Tunable, Low–Cost, Multi–Channel, Broadband Liquid Crystal Shutter for Fluorescence Imaging in Widefield Microscopy
Abstract
:1. Introduction
2. Materials and Methods
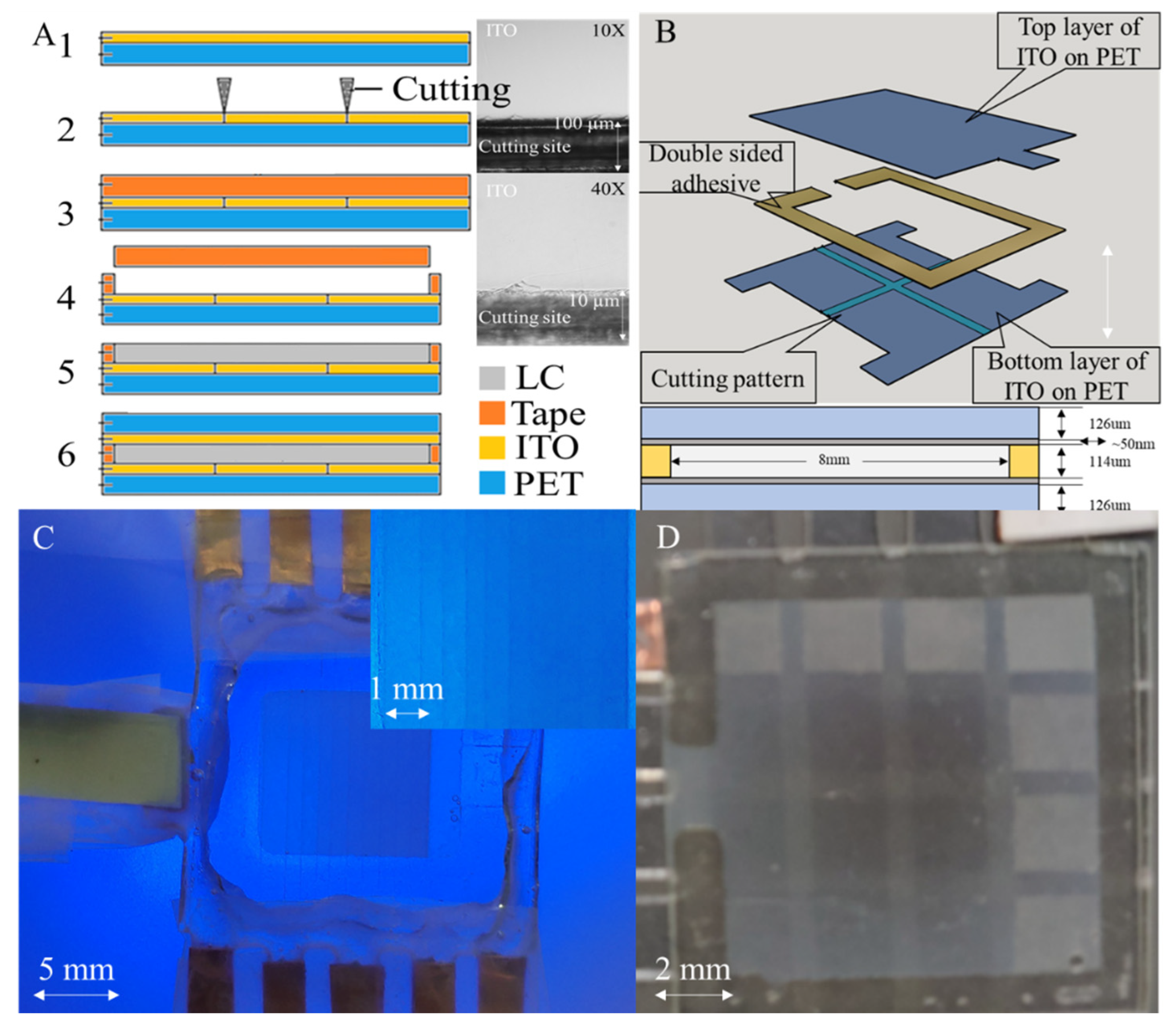
2.1. Sandwich–Structured NLC Shutter
2.2. Fluorescence Phantom and Tissue Preparation
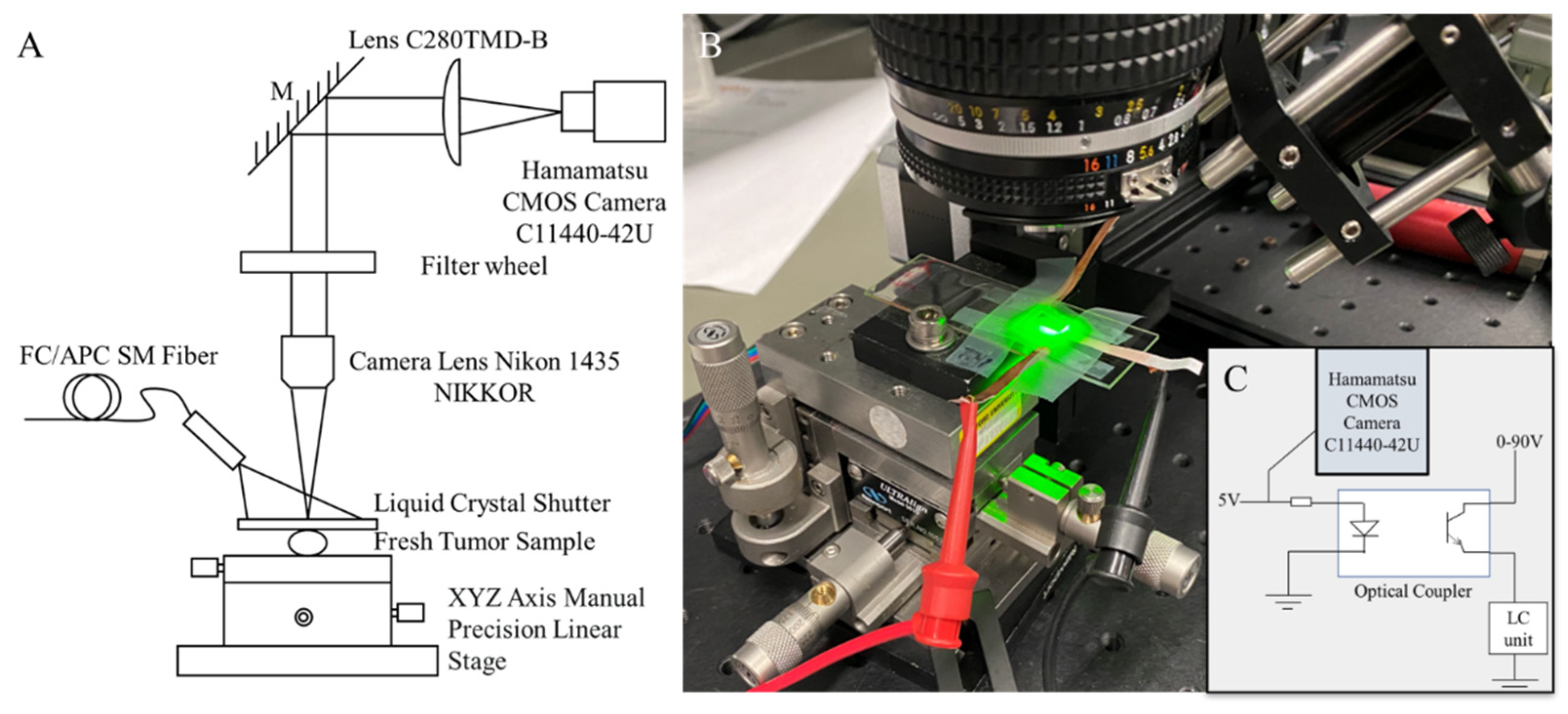
2.3. Experiment Setup
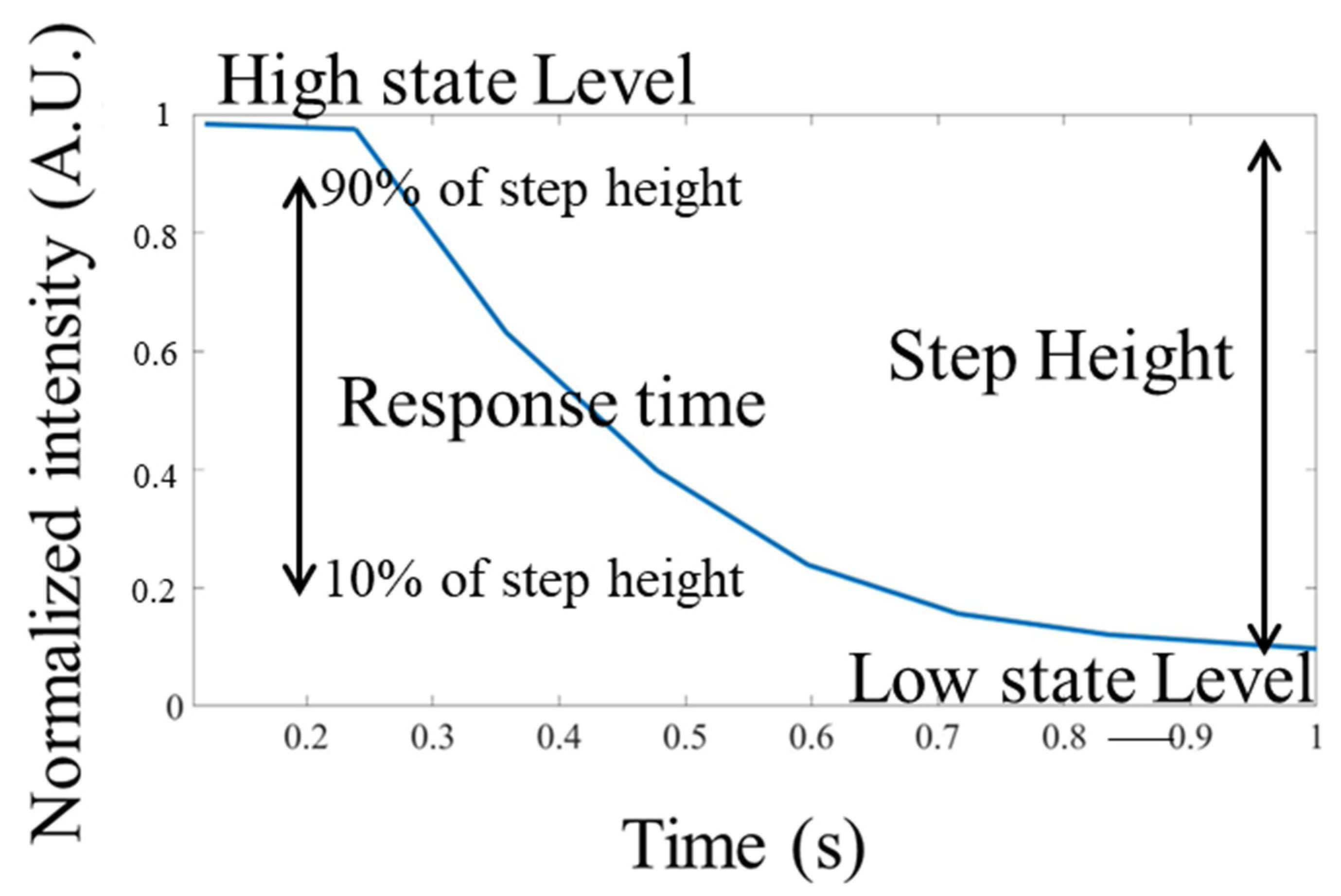
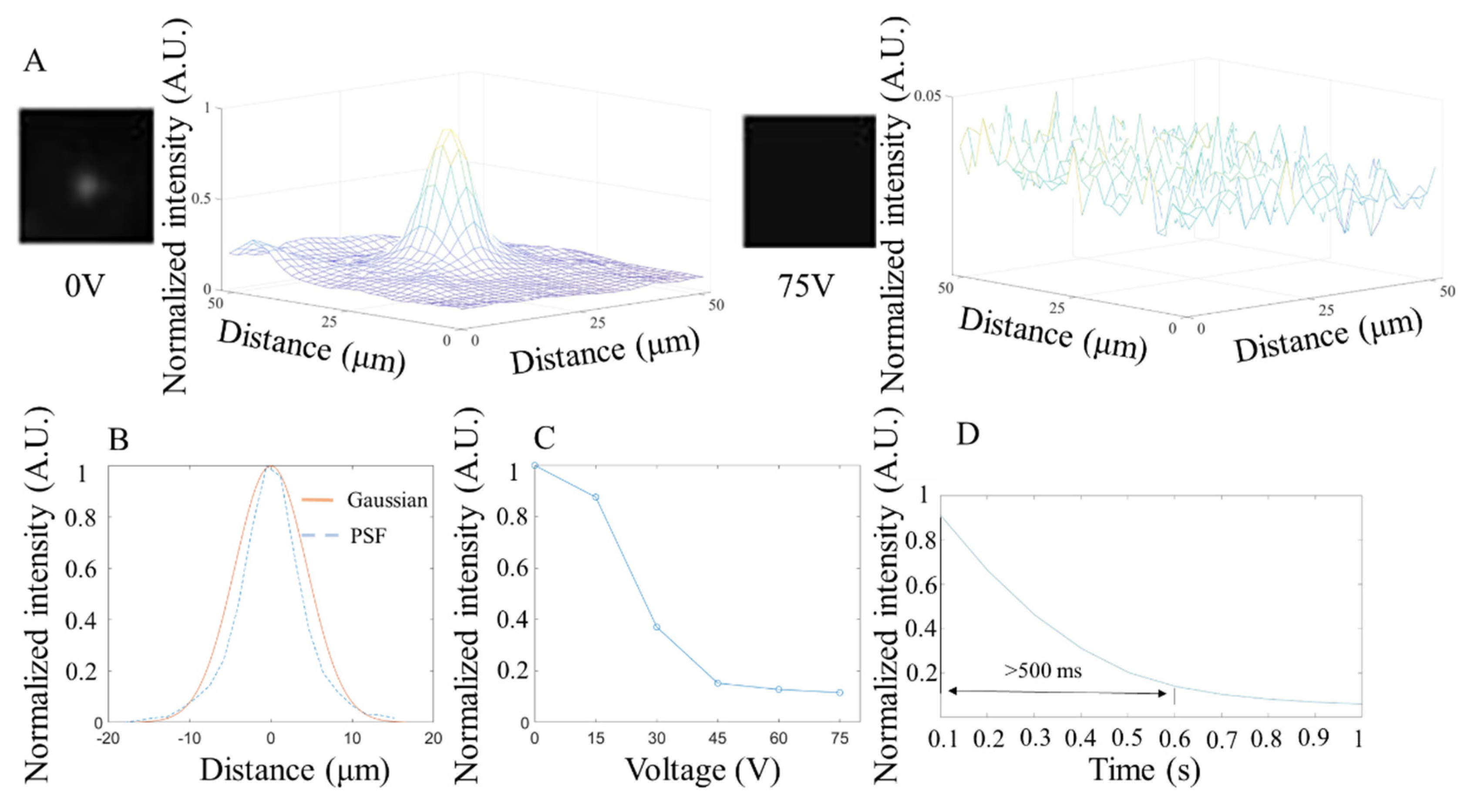
2.4. Data Acquisition and Analysis
3. Results and Discussion
3.1. General Overview of NLC
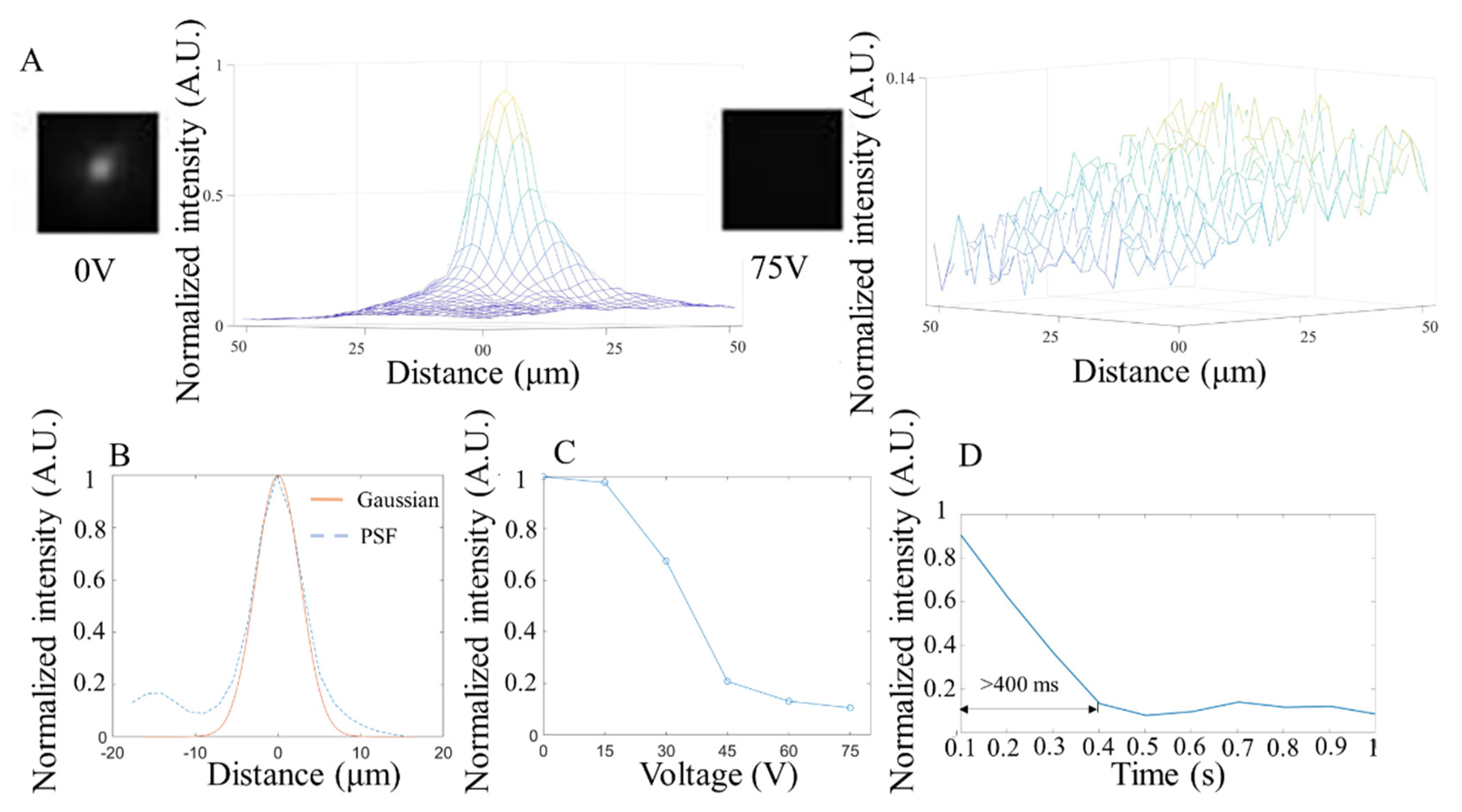
3.2. Bright Field Test
3.3. Fluorescence Test
3.4. Tissue and NIR Testing
3.5. Comparison of Shutters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, D.-K.; Wu, S.-T. Fundamentals of Liquid Crystal Devices; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Belyaev, S.V.; Malimonenko, N.V.; Miroshin, A. High–contrast and fast nematic liquid crystal shutter for projection displays. In Projection Displays II; SPIE: Bellingham, DC, USA, 1996; Volume 2650, pp. 106–109. [Google Scholar]
