Fabrication of Aluminum Oxide Thin-Film Devices Based on Atomic Layer Deposition and Pulsed Discrete Feed Method
Abstract
1. Introduction
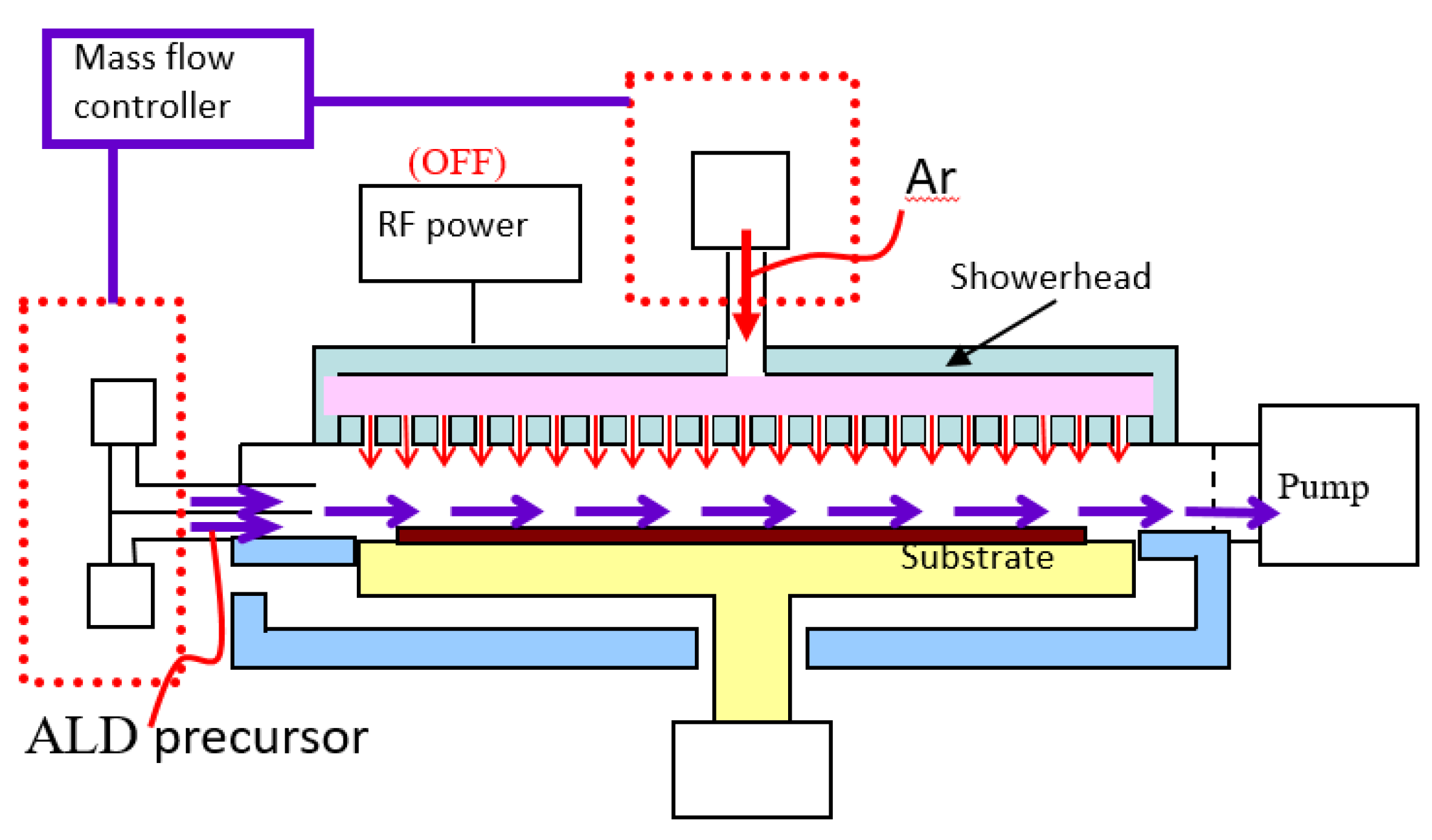
2. Materials and Methods
3. Results and Discussion
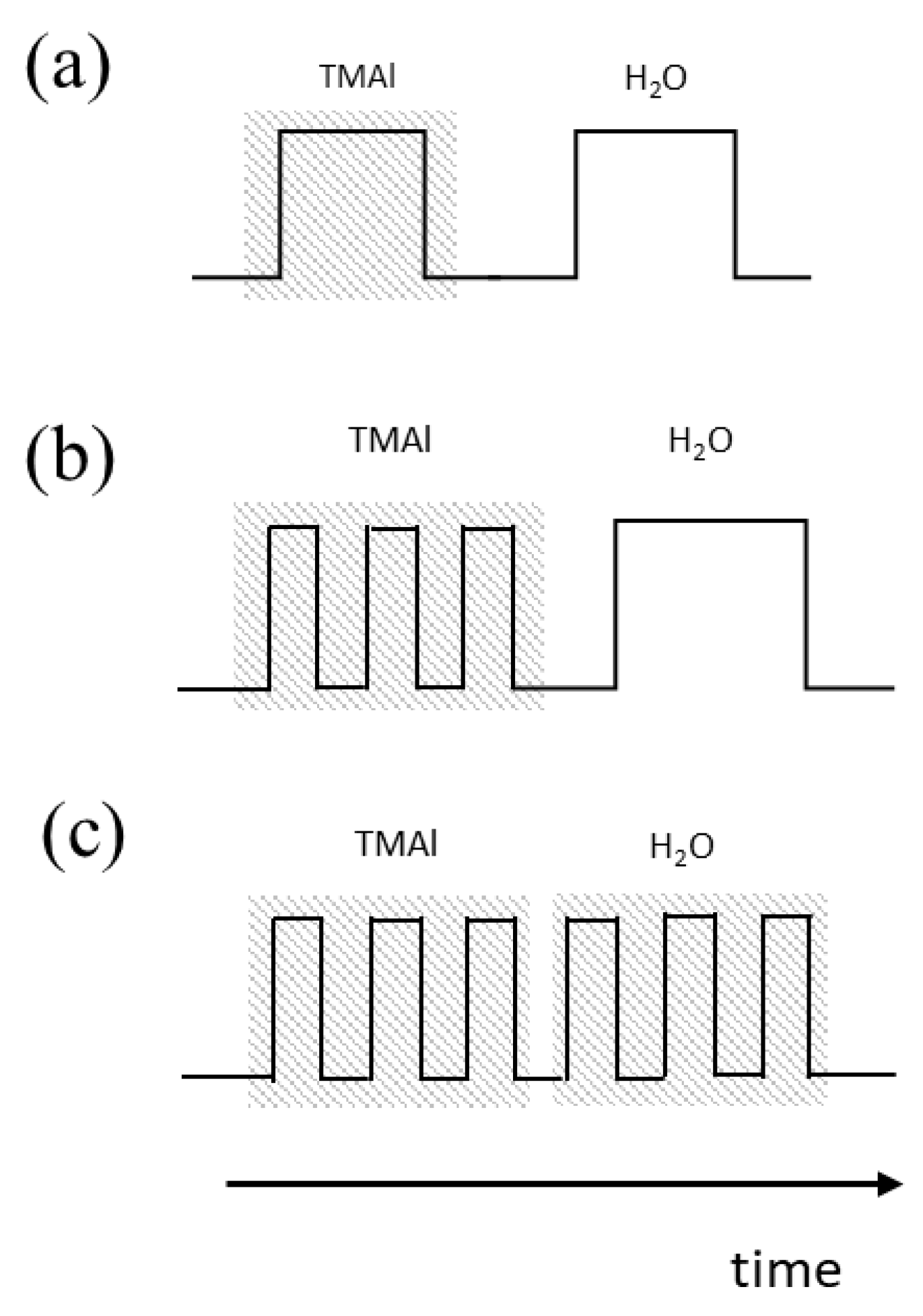
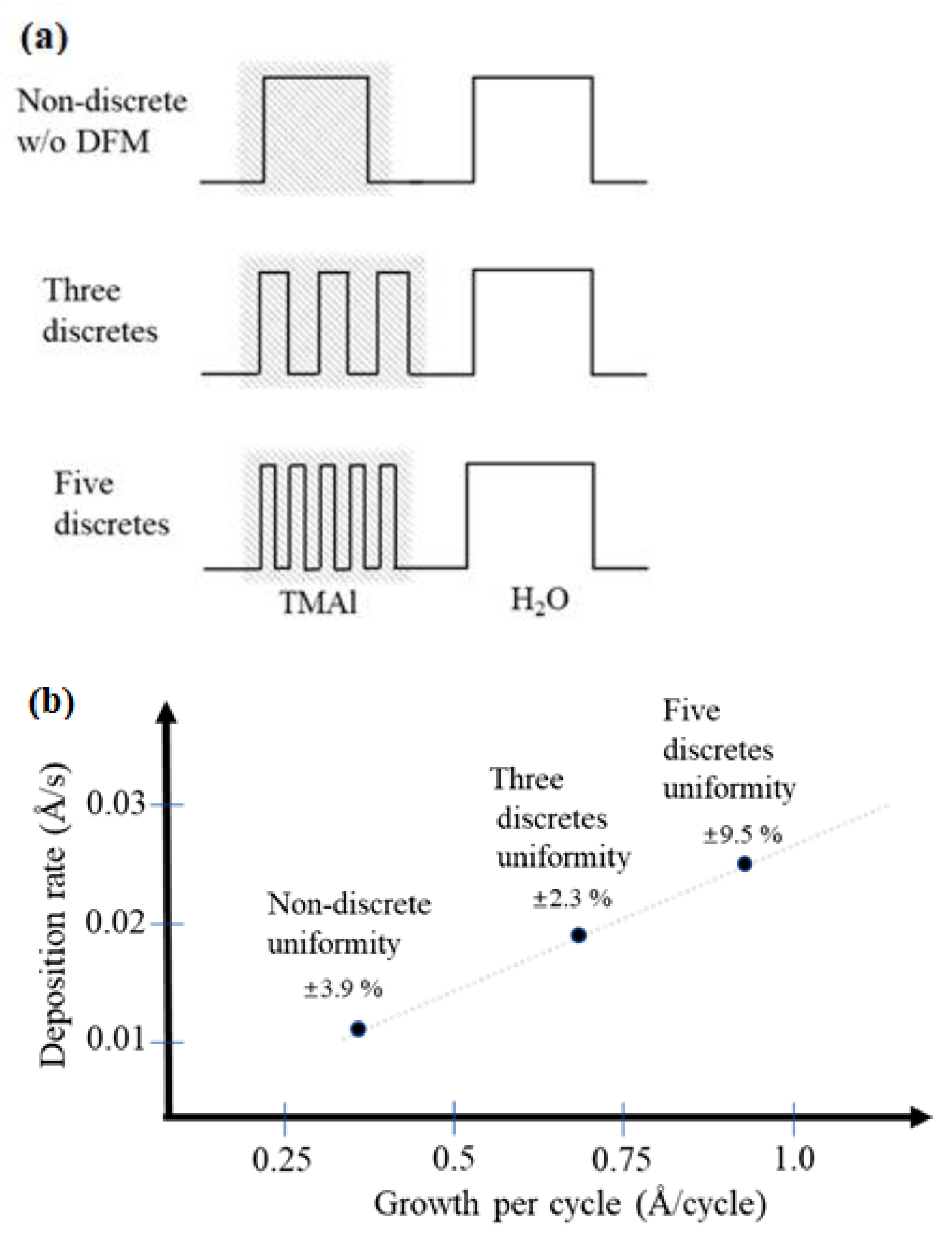
3.1. Pulsed Discrete Feed Method
3.2. Experimental Conditions and Structural Characteristics
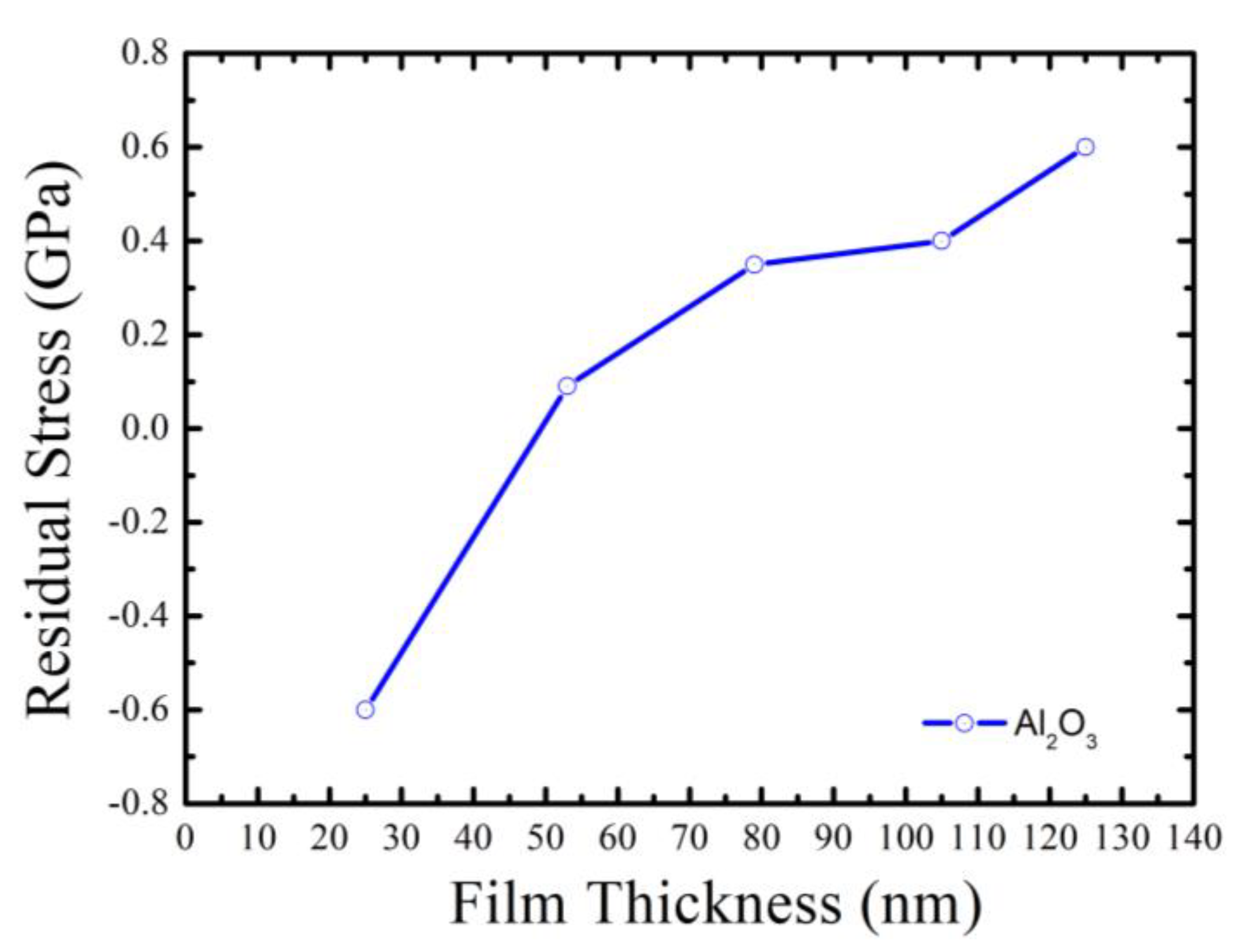
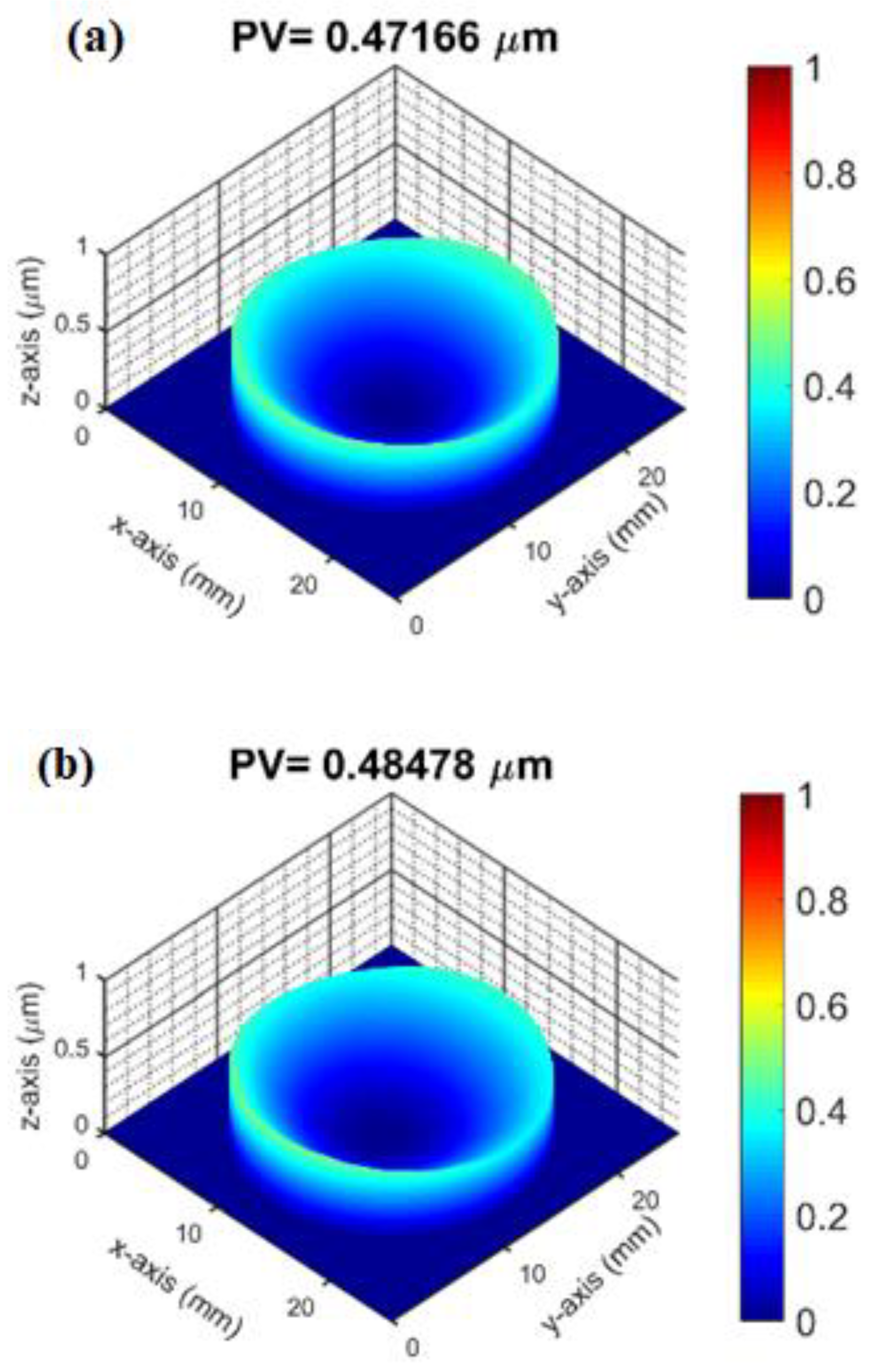
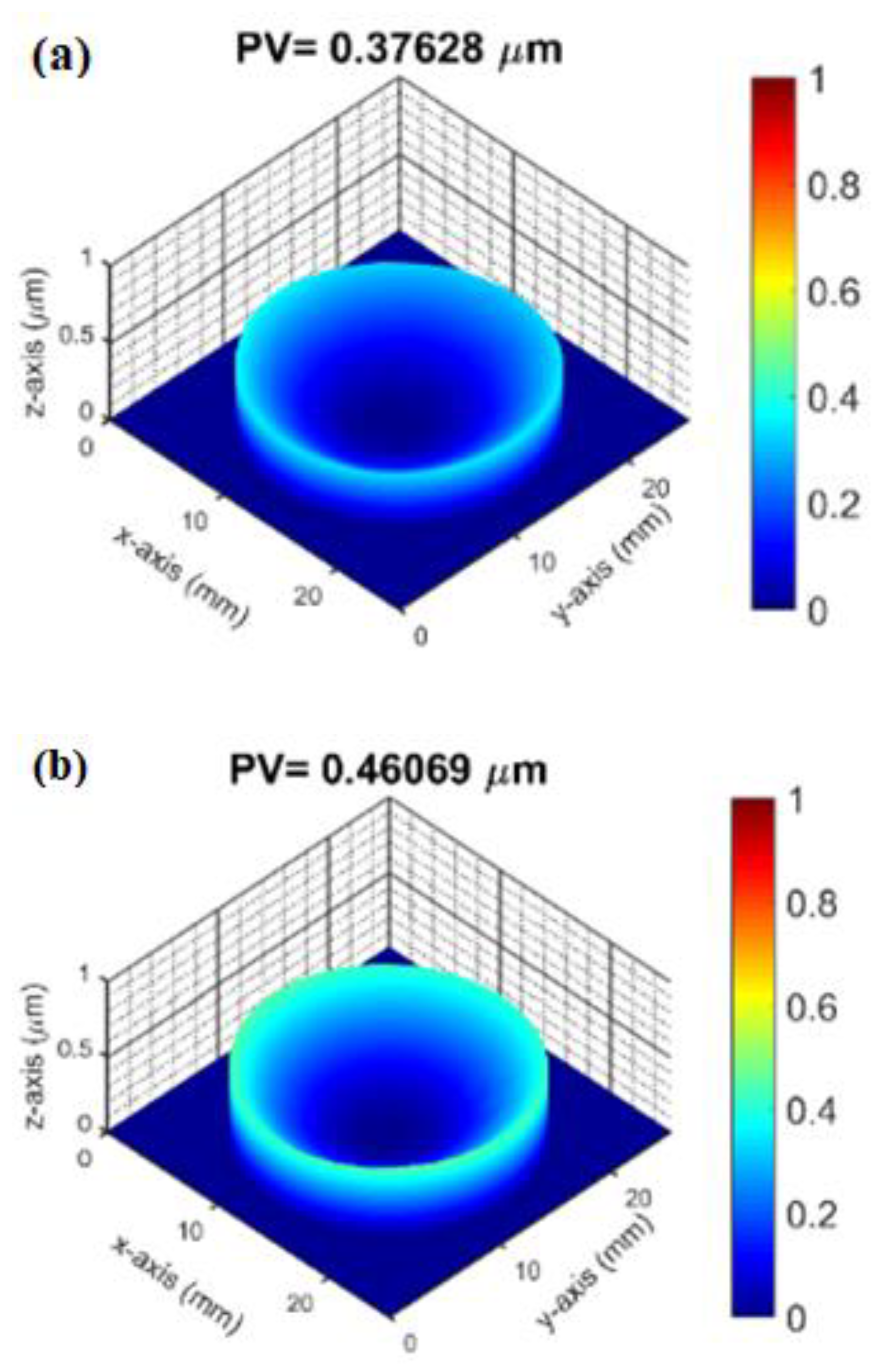
3.3. Surface Profile and Residual Stress Measurement
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gomes, T.C.; Kumar, D.; Fugikawa-Santos, L.; Alves, N.; Kettle, J. Optimization of the Anodization Processing for Aluminum Oxide Gate Dielectrics in ZnO Thin Film Transistors by Multivariate Analysis. ACS Comb. Sci. 2019, 21, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Huang, W.; Chi, L.; Al-Hashimi, M.; Marks, T.J.; Facchetti, A. High-k Gate Dielectrics for Emerging Flexible and Stretchable Electronics. Chem. Rev. 2018, 118, 5690–5754. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.Y.; Kimb, J.; Limc, S.; Hana, E.; Ho-Baillie, A.W.Y.; Park, N. Enhancing stability for organic-inorganic perovskite solar cells by atomic layer deposited Al2O3 encapsulation. Sol. Energy Mater. Sol. Cells 2018, 188, 37–45. [Google Scholar] [CrossRef]
- Hoffmann, L.; Theirich, D.; Pack, S.; Kocak, F.; Schlamm, D.; Hasselmann, T.; Fahl, H.; Raupke, A.; Gargouri, H.; Riedl, T. Gas Diffusion Barriers Prepared by Spatial Atmospheric Pressure Plasma Enhanced ALD. ACS Appl. Mater. Interfaces 2017, 9, 4171–4176. [Google Scholar] [CrossRef]
- Ha, J.W.; Ryu, H.; Lee, W.J.; Bae, J.S. Efficient photoelectrochemical water splitting using CuO nanorod/ Al2O3 heterostructure photoelectrodes with different Al layer thicknesses. Phys. B 2017, 519, 95–101. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, L.; Wang, X.; Yub, Y.; Mutzke, A. Effect of bias voltage on microstructure and optical properties of Al2O3 thin films prepared by twin targets reactive high power impulse magnetron sputtering. Vacuum 2019, 166, 88–96. [Google Scholar] [CrossRef]
