Fluid Viscosity Measurement by Means of Secondary Flow in a Curved Channel
Abstract
:1. Introduction
2. Materials and Methods
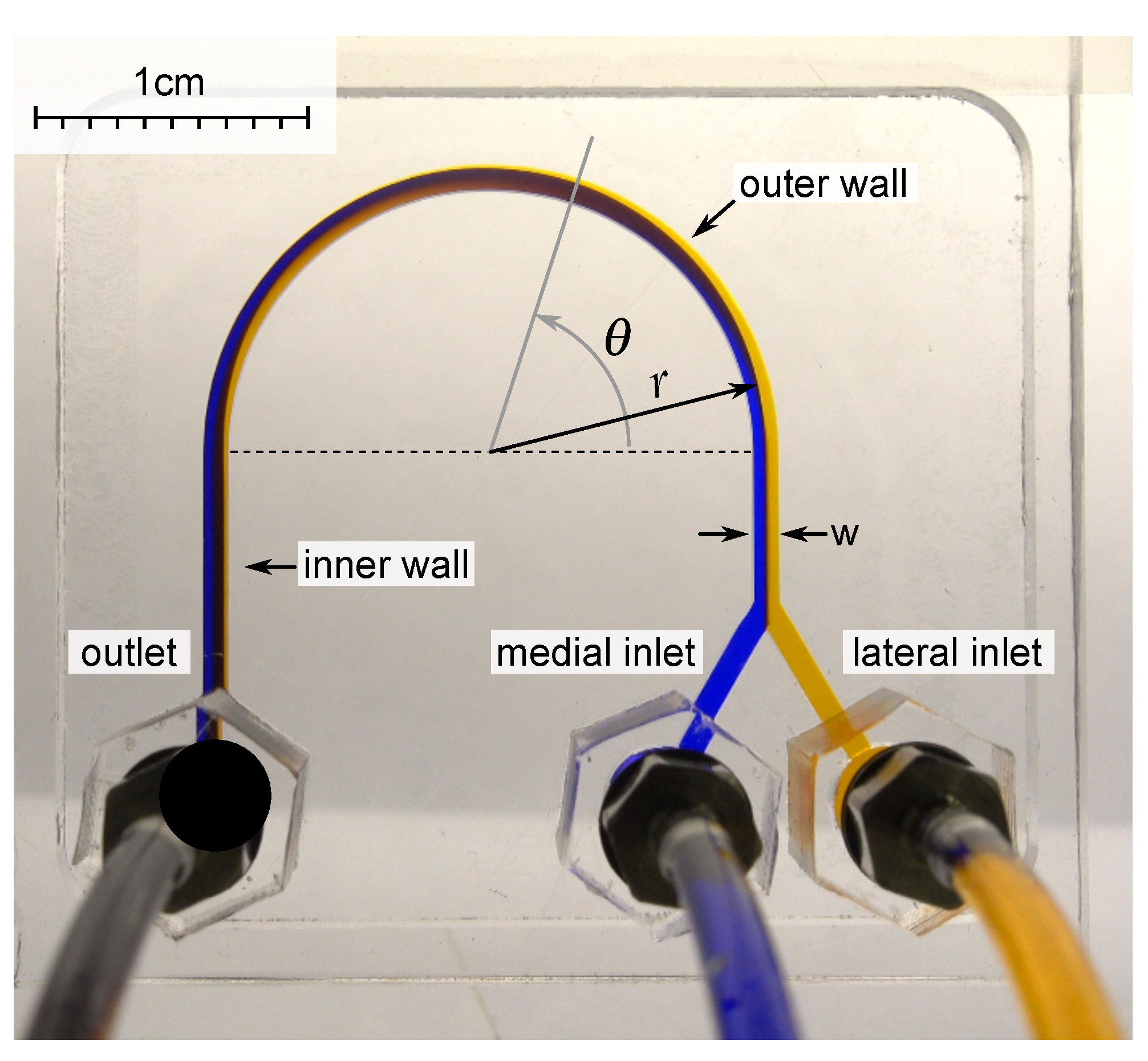
2.1. Description of the Approach
2.2. Microfluidic Device Fabrication
2.3. The Tested Liquids
2.4. Experimental Setup and Procedure
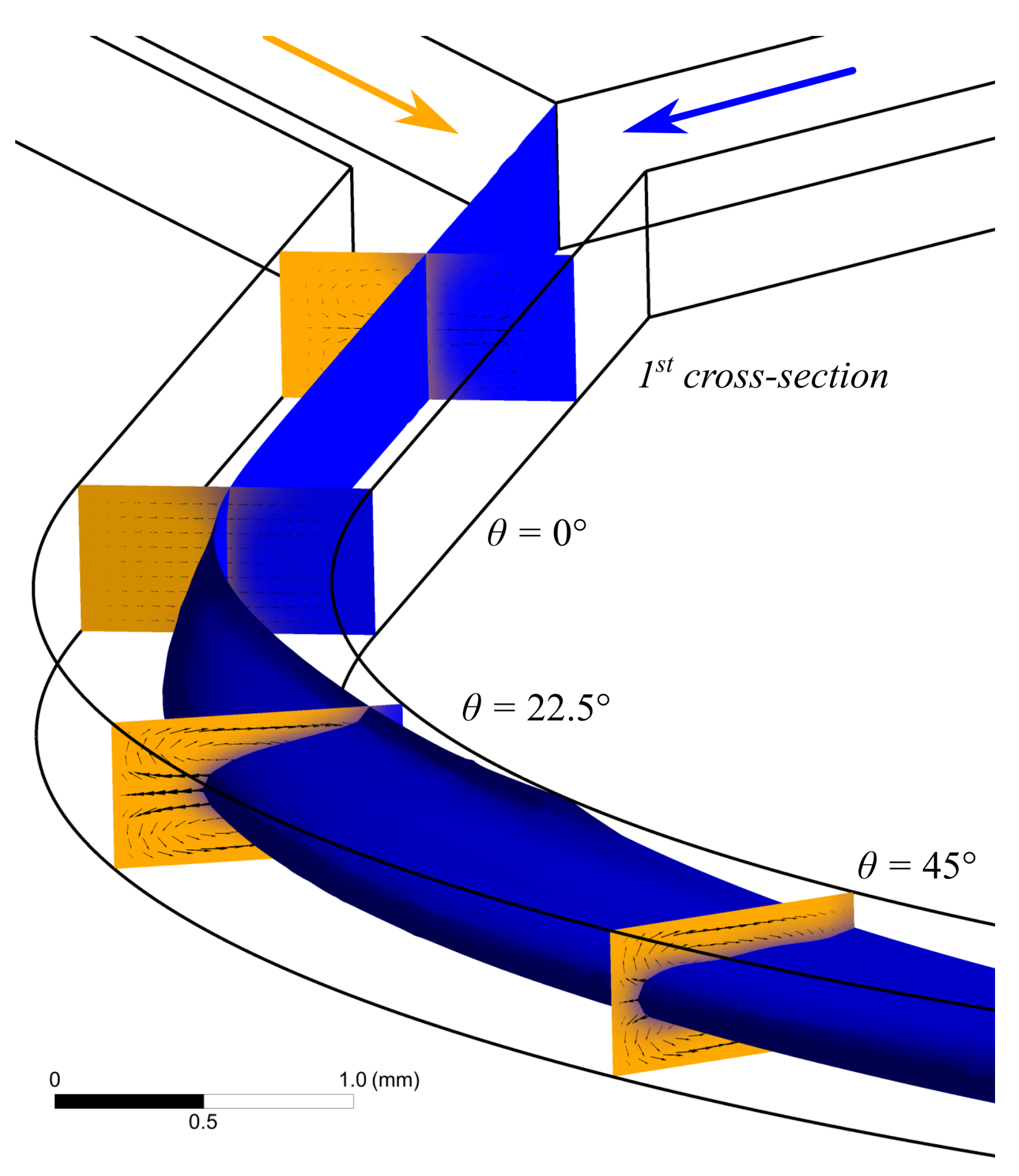
2.5. Computational Fluid Dynamics (CFD)
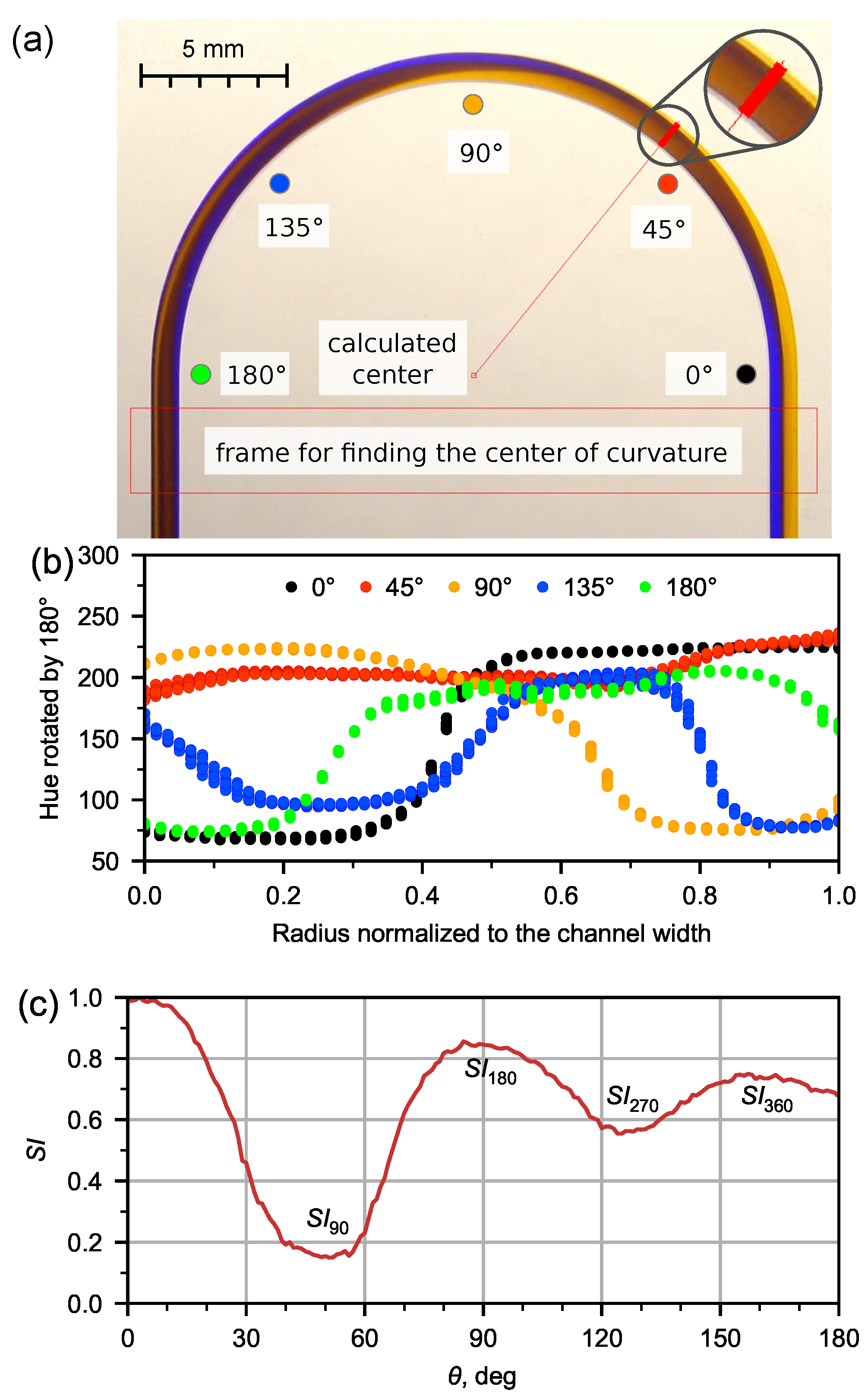
2.6. Secondary Flow Quantification
3. Results and Discussion
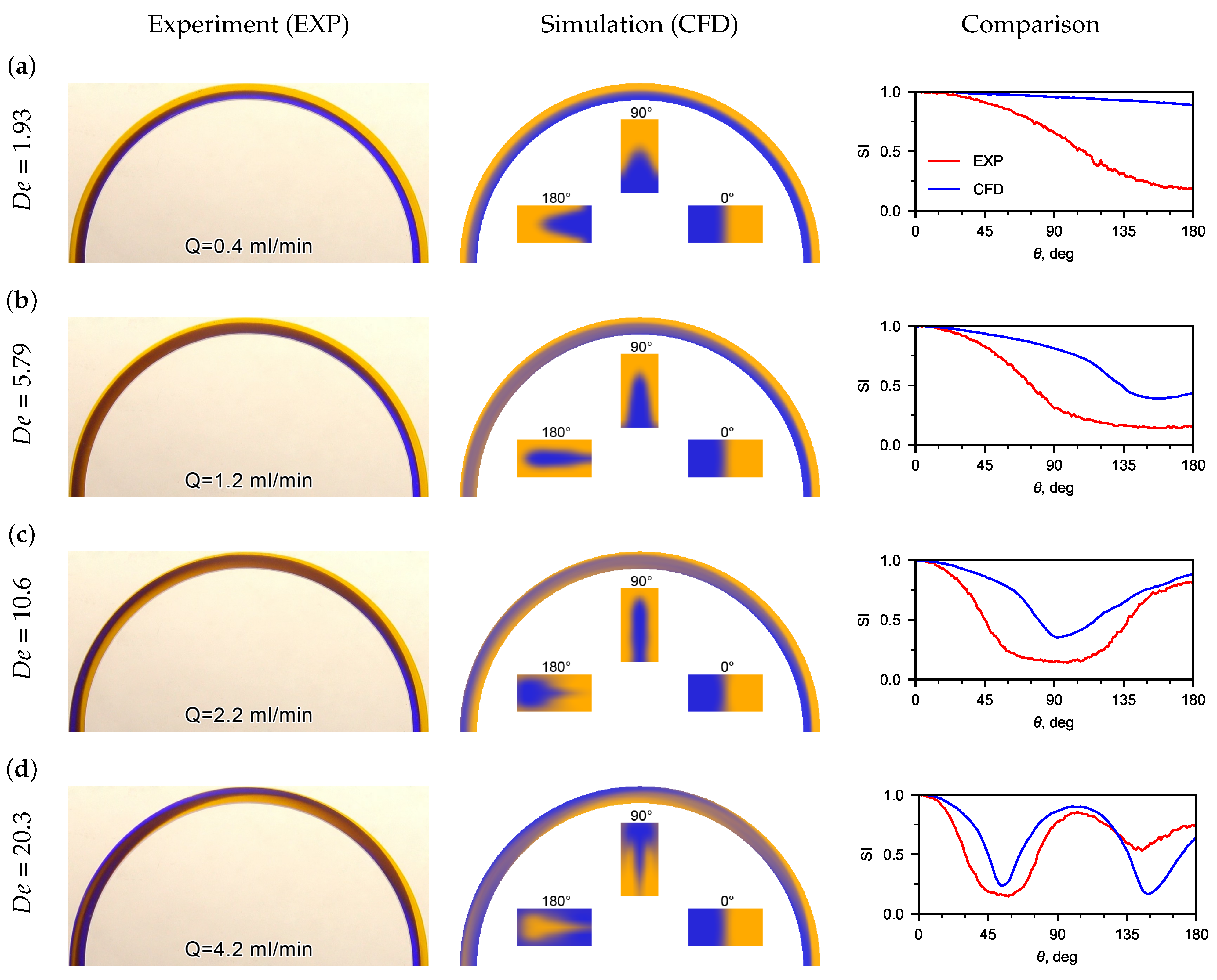
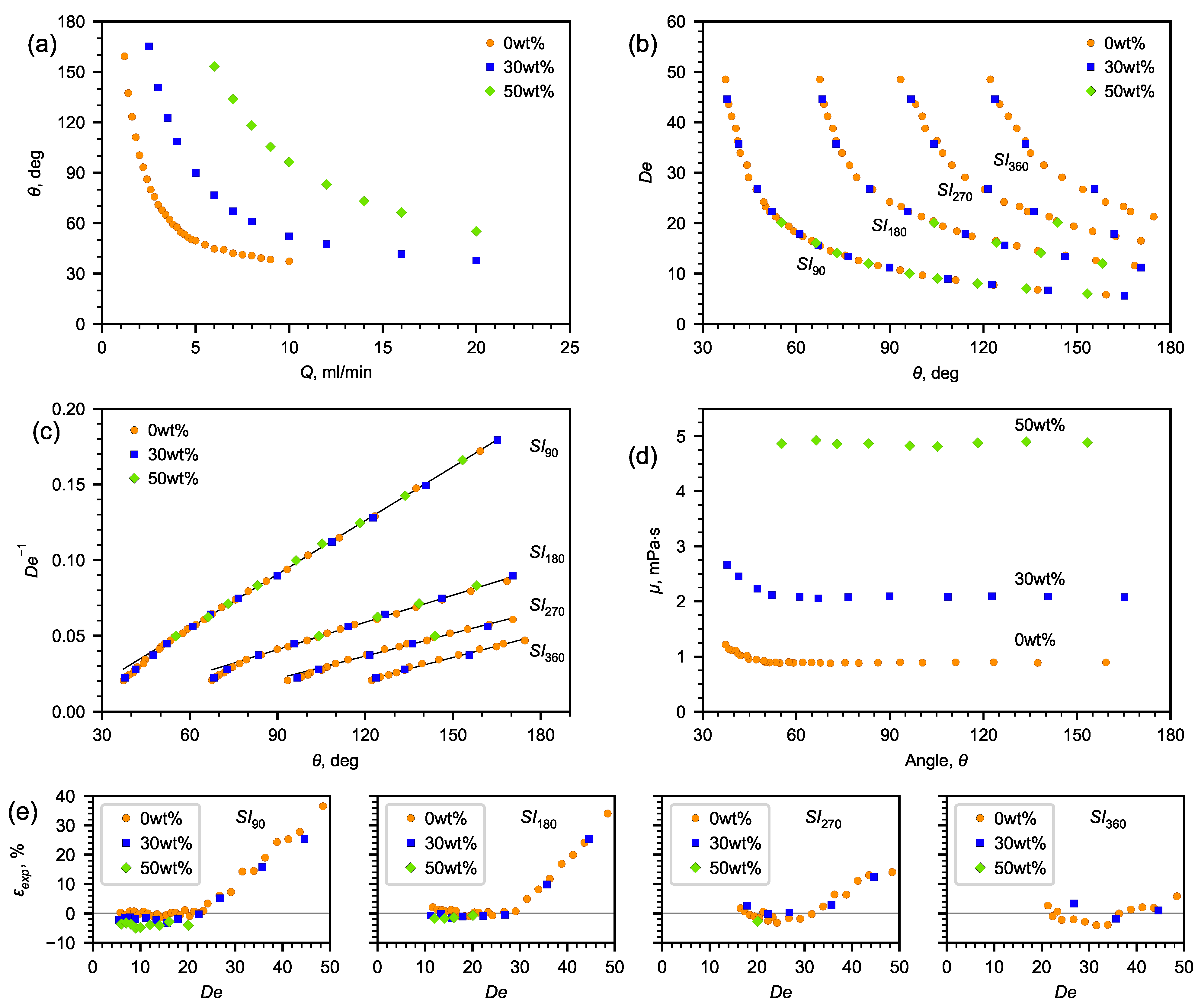
3.1. Flow Patterns Analysis
3.2. Determining Fluid Viscosity
3.3. Methodology for Design of Microfluidic Viscometer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guillot, P.; Panizza, P.; Salmon, J.; Joanicot, M.; Colin, A.; Bruneau, C.; Colin, T. Viscosimeter on a Microfluidic Chip. Langmuir 2006, 22, 6438–6445. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, J.; Ayela, F. Microfluidic on chip viscometers. Rev. Sci. Instrum. 2008, 79, 076102. [Google Scholar] [CrossRef] [PubMed]
- Bianco, M.; Zizzari, A.; Gazzera, L.; Metrangolo, P.; Gigli, G.; Viola, I.; Arima, V. Integrated microfluidic viscometer for edible oil analysis. Sens. Actuators B Chem. 2018, 265, 91–97. [Google Scholar] [CrossRef]
