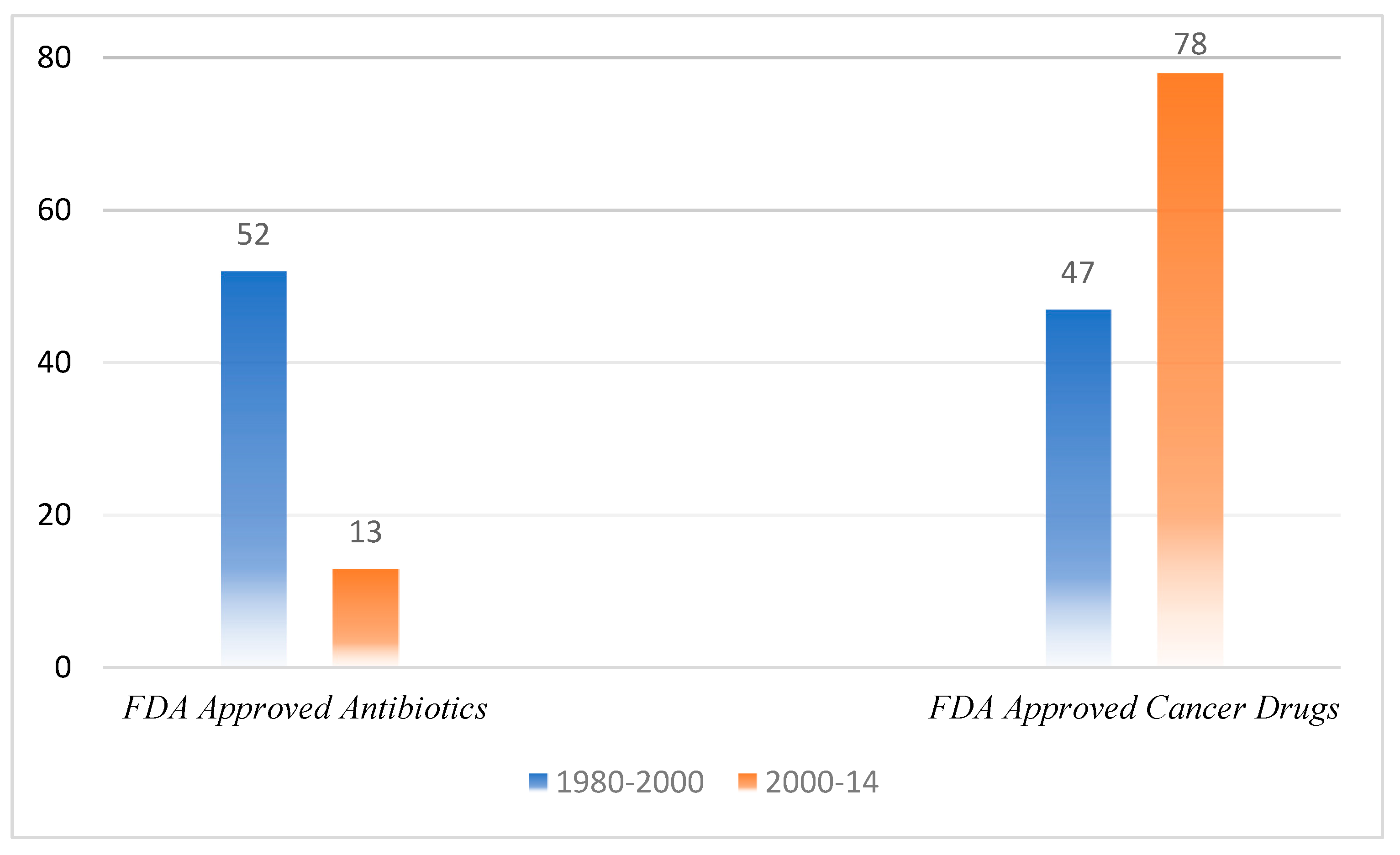
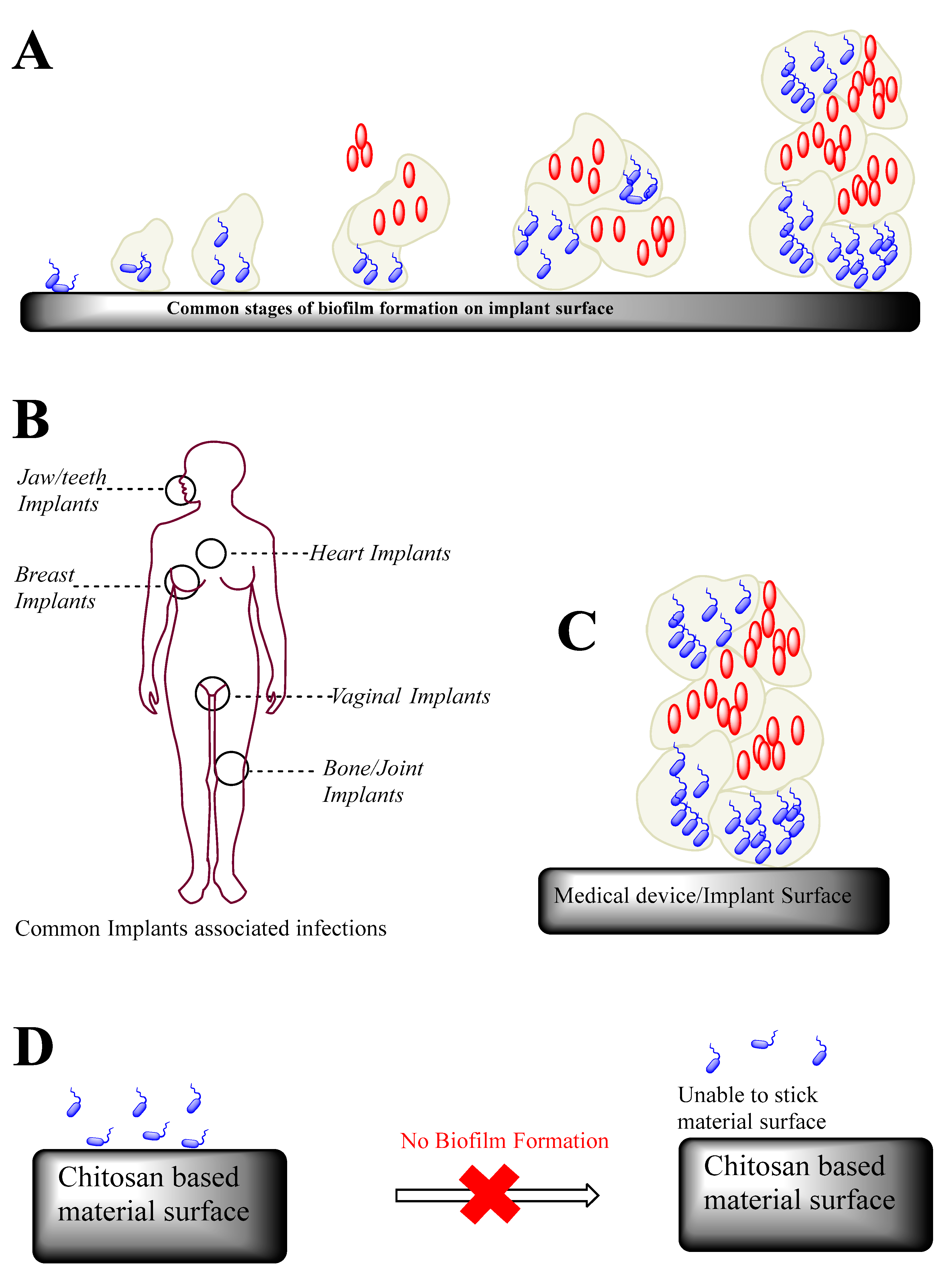
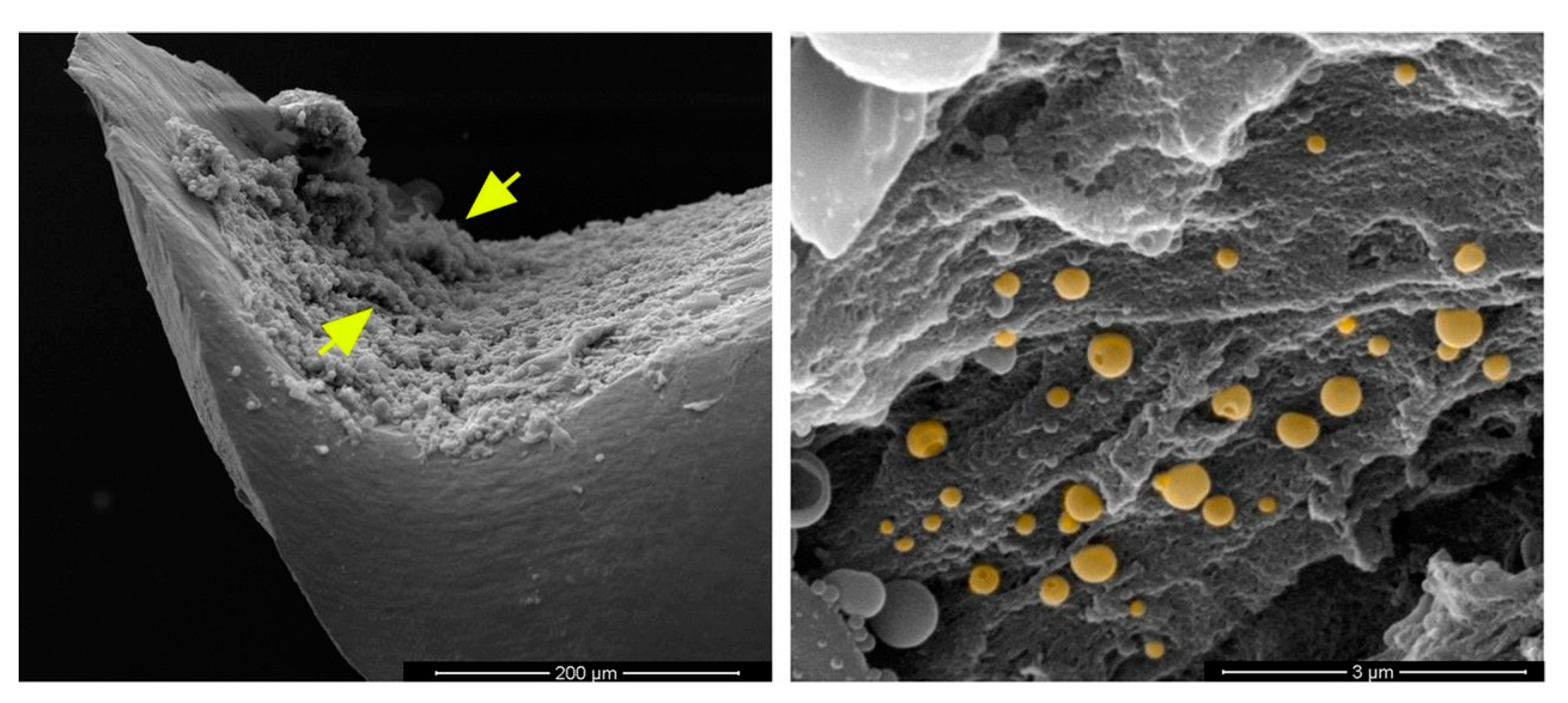
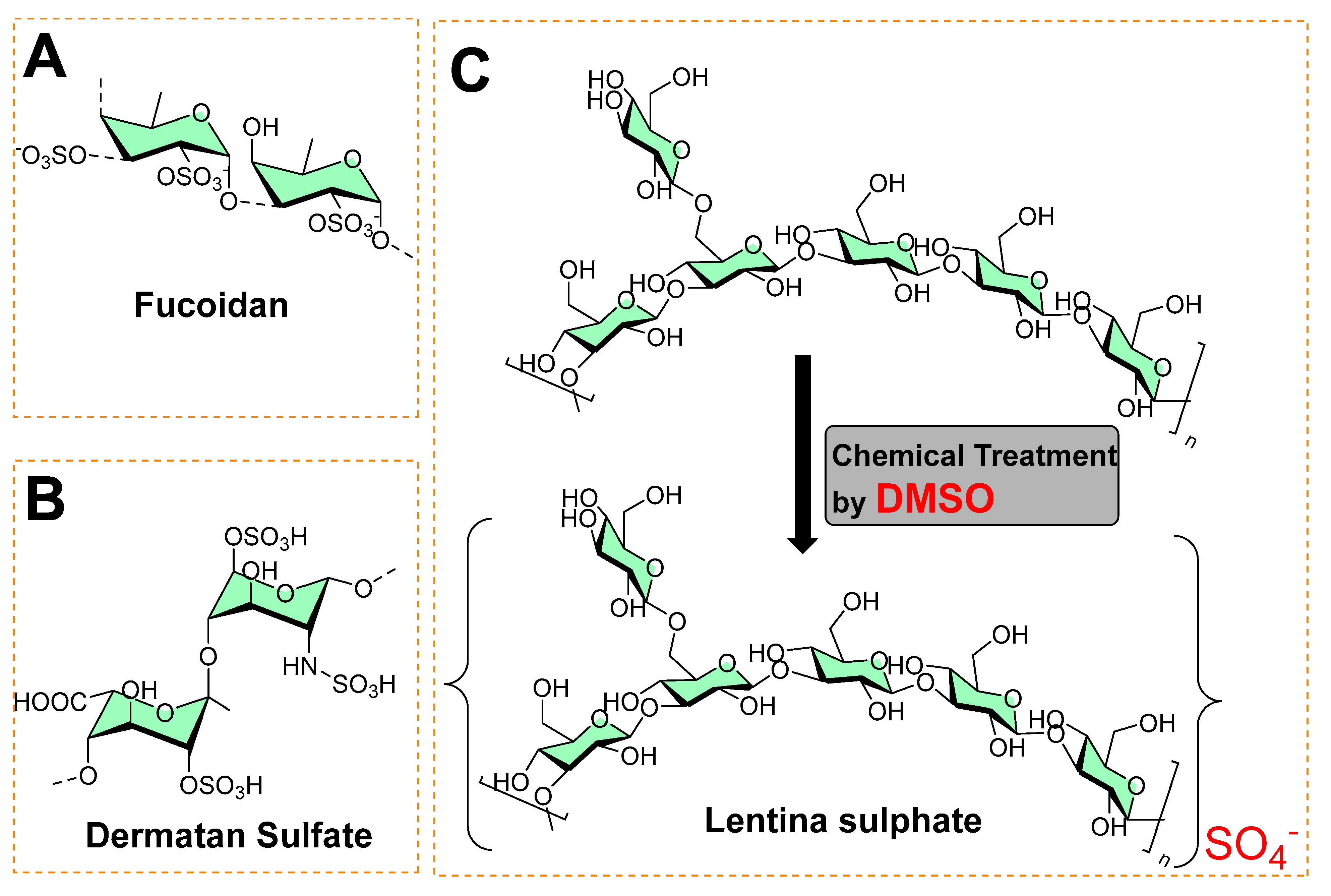
Phytochemicals always have been a source of medicinally active compounds [
48], such as anticancer indole alkaloids and antimalarial cassiarins [
49]. However, phytochemicals lack essential structural features, making their physicochemical profile challenging in terms of compliance with the “Lipinski rule of 5” (which evaluates the druggability) [
50]. In addition, most essential oils have typical hydrophobicity, restricting their direct biomedical use, and leading to the development of various formulations in recent years. One such approach is encapsulation at the microscale [
51] and nanoscale [
52]. As is typical, polymeric material is used to encapsulate these phytochemicals, and successful integration proportionally depends on particle size; therefore, nanoscale formulations were preferred over microscale formulations.