- Balakrishnana, G.; Sundari, S.T.; Ramaseshan, R.; Thirumurugesan, R.; Mohandas, E.; Sastikumar, D.; Kuppusami, P.; Kim, T.G.; Song, J.I. Effect of substrate temperature on microstructure and optical properties of nanocrystalline alumina thin films. Ceram. Int. 2013, 39, 9017–9023. [Google Scholar] [CrossRef]
- Kumar, V.S.R.S.P.; Kumar, M.; Kumari, N.; Sharma, A.L. Optical and morphological studies of aluminium oxide films fabricated at different leaning angles using ion assisted E-Beam deposition technique. Optik—Int. J. Light Electron Opt. 2020, 222, 165376. [Google Scholar] [CrossRef]
- Shamala, K.S.; Murthy, L.C.S.; Narasimha Rao, K. Studies on optical and dielectric properties of Al2O3 thin films prepared by electron beam evaporation and spray pyrolysis method. Mater. Sci. Eng. B 2004, 106, 269–274. [Google Scholar] [CrossRef]
- Seo, M.Y.; Cho, E.N.; Kim, C.E.; Moon, P.; Yun, I. Characterization of Al2O3 films grown by electron beam evaporator on Si substrates. In Proceedings of the 2010 3rd International Nanoelectronics Conference (INEC), Hong Kong, China, 3–8 January 2010; pp. 238–239. [Google Scholar]