- Srivastava, N.; Burns, M.A. Analysis of Non-Newtonian Liquids Using a Microfluidic Capillary Viscometer. Anal. Chem. 2006, 78, 1690–1696. [Google Scholar] [CrossRef]
- Kim, S.; Kim, K.C.; Yeom, E. Microfluidic method for measuring viscosity using images from smartphone. Opt. Lasers Eng. 2018, 104, 237–243. [Google Scholar] [CrossRef]
- Solomon, D.E.; Abdel-Raziq, A.; Vanapalli, S.A. A stress-controlled microfluidic shear viscometer based on smartphone imaging. Rheol. Acta 2016, 55, 727–738. [Google Scholar] [CrossRef]
- Li, Y.; Ward, K.R.; Burns, M.A. Viscosity Measurements Using Microfluidic Droplet Length. Anal. Chem. 2017, 89, 3996–4006. [Google Scholar] [CrossRef]
- Roumpea, E.; Chinaud, M.; Angeli, P. Experimental investigations of non-Newtonian/Newtonian liquid-liquid flows in microchannels. AIChE J. 2017, 63, 3599–3609. [Google Scholar] [CrossRef]
- Roy, P.; Liu, S.; Dutcher, C.S. Droplet Interfacial Tensions and Phase Transitions Measured in Microfluidic Channels. Annu. Rev. Phys. Chem. 2021, 72, 73–97. [Google Scholar] [CrossRef]
- Mustafa, A.; Eser, A.; Aksu, A.C.; Kiraz, A.; Tanyeri, M.; Erten, A.; Yalcin, O. A micropillar-based microfluidic viscometer for Newtonian and non-Newtonian fluids. Anal. Chim. Acta 2020, 1135, 107–115. [Google Scholar] [CrossRef]
