] |
| 4 | SMN-Zein/BC | DPPH ●N, ABTS●+, O2●− | ●N (DPPH method): EC50 ZeinNPs = 897.5 ± 21.4 μg/mL > EC50 SMN-ZeinNPs = 38.5 ± 1.1 μg/mL ABTS●+: EC50 ZeinNPs = 55.3 ± 2.5 μg/mL > EC50 SMN-ZeinNPs = 38.5 ± 1.1 μg/mL O2●−: EC50 ZeinNPs = 3213.5 ± 165.7 μg/mL > EC50 SMN-ZeinNPs = 214.7 ± 6.9 μg/ml | [148] |
| 5 | Au/CeO2 | ●OH | Concentration-dependant improvement/inhibition of antioxidant capacity in hybrids vs. CeO2. | [101] |
| 6 | d Fe3O4 NPs | H2O2 | Peroxidase-like activity | [150] |
| 7 | Lu2O3 NPs-doped with Eu3+ | ABTS●+ | ABTS●+: RSC = 86% | [144] |
| 8 | CuO-PEG, CuO-PVP | DPPH (●N) | TACCuO-PVP = 32.44 ± 0.1 (μg AAE/mg) > TAC CuO-PEG = 27.42 ± 0.24 (μg AAE/mg) > TACCuO = 18.94 ± 0.57 (μg AAE/mg) TRPCuO-PVP = 17.38 ± 0.15 (μg AAE/mg) > TRP CuO-PEG = 16.64 ± 0.2 (μg AAE/mg) > TRPCuO = 7.10 ± 0.3 (μg AAE/mg) ●N (DPPH method): RSCCuO-PEG = 34.14% > RSC CuO-PVP = 28.36% > RSCCuO 13.79% | [147] |
| 9 | ZnO-PEG, ZnO-PVP | DPPH (●N) | TACZnO-PEG = 22.8 ± 1.55 (μg AAE/mg) > TACZnO-PVP = 19.1 ± 1.64 (μg AAE/mg) > TACZnO = 13.1 ± 1.11 (μg AAE/mg) TRPZnO-PVP = 15.1 ± 1.65 (μg AAE/mg) > TRP ZnO-PEG = 13.5 ± 1.13 (μg AAE/mg) > TRP ZnO = 6.64 ± 0.05 (μg AAE/mg) ●N (DPPH method): RSC ZnO-PVP = 13.75% > RSCZnO-PEG = 13.66% > RSCZnO = 9.66% | [146] |
| 10 | Chi-SiO2, Chi-CMC-SiO2 | DPPH (●N) | ●N (DPPH method): RSCChi-CMC-SiO2 = 1.5 RSCChiSiO2 | [151] |
| 11 | Chi-Ppy, chi-PPy-PTDA | DPPH (●N) | ●N (DPPH method): max RSC = 86% | [152] |
| 12 | b,c MoS2@TiO2 | ROS | Strong bionic bi-enzyme activity | [153] |
| 13 | c CDs-CeO2 nanocomposites | H2O2 | Enzyme-like activity | [154] |
| 14 | a Cs-FeO | DPPH (●N), H2O2 | ●N (DPPH method): maxRSCCS-FeO ≈ 93% > RSCFeO ≈ 83% H2O2: maxRSCCS-FeO ≈ 82% > RSCFeO ≈ 72% | [43] |
| 15 | Pd-RGO-ZnO | DPPH (●N), ●NO | ●N (DPPH method): max RSCPd-RGO-ZnONPs = 58.0% > RSCRGO-ZnONPs = 45.2% > RSCRGO = 27.5%/●NO: Max RSCPd-RGO-ZnONPs= 48.6% > RSCRGO-ZnONPs = 39.3% > RSCRGO = 27.8% | [155] |
| 16 | Rpda NPs, dextran/chitosan | DPPH (●N), ABTS●+ | ●N (DPPH method): max RSCrPDA NPs = 85% ABTS●+: max RSCrPDA NPs = 90% | [156] |
| 17 | L-PDNPs | ●OH | Cell protection from/decreasing ROS-induced damages/alteration. | [157] |
| 18 | a CUR-AuNPs AuNPs and co-functionalization with Curcuma pseudomontana isolated curcumin (CUR) | DPPH (●N), H2O2, ●NO | ●N (DPPH): max RSCVitaminC = 89.6% > max RSCCUR-AuNPs = 85.2% > max RSCCUR = 84.2%, H2O2: max RSCVitamin C = 84.8% > RSCCUR-AuNPs = 83.2% > RSCCUR = 76.5%, (RP): max RSCVitaminC = 91.4% > RSCCUR-AuNPs = 87.9% > RSCCUR = 82.3%, ●NO: max RSCVitamin C = 84.8% > RSCCUR-AuNPs = 84.5% > RSCCUR = 79.5% | [158] |
| 19 | a Quercetin–linseed oil co-loaded lipid carrier (NLCS) | DPPH (●N) | ●N (DPPH): max RSCQuercetin NLCS 3 ≈ 77% | [159] |
| 20 | a Turmenic extract encapsulated in NLC, T-NLC | DPPH (●N) | ●N (DPPH): max RSCT-NLC ≈ 45% > RSC turmeric extract ≈ 40% | [160] |
| 21 | Zein-pectin NPs loaded with curcumin | DPPH (●N), ABTS+● | ●N (DPPH): SC50 Curcumin ≈ 17.5 μg/mL > SC50 Zein-pectin NPs loaded with curcumin ≈ 14.7 μg/mL > SC50 AA ≈ 5.5 μg/mL ABTS+●: TEAC Zein-pectin NPs loaded with curcumin≈14.3 mg > TEAC Curcumin ≈0.8 mg > TEAC Zein-pectin NPs ≈ 0.04 mg | [161] |
| 22 | Ti3C2 MXene nanosheets | RNS, ROS (H2O2, O2•–, and •OH) | Scavenging excessive RNS and ROS | [145] |
| Nanozymes | ||||
| 23 | CNPs (Cerium nanoparticles) | ●OH | Enzyme-like activity | [55] |
| 24 | LCNPs | DPPH (●N) | ●N (DPPH): max RSC LCNPs = 85% | [149] |
| 25 | V2O5 | NADPH | Enzyme-like activity | [104] |
5.4. Surface Chemical Modification of Nanomaterials by Grafting Natural Antioxidants or Functional Components to Produce Hybrid Nanoantioxidants
| Nanoantioxidant | Target | Evaluation Methods/Antioxidant Efficiency * | Ref. | |
|---|---|---|---|---|
| 1 | a Gallic acid at Silica NPs (SiO2@GA) | DPPH (●N) | ●N (DPPH method): nfast = 2.1 ± 0.2 | [88] |
| 2 | f Mesoporous SiO2 NPs (MSN) functionalized with morin AMSNPs-MOR | ●OH, 1O2 | ●OH: RSC AMSNPs-MOR = 57% higher than morin, 1O2:kTAMSNPs-MOR = 4.5 × 107 M−1s−1 < kTMOR = 1.3 × 108 M−1s−1 | [32] |
| 3 | MSNs (MSN-CAF), rutin (MSN-RUT), where CAF = caffeic acid, and RUT = rutin | ROO● | ORACMSN-RUT = 7.32 ± 1.93 μmol/L TE < ORACRUT = 10.92 ± 1.73 μmol/L TE | [35] |
| 4 | (Cellulose fiber)-Au NPS | DPPH (●N) | ●N (DPPH method): max RSCUBK-AuNPs = 86.05% ± 0.009% > RSCUBK = 47.7% | [169] |
| 5 | Au@PEG3SA (salvianic acid) | DPPH (●N) | ●N (DPPH method): kobs Au@PEG3SA = 65.3 ± 1.65 M−1 s−1 > kobsSA = 7.13 ± 0.55 M−1 s−1 | [173] |
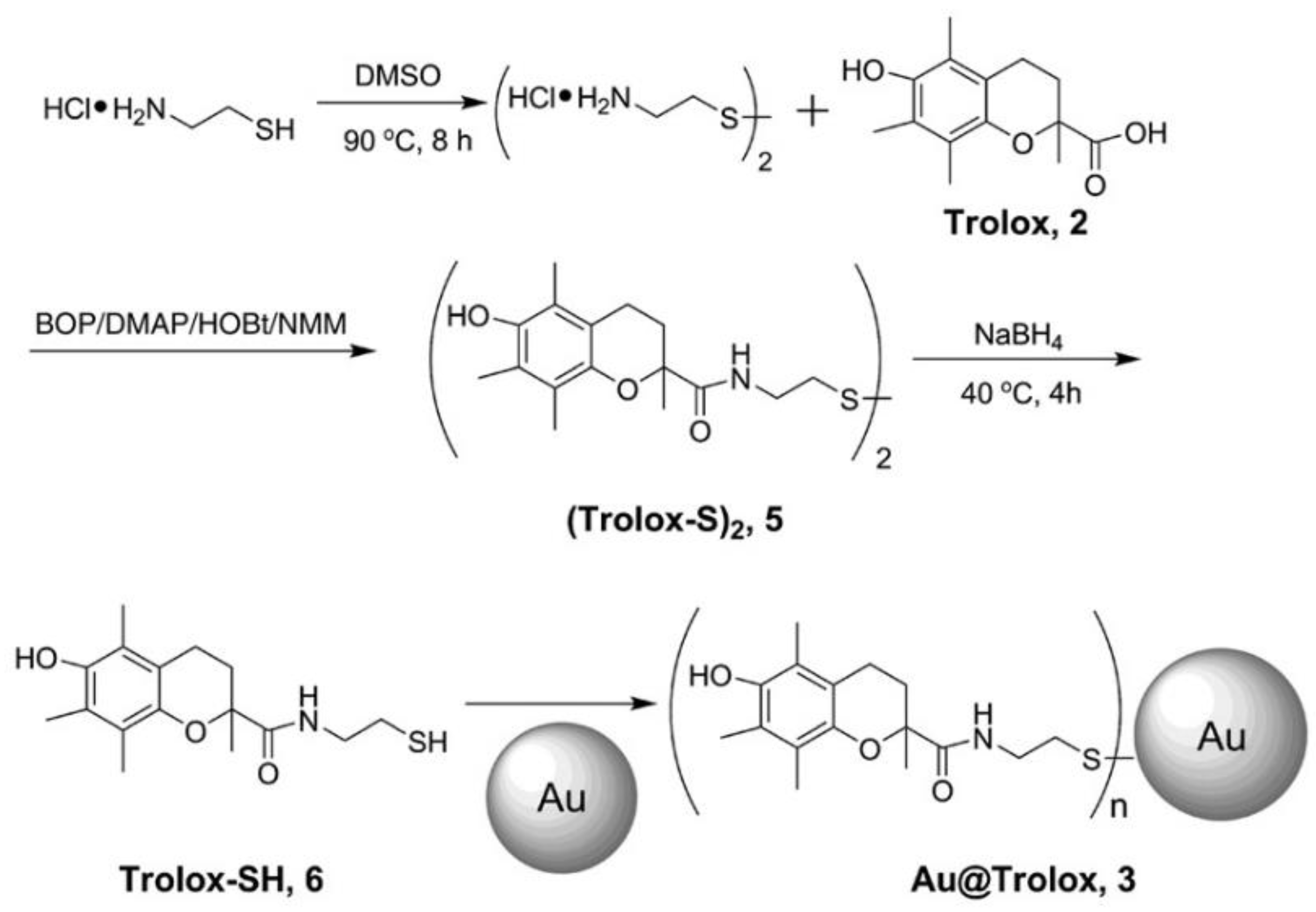
| 6 | Au@Trolox | DPPH(●N) | ●N (DPPH method): SRAu@Trolox = 8 SRTrolox | [38] |
| 7 | a Au NPs embedded 3,6 dihydroxyflavone, lutein, and selenium methyl selenocysteine | ●N, ●OH, H2O2, ●NO | ●N (DPPH method): RSC AA = 96.28% > RSC Au-triplet NPs = 87.13% RSC 3,6 dihydroxyflavone, lutein, and selenium methyl selenocysteine = 72.89% > RSC Au-3,6 dihydroxyflavone = 72.04% > RSC lutein = 65.79% > RSC 3,6 dihydroxyflavone = 65.79% > RSC selenium methyl selenocysteine = 43.85% ●OH:RSC AA = 96.18% > RSC Au-triplet NPs = 85.11% RSC 3,6 dihydroxyflavone, lutein, and selenium methyl selenocysteine = 70.63% > RSC Au-3,6 dihydroxyflavone = 70.01% > RSC lutein = 63.85% > RSC 3,6 dihydroxyflavone = 62.11% > RSC selenium methyl selenocysteine = 41.62% H2O2:RSC AA = 96.12% > RSC Au-triplet NPs = 83.10% RSC 3,6 dihydroxyflavone, lutein, and selenium methyl selenocysteine = 71.35% > RSC Au-3,6 dihydroxyflavone = 70.08% > RSC lutein = 61.85% > RSC 3,6 dihydroxyflavone = 60.11% > RSC selenium methyl selenocysteine = 40.02% ●NO: RSC AA = 96.02% > RSC Au-triplet NPs = 84.02% RSC 3,6 dihydroxyflavone, lutein, and selenium methyl selenocysteine = 69.09% > RSC Au-3,6 dihydroxyflavone = 69.01% > RSC 3,6 dihydroxyflavone =61.24% > RSClutein = 60.85% > RSC selenium methyl selenocysteine = 42.11% | [105] |
| 8 | Lignin Capped Silver NPs (LCSN) | DPPH (●N) | ●N (DPPH method): RSC = 70%, IC50 = 3360 μg/mL | [170] |
| 9 | IONP@GA | DPPH (●N) | ●N (DPPH method): RSC IONP@GA3 = 78% > RSC IONP = 50%, IC50 IONP@GA3= 1.00 ± 0.003 mg/mL > IC50 IONP = 4.7 ± 0.002 mg/mL | [23] |
| 10 | a Ag-Se bimetallic | DPPH (●N), ABTS●+ | ●N (DPPH method): RSC Trolox = 86.52 ± 0.12% > RSC Ag–Se NPs = 59 ± 0.32%, IC50 Trolox = 22.19 μg/mL < IC50 Ag–Se NPs = 31 μg/mL ABTS●+: RSC AA = 76.65 ± 0.29% > RSC Ag–Se NPs = 62.54 ± 0.21%, IC50 AA = 53.40μg/mL < IC50Ag–Se NPs = 66.38 μg/mL | [33] |
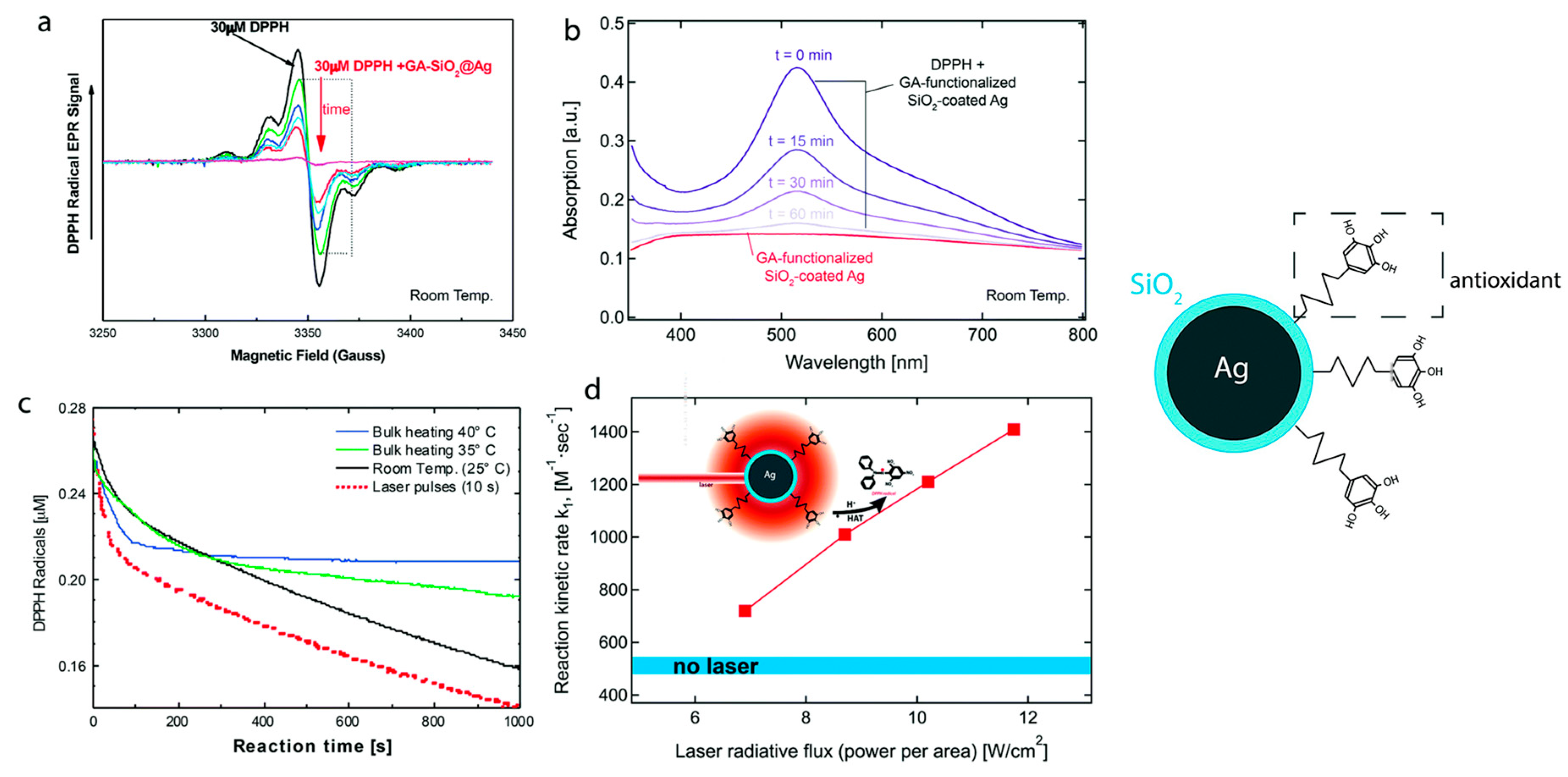
| 11 | a,f SiO2-coated Ag nanoparticles | DPPH (●N) | ●N (DPPH method): Fast phase SiO2-coated Ag n = 2 BDE SiO2-coated Ag decreases by 2 kcal/mol | [166] |
| 12 | ZnO@CA NPs | ABTS●+ | ABTS●+: RSC CA = 93.25 ± 0.43% > RSC ZnO@CA NPs = 73.68 ± 2.51% | [25] |
| 13 | poly(lactic-co-glycolic acid) (PLGA) NPs coated with polysorbate 80 (PS80) gallic acid | ABTS●+ | ABTS●+: RSC GA > RSC NP-PLGA-GA > RSC NP-PLGA/PS80-GA | [167] |
| 14 | BSA-CA | DPPH (●N) | ●N (DPPH method): RSC CA = 91.9% > RSC BSA-CA = 89.7% > RSCBSA = 9.0%, RP: RPCA >> RP BSA-CA = 0.662 > RP BSA = 0.010 ORAC: ORACCA = 4823.5 > ORAC BSA-CA = 4073.9 > ORAC BSA = 546.4 | [22] |