- Jo, J.-W.; Kim, K.-H.; Kim, J.; Ban, S.G.; Kim, Y.-H.; Park, S.K. High-Mobility and Hysteresis-Free Flexible Oxide Thin-Film Transistors and Circuits by Using Bilayer Sol−Gel Gate Dielectrics. ACS Appl. Mater. Interfaces 2018, 10, 2679–2687. [Google Scholar] [CrossRef]
- Kumar, P.; Wiedmann, M.K.; Winter, C.H.; Avrutsky, I. Optical properties of Al2O3 thin films grown by atomic layer deposition. Appl. Opt. 2009, 48, 5407–5412. [Google Scholar] [CrossRef] [PubMed]
- Barbos, C.; Blanc-Pelissier, D.; Fave, A.; Botella, C.; Regreny, P.; Grenet, G.; Blanquet, E.; Crisci, A.; Lemiti, M. Al2O3 thin films deposited by thermal atomic layer deposition: Characterization for photovoltaic applications. Thin Solid Films 2016, 617, 108–113. [Google Scholar] [CrossRef]
- Schmidt, J.; Merkle, A.; Brendel, R.; Hoex, B.; van de Sanden, M.C.M.; Kessels, W.M.M. Research Surface Passivation of High-efficiency Silicon Solar Cells by Atomic-layer-deposited Al2O3. Prog. Photovolt. Res. Appl. 2008, 16, 16461–16466. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.-H.; Jo, I.H.; Seo, J.; Yoo, Y.