- Lee, E.; Kim, B.; Choi, S. Hand-held, automatic capillary viscometer for analysis of Newtonian and non-Newtonian fluids. Sens. Actuators A Phys. 2020, 313, 112176. [Google Scholar] [CrossRef]
- Liu, L.; Hu, D.; Lam, R. Microfluidic Viscometer Using a Suspending Micromembrane for Measurement of Biosamples. Micromachines 2020, 11, 934. [Google Scholar] [CrossRef]
- Kang, Y.J.; Yoon, S.Y.; Lee, K.; Yang, S. A Highly Accurate and Consistent Microfluidic Viscometer for Continuous Blood Viscosity Measurement: Microfluidic Blood Viscometer. Artif. Organs 2010, 34, 944–949. [Google Scholar] [CrossRef]
- Kang, D.; Song, J.M.; Yeom, E. Design of microfluidic viscometer based on pressure estimation. J. Vis. 2019, 22, 25–34. [Google Scholar] [CrossRef]
- Goel, S.; Venkateswaran, P.; Prajesh, R.; Agarwal, A. Rapid and automated measurement of biofuel blending using a microfluidic viscometer. Fuel 2015, 139, 213–219. [Google Scholar] [CrossRef]
- Yang, X.; Weldetsadik, N.T.; Hayat, Z.; Fu, T.; Jiang, S.; Zhu, C.; Ma, Y. Pressure drop of single phase flow in microchannels and its application in characterizing the apparent rheological property of fluids. Microfluid. Nanofluidics 2019, 23, 75. [Google Scholar] [CrossRef]
- Hong, H.; Song, J.M.; Yeom, E. 3D printed microfluidic viscometer based on the co-flowing stream. Biomicrofluidics 2019, 13, 014104. [Google Scholar] [CrossRef]