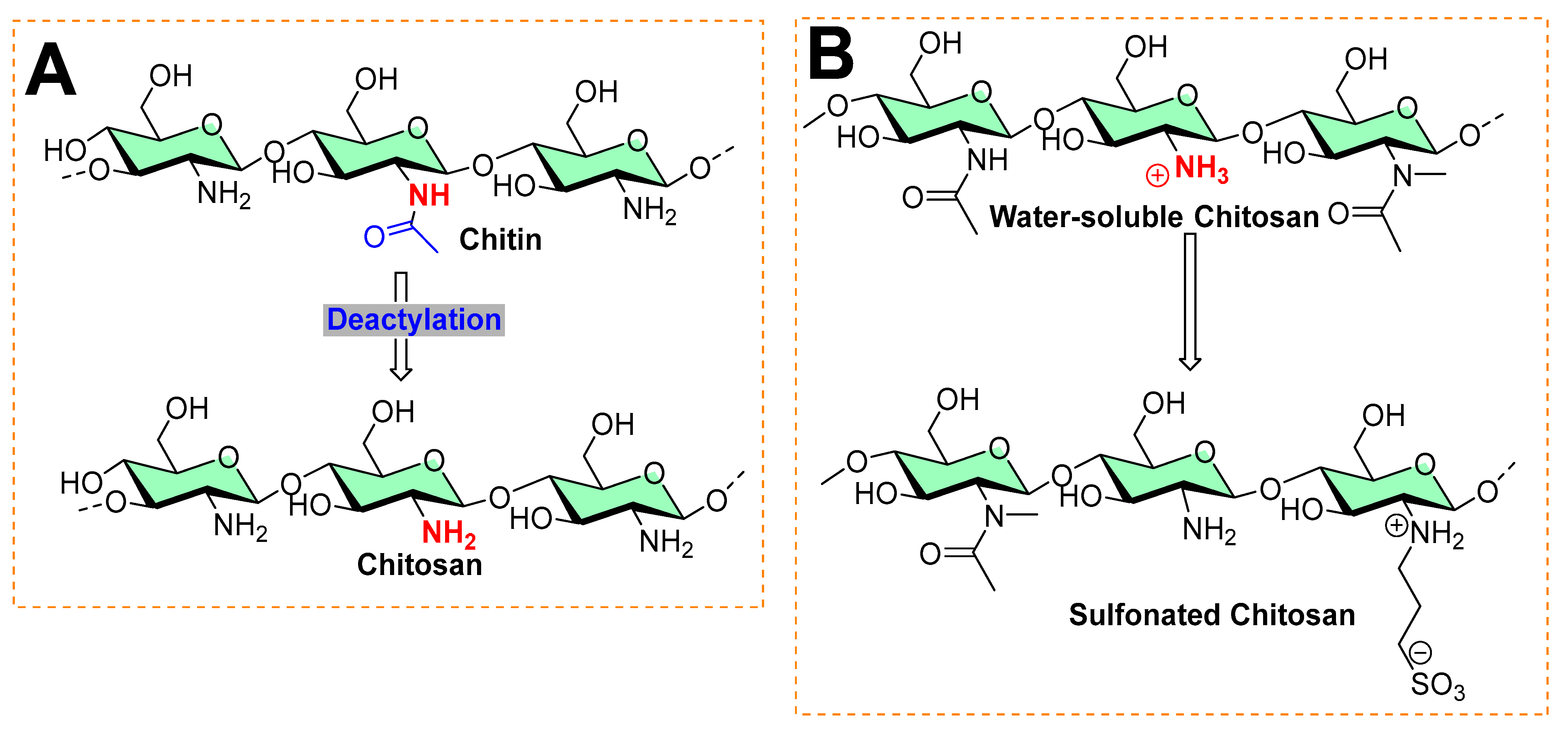
Various methods used for chitosan nanoparticles, which can be categorized based on production method and matric composition, are shown in
Table 1. The ionic gelation method is commonly practiced for the nanoencapsulation of functional compounds (essential oils, food, cosmetics, drugs, etc.). Chitosan has become the most obvious choice of many researchers because of its biodegradable nature, easy accessibility, and availability of surface functional groups (amino groups). Because of these amino groups, chitosan can be cross-linked easily to other functional materials. Key choices of crosslinkers are TPP (sodium tripolyphosphate) and HMP (hydroxymethyl melamine prepolymer). Various factors come into play to achieve effective chitosan-based nanoencapsulation, such as pH, the solvent used, mixing sequence, concentration, and molar ratios. However, the most critical aspect for succeeding in chitosan-based nanoencapsulation is measured by encapsulation efficiency (EE) and loading capacity (LC). By definition, encapsulation efficiency is measured in percentage, which indicates how much fractional amount of functional compound (essential oil, drugs, edible compounds, etc.) is entrapped (encapsulated) into the nanoparticles (called nanoencapsulation) or micelles, whereas loading capacity (LC) reflects a fraction of the amount of encapsulated functional material over the total weight of nanomaterial support used.
5.1. Ocimum basilicum L. Essential Oil
Yu et al. from Dalian Minzu University (Dalian, China) encapsulated the
Ocimum basilicum L. essential oil (BEO) into chitosan nanoparticles (CSNPs) by emulsion and ionic gelation [
93]. To their rationality, the use of chitosan serves as a carrier and improves the formulation’s biological properties (biocompatibility, safety, and degradability). Two steps (emulsification and ionic gelation) were used to prepare the BEO-loaded chitosan nanoparticles [
94]. The choice of
Escherichia coli and
Staphylococcus aureus to measure the antibiofilm activity was evident based on their high biofilm-forming tendency and higher proportional population in biofilm microcolonies. The particle size, polydispersity index (PDI), and zeta potential of BEO-loaded CSNPs were accessed through the dynamic light scattering (DLS) method.
The GC–MS data showed seventeen compounds that accounted for 95.5% of the basil essential oil, where eugenol (48.32%) and caryophyllene (26.26%) were found to be prominent ones, and the remaining ones were less than 6%. The CSNPs with no BEO had a particle size of 198.7 nm. The authors made three combinations of BEO with CSNPs, where they chose a ratio (CSNP:BEO = 1:0.5; zeta potential = 30.7 ± 0.47 mV) based on the conditional requirement of particle surface charge (zeta potential above 30 mV). A particle surface charge over CSNPs is vital to achieving specific colloidal solution characteristics (aggregation, dispersion, and flocculation) and can directly affect the bioavailability of the encapsulated essential oils [
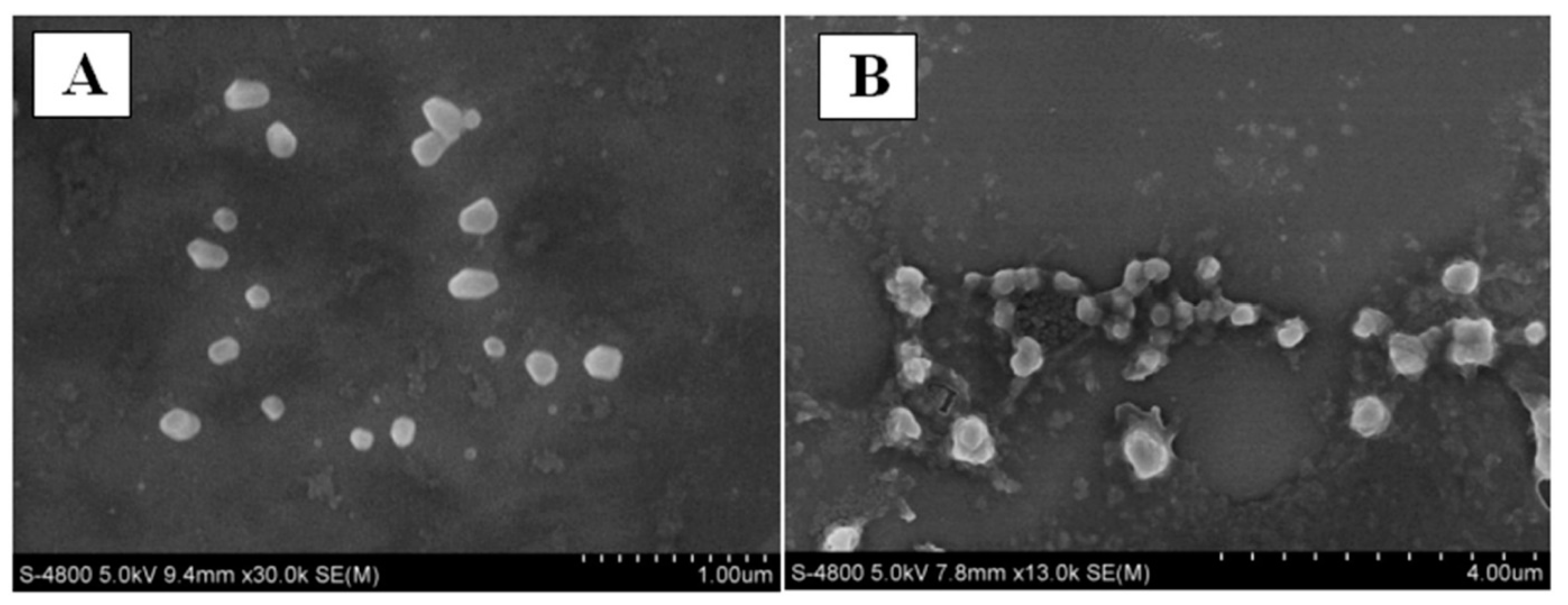
93]. Furthermore, the nanoscale characteristic was compared using scanning electron microscopy (SEM) images, as shown in
Figure 9.