-E.; Kim, J.H. Influence of growth temperature on dielectric strength of Al2O3 thin films prepared via atomic layer deposition at low temperature. Sci. Rep. 2022, 12, 5124. [Google Scholar] [CrossRef] [PubMed]
- Oviroh, P.O.; Akbarzadeh, R.; Pan, D.; Coetzee, R.A.M.; Jen, T.C. New development of atomic layer deposition: Processes, methods and applications. Sci. Technol. Adv. Mater. 2019, 20, 465–496. [Google Scholar] [CrossRef]
- Pan, D.; Ma, L.; Xie, Y. On the physical and chemical details of alumina atomic layer deposition: A combined experimental and numerical approach . J. Vac. Sci. Technol. A 2015, 33, 021511. [Google Scholar] [CrossRef]
- Lin, K.-L.; Chen, C.-C.; Tung, F.-C.; Chen, C.-Y.; Lin, S.-C.; Lin, K.-Y.; Chang, C.-H.; Wu, S.-S. Multi-mode Thin Film Deposition Apparatus and Method of Depositing a Thin Film. U.S. Patent 9023693B1, 5 May 2015. [Google Scholar]
- Song, H.; Shin, D.; Jeong, J.E.; Park, H.; Ko, D.H. Growth behavior and film properties of titanium dioxide by plasma-enhanced atomic layer deposition with discrete feeding method. AIP Adv. 2019, 9, 035333. [Google Scholar] [CrossRef]