- Bahrami, M.; Michael Yovanovich, M.; Richard Culham, J. A novel solution for pressure drop in singly connected microchannels of arbitrary cross-section. Int. J. Heat Mass Transf. 2007, 50, 2492–2502. [Google Scholar] [CrossRef]
- Carnicer, V.; Alcázar, C.; Orts, M.; Sánchez, E.; Moreno, R. Microfluidic rheology: A new approach to measure viscosity of ceramic suspensions at extremely high shear rates. Open Ceram. 2021, 5, 100052. [Google Scholar] [CrossRef]
- Lee, T.; Liao, W.; Wu, Y.; Chen, Y.; Tung, Y. Electrofluidic Circuit-Based Microfluidic Viscometer for Analysis of Newtonian and Non-Newtonian Liquids under Different Temperatures. Anal. Chem. 2018, 90, 2317–2325. [Google Scholar] [CrossRef]
- Ober, T.J.; Haward, S.J.; Pipe, C.J.; Soulages, J.; McKinley, G.H. Microfluidic extensional rheometry using a hyperbolic contraction geometry. Rheol. Acta 2013, 52, 529–546. [Google Scholar] [CrossRef]
- Del Giudice, F.; D’Avino, G.; Greco, F.; De Santo, I.; Netti, P.A.; Maffettone, P.L. Rheometry-on-a-chip: Measuring the relaxation time of a viscoelastic liquid through particle migration in microchannel flows. Lab Chip 2015, 15, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, F. Simultaneous measurement of rheological properties in a microfluidic rheometer. Phys. Fluids 2020, 32, 052001. [Google Scholar] [CrossRef]