Ultraviolet–visible (UV–Vis) spectroscopy was used to estimate the encapsulation efficiency and loading capacity. The authors chose the CSNPs loaded with BEO (1:0.5), as it exhibited excellent encapsulation efficiency (75.13 ± 0.09%) and loading capacity (18.63 ± 0.02%).
E. coli and
S. aureus were used to evaluate the antibacterial activity of chitosan powder, unloaded CSNPs, and BEO-loaded CSNPs. In comparison, unloaded CSNPs showed improvement (
E. coli = 46.67 ± 4.71%;
S. aureus = 25.76 ± 6.88%) with BEO-loaded CSNPs (
E. coli = 78.33 ± 12.96%;
S. aureus = 80.81 ± 19.99%). To understand the antibacterial mechanism of BEO-loaded CSNPs, a DNA leakage study on
E. coli and
S. aureus was performed. The change in DNA leakage posttreatment with BEO–CSNPs for 8 h increased by 60.76% against
E. coli and 50.88% against
S. aureus [
93]. While some sugars (such as iminosugars) are mechanistically found as metabolic inhibitors [
95].
5.2. Mandarin Essential Oil
Wu et al., from Shaanxi Normal University (Shaanxi, China), encapsulated mandarin essential oil (MEO) (
Citrus reticulata) with CSNPs to improve antibacterial properties and prolong pork preservation [
96]. Various loading capacities of MEO were used with CSNPs with the following CSNP:MEO ratios: 1:0, represented as CSs-C; 1:0.2, represented as CSs-L; 1:0.5, represented as CSs-M; and 1:1, represented as CSs-H. The encapsulation efficiency (EE) for MEO-CSNPs was found to be 67.32–82.35%, the mean particle size was 131.3 nm–161.9 nm, and the zeta potential was 30 mV.
The mean particle size is correlated with essential oil type, (TPP) concentration, and wall material [
97]. Zeta potentials evaluate the aggregation/dispersion between particles. As in all cases, zeta potential exceeded 30 mV, which suggested that a sufficient electrostatic repulsion among the droplets stabilized them in the MEO-loaded CSNPs [
98]. However, zeta potential and particle size directly affect the antibacterial activity of nanoencapsulation and influence its interaction with multiple molecular sites [
99]. Hence, these results demonstrate MEO-loaded CSNPs’ stability in the emulsion state, although the addition of MEO altered the nanoscale size of CSNPs.
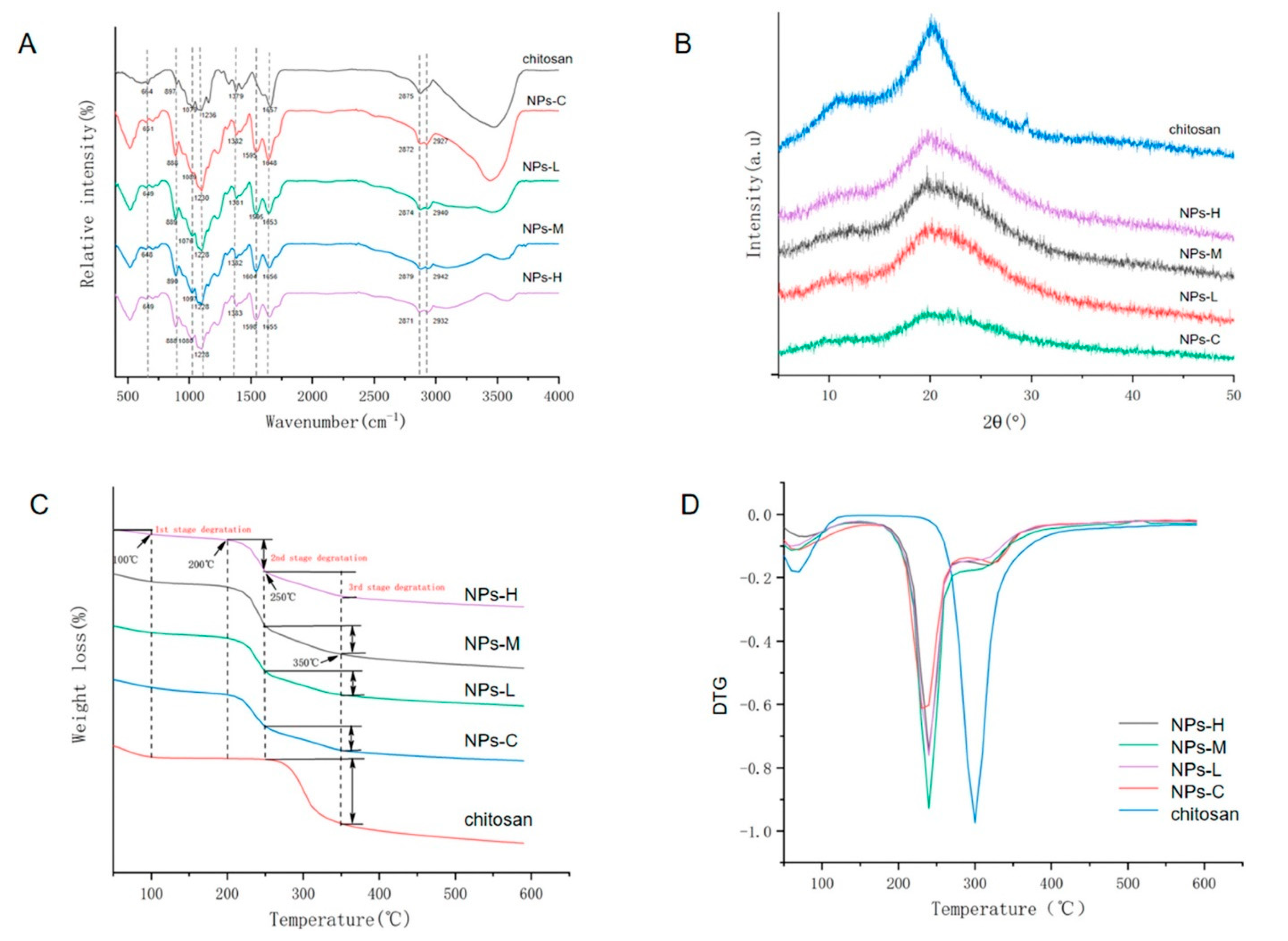
All FTIR spectra show regions affiliated with the functional groups 664 cm
−1 (pyranose ring of chitosan), 1079 cm
−1 (C–N stretching), 1379 cm
−1 (C–O–H, H–C–H), and 2875 cm
−1 (C–H stretching), as shown in
Figure 10A. The appearance of the 1230 cm
−1 (–P=O stretching) peak in the spectra indicates the TPP cross-linking in CSNP:MEO ratios, whereas XRD analysis exhibits two peaks (at 20° and 29°) representing the crystallinity of chitosan; however, the peak intensity faded in samples loaded with MEO denoting the complex structural changes in these ratios. The 1:1 ratio of chitosan:MEO shows the most potent antibacterial activity against
Staphylococcus aureus and
Escherichia coli. Furthermore, the authors evaluated the effect of such nanoencapsulation on bacterial cell morphology. It was found out that increases in MEO loading with CSNPs damage the bacterial cell morphology (
Staphylococcus aureus and
Escherichia coli) and exhibit irregular, deformed, and incomplete structures. However, the severity of damage was noticed with
Staphylococcus aureus compared to
Escherichia coli, reflecting the differences in their cellular makeup.