- Park, T.J.; Kim, J.H.; Jang, J.H.; Kim, U.K.; Lee, S.Y.; Lee, J.; Jung, H.S.; Hwang, C.S. Improved growth and electrical properties of atomic-layer-deposited metal-oxide film by discrete feeding method of metal precursor. Chem. Mater. 2011, 23, 1654–1658. [Google Scholar] [CrossRef]
- Groner, M.D.; Fabreguette, F.H.; Elam, J.W.; George, S.M. Low-temperature Al2O3 atomic layer deposition. Chem. Mater. 2004, 16, 639–645. [Google Scholar] [CrossRef]
- Takeda, M.; Ina, H.; Kobayashi, S. Fourier-transform method of fringe-pattern analysis for computer-based topography and interferometry. Appl. Opt. 1982, 72, 156–160. [Google Scholar] [CrossRef]
- Takeda, M.; Mutoh, K. Fourier transform profilometry for the automatic measurement of 3-D object shapes. Appl. Opt. 1983, 22, 3977–3982. [Google Scholar] [CrossRef] [PubMed]
- Tien, C.L.; Zeng, H.D. Measuring residual stress of anisotropic thin film by fast Fourier transform. Optics Express 2010, 18, 16594–16600. [Google Scholar] [CrossRef] [PubMed]
- Stoney, G.G. The tension of metallic films deposited by electrolysis. Proc. R. Soc. Lond. 1909, 86, 172–175. [Google Scholar]