- Tzeng, B.; Sun, Y. Design and Fabrication of a Microfluidic Viscometer Based on Electrofluidic Circuits. Micromachines 2018, 9, 375. [Google Scholar] [CrossRef]
- Jang, I.; Berg, K.E.; Henry, C.S. Viscosity measurements utilizing a fast-flow microfluidic paper-based device. Sens. Actuators B Chem. 2020, 319, 128240. [Google Scholar] [CrossRef]
- Del Giudice, F. A Review of Microfluidic Devices for Rheological Characterisation. Micromachines 2022, 13, 167. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, K.; Puchades, I.; Agarwal, P.B. A comprehensive review on MEMS-based viscometers. Sens. Actuators A Phys. 2022, 338, 113456. [Google Scholar] [CrossRef]
- Dean, W.R.; Hurst, J.M. Note on the motion of fluid in a curved pipe. Mathematika 1959, 6, 77–85. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Yuan, S.; Tan, S.K. Flow development in curved rectangular ducts with continuously varying curvature. Exp. Therm. Fluid Sci. 2016, 75, 1–15. [Google Scholar] [CrossRef]
- Zhao, Q.; Yuan, D.; Zhang, J.; Li, W. A Review of Secondary Flow in Inertial Microfluidics. Micromachines 2020, 11, 461. [Google Scholar] [CrossRef]
- Yang, W.H.; Zhang, J.Z.; Cheng, H.E. The study of flow characteristics of curved microchannel. Appl. Therm. Eng. 2005, 25, 1894–1907. [Google Scholar] [CrossRef]
