Neurotransmitter Assay for In Vivo Nerve Signal Detection Using Bismuth Immobilized on a Carbon Nanotube Paste Electrode
Abstract
1. Introduction
2. Experimental Design
2.1. Preparation of the Electrode and In Vivo Implantation
2.2. Reagents
2.3. Experimental Procedure
3. Results and Discussion
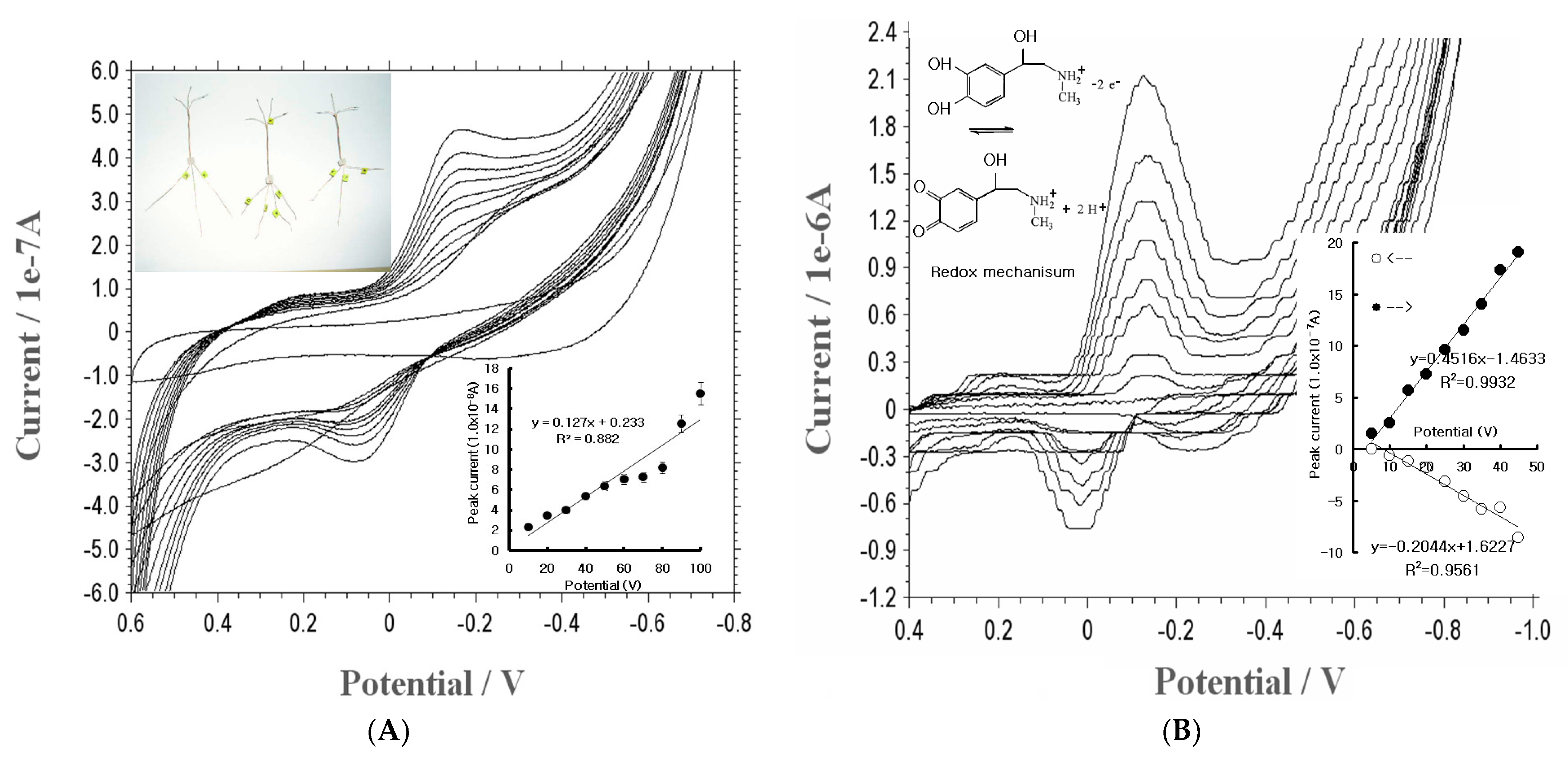
3.1. Cyclic Voltammetry
3.2. Optimization of the SW Parameters
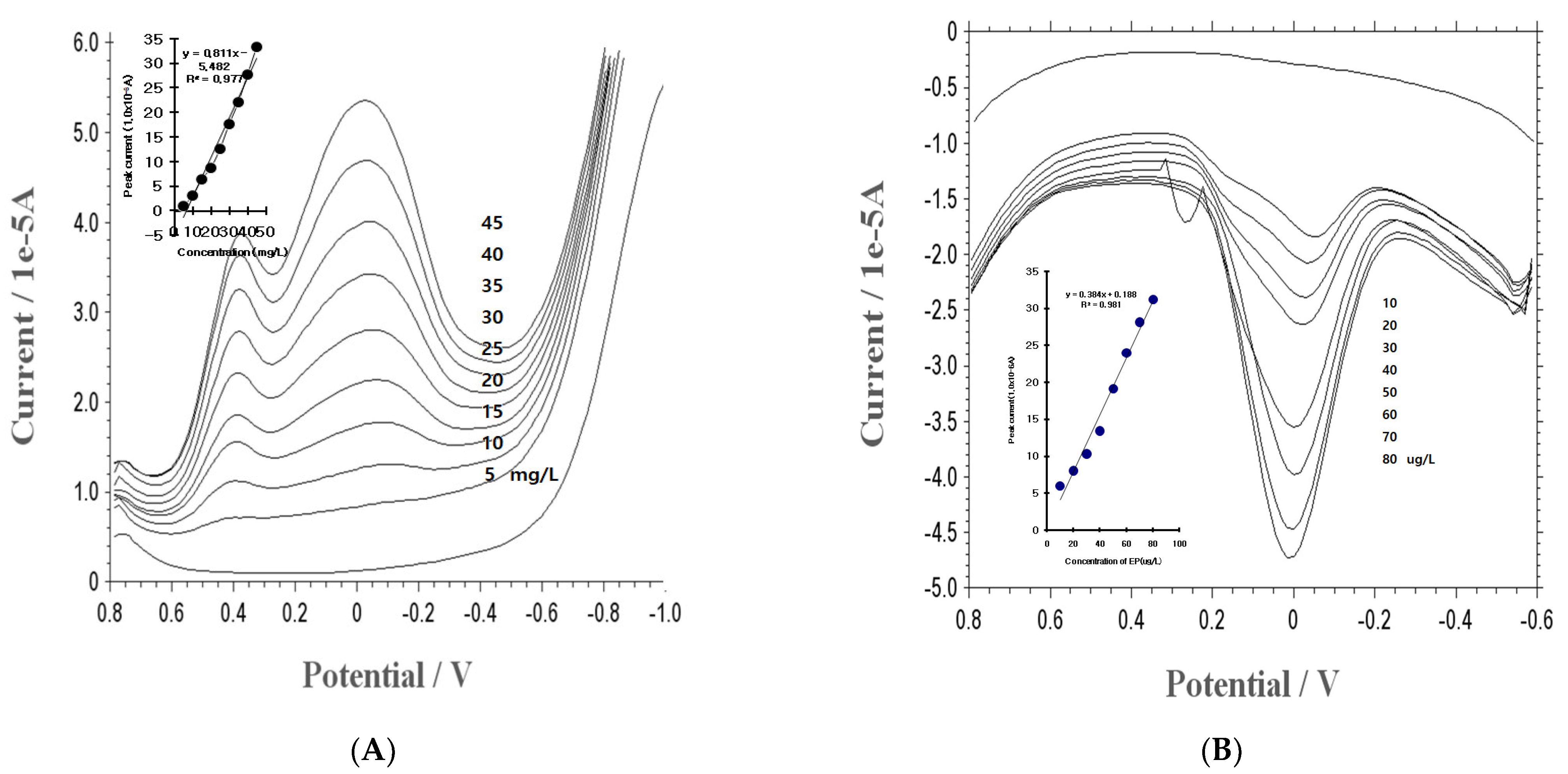
3.3. Statistics, Working Range, and Application
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EP | epinephrine |
| BCE | bismuth immobilized on a carbon nanotube paste electrode |
| CE | carbon nanotube paste electrode |
| SW | square-wave |
| SWSV | square-wave anodic stripping voltammetry |
References
- Suk, H.K.; Joon, W.L.; In, H.Y. Spectroelectrochemical and electrochemical behavior of epinephrine at a gold electrode. Electrochim. Acta 2000, 45, 2889–2895. [Google Scholar]
- Fang, X.; Mengnan, G.; Guoyue, S.; Lin, W.; Wen, Z.; Jian, X.; Litong, J.; Jiye, J. Simultaneous detection of monoamines in rat striatal microdialysate at poly(para-aminobenzoic acid) modified electrode by high-performance liquid chromatography. Anal. Chim. Acta 2001, 439, 239–246. [Google Scholar]
- Zhang, S.; Xu, Q.; Zhang, W.; Jin, L.; Jin, J.Y. In vivo monitoring of the monoamine neurotransmitters in rat brain using microdialysis sampling with liquid chromatography electrochemical detection. Anal. Chim. Acta 2000, 427, 45–53. [Google Scholar] [CrossRef]
- Tamio, K.; Tomoyuki, K.; Hirofumi, T.; Eiji, M.; Takayuki, S. Effect of the geometry of microfabricated flow reactors on chemiluminescent detection of epibephrine with lucigenin. Luminescence 2001, 16, 337–342. [Google Scholar]
- Dongchan, S.; Bulusu, V.S.; Donald, A.T.; Akira, F. Application of Diamond Microelectrodes for End-Column Electrochemical Detection in Capillary Electrophoresis. Anal. Chem. 2003, 75, 530–534. [Google Scholar]
- Hernandez, P.; Sanchez, I.; Paton, F.; Hernandez, L. Cyclic voltammetry determination of epinephrine with a carbon fiber ultramicroelectrode. Talanta 1998, 46, 985–991. [Google Scholar] [CrossRef]