- Tien, C.L.; Lee, C.C.; Jaing, C.C. The measurement of thin film stress using phase shifting interferometry. J. Modern Optics 2000, 47, 839–849. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.; Ham, S.-Y.; Ko, D.-H.; Shin, S.; Jin, Z.; Min, Y.-S. A kinetic study of zno atomic layer deposition: Effects of surface hydroxyl concentration and steric hindrance. Appl. Surf. Sci. 2019, 469, 804–810. [Google Scholar] [CrossRef]
- Adinolfi, V.; Adinolfi, V.; Cheng, L.; Laudato, M.; Clarke, R.C.; Narasimhan, V.K.; Balatti, S.; Hoang, S.; Littau, K.A. Composition-controlled atomic layer deposition of phase-change memories and ovonic threshold switches with high performance. ACS Nano 2019, 13, 10440–10447. [Google Scholar] [CrossRef]
- Krautheim, G.; Hecht, T.; Jakschik, S.; Schröder, U.; Zahn, W. Mechanical stress in ALD-Al2O3 films. Appl. Surf. Sci. 2005, 252, 200–204. [Google Scholar] [CrossRef]


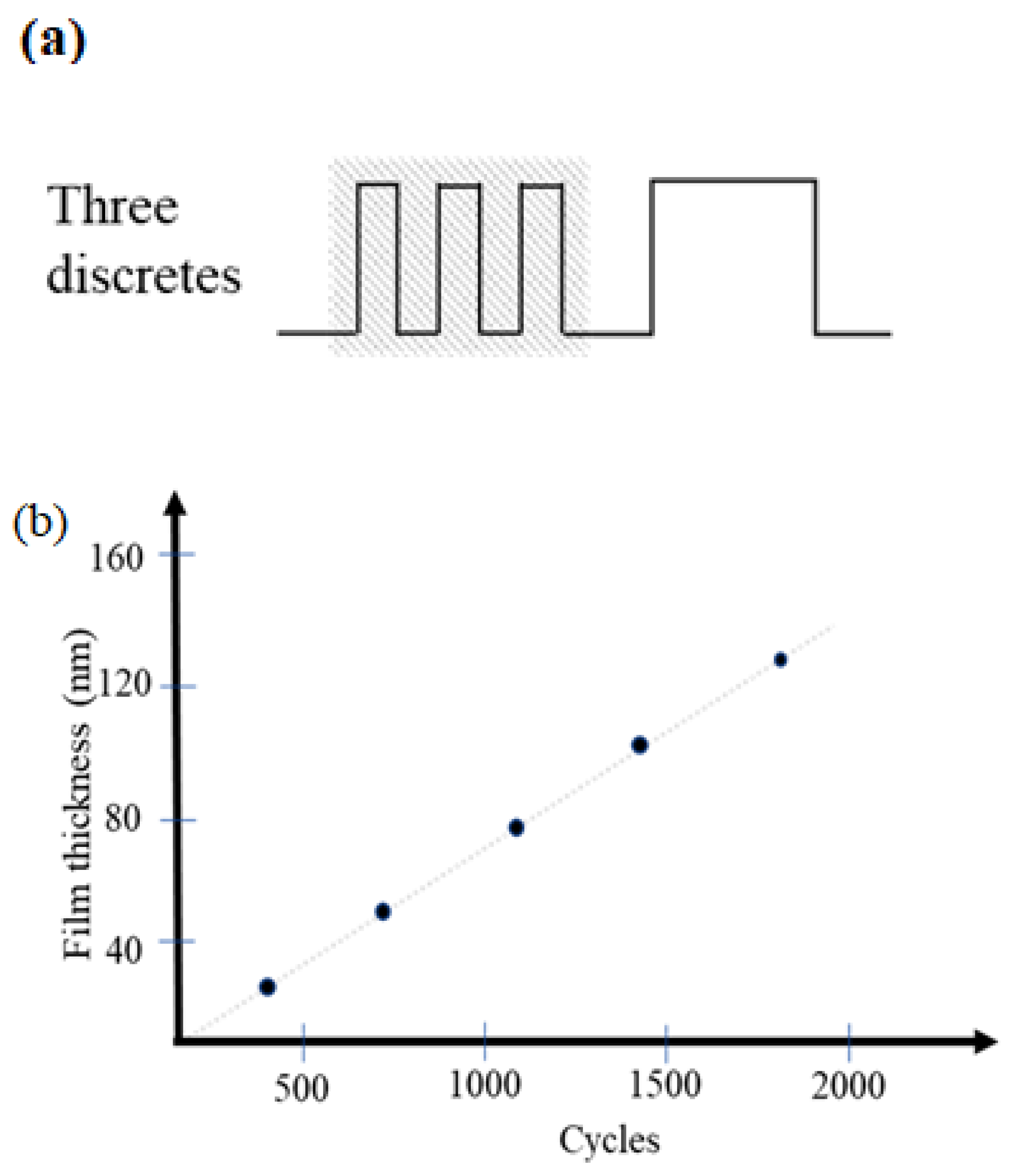

| Sample No. | Cycles | Thin Film Thickness (nm) |
|---|---|---|
| A1 | 360 | 26 |
| A2 | 720 | 52 |
| A3 | 1080 | 79 |
| A4 | 1440 | 107 |