- Suh, Y.K.; Kang, S. A Review on Mixing in Microfluidics. Micromachines 2010, 1, 82–111. [Google Scholar] [CrossRef]
- Ha, B.H.; Lee, K.S.; Jung, J.H.; Sung, H.J. Three-dimensional hydrodynamic flow and particle focusing using four vortices Dean flow. Microfluid. Nanofluidics 2014, 17, 647–655. [Google Scholar] [CrossRef]
- Martel, J.M.; Toner, M. Particle Focusing in Curved Microfluidic Channels. Sci. Rep. 2013, 3, 3340. [Google Scholar] [CrossRef]
- Bayat, P.; Rezai, P. Semi-Empirical Estimation of Dean Flow Velocity in Curved Microchannels. Sci. Rep. 2017, 7, 13655. [Google Scholar] [CrossRef]
- Howell, P.B., Jr.; Mott, D.R.; Golden, J.P.; Ligler, F.S. Design and evaluation of a Dean vortex-based micromixer. Lab Chip 2004, 4, 663. [Google Scholar] [CrossRef]
- Jung, W.; Han, J.; Choi, J.; Ahn, C.H. Point-of-care testing (POCT) diagnostic systems using microfluidic lab-on-a-chip technologies. Microelectron. Eng. 2015, 132, 46–57. [Google Scholar] [CrossRef]
- Luppa, P.B.; Müller, C.; Schlichtiger, A.; Schlebusch, H. Point-of-care testing (POCT): Current techniques and future perspectives. TrAC Trends Anal. Chem. 2011, 30, 887–898. [Google Scholar] [CrossRef]