Escherichia coli is Gram-negative, whereas
Staphylococcus aureus is Gram-positive, reflecting physicochemical property changes because of the macromolecular change of the distinctive membrane. The authors further evaluated the effect of MEO-loaded CSNPs on biofilm formation. In this test, the initial stage of adhesion of bacteria was evaluated, as it is difficult to remove the bacteria once they achieve this stage. Increased loadings of MEO:CSNPs showed potent activity against the initial adhesion stage. Environmental scanning electron microscopy (ESEM) analysis was used to compare the untreated and treated groups with MEO:CSNPs. In this analysis, the untreated group showed a typical mature biofilm, where aggregated bacteria were found in the polysaccharide structure of biofilm (higher thickness), while treated groups were found with lower thickness, showing less aggregation of bacteria. The plate counting method was further tested for MEO:CSNPs for their activity against the mature biofilm. An efficient destructive percentage of biofilm was observed for a 1:1 ratio of MEO:CSNPs and a notable reduction in the cell population.
In another study, a collaboration with the Competence Center on Agro-Food Productions, and Department of Industrial Engineering, University of Salerno (Italy) and the Canadian Irradiation Center, INRS—Institut Armand-Frappier, Institute of Nutraceutical and Functional Foods Québec, Canada modified chitosan containing a 0.05% mandarin essential oil nanoemulsion [
100]. The antibacterial testing of samples was performed with γ-irradiation, UV-C, and ozone-treated water treatments. The combined coating and γ-irradiation showed a synergistic effect on microbial growth (3.3 log CFU/g), while a 3 log CFU/g reduction of the initial
Listeria innocua population was observed when combined with UV-C irradiation. However, no antimicrobial effect of the combination with ozone-treated water was observed [
100].
5.3. Carum copticum Essential Oil
Esmaeili and Asgari from Islamic Azad University (Tehran, Iran) applied the emulsion ionic gelation method on CSNPs to encapsulate
Carum copticum essential oil (CEO) [
101]. To explore the encapsulation efficiency (EE) and loading capacity (LC), various concentrations of TPP and HMP as cross-linkers were used with chitosan. Based on EE and LC values, the authors chose a sample with a 1:1 mass ratio of chitosan to CEO and a TPP concentration of 0.5% (
w/
v).
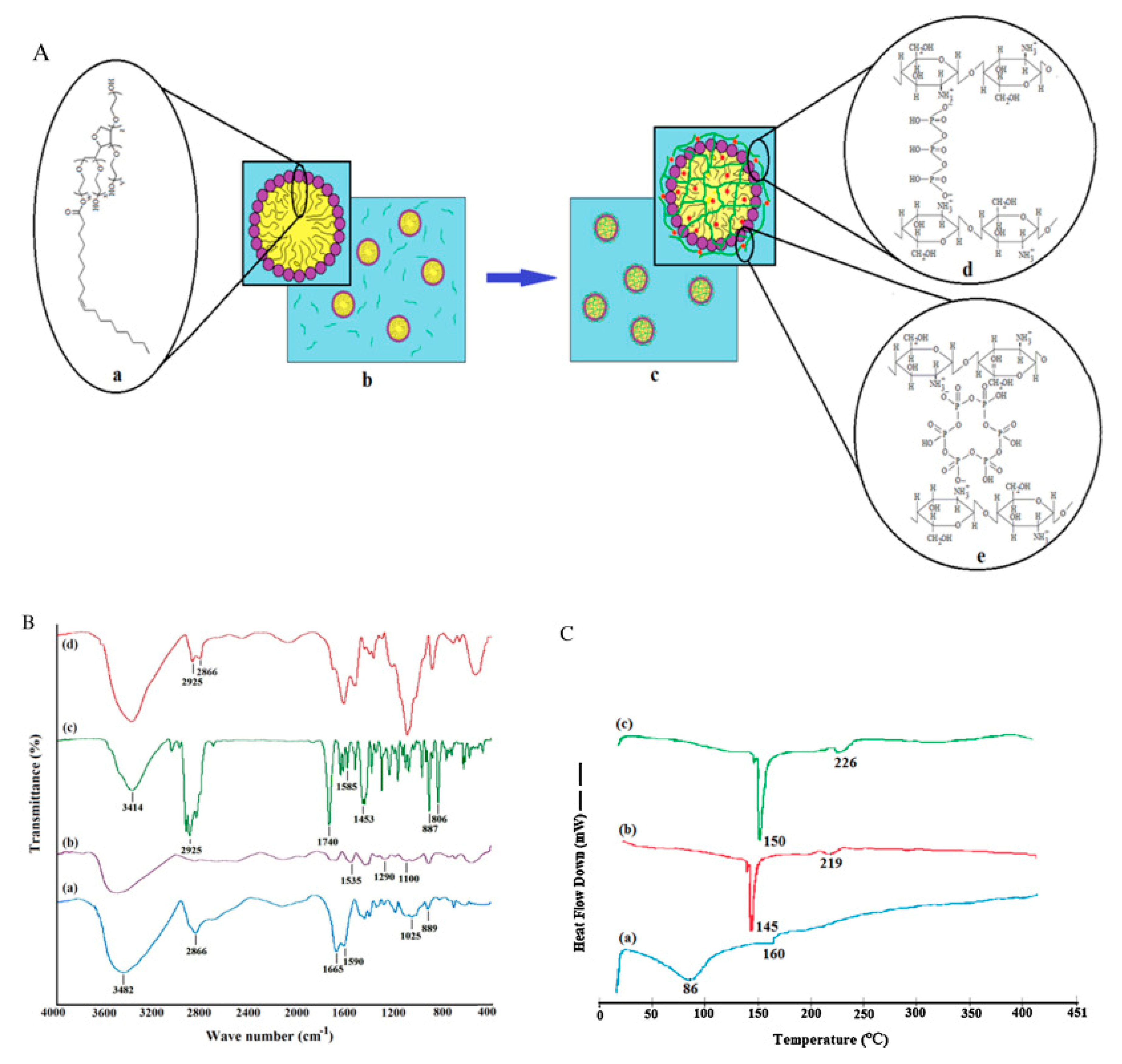
FTIR was used to characterize chitosan–TPP nanoparticles (
Figure 11B), as shown in
Table 2. The peak of amide-II (single bond NH
2 bending) shifted from 1590 to 1535 cm
−1, and new peaks appeared around 1100–1290 cm
−1 (P–O and P=O) showing the presence of phosphorus of TPP from CEO-loaded chitosan–TTP nanoparticles. Secondly, a pronounced sharpening of the C–H stretching region in FTIR further support CEO integration in chitosan nanoparticles. These changes in FTIR spectra assure the CEO encapsulation into the chitosan nanoparticles.
Thermal analysis by differential scanning calorimetry (DSC) was used to study the thermal behavior of pure CEO, chitosan nanoparticles, and CEO-loaded chitosan nanoparticles. As anticipated from the previous literature [
103], the thermogram of chitosan showed an endothermic peak (at 75 °C) related to the loss of adsorbed water (associated with hydrophilic groups of polymer), along with an exothermic peak (at 311 °C) reflecting its molecular degradation (dehydration of the anhydro-glycosidic ring, depolymerization and chemical decomposition of monosaccharide units). The thermogram of CEO exhibited two endothermic peaks (in the region of 35–128 °C) and at 160 °C, which can be correlated to its evaporation and the chemical degradation of low boiling-point components (
Figure 11C(a)) [
104,
105]. Unlike with chitosan, distinctive thermal behavior was noticed in CEO-loaded CSNPs, where exothermic peaks of 145 and 219 °C of CSNP were shifted to 150 and 226 °C (
Figure 11C(b,c)). Further conclusions can be drawn from this thermal behavior: (a) the recorded change reflected the change in molecular assembly CEO-loaded CSNPs, and (b) overall improved the thermal stability of these materials. These changes were also in parallel with the previously reported literature [
105,
106].
The SEM measurements of CSNPs and CEO-loaded CSNPs showed spherical shaped particles with an average diameter of 30–80 nm. However, a significant difference in the mean particle size and size distribution of CSNPs and CEO-loaded CSNPs by the DLS technique was found (mean diameter of CSNPs = 954 nm; average diameter of CEO-loaded CSNPs = 236.0–721.0 nm). This profound difference in the diameter indicated the method sensitivity, as the distinctive nature of SEM and DLS measurements in an aqueous solution could lead to swelling and aggregation of the CSNPs during dispersion in water, while a reduction in swelling and/or aggregation of CEO-loaded CSNPs in an aqueous solution compared to the CSNPs in an aqueous solution might be due to the hydrophobicity of CEO molecules encapsulated inside/on the nanoparticles. Similar observations were also reported in the literature [
107].