- Yuzhong, Z.; Yuejuan, C.; Shao, S. Determination of dopamine in the presence of ascorbic acid by poly(styrene sulfonic acid) sodium salt/single-wall carbonnanotube film modified glassy carbon electrode. Anal. Biochem. 2006, 350, 285–291. [Google Scholar]
- Suw, Y.L.; Yong, H.K.; In, K.H.; In, G.M.; Woon, W.J.; Sam, Y.J.; Hyun, J.S.; Tae, K.H.; Myung, H.K. Anodic square-wave stripping voltammetric analysis of epinephrine using carbon fiber microelectrode. Microchem. J. 2006, 82, 113–118. [Google Scholar]
- Legeai, S.; Vittori, O. A Cu/Nafion/Bi electrode for on-site monitoring of trace heavy metals in natural waters using anodic stripping voltammetry: An alternative to mercury-based electrodes. Anal. Chim. Acta 2006, 560, 184–190. [Google Scholar] [CrossRef]
- Minli, Y.; Zhongbo, H. Electrodeposition of bismuth onto glassy carbon electrodes from nitrate solutions. J. Electroanal. Chem. 2005, 583, 46–55. [Google Scholar]
- Sophie, L.; Stephanie, B.; Olivier, V. A copper bismuth film electrode for adsorptive cathodic stripping analysis of trace nickel using square wave voltammetry. J. Electroanal. Chem. 2006, 591, 93–98. [Google Scholar]
- Samo, H.; Bozoeidar, O.; Joseph, W.; Boris, P. A Study on Operational Parameters for Advanced Use of Bismuth Film Electrode in Anodic Stripping Voltammetry. Electroanalysis 2002, 14, 1707–1712. [Google Scholar]
- Baldrianova, L.; Svancara, I.; Economou, S.S. Anodic stripping voltammetry at in situ bismuth plated carbon and gold microdisc electrodes in variable electrolyte content unstirred solution. Anal. Chim. Acta 2006, 580, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Emily, A.H.; Samo, B.H.; Lea, M.; Bozidar, O. Bismuth film electrode for anodic stripping voltammetric determination of tin. Anal. Chim. Acta 2006, 580, 244–250. [Google Scholar]
- Emily, A.H.; Bozidar, O.; Samo, B.H.; Malcolm, R.S. Bismuth film microelectrode for direct voltammetric measurement of trace cobalt and nickel in some simulated and real body fluid samples. Anal. Chim. Acta 2006, 557, 57–63. [Google Scholar]