| 15 | d ACSSNs-CA | DPPH (●N) | ●N (DPPH method): RSC CA ≈ 95% > RSCACSSNs-CA ≈ 85%/Chelating Activity (CA): CA ACSSNs-CA ≈ 97% > CA CA ≈ 25%/1O2:kq ACSSNs-CA = 1.3 × 106 M−1·s−1> kqCA = 4.6 × 105 M−1·s−1 | [19] |
| 16 | a Se@Trolox | ABTS●+ | ABTS●+: Se@Trolox > Trolox and Se@MUN | [37] |
| 17 | Au@PEG (Au@Trolox) | DPPH (●N) | ●N (DPPH): Au@Trolox > Au@PEG + Trolox ≈ Trolox | [162] |
| 18 | ZnO@GA | ABTS●+ | ABTS●+: RSC GA ≈ 93.25 ± 0.43% > RSCZnO@GA = 69.71 ± 5.26% | [168] |
| 19 | ACSSNPs-CA (Carminic acid) | 1O2 | 1O2: KT ACSSNPs-CA = 1.30 × 108 M−1 s −1 > kTCA = 6.35 × 107 M−1·s−1 D2O: KT ACSSNPs-CA 1.67 × 108 M−1 s −1 > kT CA = 1.46 × 107 M−1·s−1 | [28] |
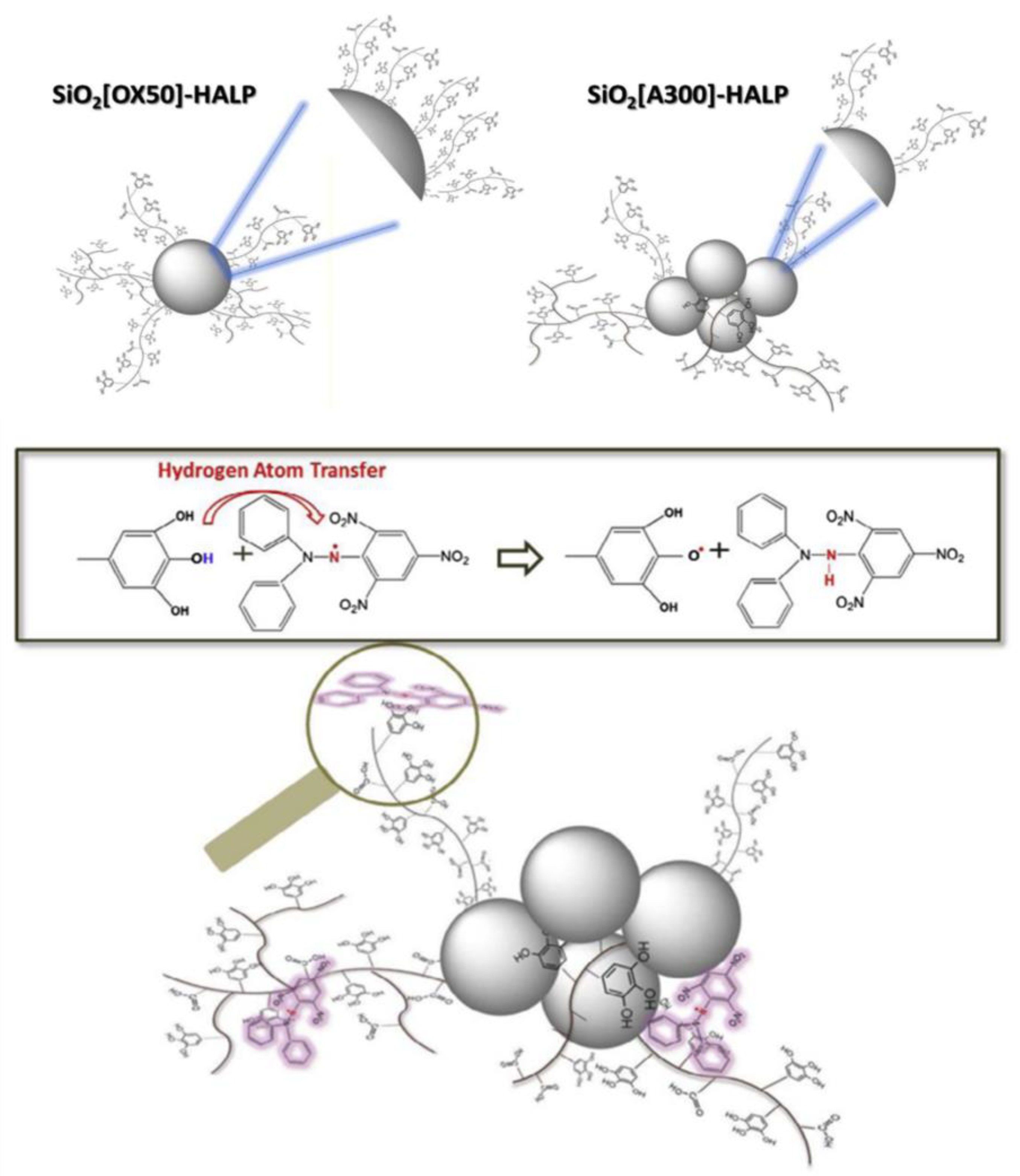
| 20 | a SiO2-HALP NPs | DPPH (●N) | ●N (DPPH): nscavenged = SiO2[A300]-HALP >> SiO2[A90]-HALP >> HALP >> SiO2[OX50]-HALP > SiO2[S300]-HALP | [29] |
| 21 | a GLA@SiO2@GLAM, SiO2@GLA, SiO2@GLAM | DPPH (●N) | ●N (DPPH): Ea (kJ/mol (±1)) Ea{GLA@SiO2@GLAM} [2:1]= 42.2 > Ea{GLA@SiO2@GLAM} [3:1]= 46.6 > EaSiO2@GLA= 65.7 > EaSiO2@GLAM = 123.3 | [15] |
| 22 | a HNT-Trolox/Que | DPPH (●N), ROO● | ●N (DPPH): nQue = 4.0 ±0.2 > nHNT/Que = 3.8 ± 0.2> nHNT-Trolox/Que = 2.8 ±0.2 > nTrolox = 2.0 ± 0.2> nHNT-Trolox = 1.3 ± 0.2 | [163] |
| 23 | C-SNPs, EC-SNPs, EGCG-SNPs, PAG-SNPs | DPPH (●N) | ●N (DPPH): IC50EGCG-SNPs = 0.59± 0.02μg/mL > IC50 EC-SNPs = 0.54 ± 0.05 μg/mL > IC50 EGCG = 0.52 ± 0.04 μg/mL > IC50 EC = 0.50 ± 0.04 μg/mL > IC50 PAC-SNPs = 0.24 ± 0.04 μg/mL > IC50 PAC = 0.23 ± 0.03 μg/mL > IC50 C = 0.22 ± 0.02 μg/mL > IC50 C-SNPs = 0.21 ± 0.03 μg/ml | [111] |
| 24 | PLA-UA NPs | HOCl | Antioxidants decrease the oxidation of TMB by HOCl | [100] |
| 25 | PLGA-Que NPs | DPPH (●N) | ●N (DPPH): RSCF3 = 80%> RSCF2 = 79% | [34] |
| 26 | PCL-Que NPs | DPPH (●N), O2●− | ●N (DPPH): EC50Quercetin-biapigenin = 5.95 ± 0.97 μg/mL > EC50 Quercetin-biapigenin PCL-loaded nanoparticles = 5.73 ± 1.20 μg/mL/O2●−: EC50Quercetin-biapigenin = 72.71 ± 4.07μg/mL/Iron (II) chelating: EC50Quercetin-biapigenin = 11.56 ± 0.44 μg/mL < EC50 Quercetin-biapigenin PCL-loaded nanoparticles = 23.50 ± 0.55μg/ml | [164] |
| 27 | Vitamin E, catechol, and Ag NPs from Hibiscus rosasinensis (HRS) extracts within a chitosan matrix | DPPH(●N), H2O2, ●NO | ●N (DPPH method): IC50Cs–AA–Glu = 13.38 ± 4.7 μg/mL/●NO: IC50Cs–AA–Glu = 1.19 ± 1.82% | [41] |
| 28 | a,e AMSN-RA | DPPH(●N) | ●N (DPPH method): max RSCAMSN-RA ≈ 97% > RSCRA ≈ 83% | [172] |
| 29 | SiO2-Que | O2●− | O2●−: RSC quercetin = over 90% > RSCSiO2-Que = 73% | [165] |
| 30 | b,c V2O5@pDA@MnO2 | ROS | Enzyme-mimicking antioxidant effect (GPx-like) | [174] |
| 31 | Nanohybrid HNT/AH2 | DPPH(●N), ROO● | DPPH method: HNT/AH2 290% vs. asc. acid (MeOH)/reaction with ROO●: rate constant 5.1 × 104 M−1 s−1 | [171] |
| 32 | Fe3O4@PDA-CuCl2 | DPPH (●N) | ●N (DPPH method): IC50Fe3O4@PDA = 258μg/mL < IC50 BHT = 386 μg/mL < IC50 Fe3O4@PDA-CuCl2 = 450 mg/mL | [175] |
| 33 | Iron oxide NPs (SPION) capped with GA, Trolox, and nordihydroguaiaretic acid | ROO• | O2 consumption ((−d[O2]/dt/µMs−1): MAG-GA,MAG-NDGA = 1.3 ± 0.2 > MAG-TX = 1.3 ± 0.2) | [58] |
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Shah, S.T.; Chowdhury, Z.Z.; Simarani, K.; Basirun, W.J.; Badruddin, I.A.; Hussien, M.; Alrobei, H.; Kamangar, S. Nanoantioxidants: The Fourth Generation of Antioxidants—Recent Research Roadmap and Future Perspectives. Coatings 2022, 12, 1568. [Google Scholar] [CrossRef]
- Halliwell, B. Antioxidants in Human Health and Disease. Annu. Rev. Nutr. 1996, 16, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef] [PubMed]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar]
- Liu, R.; Mabury, S.A. Synthetic Phenolic Antioxidants: A Review of Environmental Occurrence, Fate, Human Exposure, and Toxicity. Environ. Sci. Technol. 2020, 54, 11706–11719. [Google Scholar] [CrossRef]
- Farah, F.; Farah, F.H. Nanocarriers As Delivery Systems for Therapeutics Agents. Int. J. Pharm. Sci. Res. 2019, 10, 3487. [Google Scholar] [CrossRef]
- Gil, D.; Rodriguez, J.; Ward, B.; Vertegel, A.; Ivanov, V.; Reukov, V. Antioxidant activity of SOD and catalase conjugated with nanocrystalline ceria. Bioengineering 2017, 4, 18. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, W.; Baj, J. Antioxidants: Classification, Natural Sources, Activity / Capacity. Materials 2021, 14, 4135. [Google Scholar] [CrossRef]
- Ahmad, F.; Salem-Bekhit, M.M.; Khan, F.; Alshehri, S.; Khan, A.; Ghoneim, M.M.; Wu, H.F.; Taha, E.I.; Elbagory, I. Unique Properties of Surface-Functionalized Nanoparticles for Bio-Application: Functionalization Mechanisms and Importance in Application. Nanomaterials 2022, 12, 1333. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W.; Kammakakam, I. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.; Yehye, W.A.; Etxeberria, A.E.; Alhadi, A.A.; Dezfooli, S.M.; Julkapli, N.B.M.; Basirun, W.J.; Seyfoddin, A. Nanoantioxidants: Recent trends in antioxidant delivery applications. Antioxidants 2020, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Bhardwaj, K.; Nepovimova, E.; Kuča, K.; Dhanjal, D.S.; Bhardwaj, S.; Bhatia, S.K.; Verma, R.; Kumar, D. Antioxidant functionalized nanoparticles: A combat against oxidative stress. Nanomaterials 2020, 10, 1334. [Google Scholar] [CrossRef]
- Diniz, L.R.L.; Da Silva Maia Bezerra Filho, C.; Fielding, B.C.; De Sousa, D.P.; Gil, G. Natural Antioxidants: A Review of Studies on Human and Animal Coronavirus. Oxid. Med. Cell. Longev. 2020, 2020, 3173281. [Google Scholar] [CrossRef]
- Theofanous, A.; Sarli, I.; Fragou, F.; Bletsa, E.; Deligiannakis, Y.; Louloudi, M. Antioxidant Hydrogen-Atom-Transfer to DPPH Radicals by Hybrids of {Hyaluronic-Acid Components}@SiO2. Langmuir 2022, 38, 12333–12345. [Google Scholar] [CrossRef]
- Mayer, J.M.; Hrovat, D.A.; Thomas, J.L.; Borden, W.T. Proton-coupled electron transfer versus hydrogen atom transfer in benzyl/toluene, methoxyl/methanol, and phenoxyl/phenol self-exchange reactions. J. Am. Chem. Soc. 2002, 124, 11142–11147. [Google Scholar] [CrossRef]
- Anwar, H.; Hussain, G.; Mustafa, I. Antioxidants from Natural Sources. Antioxidants Foods Its Appl. 2018, 3–28. [Google Scholar] [CrossRef]
- Vaiserman, A.; Koliada, A.; Zayachkivska, A.; Lushchak, O. Nanodelivery of Natural Antioxidants: An Anti-aging Perspective. Front. Bioeng. Biotechnol. 2020, 7, 447. [Google Scholar] [CrossRef] [PubMed]
- Arriagada, F.; Günther, G.; Nos, J.; Nonell, S.; Olea-Azar, C.; Morales, J. Antioxidant nanomaterial based on core–shell silica nanospheres with surface-bound caffeic acid: A promising vehicle for oxidation-sensitive drugs. Nanomaterials 2019, 9, 214. [Google Scholar] [CrossRef]
- Khojasteh, A.; Mirjalili, M.H.; Alcalde, M.A.; Cusido, R.M.; Eibl, R.; Palazon, J. Powerful plant antioxidants: A new biosustainable approach to the production of rosmarinic acid. Antioxidants 2020, 9, 1273. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Miesbauer, O.; Eisner, P. Common trends and differences in antioxidant activity analysis of phenolic substances using single electron transfer based assays. Molecules 2021, 26, 1224. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Liu, Y.; Gao, L.; Zhang, Y.; Yi, J. Improved chemical stability and cellular antioxidant activity of resveratrol in zein nanoparticle with bovine serum albumin-caffeic acid conjugate. Food Chem. 2018, 261, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.T.; Yehye, W.A.; Saad, O.; Simarani, K.; Chowdhury, Z.Z.; Alhadi, A.A.; Al-Ani, L.A. Surface functionalization of iron oxide nanoparticles with gallic acid as potential antioxidant and antimicrobial agents. Nanomaterials 2017, 7, 306. [Google Scholar] [CrossRef] [PubMed]