- Behroodi, E.; Latifi, H.; Bagheri, Z.; Ermis, E.; Roshani, S.; Salehi Moghaddam, M. A combined 3D printing/CNC micro-milling method to fabricate a large-scale microfluidic device with the small size 3D architectures: An application for tumor spheroid production. Sci. Rep. 2020, 10, 22171. [Google Scholar] [CrossRef]
- Guckenberger, D.J.; de Groot, T.E.; Wan, A.M.D.; Beebe, D.J.; Young, E.W.K. Micromilling: A method for ultra-rapid prototyping of plastic microfluidic devices. Lab Chip 2015, 15, 2364–2378. [Google Scholar] [CrossRef] [Green Version]
- Denisov, I.; Lukyanenko, K.; Yakimov, A.; Kukhtevich, I.; Esimbekova, E.; Belobrov, P. Disposable luciferase-based microfluidic chip for rapid assay of water pollution. Luminescence 2018, 33, 1054–1061. [Google Scholar] [CrossRef]
- Cheng, N. Formula for the Viscosity of a Glycerol-Water Mixture. Ind. Eng. Chem. Res. 2008, 47, 3285–3288. [Google Scholar] [CrossRef]


| Property | Water | 30 wt% | 50 wt% |
|---|---|---|---|
| Viscosity [mPa·s] | 0.94 ± 0.03 | 2.09 ± 0.03 | 5.11 ± 0.03 |
| Density [kg·m] | 997 | 1068 | 1137 |
| AAD | MAD | ||||||
|---|---|---|---|---|---|---|---|
| 6.787 | −1.604 | 0.999 | 0.56% | 1.25% | 5 ÷ 20 | 45 | |
| 3.414 | −1.261 | 0.997 | 1.01% | 2.18% | 10 ÷ 30 | 80 | |
| 2.843 | −2.283 | 0.992 | 1.35% | 3.18% | 15 ÷ 35 | 110 | |
| 2.874 | −3.942 | 0.990 | 2.08% | 4.06% | 20 ÷ 45 | 125 |
