Green Production of Functionalized Few-Layer Graphene–Silver Nanocomposites Using Gallnut Extract for Antibacterial Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
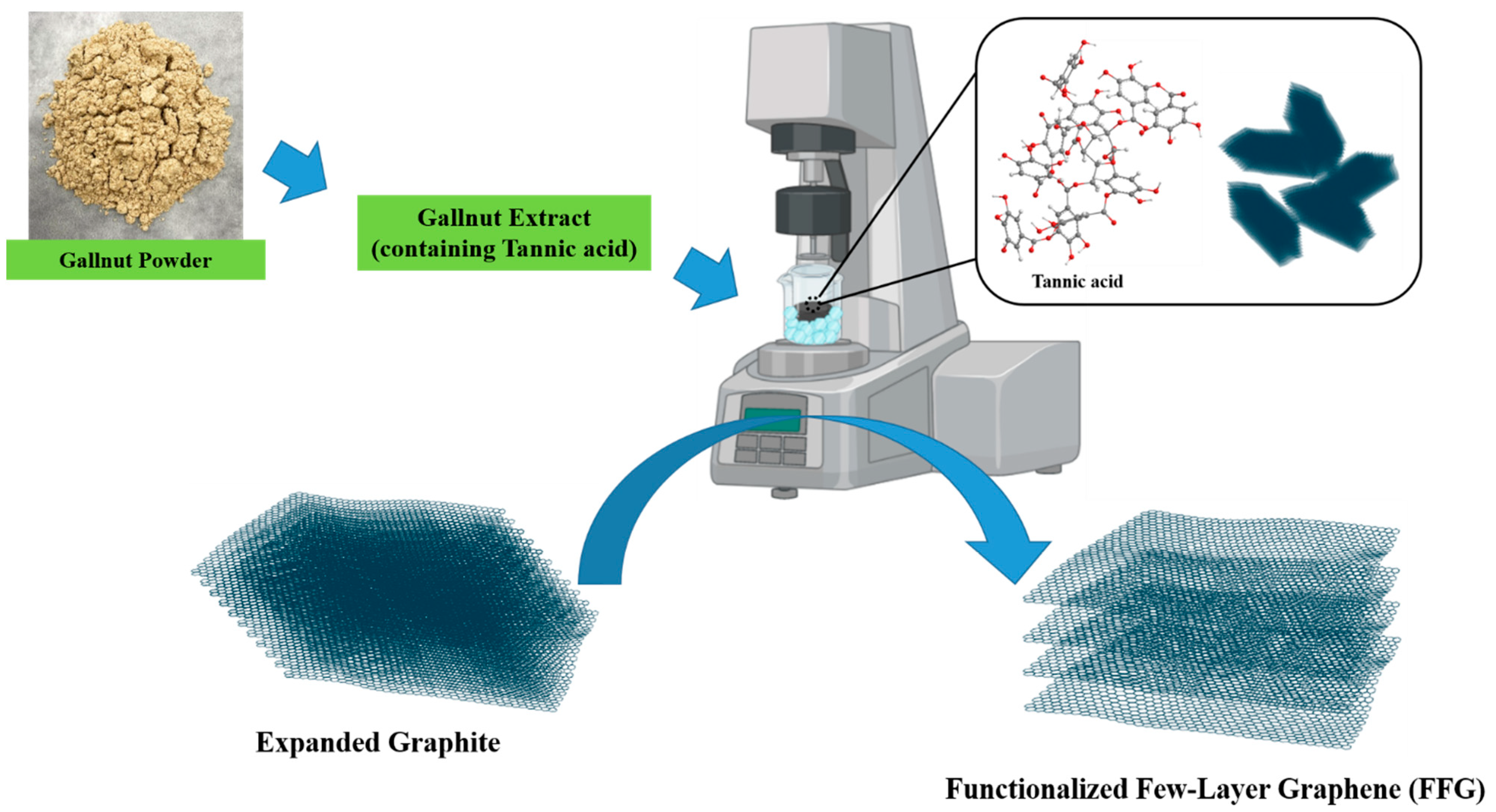
2.2. Synthesis of FFG
2.2.1. Preparation of Gallnut Extract
2.2.2. Exfoliation of FFG Using Gallnut Extract
2.3. Preparation of Ag–FFG Nanocomposites and Their Antibacterial Activity
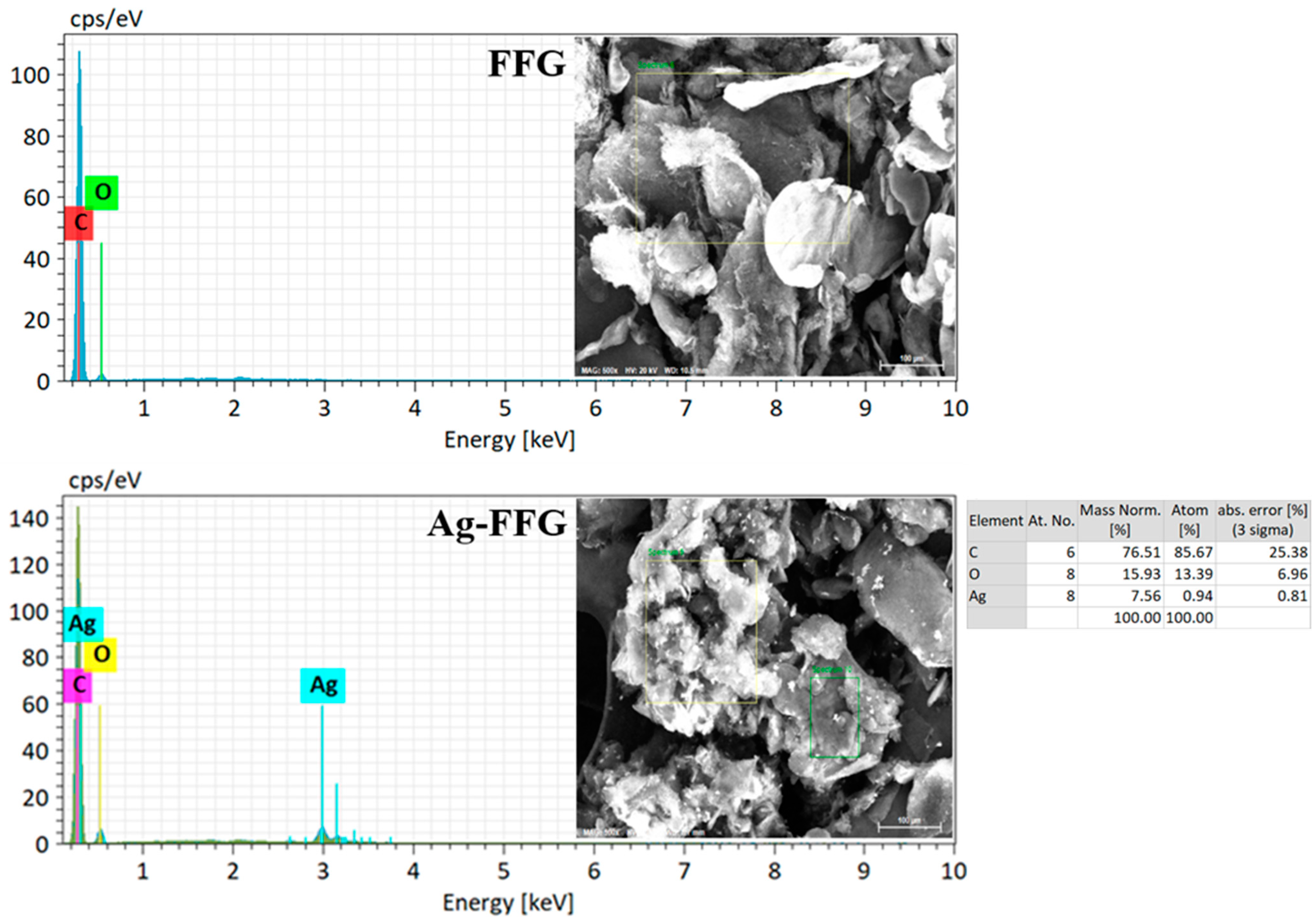
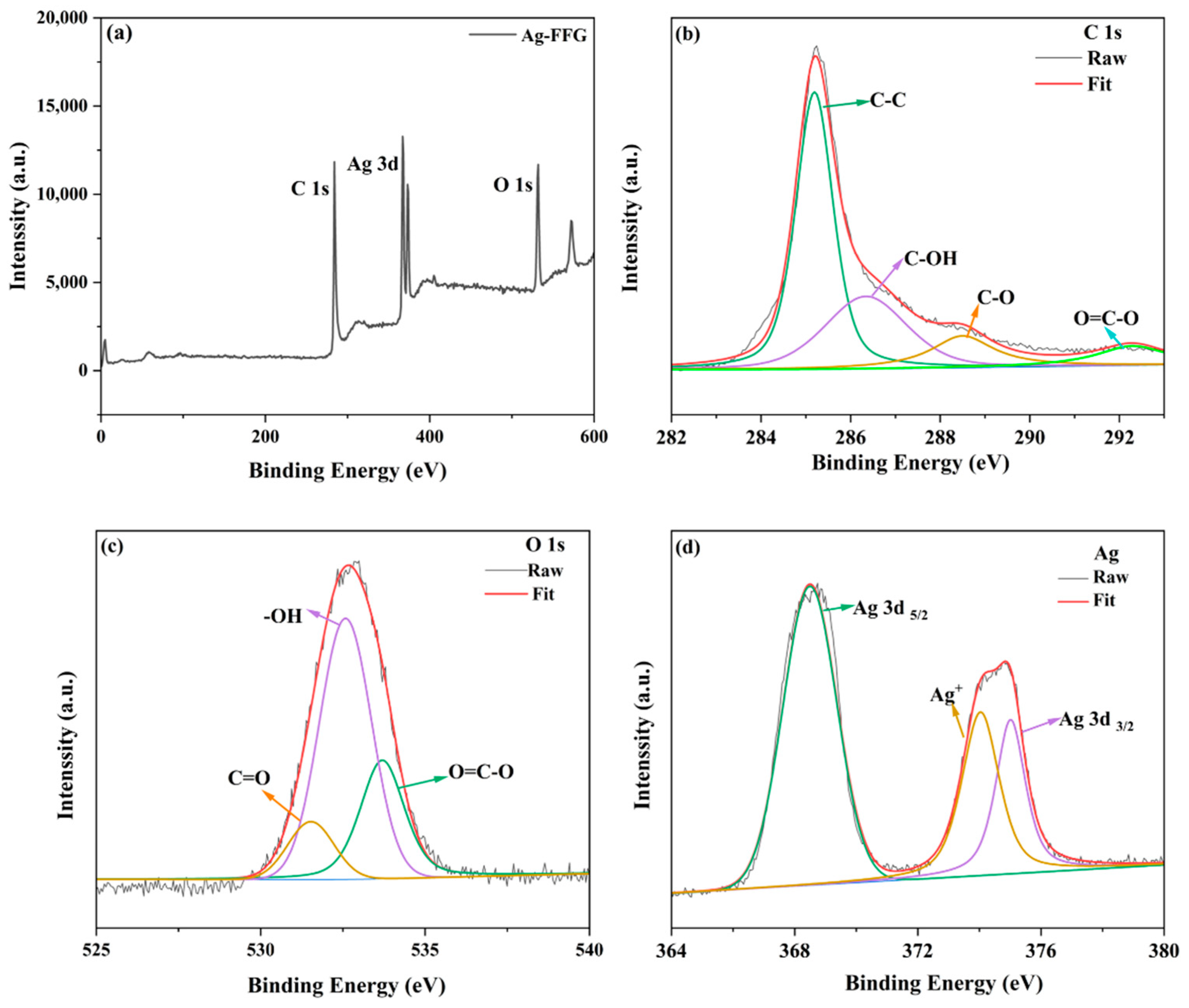
2.4. Characterization of FFG and Ag–FFG Nanocomposites
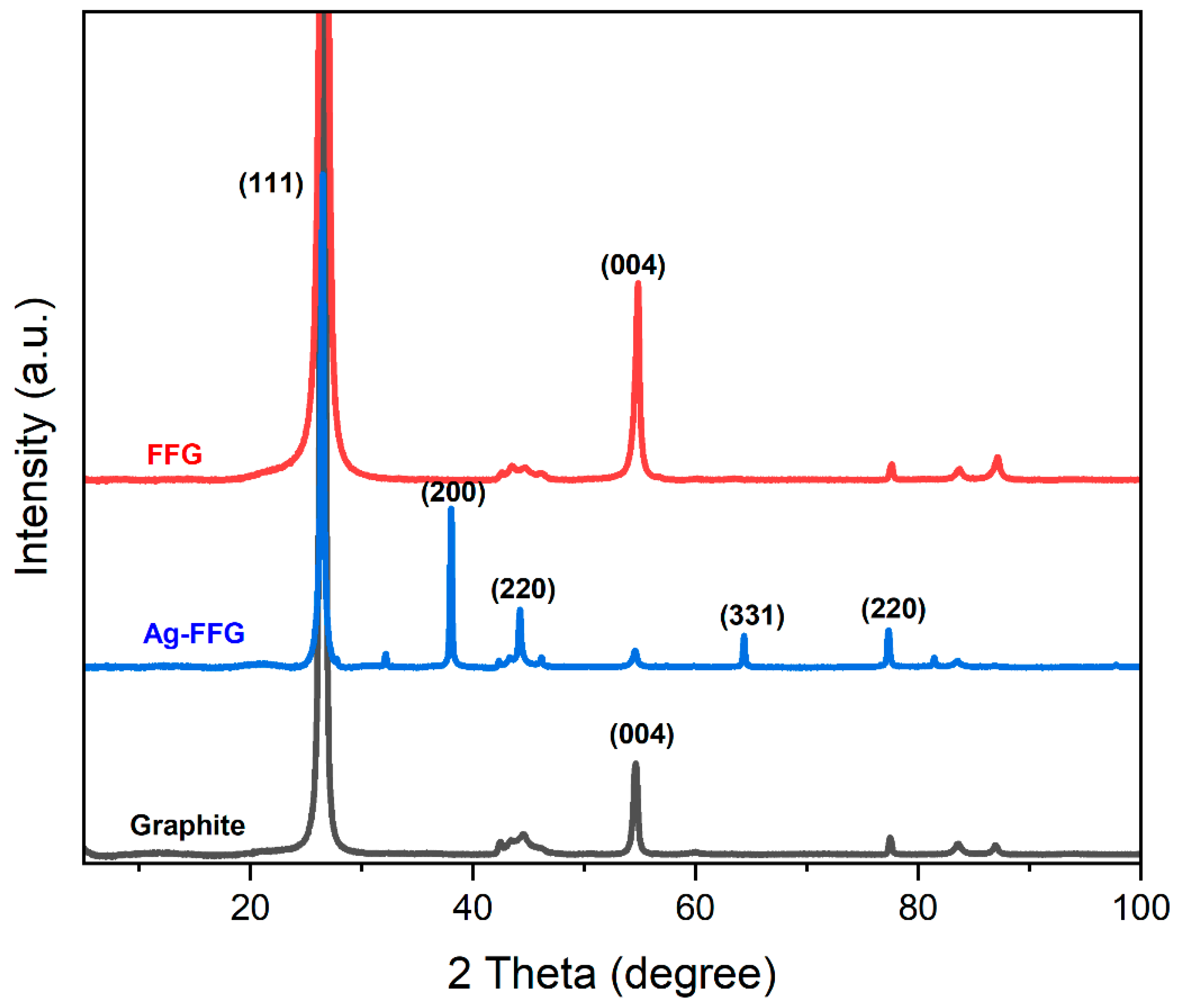
2.4.1. Raman Spectroscopy and X-ray Diffraction Analysis