- Badhani, B.; Sharma, N.; Kakkar, R. RSC Advances. R. Soc. Chem. Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Choi, K.H.; Nam, K.C.; Lee, S.Y.; Cho, G.; Jung, J.S.; Kim, H.J.; Park, B.J. Antioxidant potential and antibacterial efficiency of caffeic acid-functionalized ZnO nanoparticles. Nanomaterials 2017, 7, 148. [Google Scholar] [CrossRef]
- Cao, H.; Cheng, W.X.; Li, C.; Pan, X.L.; Xie, X.G.; Li, T.H. DFT study on the antioxidant activity of rosmarinic acid. J. Mol. Struct. Theochem 2005, 719, 177–183. [Google Scholar] [CrossRef]
- Sahiner, N.; Sagbas, S.; Aktas, N. Preparation and characterization of monodisperse, mesoporous natural poly(tannic acid)-silica nanoparticle composites with antioxidant properties. Microporous Mesoporous Mater. 2016, 226, 316–324. [Google Scholar] [CrossRef]
- Arriagada, F.; Ugarte, C. Carminic Acid Linked to Silica Nanoparticles as Pigment / Antioxidant Bifunctional Excipient for Pharmaceutical Emulsions. Pharmaceutics 2020, 12, 376. [Google Scholar] [CrossRef]
- Bletsa, E.; Stathi, P.; Dimos, K.; Louloudi, M.; Deligiannakis, Y. Interfacial Hydrogen Atom Transfer by nanohybrids based on Humic Acid Like Polycondensates. J. Colloid Interface Sci. 2015, 455, 163–171. [Google Scholar] [CrossRef]
- Shah, B.R.; Zhang, C.; Li, Y.; Li, B. Bioaccessibility and antioxidant activity of curcumin after encapsulated by nano and Pickering emulsion based on chitosan-tripolyphosphate nanoparticles. Food Res. Int. 2016, 89, 399–407. [Google Scholar] [CrossRef]
- Menaga, D.; Rahman, P.K.S.M.; Rajakumar, S.; Ayyasamy, P.M. Antioxidant and Cytotoxic Activities of A Novel Isomeric Molecule (PF5) Obtained from Methanolic Extract of Pleurotus Florida Mushroom. J. Bioresour. Bioprod. 2021, 6, 338–349. [Google Scholar] [CrossRef]
- Arriagada, F.; Correa, O.; Günther, G.; Nonell, S.; Mura, F.; Olea-Azar, C.; Morales, J. Morin flavonoid adsorbed on mesoporous silica, a novel antioxidant nanomaterial. PLoS ONE 2016, 11, e0164507. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.K.; Kumar, S.; Banerjee, U.C. Quercetin and gallic acid mediated synthesis of bimetallic (silver and selenium) nanoparticles and their antitumor and antimicrobial potential. J. Colloid Interface Sci. 2014, 431, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Anwer, M.K.; Al-Mansoor, M.A.; Jamil, S.; Al-Shdefat, R.; Ansari, M.N.; Shakeel, F. Development and evaluation of PLGA polymer based nanoparticles of quercetin. Int. J. Biol. Macromol. 2016, 92, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Ebabe Elle, R.; Rahmani, S.; Lauret, C.; Morena, M.; Bidel, L.P.R.; Boulahtouf, A.; Balaguer, P.; Cristol, J.P.; Durand, J.O.; Charnay, C.; et al. Functionalized Mesoporous Silica Nanoparticle with Antioxidants as a New Carrier That Generates Lower Oxidative Stress Impact on Cells. Mol. Pharm. 2016, 13, 2647–2660. [Google Scholar] [CrossRef]
- Das, S.; Batuta, S.; Alam, M.N.; Fouzder, C.; Kundu, R.; Mandal, D.; Begum, N.A. Antioxidant flavone analog functionalized fluorescent silica nanoparticles: Synthesis and exploration of their possible use as biomolecule sensor. Colloids Surfaces B Biointerfaces 2017, 157, 286–296. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Zheng, W.; Fan, C.; Zhang, Y.; Chen, T. Functionalized selenium nanoparticles with nephroprotective activity, the important roles of ROS-mediated signaling pathways. J. Mater. Chem. B 2013, 1, 6365–6372. [Google Scholar] [CrossRef]
- Nie, Z.; Liu, K.J.; Zhong, C.J.; Wang, L.F.; Yang, Y.; Tian, Q.; Liu, Y. Enhanced radical scavenging activity by antioxidant-functionalized gold nanoparticles: A novel inspiration for development of new artificial antioxidants. Free Radic. Biol. Med. 2007, 43, 1243–1254. [Google Scholar] [CrossRef]
- Brown, M.B.; Jones, S.A. Hyaluronic acid: A unique topical vehicle for the localized delivery of drugs to the skin. J. Eur. Acad. Dermatology Venereol. 2005, 19, 308–318. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Liu, J.; Ji, N.; Liang, C.; Sun, Q.; Xiong, L. Preparation of Hollow Biopolymer Nanospheres Employing Starch Nanoparticle Templates for Enhancement of Phenolic Acid Antioxidant Activities. J. Agric. Food Chem. 2017, 65, 3868–3882. [Google Scholar] [CrossRef]
- Nayak, D.; Minz, A.P.; Ashe, S.; Rauta, P.R.; Kumari, M.; Chopra, P.; Nayak, B. Synergistic combination of antioxidants, silver nanoparticles and chitosan in a nanoparticle based formulation: Characterization and cytotoxic effect on MCF-7 breast cancer cell lines. J. Colloid Interface Sci. 2016, 470, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, production and commercial applications of fungal chitosan: A review. J. Bioresour. Bioprod. 2022, 7, 85–98. [Google Scholar] [CrossRef]
- Bharathi, D.; Ranjithkumar, R.; Vasantharaj, S.; Chandarshekar, B.; Bhuvaneshwari, V. Synthesis and characterization of chitosan/iron oxide nanocomposite for biomedical applications. Int. J. Biol. Macromol. 2019, 132, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Wang, Z.; Owens, A.C.E.; Kulaots, I.; Chen, Y.; Kane, A.B.; Hurt, R.H. Antioxidant chemistry of graphene-based materials and its role in oxidation protection technology. Nanoscale 2014, 6, 11744–11755. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Varillas, S.; Fontanil, T.; Obaya, Á.J.; Fernández-González, A.; Murru, C.; Badía-Laíño, R. Biocompatibility and Antioxidant Capabilities of Carbon Dots Obtained from Tomato (Solanum lycopersicum). Appl. Sci. 2022, 12, 773. [Google Scholar] [CrossRef]
- Liberti, D.; Alfieri, M.L.; Monti, D.M.; Panzella, L.; Napolitano, A. A melanin-related phenolic polymer with potent photoprotective and antioxidant activities for dermo-cosmetic applications. Antioxidants 2020, 9, 270. [Google Scholar] [CrossRef]
- De Goncalves, R.C.R.; Pombeiro-Sponchiado, S.R. Antioxidant activity of the melanin pigment extracted from Aspergillus nidulans. Biol. Pharm. Bull. 2005, 28, 1129–1131. [Google Scholar] [CrossRef]
- P’yanova, L.G.; Drozdov, V.A.; Sedanova, A.V.; Drozdetskaya, M.S.; Glyzdova, M.V.; Kravchenko, E.A. Synthesis of Modified Carbon Sorbents and a Study of Their Antioxidant Properties. Prot. Met. Phys. Chem. Surfaces 2018, 54, 1010–1014. [Google Scholar] [CrossRef]
- Skrypnik, L.; Babich, O.; Sukhikh, S.; Shishko, O.; Ivanova, S.; Mozhei, O.; Kochish, I.; Nikonov, I. A study of the antioxidant, cytotoxic activity and adsorption properties of karelian shungite by physicochemical methods. Antioxidants 2021, 10, 1121. [Google Scholar] [CrossRef]
- Sajo, M.E.J.; Kim, C.S.; Kim, S.K.; Shim, K.Y.; Kang, T.Y.; Lee, K.J. Antioxidant and Anti-Inflammatory Effects of Shungite against Ultraviolet B Irradiation-Induced Skin Damage in Hairless Mice. Oxid. Med. Cell. Longev. 2017, 2017, 7340143. [Google Scholar] [CrossRef]