- Suw, Y.L.; Jin, H.L. Human-Urine Diabetes Assay and In Vivo Rat Bladder Assay Using a Fluorine-Doped Carbon Nanotube Catheter Sensor. Ann. Biomed. Eng. 2009, 37, 2028–2033. [Google Scholar]
- Suw, Y.L. Diagnosis of Diabetes in a Diabetic Patient’s Urine and Blood Using a Combination Electrode with a Ubiquitous Handheld Analyzer. Curr. Anal. Chem. 2009, 5, 59–64. [Google Scholar]
- Williams, J.H.; Barnes, W.S. The positive inotropic effect of epinephrine on skeletal muscle: A brief review. Muscle Nerve 1989, 12, 968–975. [Google Scholar] [CrossRef]
- Febbraio, M.A.; Lambert, D.L.; Starkie, R.L.; Proietto, J.; Hargreaves, M. Effect of epinephrine on muscle glycogenolysis during exercise in trained men. J. Appl. Physiol. 1998, 84, 465–470. [Google Scholar] [CrossRef]
- Baker, T.W.; Webber, C.M.; Bush, A.C.; Gonzalez-Reyes, E. The Ten Study: Time Epinephrine Needs to Reach Muscle. J. Allergy Clin. Immunol. 2011, 127, AB247. [Google Scholar] [CrossRef]
- Johnson, T.P.; Moon, T.W.; Johnston, I.A. Actions of epinephrine on the contractility of fast and slow skeletal muscle fibres in teleosts. Fish Physiol. Biochem. 1991, 9, 83–89. [Google Scholar] [CrossRef]
- Bacil, R.P.; Garcia, P.H.; Serrano, S.H.P. New insights on the electrochemical mechanism of epinephrine on glassy carbon electrode. J. Electroanal. Chem. 2022, 908, 116111. [Google Scholar] [CrossRef]
- Álvarez-Diduk, R.; Galano, A. Adrenaline and Noradrenaline: Protectors against Oxidative Stress or Molecular Targets? J. Phys. Chem. B 2015, 119, 3479–3491. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Tsai, K.; Ruhunage, C.K.; Gupta, V.K.; Rahm, C.E.; Jiang, D.; Alvarez, N.T. True Picomolar Neurotransmitter Sensor Based on Open-Ended Carbon Nanotubes. Anal. Chem. 2020, 92, 8536–8545. [Google Scholar] [CrossRef]
- Levy, B.; Mansart, A.; Bollaert, P.-E.; Franck, P.; Mallie, J.-P. Effects of epinephrine and norepinephrine on hemodynamics, oxidative metabolism, and organ energetics in endotoxemic rats. Intensiv. Care Med. 2003, 29, 292–300. [Google Scholar] [CrossRef]