| Mass | [42] | ||||
|---|---|---|---|---|---|
| Fraction | [mPa·s] | [mPa·s] | [mPa·s] | [%] | [%] |
| 0 | 0.8897 | 0.94 | 0.8927 | −0.34 | 5.3 |
| 0.3 | 2.081 | 2.09 | 2.124 | −2.0 | −1.1 |
| 0.5 | 4.864 | 5.11 | 5.004 | −2.8 | 1.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pryazhnikov, M.I.; Yakimov, A.S.; Denisov, I.A.; Pryazhnikov, A.I.; Minakov, A.V.; Belobrov, P.I. Fluid Viscosity Measurement by Means of Secondary Flow in a Curved Channel. Micromachines 2022, 13, 1452. https://doi.org/10.3390/mi13091452
Pryazhnikov MI, Yakimov AS, Denisov IA, Pryazhnikov AI, Minakov AV, Belobrov PI. Fluid Viscosity Measurement by Means of Secondary Flow in a Curved Channel. Micromachines. 2022; 13(9):1452. https://doi.org/10.3390/mi13091452
Chicago/Turabian StylePryazhnikov, Maxim I., Anton S. Yakimov, Ivan A. Denisov, Andrey I. Pryazhnikov, Andrey V. Minakov, and Peter I. Belobrov. 2022. "Fluid Viscosity Measurement by Means of Secondary Flow in a Curved Channel" Micromachines 13, no. 9: 1452. https://doi.org/10.3390/mi13091452
APA StylePryazhnikov, M. I., Yakimov, A. S., Denisov, I. A., Pryazhnikov, A. I., Minakov, A. V., & Belobrov, P. I. (2022). Fluid Viscosity Measurement by Means of Secondary Flow in a Curved Channel. Micromachines, 13(9), 1452. https://doi.org/10.3390/mi13091452