- Pishnyak, O.; Sato, S.; Lavrentovich, O.D. Electrically tunable lens based on a dual–frequency nematic liquid crystal. Appl. Opt. 2006, 45, 4576–4582. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Akira, S.; Rumiko, S. Variable–focus liquid–crystal Fresnel lens. Jpn. J. Appl. Phys. 1985, 24, L626. [Google Scholar] [CrossRef]
- Verwoerd, N.P.; Hennink, E.J.; Bonnet, J.; Van der Geest, C.R.G.; Tanke, H.J. Use of ferro-electric liquid crystal shutters for time-resolved fluorescence microscopy. Cytom. J. Int. Soc. Anal. Cytol. 1994, 16, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Lee, S.A.; Kang, D.G.; Park, M.; Choi, Y.J.; Jeong, K.U. Photoresponsive carbohydrate–based giant surfactants: Automatic vertical alignment of nematic liquid crystal for the remote–controllable optical device. ACS Appl. Mater. Interfaces 2015, 7, 6195–6204. [Google Scholar] [CrossRef] [PubMed]
- Kelker, H.; Knoll, P.M. Some Pictures of the History of Liquid Crystals. Liq. Cryst. 1989, 5, 19–42. [Google Scholar] [CrossRef]
- Jeong, K. –U.; Jing, A.J.; Mansdorf, B.; Graham, M.J.; Yang, D.–K.; Harris, F.W.; Cheng, S.Z.D. Biaxial Molecular Arrangement of Rod–Disc Molecule under an Electric Field. Chem. Mater. 2007, 19, 2921–2923. [Google Scholar] [CrossRef]