- Booth, J.H. The Effects of Oxygen Supply, Epinephrine, and Acetylcholine on the Distribution of Blood Flow in Trout Gills. J. Exp. Biol. 1979, 83, 31–39. [Google Scholar] [CrossRef]
- Routledge, C.; Marsden, C. Adrenaline in the CNS: In vivo evidence for a functional pathway innervating the hypothalamus. Neuropharmacology 1987, 26, 823–830. [Google Scholar] [CrossRef]
- Pedersen, S.B.; Bak, J.F.; Holck, P.; Schmitz, O.; Richelsen, B. Epinephrine stimulates human muscle lipoprotein lipase activity in vivo. Metabolism 1999, 48, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Bernards, C.M.; Kopacz, D.J. Effect of Epinephrine on Lidocaine Clearance In Vivo: A Microdialysis Study in Humans. Anesthesiology 1999, 91, 962–968. [Google Scholar] [CrossRef]
- Rue, E.Y.; Chae, J.S. Comparison of Epinephrine and Epinephrine with Vasopressin Mixture on the Cerebral “No-Reflow” in a Rabbit Model of Cardiopulmonary Resuscitation. J. Korean Soc. Emerg. Med. 1997, 8, 490–498. [Google Scholar]
- Lin, L.; Thongngamdee, S.; Wang, J.; Lin, Y.; Sadik, O.A.; Ly, S.Y. Adsorptive stripping voltammetric measurements of trace uranium at the bismuth film electrode. Anal. Chim. Acta 2005, 11, 9–13. [Google Scholar] [CrossRef]
- Li, X.; Chen, M.; Xinying, M.A. Selective Determination of Epinephrine in the Presence of Ascorbic Acid Using a Glassy Carbon Electrode Modified with Graphene. Anal. Sci. 2012, 28, 147. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Erdogduğ, G. Selective Voltammetric Detection of Epinephrine in the Presence of Ascorbic Acid at Poly(4-Methoxyphenol)-Modified Electrode. J. Anal. Chem. 2002, 57, 620–623. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Alvansaz-Yazdi, F.; Hoseynidokht, F.; Khoshroo, A. Fabrication of an Electrochemical Sensor for Determination of Epinephrine Using a Glassy Carbon Electrode Modified with Catechol. Anal. Bioanal. Chem. Res. 2023, 10, 387–394. [Google Scholar]
- Ülker, E.; Kavanoz, M. Determination of epinephrine in human serum using a Pt electrode modified by polyaniline–poly(3-methylthiophene)–poly(3,3′-diaminobenzidine). Can. J. Chem. 2015, 93, 1239–1244. [Google Scholar] [CrossRef]