| A5 | 1800 | 133 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.-C.; Wang, C.-C.; Tien, C.-L.; Tung, F.-C.; Wang, H.-F.; Lai, S.-H. Fabrication of Aluminum Oxide Thin-Film Devices Based on Atomic Layer Deposition and Pulsed Discrete Feed Method. Micromachines 2023, 14, 279. https://doi.org/10.3390/mi14020279
Lin S-C, Wang C-C, Tien C-L, Tung F-C, Wang H-F, Lai S-H. Fabrication of Aluminum Oxide Thin-Film Devices Based on Atomic Layer Deposition and Pulsed Discrete Feed Method. Micromachines. 2023; 14(2):279. https://doi.org/10.3390/mi14020279
Chicago/Turabian StyleLin, Shih-Chin, Ching-Chiun Wang, Chuen-Lin Tien, Fu-Ching Tung, Hsuan-Fu Wang, and Shih-Hsiang Lai. 2023. "Fabrication of Aluminum Oxide Thin-Film Devices Based on Atomic Layer Deposition and Pulsed Discrete Feed Method" Micromachines 14, no. 2: 279. https://doi.org/10.3390/mi14020279
APA StyleLin, S.-C., Wang, C.-C., Tien, C.-L., Tung, F.-C., Wang, H.-F., & Lai, S.-H. (2023). Fabrication of Aluminum Oxide Thin-Film Devices Based on Atomic Layer Deposition and Pulsed Discrete Feed Method. Micromachines, 14(2), 279. https://doi.org/10.3390/mi14020279