- Kim, J.; Khan, M.; Park, S.-Y. Glucose Sensor Using LiquidCrystal Droplets Made by Microfluidics. ACS Appl. Mater. Interfaces 2013, 5, 13135–13139. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, W.; Abbott, N.L.; Mann, E.K.; Jakli, A. Improving’ Liquid–Crystal–based Biosensing in Aqueous Phases. ACS Appl. Mater. Interfaces 2012, 4, 6884–6890. [Google Scholar] [CrossRef]
- Goodby, J.W.; Waugh, M.A.; Stein, S.M.; Chin, E.; Pindak, R.; Patel, J.S. Characterization of a New Helical Smectic Liquid Crystal. Nature 1989, 337, 449–452. [Google Scholar] [CrossRef]
- Kikuchi, H.; Yokota, M.; Hisakado, Y.; Yang, H.; Kajiyama, T. Polymer–Stabilized Liquid Crystal Blue Phases. Nat. Mater. 2002, 1, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Fang, G.; Glaser, M.A.; Maclennan, J.E.; Korblova, E.; Walba, D.M.; Clark, N.A. Phase Winding of a Nematic Liquid Crystal by Dynamic Localized Reorientation of an Azo–based Self–Assembled Monolayer. Langmuir 2014, 30, 9560–9566. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Kobayashi, T. Fabrication of Stable Nanocylinder Arrays in Highly Birefringent Films of an Amphiphilic Liquid–Crystalline Diblock Copolymer. ACS Appl. Mater. Interfaces 2009, 1, 2755–2762. [Google Scholar] [CrossRef] [PubMed]