- Teradale, A.B.; Lamani, S.D.; Ganesh, P.S.; Swamy, B.E.K.; Das, S.N. Niacin Film Coated Carbon Paste Electrode Sensor for the Determination of Epinephrine in Presence of Uric Acid: A Cyclic Voltammetric Study. Anal. Chem. Lett. 2017, 7, 748–764. [Google Scholar] [CrossRef]
- Lu, H.; Huang, Y.; Zhu, X.; Heineman, W.R. Sensitive Detection of Adrenaline using Electrochemically Surface-Treated rGO-AuNPs Electrode. In Proceedings of the 2022 IEEE Sensors, Dallas, TX, USA, 30 October–2 November 2022; pp. 1–4. [Google Scholar] [CrossRef]


| This Method: Bismuth Immobilized on a Carbon Nanotube Paste Electrode | 0.45 ug/L (2.45 × 10−9 M) | References |
|---|---|---|
| Graphene-modified glassy carbon electrode | 8.9 × 10−8 mol L−1 | [32] |
| A gold electrode | 0.1 μM | [33] |
| Glassy carbon electrode modified with catechol | 1.6 µM | [34] |
| Pt electrode modified by polyaniline–poly(3-methylthiophene)–poly(3,3′-diaminobenzidine) | 1.23 × 10−4 mmol/L | [35] |
| Niacin film-coated carbon paste electrode | 11.3 nM | [36] |
| rGO-AuNPs electrode | 0.45 µM | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.; Min, J.; Kim, J.S.; Park, J.H.; Ly, S. Neurotransmitter Assay for In Vivo Nerve Signal Detection Using Bismuth Immobilized on a Carbon Nanotube Paste Electrode. Micromachines 2023, 14, 1899. https://doi.org/10.3390/mi14101899
Choi J, Min J, Kim JS, Park JH, Ly S. Neurotransmitter Assay for In Vivo Nerve Signal Detection Using Bismuth Immobilized on a Carbon Nanotube Paste Electrode. Micromachines. 2023; 14(10):1899. https://doi.org/10.3390/mi14101899
Chicago/Turabian StyleChoi, Jongwan, Jiwon Min, Jason Sahngwook Kim, Jung Hyun Park, and SuwYoung Ly. 2023. "Neurotransmitter Assay for In Vivo Nerve Signal Detection Using Bismuth Immobilized on a Carbon Nanotube Paste Electrode" Micromachines 14, no. 10: 1899. https://doi.org/10.3390/mi14101899
APA StyleChoi, J., Min, J., Kim, J. S., Park, J. H., & Ly, S. (2023). Neurotransmitter Assay for In Vivo Nerve Signal Detection Using Bismuth Immobilized on a Carbon Nanotube Paste Electrode. Micromachines, 14(10), 1899. https://doi.org/10.3390/mi14101899