- Seal, S.; Jeyaranjan, A.; Neal, C.J.; Kumar, U.; Sakthivel, T.S.; Sayle, D.C. Engineered defects in cerium oxides: Tuning chemical reactivity for biomedical, environmental, & energy applications. Nanoscale 2020, 12, 6879–6899. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.C.; Johnson, M.E.; Walker, M.L.; Riley, K.R.; Sims, C.M. Antioxidant cerium oxide nanoparticles in biology and medicine. Antioxidants 2016, 5, 15. [Google Scholar] [CrossRef]
- Sharpe, E.; Andreescu, D.; Andreescu, S. Artificial nanoparticle antioxidants. ACS Symp. Ser. 2011, 1083, 235–253. [Google Scholar] [CrossRef]
- Matter, M.T.; Furer, L.A.; Starsich, F.H.L.; Fortunato, G.; Pratsinis, S.E.; Herrmann, I.K. Engineering the Bioactivity of Flame-Made Ceria and Ceria/Bioglass Hybrid Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 2830–2839. [Google Scholar] [CrossRef]
- Caputo, F.; Mameli, M.; Sienkiewicz, A.; Licoccia, S.; Stellacci, F.; Ghibelli, L.; Traversa, E. A novel synthetic approach of cerium oxide nanoparticles with improved biomedical activity. Sci. Rep. 2017, 7, 4636. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Mishra, S.; Manna, K.; Saha, K.D.; Mukherjee, S.; Roy, S. Pro-Oxidant Therapeutic Activities of Cerium Oxide Nanoparticles in Colorectal Carcinoma Cells. ACS Omega 2020, 5, 9714–9723. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Aker, W.G.; Fu, P.P.; Hwang, H.M. Toxicity of engineered metal oxide nanomaterials mediated by nano-bio-eco-interactions: A review and perspective. Environ. Sci. Nano 2015, 2, 564–582. [Google Scholar] [CrossRef]
- Scurti, S.; Caretti, D.; Mollica, F.; Di Antonio, E.; Amorati, R. Chain-Breaking Antioxidant and Peroxyl Radical Trapping Activity of Phenol-Coated Magnetic Iron Oxide Nanoparticles. Antioxidants 2022, 11, 1163. [Google Scholar] [CrossRef]
- Viglianisi, C.; Scarlini, A.; Tofani, L.; Menichetti, S.; Baschieri, A.; Amorati, R. Magnetic nanoantioxidants with improved radical-trapping stoichiometry as stabilizers for inhibition of peroxide formation in ethereal solvents. Sci. Rep. 2019, 9, 17219. [Google Scholar] [CrossRef]
- Feng, L.; Liu, Z. Biomedical Applications of Carbon Nanomaterials. Biomed. Appl. Toxicol. Carbon Nanomater. 2016, 234, 131–162. [Google Scholar] [CrossRef]
- Fragou, F.; Stathi, P.; Deligiannakis, Y.; Louloudi, M. Safe-by-Design Flame Spray Pyrolysis Nano-SiO2: Minimizing the ROS generation and acute toxicity by design. ACS Appl. Nano Mater. 2022, 5, 8184–8195. [Google Scholar] [CrossRef]
- Helberg, J.; Pratt, D.A. Autoxidation vs. antioxidants-the fight for forever. Chem. Soc. Rev. 2021, 50, 7343–7358. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, A.; Dizaj, S.M.; Chodari, L.; Sunar, S.; Hasanzadeh, A.; Ahmadian, E.; Hasanzadeh, M. The promising future of nano-antioxidant therapy against environmental pollutants induced-toxicities. Biomed. Pharmacother. 2018, 103, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Çalişkan, B.; Çalişkan, A.C. EPR Analysis of Antioxidant Compounds. Free Radicals, Antioxidants Dis. 2018. [Google Scholar] [CrossRef]
- Nauser, T.; Gebicki, J.M. Fast reaction of carbon free radicals with flavonoids and other aromatic compounds. Arch. Biochem. Biophys. 2019, 674, 108107. [Google Scholar] [CrossRef]
- Das, B.; Makol, A.; Kundu, S. Phosphorus radicals and radical ions. Dalt. Trans. 2022, 51, 12404–12426. [Google Scholar] [CrossRef]
- Ulas, G.; Lemmin, T.; Wu, Y.; Gassner, G.T.; DeGrado, W.F. Designed metalloprotein stabilizes a semiquinone radical. Nat. Chem. 2016, 8, 354–359. [Google Scholar] [CrossRef]
- Shafirovich, V.; Dourandin, A.; Huang, W.; Geacintov, N.E. The Carbonate Radical Is a Site-selective Oxidizing Agent of Guanine in Double-stranded Oligonucleotides. J. Biol. Chem. 2001, 276, 24621–24626. [Google Scholar] [CrossRef]
- Bühl, M.; Dabell, P.; Manley, D.W.; McCaughan, R.P.; Walton, J.C. Bicarbonate and Alkyl Carbonate Radicals: Structural Integrity and Reactions with Lipid Components. J. Am. Chem. Soc. 2015, 137, 16153–16162. [Google Scholar] [CrossRef]
- Stathi, P.; Fotou, E.; Moussis, V.; Tsikaris, V.; Louloudi, M.; Deligiannakis, Y. Control of Tyrosyl Radical Stabilization by {SiO2@Oligopeptide} Hybrid Biomimetic Materials. Langmuir 2022, 38, 9799–9809. [Google Scholar] [CrossRef] [PubMed]
- Dixon, W.T.; Murphy, D. Determination of the acidity constants of some phenol radical cations by means of electron spin resonance. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1976, 72, 1221–1230. [Google Scholar] [CrossRef]
- Karoui, H.; Hogg, N.; Fréjaville, C.; Tordo, P.; Kalyanaraman, B. Characterization of sulfur-centered radical intermediates formed during the oxidation of thiols and sulfite by peroxynitrite: ESR-spin trapping and oxygen uptake studies. J. Biol. Chem. 1996, 271, 6000–6009. [Google Scholar] [CrossRef]
- Xia, X.; Zhu, F.; Li, J.; Yang, H.; Wei, L.; Li, Q.; Jiang, J.; Zhang, G.; Zhao, Q. A Review Study on Sulfate-Radical-Based Advanced Oxidation Processes for Domestic/Industrial Wastewater Treatment: Degradation, Efficiency, and Mechanism. Front. Chem. 2020, 8, 592056. [Google Scholar] [CrossRef]
- Martínez, M.C.; Andriantsitohaina, R. Reactive nitrogen species: Molecular mechanisms and potential significance in health and disease. Antioxidants Redox Signal. 2009, 11, 669–702. [Google Scholar] [CrossRef]
- Taub, T.; Ruthstein, S.; Cohen, H. The involvement of carbon-centered radicals in the aging process of coals under atmospheric conditions: An EPR study. Phys. Chem. Chem. Phys. 2018, 20, 27025–27035. [Google Scholar] [CrossRef]
- Lednor, P.W.; Versloot, P.C. Radical-anion chemistry of carbon monoxide. J. Chem. Soc. Chem. Commun. 1983, 983, 284–285. [Google Scholar] [CrossRef]
- Pokrovski, G.S.; Kokh, M.A.; Desmaele, E.; Laskar, C.; Bazarkina, E.F.; Borisova, A.Y.; Testemale, D.; Hazemann, J.L.; Vuilleumier, R.; Ferlat, G.; et al. The trisulfur radical ion S3•− controls platinum transport by hydrothermal fluids. Proc. Natl. Acad. Sci. USA 2021, 118, e2109768118. [Google Scholar] [CrossRef]
- Lei, Y.; Lei, X.; Westerhoff, P.; Zhang, X.; Yang, X. Reactivity of Chlorine Radicals (Cl•and Cl2•-) with Dissolved Organic Matter and the Formation of Chlorinated Byproducts. Environ. Sci. Technol. 2021, 55, 689–699. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Rubio, L.; Pyrgiotakis, G.; Beltran-Huarac, J.; Zhang, Y.; Gaurav, J.; Deloid, G.; Spyrogianni, A.; Sarosiek, K.A.; Bello, D.; Demokritou, P. Safer-by-design flame-sprayed silicon dioxide nanoparticles: The role of silanol content on ROS generation, surface activity and cytotoxicity. Part. Fibre Toxicol. 2019, 16, 14. [Google Scholar] [CrossRef]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stresses and their classifications Chemico-Biological Interactions Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2016, 224, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Dad, S.; Bisby, R.H.; Clark, I.P.; Parker, A.W. Europe PMC Funders Group Formation of singlet oxygen from solutions of vitamin E. Free Radic Res. 2006, 40, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Cao, C.; Chen, Y.; Wu, Y.; Deumens, E.; Cheng, H.P. OPAL: A Multiscale Multicenter Simulation Package Based on MPI-2 Protocol. Int. J. Quantum Chem. 2011, 111, 4020–4029. [Google Scholar] [CrossRef]