- Doane, J.W.; Vaz, N.A.; Wu, B.G.; Žumer, S. Field controlled light scattering from nematic microdroplets. Appl. Phys. Lett. 1986, 48, 269–271. [Google Scholar] [CrossRef]
- Hikmet, R.A.M. Electrically induced light scattering from anisotropic gels. J. Appl. Phys. 1990, 68, 4406–4412. [Google Scholar] [CrossRef]
- Yang, D.K.; West, J.L.; Chien, L.C.; Doane, J.W. Control of reflectivity and bistability in displays using cholesteric liquid crystals. J. Appl. Phys. 1994, 76, 1331–1333. [Google Scholar] [CrossRef]
- Ren, H.; Lin, Y.H.; Fan, Y.H.; Wu, S.T. In–plane switching liquid crystal gel for polarization–independent light switch. J. Appl. Phys. 2004, 96, 3609–3611. [Google Scholar] [CrossRef]
- Love, G.D.; Kirby, A.K.; Ramsey, R.A. Sub–millisecond, high stroke phase modulation using polymer network liquid crystals. Opt. Express 2010, 18, 7384–7389. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Hwang, K.Y.; Jang, J.E.; Jin, Y.W.; Lee, S.Y.; Jung, J.E. Characteristics of color optical shutter with dye–doped polymer network liquid crystal. Opt. Lett. 2011, 36, 754–756. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ramsey, R.A.; Chen, Y.; Wu, S.T. Submillisecond–response sheared polymer network liquid crystals for display applications. J. Disp. Technol. 2012, 8, 87–90. [Google Scholar] [CrossRef]
- Yu, B.H.; Huh, J.W.; Kim, K.H.; Yoon, T.H. Light shutter using dichroic–dye–doped long–pitch cholesteric liquid crystals. Opt. Express 2013, 21, 29332–29337. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Huh, J.W.; Yoon, T.H. Fast–switching initially–transparent liquid crystal light shutter with crossed patterned electrodes. AIP Adv. 2015, 5, 047118. [Google Scholar] [CrossRef]
- Fratalocchi, A.; Asquini, R.; Assanto, G. Integrated electro–optic switch in liquid crystals. Opt. Express 2005, 13, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Goebbert, C.; Nonninger, R.; Aegerter, M.A.; Schmidt, H. Wet chemical deposition of ATO and ITO coatings using crystalline nanoparticles redispersable in solutions. Thin Solid Film. 1999, 351, 79–84. [Google Scholar] [CrossRef]
- Shigesato, Y.; Koshi–Ishi, R.; Kawashima, T.; Ohsako, J. Early stages of ITO deposition on glass or polymer substrates. Vacuum 2000, 59, 614–621. [Google Scholar] [CrossRef]