The in vitro release study of CEO from CEO-loaded CSNPs was evaluated with varying pH using buffers of pH 3 and 5 (acetate buffer), pH 7.4 (phosphate buffer), and pH 10 (phosphate buffer) for 4 days. The in vitro release studies of CEO were conducted from the prepared sample with a mass ratio of chitosan to CEO of 1:1 and a TPP concentration of 0.5% (
w/
v). Generally, the release of encapsulated compound uses some or all of the following mechanism: diffusion, desorption, disintegration, and surface erosion [
94]. However, diffusion followed by polymer matrix degradation is commonly observed in CSNP-based encapsulation [
94,
102]. A biphasic process where an initial burst (for 5 h) was followed by a slow declining release at all pH values was recorded until 24 h. The observed initial burst could be related to the facile release of CEO molecules either loosely bound or superficially encapsulated [
94]. After 24 h, a steady release was observed in all the pH cases until 72 h. After 72 h, a significant decrease was noted in release levels, as a plateau stage was achieved. As in previous reports [
108] of pH affecting the in vitro release of the encapsulated materials or compounds from the nanoparticles, the authors noticed that the CEO released in lower pH (3 and 5) buffers was significantly higher (
p < 0.05) than that in the saline (pH 7.4) and basic buffer (pH 10). The observation that having a higher diffusion rate of CEO in an acidic medium could be correlated with the strength of ionic repulsions of protonated NH
2 groups of chitosan with each other contributed to the partial dissolution and swelling of CSNPs [
106,
109]. Furthermore, as the acidity of the solution increases (pH 3 buffer), a proportional rise in the swelling will also be observed; therefore, a greater CEO release was observed in pH = 3 buffer than in pH = 5 buffer. Such observations were also reported with an in vitro release study of curcumin-loaded dextran sulphate–chitosan nanoparticles systems as well [
109]. As anticipated, the swelling property of chitosan decreased with an increase in alkalinity of the solution, and a lower released amount of CEO was expected.
Contrary to the previous statement, the in vitro release of CEO found at pH = 10 buffer was found to be significantly higher (
p < 0.05) than that at pH = 7.4 (saline buffer), reasonably due to the deprotonation of NH
2 groups of chitosan and therefore a decline in strength of ionic repulsion [
107]. Comparatively, more release of essential oil (CEO) was observed in acidic buffers than in alkaline buffers, supporting chitosan as a suitable material scaffold to control the release of the essential oils. In conclusion, the in vitro release demonstrates the role of chitosan’s physicochemical parameters, which affect its material properties as a polymer.
The antibacterial study of CSNPs and CEO-encapsulated CSNPs was studied by the agar disk diffusion method on
Staphylococcus aureus,
Staphylococcus epidermidis,
Bacillus cereus,
Escherichia coli,
Salmonella typhimurium, and
Proteus vulgaris, as summarized in
Table 3. The opted controls (phosphate buffer saline and dimethyl sulphoxide, negative control) showed no antibacterial activity. The non-encapsulated CEO showed antibacterial activity against all the strains, as suggested in the previous study [
110] (as shown in
Table 3). The antibacterial activity of CEO was due to the presence of critical essential oils (thymol, γ-terpinene, and ρ-cymene) [
111]. These essential oils cross the bacterial membrane and alter the cytoplasm’s pH and equilibrium of ionic concentration, leading to their antibacterial activity [
112]. Compared with non-encapsulated CEO or chitosan nanoparticles (CSNPs), CEO-loaded CSNPs exhibited improved antibacterial activity, further justifying the suitability of chitosan-based material support for essential oil nanoencapsulation. Furthermore, these observations suggested a synergistic antibacterial mechanism, where positively charged pronated NH2 terminals of chitosan could easily form complexations with the negatively charged components of bacterial membrane, which leads to the swelling of the CSNP encapsulation and release of essential oils that further damage the bacterial cell [
113].
5.6. Nanoencapsulation of Melissa officinalis L.
The Romanian research team extended the scope of encapsulating polymeric supports (HCB-CS or HCR-CS) to lemon balm essential oil (
Melissa officinalis L.; MOEO) to form nanofibers. The FTIR studies provided more insights into the chemical composition, as shown in
Figure 13. In this figure, researchers compared the various possible versions of nanoencapsulation with/without loading of AGEO or MOEO.
Based on observations from
Figure 13, the following conclusions can be drawn: (a) the amide-I functionality of chitosan (stretching vibrations of C=O groups, ν = 1639 cm
−1) was shifted for HCB-CS (ν = 1663 cm
−1) and HCR-CS (ν = 1635 cm
−1); (b) amide-II (ν = 1545 cm
−1) associated with the secondary structure in chitosan was not found in collagen–chitosan mixtures and encapsulated essential oil samples, providing evidence of participation of chitosan’s –NH
2 and –OH groups in chemical reactions [
117]; and (c) the appearance of a peak (ν = 1635 cm
−1) in encapsulated samples suggested the presence of essential oils (AGEO and MOEO) within the electrospun collagen–chitosan nanofiber complex.
Antimicrobial testing showed a broader spectrum for encapsulated samples than unloaded essential oils (L, D&L vs. HCB-CS/L, HCB-CS/D&L, HCR-CS/L, HCR-CS/D&L), as shown in
Table 5.
The in vivo studies on nanofiber samples were performed on 3-month-old white Swiss adult mice (25–30 g in weight with uniform sex distribution). Animals were supervised for a week and anesthetized using an intraperitoneal route with ketamine (50 mg/kg) and xylazine (10 mg/kg). Later, the dorsal on the left side was shaved, and a superficial incision (1 cm parallel to backbone) was made. The nanofiber textile material, as shown in
Figure 14 (size: 1 × 0.5 cm), was fixed on the incision area, while a dry sterile patch was used for the control group animals.
After animals were fed and watered, personal hygiene was maintained until the 7th day. On the 7th day, patches were removed, and the incision area was evaluated under a microscope. Both animal groups (treated and control) did not show any appearance of inflammation, and the incision area was scarred. Furthermore, no hematology differences (%) were recorded for the treated group compared to the control group, as shown in
Table 6. The obtained in vivo results indicate reasonable biocompatibility of encapsulated essential oils within chitosan–collagen polymeric material as a putative biomedical wound dressing.
5.7. Nanoencapsulation of Peppermint Oil and Green Tea Oil
A collaboration of Yue and co-workers from the Jiangxi University of Chinese Medicine (Nanchang, China) and the Department of Pharmacy at the 908th Hospital of People’s Liberation Army (Nanchang, China) utilized chitosan-based silica nanoparticles (CS–SiNPs) to encapsulate the peppermint oil.
Mean particle size, zeta potential, polydispersity index (PDI), and contact angles for the various ratios of chitosan within chitosan-decorated silica nanoparticles samples were estimated. The mean particle size of the silica nanoparticles (SiNPs) was 116.86 nm. However, the mean particle sizes of chitosan–SiNPs (CS–SiNPs) proportionally increased (118.12–152.5 nm) with increases in chitosan concentration, which could be due to electrostatic adsorption of chitosan over the silica nanoparticle surface. Furthermore, a significant rise in zeta potential () with the addition of chitosan from −41.8 mV (SiNPs) to 42.5 mV (SiNPs with 5% of chitosan) was also observed. The alteration of zeta potential was anticipated, as numerous hydroxyl groups on silica nanoparticles possess negative charges after deprotonation [
118]. In comparison, the free amino groups of chitosan are generally protonated and therefore possess positive charges [
119,
120]. These opposite charges on the silica nanoparticles and chitosan facilitate their homogenized adsorption.
The 1% chitosan with silica nanoparticles had a regular sphere-shaped morphology. Scanning transmission electron microscopy (STEM) coupled with energy dispersive X-ray spectroscopy (EDS) helped in mapping the elemental composition (C, N, O, and Si), suggesting a uniform spherical distribution of carbon and nitrogen from chitosan. This observation suggested an absorption of chitosan onto the negatively charged surface of silica nanoparticles and within the agreement of zeta potential results.