- Capaldo, L.; Ravelli, D. Hydrogen Atom Transfer (HAT): A Versatile Strategy for Substrate Activation in Photocatalyzed Organic Synthesis. European J. Org. Chem. 2017, 2017, 2056–2071. [Google Scholar] [CrossRef]
- Deligiannakis, Y.; Sotiriou, G.A.; Pratsinis, S.E. Antioxidant and antiradical SiO2 nanoparticles covalently functionalized with gallic acid. Tech. Proc. 2013 NSTI Nanotechnol. Conf. Expo, NSTI-Nanotech 2013 2013, 1, 630–633. [Google Scholar] [CrossRef]
- Di Meo, F.; Lemaur, V.; Cornil, J.; Lazzaroni, R.; Duroux, J.L.; Olivier, Y.; Trouillas, P. Free radical scavenging by natural polyphenols: Atom versus electron transfer. J. Phys. Chem. A 2013, 117, 2082–2092. [Google Scholar] [CrossRef]
- Hammes-Schiffer, S. Comparison of hydride, hydrogen atom, and proton-coupled electron transfer reactions. ChemPhysChem 2002, 3, 33–42. [Google Scholar] [CrossRef]
- Katarina, N.M. Mechanistic studies of phenolic antioxidants in reaction with nitrogen- and oxygen-centered radicals. J. Mol. Struct. Theochem. 2007, 818, 141–150. [Google Scholar] [CrossRef]
- Mayer, J.M. Understanding hydrogen atom transfer: From bond strengths to marcus theory. Acc. Chem. Res. 2011, 44, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M. On the antioxidant activity of the Ortho and Meta substituted Daidzein derivatives in the gas phase and solvent environment. J. Mex. Chem. Soc. 2014, 58, 36–45. [Google Scholar]
- Zobo Mfomo, J.; Bikele Mama, D.; Lissouck, D.; Younang, E.; N’sikabaka, S.; Mbouombouo Ndassa, I.; Mbaze Meva’à, L. Thermodynamics-antioxidant activity relationships of some 4-benzylidenamino-4, 5-dihydro-1h-1,2,4-triazol-5-one derivatives: Theoretical evaluation. Int. J. Food Prop. 2017, 20, 1935–1948. [Google Scholar] [CrossRef]
- Clément, J.L.; Ferré, N.; Siri, D.; Karoui, H.; Rockenbauer, A.; Tordo, P. Assignment of the EPR spectrum of 5,5-dimethyl-1-pyrroline N-oxide (DMPO) superoxide spin adduct. J. Org. Chem. 2005, 70, 1198–1203. [Google Scholar] [CrossRef]
- Diamantis, D.A.; Oblukova, M.; Chatziathanasiadou, M.V.; Gemenetzi, A.; Papaemmanouil, C.; Gerogianni, P.S.; Syed, N.; Crook, T.; Galaris, D.; Deligiannakis, Y.; et al. Bioinspired tailoring of fluorogenic thiol responsive antioxidant precursors to protect cells against H2O2-induced DNA damage. Free Radic. Biol. Med. 2020, 160, 540–551. [Google Scholar] [CrossRef]
- Boligon, A.A. Technical Evaluation of Antioxidant Activity. Med. Chem. 2014, 4, 517–522. [Google Scholar] [CrossRef]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef]
- Youssef, M.M. Methods for Determining the Antioxidant Activity: A Review. Alexandria J. Food Sci. Technol. 2014, 11, 31–42. [Google Scholar] [CrossRef]
- Antônio, E.; Antunes, O. dos, R.; de Araújo, I.S.; Khalil, N.M.; Mainardes, R.M. Poly(lactic acid) nanoparticles loaded with ursolic acid: Characterization and in vitro evaluation of radical scavenging activity and cytotoxicity. Mater. Sci. Eng. C 2017, 71, 156–166. [Google Scholar] [CrossRef]
- Fa, M.; Yang, D.; Gao, L.; Zhao, R.; Luo, Y.; Yao, X. The effect of AuNP modification on the antioxidant activity of CeO2 nanomaterials with different morphologies. Appl. Surf. Sci. 2018, 457, 352–359. [Google Scholar] [CrossRef]
- Silveri, F.; Della Pelle, F.; Scroccarello, A.; Mazzotta, E.; Di Giulio, T.; Malitesta, C.; Compagnone, D. Carbon Black Functionalized with Naturally Occurring Compounds in Water Phase for Electrochemical Sensing of Antioxidant Compounds. Antioxidants 2022, 11, 2008. [Google Scholar] [CrossRef] [PubMed]
- Teerasong, S.; Jinnarak, A.; Chaneam, S.; Wilairat, P.; Nacapricha, D. Poly(vinyl alcohol) capped silver nanoparticles for antioxidant assay based on seed-mediated nanoparticle growth. Talanta 2017, 170, 193–198. [Google Scholar] [CrossRef]
- Vernekar, A.A.; Sinha, D.; Srivastava, S.; Paramasivam, P.U.; D’Silva, P.; Mugesh, G. An antioxidant nanozyme that uncovers the cytoprotective potential of vanadia nanowires. Nat. Commun. 2014, 5, 5301. [Google Scholar] [CrossRef] [PubMed]
- Medhe, S.; Bansal, P.; Srivastava, M.M. Enhanced antioxidant activity of gold nanoparticle embedded 3,6-dihydroxyflavone: A combinational study. Appl. Nanosci. 2014, 4, 153–161. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Gogoi, B.; Buragohain, A.K.; Deb, P. Fe2O3/C nanocomposites having distinctive antioxidant activity and hemolysis prevention efficiency. Mater. Sci. Eng. C 2014, 42, 595–600. [Google Scholar] [CrossRef]
- Leaves, L. Antioxidant Activity by DPPH Radical Scavenging Method of Ageratum conyzoides. Orient 2014, 1, 244–249. [Google Scholar]
- Foti, M.C. Use and Abuse of the DPPH• Radical. J. Agric. Food Chem. 2015, 63, 8765–8776. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Rafiullah, M.; Shehata, W.A.; Hossain, A.; Ali, M. Comparative phytochemical, thin layer chromatographic profiling and antioxidant activity of extracts from some Indian herbal drugs. J. Bioresour. Bioprod. 2022, 7, 128–134. [Google Scholar] [CrossRef]
- Chen, Z.; Bertin, R.; Froldi, G. EC50 estimation of antioxidant activity in DPPH* assay using several statistical programs. Food Chem. 2013, 138, 414–420. [Google Scholar] [CrossRef]
- Liu, C.; Ge, S.; Yang, J.; Xu, Y.; Zhao, M.; Xiong, L.; Sun, Q. Adsorption mechanism of polyphenols onto starch nanoparticles and enhanced antioxidant activity under adverse conditions. J. Funct. Foods 2016, 26, 632–644. [Google Scholar] [CrossRef]
- Logan, S.R. The Origin and Status of the Arrhenius Equation. J. Chem. Educ. 1982, 59, 279. [Google Scholar] [CrossRef]
- Ganguly, P.; Breen, A.; Pillai, S.C. Toxicity of Nanomaterials: Exposure, Pathways, Assessment, and Recent Advances. ACS Biomater. Sci. Eng. 2018, 4, 2237–2275. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.P.; Xia, Q.; Hwang, H.M.; Ray, P.C.; Yu, H. Mechanisms of nanotoxicity: Generation of reactive oxygen species. J. Food Drug Anal. 2014, 22, 64–75. [Google Scholar] [CrossRef]
- Zhang, H.; Dunphy, D.R.; Jiang, X.; Meng, H.; Sun, B.; Tarn, D.; Xue, M.; Wang, X.; Lin, S.; Ji, Z.; et al. Processing Pathway Dependence of Amorphous Silica Nanoparticle Toxicity: Colloidal vs. Pyrolytic. J. Am. Chem. Soc. 2012, 134, 15790–15804. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhao, F.; Wang, J.; Zu, Y.; Gu, Z.; Zhao, Y. A Safe-by-Design Strategy towards Safer Nanomaterials in Nanomedicines. Adv. Mater. 2019, 31, 1805391. [Google Scholar] [CrossRef]
- Nath, D.; Banerjee, P. Green Nanotechnology–A New Hope for Medical Biology; Elsevier B.V.: Amsterdam, The Netherlands, 2013; Volume 36, ISBN 9133943338457. [Google Scholar]
- Quester, K.; Avalos-Borja, M.; Castro-Longoria, E. Biosynthesis and microscopic study of metallic nanoparticles. Micron 2013, 54–55, 1–27. [Google Scholar] [CrossRef]
- Sriranjani, R.; Srinithya, B.; Vellingiri, V.; Brindha, P.; Anthony, S.P.; Sivasubramanian, A.; Muthuraman, M.S. Silver nanoparticle synthesis using Clerodendrum phlomidis leaf extract and preliminary investigation of its antioxidant and anticancer activities. J. Mol. Liq. 2016, 220, 926–930. [Google Scholar] [CrossRef]