- Chou, T.R.; Chen, S.H.; Chiang, Y.T.; Lin, Y.T.; Chao, C.Y. Highly conductive PEDOT: PSS films by post–treatment with dimethyl sulfoxide for ITO–free liquid crystal display. J. Mater. Chem. C 2015, 3, 3760–3766. [Google Scholar] [CrossRef]
- Ibragimov, T.D.; Hashimov, A.M.; Ibragimov, G.B.; Rzayev, R.M. Decrease in negative dielectric anisotropy and electric conductivity of 4–methoxybenzylidene–4′–butylaniline doped with fullerenes C60. Fuller. Nanotub. Carbon Nanostructures 2021, 29, 951–955. [Google Scholar] [CrossRef]
- Dumitrascu, I.; Dumitrascu, L.; Dorohoi, D.O. The influence of the external electric field on the birefringence of nematic liquid crystal layers. J. Optoelectron. Adv. Mater. 2006, 8, 1028. [Google Scholar]
- Kneppe, H.; Schneider, F. Determination of the viscosity coefficients of the liquid crystal MBBA. Mol. Cryst. Liq. Cryst. 1981, 65, 23–37. [Google Scholar] [CrossRef]
- Huh, J.W.; Oh, S.W.; Seo, J.H.; Sohn, H.J.; Yoon, T.H. Filtering of yellow light in a liquid–crystal light shutter for higher color contrast and reduced glare. J. Mol. Liq. 2021, 327, 114846. [Google Scholar] [CrossRef]