The authors measured the samples for their wettability, as it was required to prepare a stable Pickering emulsion. As a general statement about the wettability, “An appropriate wettability of solid particles facilitates their absorption at the oil/water interface and provides enough steric hindrance that abolishes the droplet coalescence of Pickering emulsions” [
121]. The contact angle (39.4°) of SiNPs indicated its excessive hydrophilicity and an obstacle in using it for a stable Pickering emulsion [
122]. However, chitosan (from 1 to 5%
w/
w relative silica weight%) loaded to SiNPs showed an increase in contact angle from 41.1° to 67.4°, implying the significance of chitosan loading with SiNPs as an essential component for achieving a stable Pickering emulsion. A contact angle of 90° is considered optimum to enable absorption on the oil/water interface and to have steric hindrance against the aggregation of oil droplets; therefore, the highest contact angle of chitosan-loaded SiNPs (5%
w/
w relative to silica weight%) was selected for preparing a Pickering emulsion and investigated for further chemical characterization [
123].
The chemical characterization was performed using XRD and FTIR analyses. The SiNPs demonstrated characteristic broad peaks at 2θ of 22°, which was in agreement with the previous literature [
124]. Similarly, chitosan demonstrated broad characteristic peaks at 2θ of 10.6 and 23.2°. However, a mixture of chitosan and SiNPs, when compared with chitosan-loaded SiNPs, showed similar characteristic peaks, except for a missing peak at 2θ of 11.6° for chitosan-loaded SiNPs [
123]. In FTIR, chitosan powder exhibited typical peaks, such as stretching vibrations for C–H (2939.83 cm
−1), –OH bond (3431.15 cm
−1), and C=O of amide-I (1633.94 cm
−1); a bending vibration of N–H of primary amine (1528.97 cm
−1) [
125]; CH
2 bending (1383.11) and CH
3 symmetrical deformations (1321.56 cm
−1); and asymmetric stretching of C–O–C (1151.90 cm
−1) [
126]. For silica, the characteristic broad peaks related to O–H stretching of silanol groups (3446.53 and 1634.79 cm
−1) [
127], Si–O–Si stretching (807.02 cm
−1), Si–O–Si bending (469.87 cm
−1) [
128,
129], and vibrations of siloxane of (SiO)
n groups (1112 cm
−1) [
130] were observed. However, the presence of peaks for a mixture of chitosan and unloaded SiNPs for silica (3449.10 cm
−1, 1635.60 cm
−1, 1102.30 cm
−1, 805.69 cm
−1, and 470.06 cm
−1) and chitosan (2650.63 cm
−1 and 1489.26 cm
−1), and a merging of certain peaks (383.11 cm
−1 and 1321.56 cm
−1) confirmed their presence as components in the composition. However, the appearance or disappearance of no peaks with chitosan-loaded SiNPs were observed when compared to the mixture of chitosan and unloaded SiNPs, indicated a possible explanation that the interaction within chitosan-loaded SiNPs are of an electrostatic nature [
131].
The 5% chitosan-loaded SiNPs (CS–SiNPs) were used in different proportions to prepare peppermint oil Pickering emulsions (PO-PE). The estimated particle sizes (D
50) for 0.5% and 1% of CS–SiNPs with PO-PE were found to be large enough (6.61 ± 0.31 μm and 5.42 ± 0.25 μm), making them not ideal for stabilizing Pickering emulsions, as they were susceptible to creaming after 24 h. With further increases in the CS–SiNPs proportions (1.5 and 2% relative to PO-PE), a decrease in particle size (D
50) was observed. The 2% CS–SiNPs/PO-PE exhibited a particle size (3.73 ± 0.213 μm) that did not show creaming during storage, indicating an efficient absorption of CS–SiNPs onto the oil–water interface of Pickering emulsions droplets. Furthermore, a sphere-shaped oil droplet morphology was observed by confocal laser scanning microscopy and cryo-SEM, indicating peppermint oil encapsulation into the core of Pickering emulsions. Additionally, these images showed silica nanoparticles at the surface of droplets, leading to speculation that CS–SiNPs make a steric barrier shell and therefore improve the stability of PO-PE [
123].
Different concentrations of hydroxypropyl methyl cellulose (HPMC) was used to encapsulate the prepared material (CS–SiNPs-encapsulated PO-PE) for sustained release studies [
123]. The loading capacities of CS–SiNPs-encapsulated PO-PE with HPMC (50 wt%, 75 wt%, and 100.0 wt%) were estimated at 16.7 ± 0.9%, 28.4 ± 0.7%, and 25.5 ± 1.1%, respectively. Later, the release of peppermint oil was studied with different concentrations of HPMC at varied temperatures (4, 25, 35, and 50 °C). In the initial 4 h, a slow release was noted for 25, 35, and 50 °C, with a relatively slower release for 4 °C, indicating that an increase in temperature increases the release kinetics of peppermint oil. This could arguable be because of the volatile nature of peppermint oil, which is attributed to its lower boiling point. Additionally, it was noticed that the release rate of peppermint oil from CS–SiNPs-encapsulated PO-PE was higher in lower HPMC concentrations (50 and 75%) than in 100% HPMC, directing the performance of HPMC as an encapsulating agent. This led the authors to choose the 100% HPMC-based CS–SiNPs-encapsulated PO-PE to evaluate the antibacterial activity. The disk diffusion method was used to determine the antibacterial activity, as shown in
Table 7 [
123].
In another study, Mamdouh’s research group from the School of Sciences and Engineering, The American University in Cairo (New Cairo, Egypt), performed a comparative study of encapsulated peppermint and green tea essential oils in chitosan nanoparticles [
132]. The zeta-potentials of CSNPs with varied concentrations of peppermint essential oil (CSNPs–PE) and green tea oil (CSNPs–GTO) were found in the range of 20.9 ± 0.66 to 23.1 ± 0.4 mV and 24.2 ± 0.3 to 29.0 ± 0.2 mV, respectively. The highest zeta-potentials in both encapsulated formations (CSNPs–PO and CSNPs–GTO) were found with a 1:1 ratio; therefore, these combinations were chosen for further investigations. Additionally, the zeta potential of CSNPs was found to 24.9 ± 0.95 mV, in agreement with the previous literature [
133]. Based on DLVO theory, an equilibrium between attractive van der Waals’ forces and the electrical repulsion is required. For example, a high charge density over the surface of nanoparticles abolishes their aggregation because of repulsion among them [
134]. Additionally, the zeta potential value of 30.0 mV is considered ideal for stability, 20.0 mV indicates short-time stability, and ~5 mV indicates quick aggregation [
135]. Although the authors did not achieve a zeta potential value of 30.0 mV for their CSNP-encapsulated essential oil samples, no aggregation was observed. This observation indicates that the zeta potential is not the only parameter that decides the stability of nanoparticles. However, a higher zeta potential of CSNPs–GTO than CSNPs–PO evidently shows a higher stability of CSNPs–GTO. A spherical particle morphology was found using TEM microscopy for 1:1
w/
w ratios of CSNPs–GTO and CSNPs–PO with a size range of 20–60 nm. Furthermore, estimations of average particle sizes of CSNPs, CSNPs–PO, and CSNPs–GTO were found to be 36.1 ± 0.88 nm, 43.5 ± 1.97 nm, and 30.7 ± 1.13 nm, respectively [
132]. Later, the authors determined the encapsulation efficiency (EE%) and loading capacity (LC%) for both essential oils in their nanoencapsulated forms with the help of the following equation:
The loading capacities (LC%) of the encapsulated peppermint oil and green tea oil were found in the range of 8.15–22.2% and 2.2–23.1%, respectively. Additionally, the encapsulation efficiencies (EE%) of CSNPs–PO and CSNPs–GTO were determined as 78–82% and 22–81%, respectively, in agreement with the previous literature [
106].
A 72 h in vitro release study of CSNPs–PO and CSNPs–GTO was performed in buffer (pH = 3 (acetate buffer) and 7.4 (phosphate-buffered saline)). After an initial 12 h period phase, 45.7% and 74.5% for peppermint essential oil and green tea oil, respectively, were released in acidic acetate buffer, while a slow release was noticed in acidic acetate buffer until 72 h (61.3% and 74.9% for peppermint essential oil and green tea oil, respectively). Similar observations were also made with saline buffer (pH = 7.4), where the initial 12 h release (35.8% and 57.4% for peppermint essential oil and green tea oil, respectively) and until 72 h (50.7% and 62.9% for peppermint essential oil and green tea oil, respectively) were recorded. In our opinion, based on the results reported by the authors, a more controlled in vitro release of green tea oil than peppermint oil was observed in both buffers after 12 h [
132].