- Nichita, C.; Stamatin, I. The antioxidant activity of the biohybrides based on carboxylated/hydroxylated carbon nanotubes-flavonoid compounds. Dig. J. Nanomater. Biostruct. 2013, 8, 445–455. [Google Scholar]
- Das, D.; Bhattacharyya, S.; Bhattacharyya, M.; Mandal, P. Green chemistry inspired formation of bioactive stable colloidal nanosilver and its wide-spectrum functionalised properties for sustainable industrial escalation. Results Chem. 2022, 4, 100533. [Google Scholar] [CrossRef]
- Nagaich, U.; Gulati, N.; Chauhan, S. Antioxidant and Antibacterial Potential of Silver Nanoparticles: Biogenic Synthesis Utilizing Apple Extract. J. Pharm. 2016, 2016, 7141523. [Google Scholar] [CrossRef] [PubMed]
- Fafal, T.; Taştan, P.; Tüzün, B.S.; Ozyazici, M.; Kivcak, B. Synthesis, characterization and studies on antioxidant activity of silver nanoparticles using Asphodelus aestivus Brot. aerial part extract. S. Afr. J. Bot. 2017, 112, 346–353. [Google Scholar] [CrossRef]
- Sudha, A.; Jeyakanthan, J.; Srinivasan, P. Green synthesis of silver nanoparticles using Lippia nodiflora aerial extract and evaluation of their antioxidant, antibacterial and cytotoxic effects. Resour. Technol. 2017, 3, 506–515. [Google Scholar] [CrossRef]
- AlSalhi, M.S.; Elangovan, K.; Ranjitsingh, A.J.A.; Murali, P.; Devanesan, S. Synthesis of silver nanoparticles using plant derived 4-N-methyl benzoic acid and evaluation of antimicrobial, antioxidant and antitumor activity. Saudi J. Biol. Sci. 2019, 26, 970–978. [Google Scholar] [CrossRef]
- Govindappa, M.; Hemashekhar, B.; Arthikala, M.K.; Ravishankar Rai, V.; Ramachandra, Y.L. Characterization, antibacterial, antioxidant, antidiabetic, anti-inflammatory and antityrosinase activity of green synthesized silver nanoparticles using Calophyllum tomentosum leaves extract. Results Phys. 2018, 9, 400–408. [Google Scholar] [CrossRef]
- Das, D.; Ghosh, R.; Mandal, P. Biogenic synthesis of silver nanoparticles using S1 genotype of Morus alba leaf extract: Characterization, antimicrobial and antioxidant potential assessment. SN Appl. Sci. 2019, 1, 498. [Google Scholar] [CrossRef]
- Oueslati, M.H.; Tahar, L.B.; Harrath, A.H. Catalytic, antioxidant and anticancer activities of gold nanoparticles synthesized by kaempferol glucoside from Lotus leguminosae. Arab. J. Chem. 2020, 13, 3112–3122. [Google Scholar] [CrossRef]
- Priya Velammal, S.; Devi, T.A.; Amaladhas, T.P. Antioxidant, antimicrobial and cytotoxic activities of silver and gold nanoparticles synthesized using Plumbago zeylanica bark. J. Nanostructure Chem. 2016, 6, 247–260. [Google Scholar] [CrossRef]
- Selvi, A.M.; Palanisamy, S.; Jeyanthi, S.; Vinosha, M.; Mohandoss, S.; Tabarsa, M.; You, S.G.; Kannapiran, E.; Prabhu, N.M. Synthesis of Tragia involucrata mediated platinum nanoparticles for comprehensive therapeutic applications: Antioxidant, antibacterial and mitochondria-associated apoptosis in HeLa cells. Process Biochem. 2020, 98, 21–33. [Google Scholar] [CrossRef]
- Zangeneh, M.M.; Ghaneialvar, H.; Akbaribazm, M.; Ghanimatdan, M.; Abbasi, N.; Goorani, S.; Pirabbasi, E.; Zangeneh, A. Novel synthesis of Falcaria vulgaris leaf extract conjugated copper nanoparticles with potent cytotoxicity, antioxidant, antifungal, antibacterial, and cutaneous wound healing activities under in vitro and in vivo condition. J. Photochem. Photobiol. B Biol. 2019, 197, 111556. [Google Scholar] [CrossRef]
- Venugopalan, R.; Pitchai, S.; Devarayan, K.; Swaminathan, V.C. Biogenic synthesis of copper nanoparticles using Borreria hispida (Linn.) extract and its antioxidant activity. Mater. Today Proc. 2020, 33, 4023–4025. [Google Scholar] [CrossRef]
- Merugu, R.; Gothalwal, R.; Kaushik Deshpande, P.; De Mandal, S.; Padala, G.; Latha Chitturi, K. Synthesis of Ag/Cu and Cu/Zn bimetallic nanoparticles using toddy palm: Investigations of their antitumor, antioxidant and antibacterial activities. Mater. Today Proc. 2021, 44, 99–105. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Oladipo, A.O.; Msagati, T.A.M.; Lebelo, S.L.; Meddows-Taylor, S.; More, G.K. Novel silver-platinum bimetallic nanoalloy synthesized from Vernonia mespilifolia extract: Antioxidant, antimicrobial, and cytotoxic activities. Arab. J. Chem. 2020, 13, 6639–6648. [Google Scholar] [CrossRef]
- Sharma, C.; Ansari, S.; Ansari, M.S.; Satsangee, S.P.; Srivastava, M.M. Single-step green route synthesis of Au/Ag bimetallic nanoparticles using clove buds extract: Enhancement in antioxidant bio-efficacy and catalytic activity. Mater. Sci. Eng. C 2020, 116, 111153. [Google Scholar] [CrossRef] [PubMed]
- Velsankar, K.; Sudhahar, S.; Maheshwaran, G. Effect of biosynthesis of ZnO nanoparticles via Cucurbita seed extract on Culex tritaeniorhynchus mosquito larvae with its biological applications. J. Photochem. Photobiol. B Biol. 2019, 200, 111650. [Google Scholar] [CrossRef]
- Sharmila, G.; Muthukumaran, C.; Sangeetha, E.; Saraswathi, H.; Soundarya, S.; Kumar, N.M. Green fabrication, characterization of Pisonia alba leaf extract derived MgO nanoparticles and its biological applications. Nano-Struct. Nano-Objects 2019, 20, 100380. [Google Scholar] [CrossRef]
- Sharmila, G.; Thirumarimurugan, M.; Muthukumaran, C. Green synthesis of ZnO nanoparticles using Tecoma castanifolia leaf extract: Characterization and evaluation of its antioxidant, bactericidal and anticancer activities. Microchem. J. 2019, 145, 578–587. [Google Scholar] [CrossRef]
- Loganathan, S.; Shivakumar, M.S.; Karthi, S.; Nathan, S.S.; Selvam, K. Metal oxide nanoparticle synthesis (ZnO-NPs) of Knoxia sumatrensis (Retz.) DC. Aqueous leaf extract and It’s evaluation of their antioxidant, anti-proliferative and larvicidal activities. Toxicol. Reports 2021, 8, 64–72. [Google Scholar] [CrossRef]
- Thakar, M.A.; Saurabh Jha, S.; Phasinam, K.; Manne, R.; Qureshi, Y.; Hari Babu, V.V. X ray diffraction (XRD) analysis and evaluation of antioxidant activity of copper oxide nanoparticles synthesized from leaf extract of Cissus vitiginea. Mater. Today Proc. 2021, 51, 319–324. [Google Scholar] [CrossRef]
- Akinola, P.O.; Lateef, A.; Asafa, T.B.; Beukes, L.S.; Hakeem, A.S.; Irshad, H.M. Multifunctional titanium dioxide nanoparticles biofabricated via phytosynthetic route using extracts of Cola nitida: Antimicrobial, dye degradation, antioxidant and anticoagulant activities. Heliyon 2020, 6, e04610. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Sathiyaseelan, A.; Mariadoss, A.V.A.; Wang, M.H. Antioxidant and antidiabetic properties of biocompatible ceria oxide (CeO2) nanoparticles in mouse fibroblast NIH3T3 and insulin resistant HepG2 cells. Ceram. Int. 2021, 47, 8618–8626. [Google Scholar] [CrossRef]
- Dutta, D.; Mukherjee, R.; Patra, M.; Banik, M.; Dasgupta, R.; Mukherjee, M.; Basu, T. Green synthesized cerium oxide nanoparticle: A prospective drug against oxidative harm. Colloids Surf. B Biointerfaces 2016, 147, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Olvera Salazar, A.; García Hernández, M.; López Camacho, P.Y.; López Marure, A.; Reyes de la Torre, A.I.; Morales Ramírez, Á.; De, J.; Hernández Santiago, F.; Aguilera Vázquez, L. Influence of Eu3 + doping content on antioxidant properties of Lu2O3 sol-gel derived nanoparticles. Mater. Sci. Eng. C 2016, 69, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, R.; Duan, Z.; Zhu, C.; Fan, D. Artificial Nonenzymatic Antioxidant MXene Nanosheet-Anchored Injectable Hydrogel as a Mild Photothermal-Controlled Oxygen Release Platform for Diabetic Wound Healing. ACS Nano 2022, 16, 7486–7502. [Google Scholar] [CrossRef] [PubMed]