- Du, X.; Li, Y.; Liu, Y.; Wang, F.; Luo, D. Electrically switchable bistable dual frequency liquid crystal light shutter with hyper–reflection in near infrared. Liq. Cryst. 2019, 46, 1727–1733. [Google Scholar] [CrossRef]
- Oh, S.W.; Baek, J.M.; Heo, J.; Yoon, T.H. Dye–doped cholesteric liquid crystal light shutter with a polymer–dispersed liquid crystal film. Dye. Pigment. 2016, 134, 36–40. [Google Scholar] [CrossRef]
- De Filpo, G.; Formoso, P.; Mashin, A.I.; Nezhdanov, A.; Mochalov, L.; Nicoletta, F.P. A new reverse mode light shutter from silica–dispersed liquid crystals. Liq. Cryst. 2018, 45, 721–727. [Google Scholar] [CrossRef]
- Emek, M.; Besli, N.; Yildirim, A.; Yilmaz, S. Optical properties of nematic liquid crystal (C21H27NO2S) under AC/DC electric fields. Can. J. Phys. 2009, 87, 293–298. [Google Scholar] [CrossRef]




| LC Type | Working Range | Deactivated Transmittance (%) | Activated Transmittance (%) | Reference |
|---|---|---|---|---|
| NLC (This article) | 515 nm to 1100 nm | >60% | >5% | NA |
| Dye–doped LC | 590 nm | 50.2% | 5.1% | [31] |
| Cholesteric (Ch) LC | 750 nm to 1120 nm | >60% | 35% to 1.04% | [32] |
| Dye–doped ChLC | 1000 nm/2000 nm | 0.795%/1.94% | 72.7%/34.7% | [33] |
| polymer–networked LC | 400 nm to 650 nm | 65.5% | 2.3% | [23] |