The agar dilution and colony counting methods were used to evaluate the antibacterial activity of peppermint oil and green tea oil before and after their encapsulation with CSNPs. The antibacterial activity was measured as minimum bactericidal concentration (MBC), which is defined as “the lowest concentration of sample/agent that kills 99.9% or more of the initial inoculum”. The bacteria used in antibacterial assays were
Staphylococcus aureus (as a representative Gram-positive bacteria) and
Escherichia coli (as a representative Gram-negative bacteria). In both cases, encapsulated essential oil samples were found with more anti-staphylococcal activity (MBC values: CSNPs–PO = 1.11 mg·mL
−1, versus peppermint oil = 1.36 mg·mL
−1; CSNPs–GTO = 0.57 mg·mL
−1, versus green tea oil ≥ 5.44 mg·mL
−1). On the contrary, CSNPs exhibited 5.0 mg·mL
−1 of anti-staphylococcal activity. These results exemplify an example of the decisive role of chitosan nanoencapsulation in enhancing the antibacterial activity of essential oil [
132].
Antibacterial testing on
Escherichia coli (MBC values: CSNPs–PO ≥ 2.72 mg·mL
−1, versus peppermint oil = 2.72 mg·mL
−1; CSNPs–GTO = 1.15 mg·mL
−1, versus green tea oil = 5.44 mg·mL
−1) exhibited a similar trend in the case of green tea oil. The antibacterial (
Escherichia coli) activity for CSNPs was measured at 7.50 mg·mL
−1 [
132].
5.9. Nanoencapsulation of Clove Essential Oil
Hadidi et al. reported clove essential oil-loaded CSNPs as antibacterial material for
Listeria monocytogenes and
Staphylococcus aureus. The authors extracted the clove essential oil, where GC–MS was used to characterize the 23 compounds. The major components of essential oil were eugenol (89.86%) and β-caryophyllene (5.40%), which is in agreement with the previous literature [
137,
138]. Various concentrations of essential oil loading were used with chitosan. The clove essential oil-loaded CSNP ratios were evaluated for their physicochemical characterization, as shown in
Table 8.
The chemical characterization was performed with FTIR. The unloaded CSNP showed characteristic peaks at 3445 cm
−1 (O–H), 3298 cm
−1 (N–H
2 stretching), 2991 cm
−1 (C–H stretching), 1546 cm
−1(–CONH
2 of amide-II), 1367 cm
−1 (C–N stretching), 1201 cm
−1 (β-(1−4) glycosidic linkage), 1065 cm
−1 (C–O–C stretching of glucose ring), 997 cm
−1 (C–O stretching), and 904 cm
−1 (vibration of the pyranose ring). Surprisingly, C=O stretching of amide-I and the other two peaks (1065 cm
−1 of C–O–C stretching of glucose ring; 1545 cm
−1 of amide-II) did not appear, suggesting ionic crosslinking between the PO
43− group of TPP and NH
3+ group of chitosan [
94,
107]. However, the increase in the intensity of the C–H stretching (2991 cm
−1) peak for clove essential oil-loaded CSNPs suggested encapsulation [
139].
The inhibition halo (cm) of clove essential oil CSNPs (CSNP:essential oil ratio of 1:0.5) on
Staphylococcus aureus,
Listeria monocytogenes,
Salmonella Typhi, and
Escherichia coli for 32 μL of minimum inhibitory volume (MIV), was found to be 4.8, 4.78, 4.49, and 3.95 cm, respectively [
139].
5.11. Nanoencapsulation of Carvacrol
Keawchaoon et al. from the Department of Packaging and Materials Technology, Faculty of Agro-Industry, Kasetsart University (Bangkok, Thailand), studied the chitosan-based nanoencapsulation of carvacrol. Carvacrol is considered as a safe food additive and is mainly derived from the essential oils of marjoram, oregano, summer savory, and thyme [
141,
142]. Although carvacrol has broad applications in the cosmetic, drug, and food industries, it is sensitive towards heat, light, and oxygen. To enhance its stability and shelf-life, the authors attempted a nanoencapsulation strategy. A two-step process (droplet formation and droplet solidification) was used to prepare the carvacrol-loaded CSNPs. The oil-in-water emulsion technique was implemented for carvacrol droplet formation in chitosan solution, while droplet solidification was conducted by the cross-linking of polyphosphate groups (P3O105−) of TPP molecules with protonated NH
2 groups (NH
3+) of chitosan molecules enclosing the carvacrol droplet [
106].
Chemical characterization was performed by FTIR. Carvacrol showed peaks at 3378 (–OH); 2960 (C–H stretching); 1459, 1382, and 1346 (C–H deformation); and 866 and 812 cm
−1 (aromatic ring) [
106]. CSNP peaks were found at 3500–3250 (–OH), 2927 (C–H stretching), 1634 (–CONH
2, amide-I), 1539 (–CONH
2, amide-II), 1155 (P=O) [
70,
143,
144], 1072 (C–O–C), and 890 cm
−1 (pyranose ring). There were no substantial differences found in carvacrol-loaded CSNPs, except the pronounced increase in the C–H stretching peak at 2870–2959 cm
−1. This evidently provided a clue of carvacrol presence in the chitosan matrix.
In TGA analysis, mass losses of samples were studied as functions of temperature and to evaluate the thermal stability of the respective samples. During this study, a DTG thermogram was plotted, where the decomposition temperature (T
d) represented a peak as a corresponding temperature to a maximum mass loss of sample [
106]. Carvacrol showed a one-step mass loss from 183.8 °C (peaks at 213.9 °C), while CSNPs showed a two-step mass loss from 90.5 °C and 231.3 °C. A two-step mass loss in CSNPs reflected moisture evaporation followed by the decomposition of chitosan. However, carvacrol-loaded CSNPs showed two new T
d values (183.5–186.4 °C; 322.2–340.6 °C), indicating the consequent loss of free carvacrol and encapsulated carvacrol. Interestingly, the significant increase of T
d values of carvacrol to carvacrol-loaded CSNPs showed an enhancement of thermal stability.
The encapsulation efficiency (EE) and loading capacity (LC) of carvacrol-loaded CSNPs were evaluated for various combinations of carvacrol with CSNPs (CSNP:carvacrol = 1:0, 1:0.25, 1:05, 1.0.75, 1:1, 1:1.25). A proportional increasing encapsulation efficiency trend was observed up to a 1:1 ratio (EE = 31.4 ± 1.3), which could be CSNPs saturated with carvacrol. For the ratio of 1:1 of CSNP:carvacrol loading capacity (from UV–Vis spectroscopy = 18.9 ± 0.8; from TGA analysis = 18.9; from FTIR, as a comparison of intensity peak ratio at 2959 (for C–H stretching) and 890 (for pyranose ring) = 1.6), Z-average diameter (= 695.9 ± 48.8 nm) and zeta potential (= 29.3 ± 0.9 mV) were estimated.
Later, carvacrol-loaded CSNPs (1:1) were tested against the three strains of bacteria (Escherichia coli, Staphylococcus aureus, and Bacillus cereus) using a broth dilution assay. Unloaded CSNPs when evaluated for antibacterial activity were found to be 8.225 mg/mL and unable to prevent the growth of these three bacteria strains. However, there were minimum bactericidal concentrations (MBCs) for carvacrol-loaded CSNPs of Staphylococcus aureus (= 4.113 mg/mL), Bacillus cereus (= 2.056 mg/mL), and Escherichia coli (= 8.225 mg/mL).
In another study, Mexican researchers (collaboration of Universidad de Sonora and Centro de Investigación en Alimentación y Desarrollo) nanoencapsulated the carvacrol into CSNPs against
Pseudomonas aeruginosa biofilms [
145]. The carvacrol-loaded CSNPs prevented the growth of
Pseudomonas aeruginosa in biofilms (0.078–2.0 log CFU·cm
−2) and reduced the swarming motility (40–60%). Additionally, reduced quorum sensing in
Chromobacterium violaceum was observed [
145].