- Javed, R.; Usman, M.; Tabassum, S.; Zia, M. Effect of capping agents: Structural, optical and biological properties of ZnO nanoparticles. Appl. Surf. Sci. 2016, 386, 319–326. [Google Scholar] [CrossRef]
- Javed, R.; Ahmed, M.; Haq, I.; Nisa, S.; Zia, M. PVP and PEG doped CuO nanoparticles are more biologically active: Antibacterial, antioxidant, antidiabetic and cytotoxic perspective. Mater. Sci. Eng. C 2017, 79, 108–115. [Google Scholar] [CrossRef]
- Tsai, Y.H.; Yang, Y.N.; Ho, Y.C.; Tsai, M.L.; Mi, F.L. Drug release and antioxidant/antibacterial activities of silymarin-zein nanoparticle/bacterial cellulose nanofiber composite films. Carbohydr. Polym. 2018, 180, 286–296. [Google Scholar] [CrossRef]
- Kim, S.J.; Chung, B.H. Antioxidant activity of levan coated cerium oxide nanoparticles. Carbohydr. Polym. 2016, 150, 400–407. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Alvarez Echazú, M.I.; Olivetti, C.E.; Peralta, I.; Alonso, M.R.; Anesini, C.; Perez, C.J.; Alvarez, G.S.; Desimone, M.F. Development of pH-responsive biopolymer-silica composites loaded with Larrea divaricata Cav. extract with antioxidant activity. Colloids Surf. B Biointerfaces 2018, 169, 82–91. [Google Scholar] [CrossRef]
- Lee, R.J.; Tamm, T.; Temmer, R.; Aabloo, A.; Kiefer, R. Two formation mechanisms and renewable antioxidant properties of suspensible chitosan-PPy and chitosan-PPy-BTDA composites. Synth. Met. 2013, 164, 6–11. [Google Scholar] [CrossRef]
- Liu, X.; Li, D.; Liang, Y.; Lin, Y.; Liu, Z.; Niu, H.; Xu, Y. Establishment of anti-oxidation platform based on few-layer molybdenum disulfide nanosheet-coated titanium dioxide nanobelt nanocomposite. J. Colloid Interface Sci. 2021, 601, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, A.; Gopinath, P. Monitoring the Intracellular Distribution and ROS Scavenging Potential of Carbon Dot–Cerium Oxide Nanocomposites in Fibroblast Cells. ChemNanoMat 2016, 2, 226–235. [Google Scholar] [CrossRef]
- Rajeswari, R.; Gurumallesh Prabu, H. Palladium–Decorated reduced graphene oxide/zinc oxide nanocomposite for enhanced antimicrobial, antioxidant and cytotoxicity activities. Process Biochem. 2020, 93, 36–47. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, J.; Wang, Y.; Li, J.; Bao, J.; Xu, X.; Zhang, C.; Li, Y.; Wu, H.; Gu, Z. Reduced polydopamine nanoparticles incorporated oxidized dextran/chitosan hybrid hydrogels with enhanced antioxidative and antibacterial properties for accelerated wound healing. Carbohydr. Polym. 2021, 257, 117598. [Google Scholar] [CrossRef]
- Battaglini, M.; Marino, A.; Carmignani, A.; Tapeinos, C.; Cauda, V.; Ancona, A.; Garino, N.; Vighetto, V.; La Rosa, G.; Sinibaldi, E.; et al. Polydopamine Nanoparticles as an Organic and Biodegradable Multitasking Tool for Neuroprotection and Remote Neuronal Stimulation. ACS Appl. Mater. Interfaces 2020, 12, 35782–35798. [Google Scholar] [CrossRef]
- Muniyappan, N.; Pandeeswaran, M.; Amalraj, A. Green synthesis of gold nanoparticles using Curcuma pseudomontana isolated curcumin: Its characterization, antimicrobial, antioxidant and anti- inflammatory activities. Environ. Chem. Ecotoxicol. 2021, 3, 117–124. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Q.; Li, T.; Xia, N.; Xia, Q. Nanostructured lipid carrier (NLC) as a strategy for encapsulation of quercetin and linseed oil: Preparation and in vitro characterization studies. J. Food Eng. 2017, 215, 1–12. [Google Scholar] [CrossRef]
- Karimi, N.; Ghanbarzadeh, B.; Hamishehkar, H.; Mehramuz, B.; Kafil, H.S. Antioxidant, Antimicrobial and Physicochemical Properties of Turmeric Extract-Loaded Nanostructured Lipid Carrier (NLC). Colloids Interface Sci. Commun. 2018, 22, 18–24. [Google Scholar] [CrossRef]
- Huang, X.; Huang, X.; Gong, Y.; Xiao, H.; McClements, D.J.; Hu, K. Enhancement of curcumin water dispersibility and antioxidant activity using core-shell protein-polysaccharide nanoparticles. Food Res. Int. 2016, 87, 1–9. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, X.; Kang, X.; Yang, H.; Guo, W.; Guan, L.; Wu, H.; Du, L. Surface functionalization of pegylated gold nanoparticles with antioxidants suppresses nanoparticle-induced oxidative stress and neurotoxicity. Chem. Res. Toxicol. 2020, 33, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Riela, S.; Guernelli, S.; Parisi, F.; Lazzara, G.; Baschieri, A.; Valgimigli, L.; Amorati, R. A synergic nanoantioxidant based on covalently modified halloysite–trolox nanotubes with intra-lumen loaded quercetin. J. Mater. Chem. B 2016, 4, 2229–2241. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.I.; Pinho, C.; Fonte, P.; Sarmento, B.; Dias, A.C.P. Development, characterization, antioxidant and hepatoprotective properties of poly(Ɛ-caprolactone) nanoparticles loaded with a neuroprotective fraction of Hypericum perforatum. Int. J. Biol. Macromol. 2018, 110, 185–196. [Google Scholar] [CrossRef]
- Lee, G.H.; Lee, S.J.; Jeong, S.W.; Kim, H.C.; Park, G.Y.; Lee, S.G.; Choi, J.H. Antioxidative and antiinflammatory activities of quercetin-loaded silica nanoparticles. Colloids Surf. B Biointerfaces 2016, 143, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, G.A.; Blattmann, C.O.; Deligiannakis, Y. Nanoantioxidant-driven plasmon enhanced proton-coupled electron transfer. Nanoscale 2016, 8, 796–803. [Google Scholar] [CrossRef]
- De Cristo Soares Alves, A.; Mainardes, R.M.; Khalil, N.M. Nanoencapsulation of gallic acid and evaluation of its cytotoxicity and antioxidant activity. Mater. Sci. Eng. C 2016, 60, 126–134. [Google Scholar] [CrossRef]
- Lee, J.; Choi, K.H.; Min, J.; Kim, H.J.; Jee, J.P.; Park, B.J. Functionalized ZnO nanoparticles with gallic acid for antioxidant and antibacterial activity against methicillin-resistant S. aureus. Nanomaterials 2017, 7, 365. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Choi, J.; Park, I.; Ko, S. Facile Biosynthesis and Antioxidant Property of Nanogold-Cellulose Fiber Composite. J. Nanomater. 2015, 2015, 146460. [Google Scholar] [CrossRef]
- Marulasiddeshwara, M.B.; Dakshayani, S.S.; Sharath Kumar, M.N.; Chethana, R.; Raghavendra Kumar, P.; Devaraja, S. Facile-one pot-green synthesis, antibacterial, antifungal, antioxidant and antiplatelet activities of lignin capped silver nanoparticles: A promising therapeutic agent. Mater. Sci. Eng. C 2017, 81, 182–190. [Google Scholar] [CrossRef]
- Baschieri, A.; Amorati, R.; Benelli, T.; Mazzocchetti, L.; D’angelo, E.; Valgimigli, L. Enhanced antioxidant activity under biomimetic settings of ascorbic acid included in halloysite nanotubes. Antioxidants 2019, 8, 30. [Google Scholar] [CrossRef]