| Dye–doped NLC | 400 nm to 650 nm | 84% (35 wt%) | 10% (35 wt%) | [34] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Y.; Li, B.; Yao, C.-Y.; Yang, W.; Fan, Q.H.; Qiu, Z.; Li, W. Tunable, Low–Cost, Multi–Channel, Broadband Liquid Crystal Shutter for Fluorescence Imaging in Widefield Microscopy. Micromachines 2022, 13, 1310. https://doi.org/10.3390/mi13081310
Gong Y, Li B, Yao C-Y, Yang W, Fan QH, Qiu Z, Li W. Tunable, Low–Cost, Multi–Channel, Broadband Liquid Crystal Shutter for Fluorescence Imaging in Widefield Microscopy. Micromachines. 2022; 13(8):1310. https://doi.org/10.3390/mi13081310
Chicago/Turabian StyleGong, Yan, Bo Li, Cheng-You Yao, Weiyang Yang, Qi Hua Fan, Zhen Qiu, and Wen Li. 2022. "Tunable, Low–Cost, Multi–Channel, Broadband Liquid Crystal Shutter for Fluorescence Imaging in Widefield Microscopy" Micromachines 13, no. 8: 1310. https://doi.org/10.3390/mi13081310
APA StyleGong, Y., Li, B., Yao, C.-Y., Yang, W., Fan, Q. H., Qiu, Z., & Li, W. (2022). Tunable, Low–Cost, Multi–Channel, Broadband Liquid Crystal Shutter for Fluorescence Imaging in Widefield Microscopy. Micromachines, 13(8), 1310. https://doi.org/10.3390/mi13081310