- Arriagada, F.; Günther, G.; Morales, J. Nanoantioxidant–based silica particles as flavonoid carrier for drug delivery applications. Pharmaceutics 2020, 12, 302. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Suo, S.; Wang, G.; Jia, H.; Liu, K.J.; Zhao, B.; Liu, Y. Mechanism and cellular kinetic studies of the enhancement of antioxidant activity by using surface-functionalized gold nanoparticles. Chem. A Eur. J. 2013, 19, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, Z.; Liu, C.; Ju, E.; Zhang, Y.; Ren, J.; Qu, X. Self-Assembly of Multi-nanozymes to Mimic an Intracellular Antioxidant Defense System. Angew. Chemie 2016, 128, 6758–6762. [Google Scholar] [CrossRef]
- Hemmati, S.; Zangeneh, M.M.; Zangeneh, A. CuCl2 anchored on polydopamine coated-magnetic nanoparticles (Fe3O4@PDA/Cu(II)): Preparation, characterization and evaluation of its cytotoxicity, antioxidant, antibacterial, and antifungal properties. Polyhedron 2020, 177, 114327. [Google Scholar] [CrossRef]





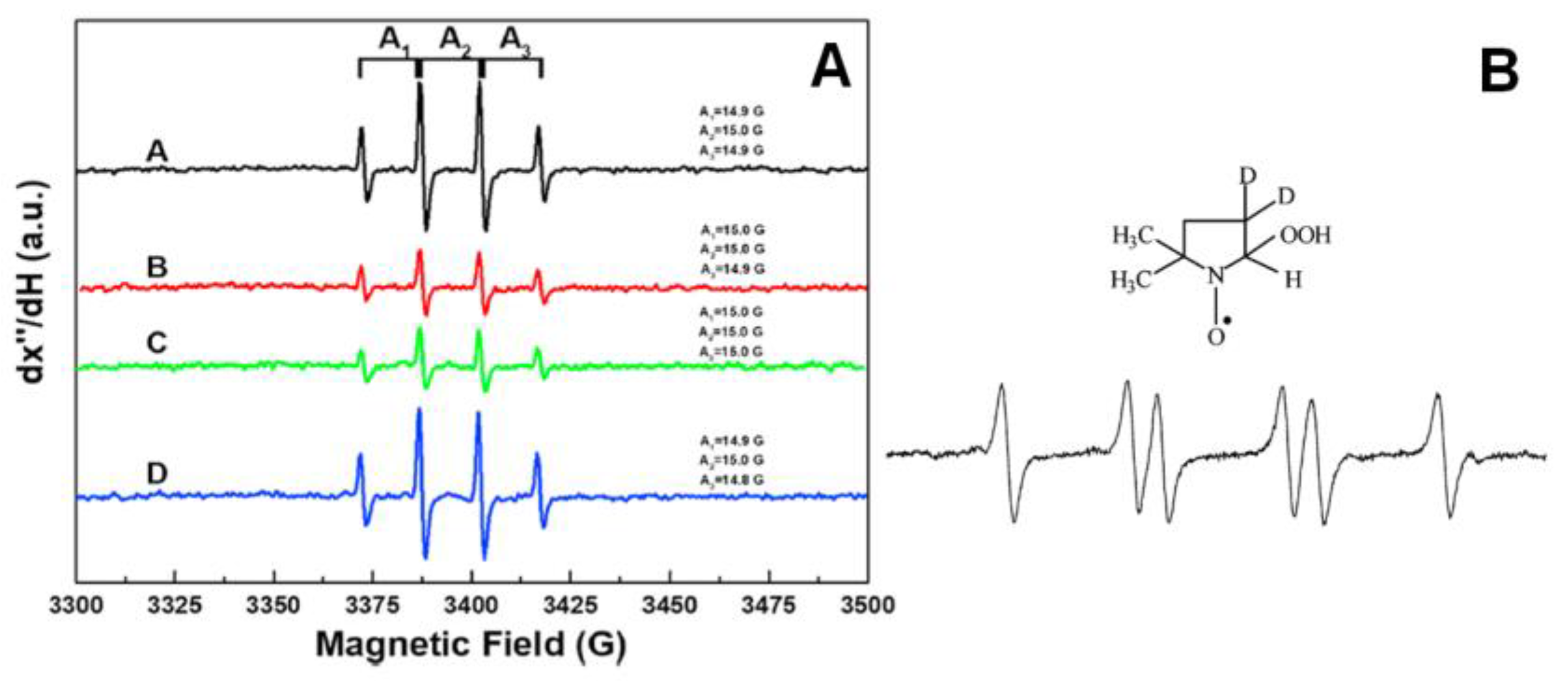

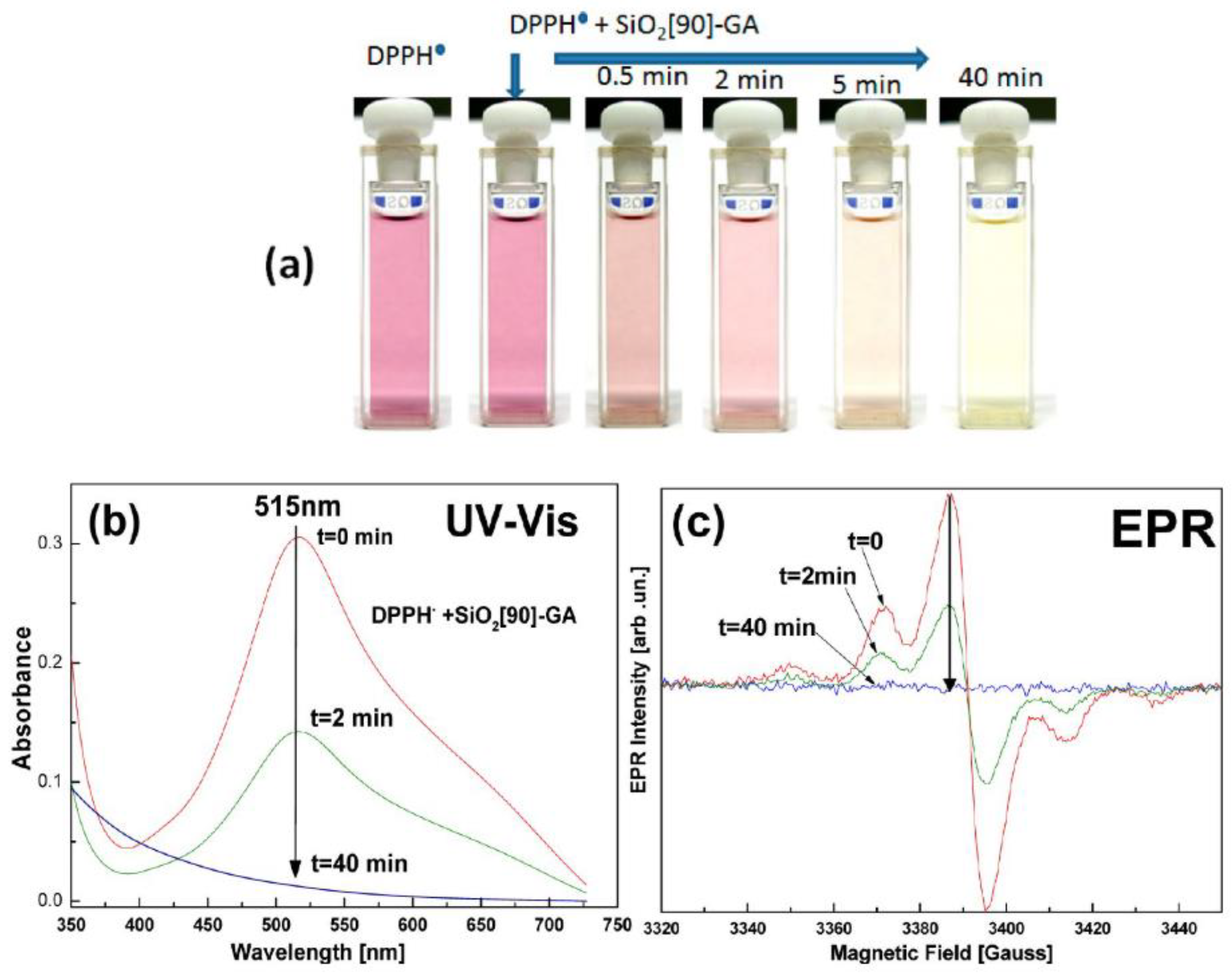






| Oxygen-Centered Radicals | Non-Oxygen-Centered Radicals | ||
|---|---|---|---|
| Hydroxyl radical | •OH | Nitric oxide radical | •NO |
| Superoxide anion radical | •O2− | Nitrogen dioxide | •NO2 |
| Peroxyl radicals | HO2•, ROO•, LOO• | Carbon monoxide anion | CO•− |
| Alkoxyl/Phenoxyl radicals | RO•, LO•, Tyr• | Trisulfur radical | S3•− |
| Semiquinone radical | SQ•− | Chlorine radicals | Cl•, Cl2•− |
| Carbonate radical | CO3• | ||
| Sulfate/Phosphate radical | SO4•−, PO42−• | ||
| Nanoantioxidant | Target | Evaluation Methods/Antioxidant Efficiency * | Ref. | |
|---|---|---|---|---|
| 1 | Ag NPs from clerodendrum phlomidis leaf extract | ●N, O2●− | ●N (DPPH method): IC50 = 55.86 μg/mL < IC50 = 202.2 μg/mL O2●−: IC50 = 9.12 μg/mL < IC50 = 182.8 μg/mL FRAP: 1.63 AU < FA ≈ 1.8 AU Phosphomolybdate assay: 910 AEAA > FA ≈ 710 AEAA | [119] |
| 2 | SLG/GA | DPPH (●N), ●OH | enhanced the antioxidant activity of phenolic acids | [40] |
| 3 | Ag NPs, Malus domestica | DPPH (●N) | DPPH method/RSC = 75.16% | [122] |
| 4 | Ag NPs, Asphodelus aestivus Brot. | DPPH (●N), ABTS●+, H2O2 | ●N (DPPH): RSC Ag NPs = 67.54 ± 5.49 > RSC ASP = 31.82 ± 4.04 ABTS●+: RSC Ag NPs = 79.94 ± 0.02 > RSC ASP = 39.62 ± 0.02 H2O2/RSC Ag NPs = 31.67 ± 0.06 < RSC ASP = 55.86 ± 0.14 | [123] |
| 5 | a Ag NPs, Lippia Nodiflora (ASP) | DPPH (●N), O2●−, ●OH | ●N: (DPPH method)/RSC Ag NPs = 67% < RSC BHT = 83% O2●−: RSC Ag NPs = 70% < RSC BHT = 84% ●OH: RSC Ag NPs = 69% < RSC BHT = 75% Reducing power: RSC Ag NPs = 0.115 < RSC BHT = 0.095 H2O2: RSC Ag NPs = 71.1% > RSC BHT = 68.2% | [124] |
| 6 | Ag NPs, Memecylon umbellatum Burm | DPPH (●N), O2●− | ●N: (DPPH method): RSC Ag NPs = 81.57% < RSC BHT = 85.39%, EC50 Ag NPs = 53.46 μg/mL > EC50 BHT = 37.92 μg/mL O2●−: RSC Ag NPs = 74.76% < RSC BHT = 80.71%, EC50 Ag NPs = 66.68 μg/mL > EC50 BHT = 53.39 μg/mL | [125] |
| 7 | aCt Ag NPs, Calophyllum tomentosum | DPPH (●N), H2O2, ●NO | ●N: (DPPH method): RSC CtAg NPs = 90% > RSCBHT H2O2: RSC CtAg NPs = 83.94% > RSC AA ON●: RSC CtAg NPs = 78.46% < RSCBHT = 79.11% Reducing power: RSC CtAg NPs = 74% < RSCBHT = 83% | [126] |
| 8 | a Ag NPs, Morus alba (Mulberry) | DPPH, ABTS+●, O2●−, ●NO, Metal chelation | ●N: (DPPH method): IC50 AgNPs = 97.273 μg/mL < IC50 plant extract = 143.967 μg/mL ABTS+: IC50 AgNPs = 25.929 μg/mL < IC50 plant extract = 53.832 μg/mL O2●−: IC50 AgNPs = 37.097 μg/mL < IC50 plant extract = 77.479 μg/mL ON●: IC50 AgNPs = 70.992 μg/mL < IC50 plant extract = 101.587 μg/mL Metal chelation: IC50 AgNPs = 54.325 μg/mL < IC50 plant extract = 73.837 μg/mL | [127] |
| 9 | a AuNPs, from KG, Lotus leguminosae | DPPH (●N) | ●N: (DPPH method): EC50 GA = 11.92 μg/mL > EC50 Au NPs = 30.54 μg/mL > EC50 KG = 48.9 μg/ml | [128] |
| 10 | Au, Ag NPs, Plumbago zeylanica | DPPH (●N) | ●N: (DPPH method): RSCAuNPs = 87.34% > RSCAgNPs = 78.17% > RSCBHT = 74.88% > RSCextract = 71.16% | [129] |
| 11 | Ti-Pt NPs from Tragia involucrata | DPPH (●N) | ●N (DPPH method): RSCTi-Pt NPs = 64 ± 0.43% > RSCAE-Ti Reducing Power (RP): RSCTi-Pt NPs = 13.45 ± 0.23% > RSCAE-Ti Total Antioxidant Properties:RSCTi-Pt NPs = 15.85 ± 0.22% > RSCAE-Ti | [130] |
| 12 | a Cu NPs, Falcaria vulgaris | DPPH (●N) | ●N (DPPH method): IC50F.Vulgaris = 392 μg/mL > IC50 BHT = 314 μg/mL > IC50 CuNPs = 190 μg/ml | [131] |
| 13 | Cu NPs, Borreria hispida (Linn.) | DPPH (●N) | ●N (DPPH method): IC50 crude extract = 1.5 μg/mL > IC50 CuNPs = 0.6 μg/mL. | [132] |
| 14 | Ag/Cu, Cu/Zn NPs, Borassus flabellife | DPPH (●N), ●OH, H2O2 | ●N (DPPH method): C = 100 μg/mL RSCAA = 72% > RSCAg/CuNPs = 58% > RSCCu/ZnNPs = 40% ●OH: C = 100 μg/mL, RSCAA = 74% > RSCAg/CuNPs = 48% > RSCCu/ZnNPs = 38% H2O2: C = 100 μg/mL, RSCAA = 74% > RSCAg/CuNPs = 42% > RSCCu/ZnNPs = 28% | [133] |
| 15 | AgPt NPs, Vernonia mespilifolia plant | DPPH (●N), ABTS+● | ●N (DPPH method): IC50 AA = 131.8 ± 0.4 μg/mL > IC50 AgNPs = 28.5 ± 0.1 μg/mL > IC50 AgPt NPs = 19.5 ± 0.2 μg/mL ABTS+●: IC50 AgNPs = 302.7 ± 2.8 μg/mL > IC50 AA = 210.7 ± 1.0 μg/mL > IC50 AgPt NPs = 21.6 ± 2.1 μg/mL FRAPAgPt NPs = 44.1 ± 2.7 mg GAE/g > FRAP AgNPs = 18.5 ± 0.2 mg GAE/g | [134] |
| 16 | Au/Ag (BM NPs), Clove buds | DPPH (●N), ABTS+●, ●OH | ●N (DPPH method): IC50 Au/Ag BMNPs= 0.5 IC50 AgNPs ABTS+●: IC50 = 18.27 μg/mL. ●OH:IC50 = 30.59 μg/ml | [135] |
| 17 | ZnO NPs, Cucurbita seed | DPPH (●N) | ●N (DPPH method): RSCZnONPs = 91.37 ± 6.39% > RSCAA = 83.68 ± 5.85%, IC50AA = 45.33 μg/mL > IC50ZnONPs = 40.81 μg/ml | [136] |
| 18 | MONPs (Magnesium oxide), Pisonia Alba | DPPH (●N) | ●N (DPPH method): RSC = 65%/FRAP: RSC = 69.3% | [137] |
| 19 | ZnO NPs, Tecoma castanifolia leaf | DPPH (●N) | ●N (DPPH method): RSC = 67%, at 100 μg/mL | [138] |
| 20 | ZnO NPs, Knoxia sumatrensis aqueous (Ks-ALE) | DPPH (●N), ABTS+●, H2O2 | ●N (DPPH method): IC50 = 95.80 μg/mL ABTS+●: IC50 = 92.29 μg/mL/H2O2: IC50 = 98.92 μg/ml | [139] |
| 21 | CuNPs, Cissus vitiginea | DPPH (●N) | ●N (DPPH method): C = 80 μg/mL, RSCAA = 90.31 ± 6.32% >RSCCuONPs = 86.78 ± 6.07% > RSCCissus Vitiginea = 82.37 ± 5.76%, IC50 Cissus Vitiginea = 50.51μg/mL > IC50 CuONPs = 45.29 μg/mL > IC50 AA = 41.33 μg/ml | [140] |
| 22 | TiO2 NPs, Cola nitida | DPPH (●N) | ●N (DPPH method): RSC = 60.08%/H2O2: RSC = 99.23% | [141] |
| 23 | b CeO2 NPs, Stachys japonica | DPPH (●N), ABTS+● | ●N (DPPH method): IC50 = 109.5 ± 0.26 μg/mL ABTS+●: IC50 = 12.16 ± 0.12 μg/ml | [142] |
| 24 | b CeONP, Aloe Vera | DPPH (●N) | ●N (DPPH method): RSC ≈ 83% | [143] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fragou, F.; Theofanous, A.; Deligiannakis, Y.; Louloudi, M. Nanoantioxidant Materials: Nanoengineering Inspired by Nature. Micromachines 2023, 14, 383. https://doi.org/10.3390/mi14020383
Fragou F, Theofanous A, Deligiannakis Y, Louloudi M. Nanoantioxidant Materials: Nanoengineering Inspired by Nature. Micromachines. 2023; 14(2):383. https://doi.org/10.3390/mi14020383
Chicago/Turabian StyleFragou, Fotini, Annita Theofanous, Yiannis Deligiannakis, and Maria Louloudi. 2023. "Nanoantioxidant Materials: Nanoengineering Inspired by Nature" Micromachines 14, no. 2: 383. https://doi.org/10.3390/mi14020383
APA StyleFragou, F., Theofanous, A., Deligiannakis, Y., & Louloudi, M. (2023). Nanoantioxidant Materials: Nanoengineering Inspired by Nature. Micromachines, 14(2), 383. https://doi.org/10.3390/mi14020383