2.4.2. Microscopy Analysis
3. Results and Discussion
3.1. Preparation and Characterization of FFG
3.2. Optimization of Exfoliation Process
3.2.1. Effect of Rotational Speed
3.2.2. Effect of Exfoliation Time
3.2.3. Effect of Initial Graphite Concentration and GNE Concentration
3.3. Antibacterial Activity of the Prepared Ag–FFG
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhuo, H.; Zhang, X.; Wang, L.; Lu, Q.; Kaplan, D.L. Sonication exfoliation of defect-free graphene in aqueous silk nanofiber solutions. ACS Sustain. Chem. Eng. 2018, 6, 12261–12267. [Google Scholar] [CrossRef]
- Rostami, S.; Mehdinia, A.; Jabbari, A. Intrinsic peroxidase-like activity of graphene nanoribbons for label-free colorimetric detection of dopamine. Mater. Sci. Eng. C 2020, 114, 111034. [Google Scholar] [CrossRef] [PubMed]
- Maharana, M.; Baruah, N.; Nayak, S.K.; Sahoo, N.; Wu, K.; Goswami, L. Electrohydrodynamics Analysis of Dielectric 2D Nanofluids. Nanomaterials 2022, 12, 1489. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Young, R.J.; Backes, C.; Zhao, W.; Zhang, X.; Zhukov, A.A.; Coleman, J.N. Mechanisms of liquid-phase exfoliation for the production of graphene. ACS Nano 2020, 14, 10976–10985. [Google Scholar] [CrossRef]
- Tang, Y.B.; Lee, C.S.; Xu, J.; Liu, Z.T.; Chen, Z.H.; He, Z.; Lee, S.T. Incorporation of graphenes in nanostructured TiO2 films via molecular grafting for dye-sensitized solar cell application. ACS Nano 2010, 4, 3482–3488. [Google Scholar] [CrossRef]
- Randviir, E.P.; Brownson, D.A.; Banks, C.E. A decade of graphene research: Production, applications and outlook. Mater. Today 2014, 17, 426–432. [Google Scholar] [CrossRef]
- Carey, T.; Alhourani, A.; Tian, R.; Seyedin, S.; Arbab, A.; Maughan, J.; Šiller, L.; Horvath, D.; Kelly, A.; Kaur, H.; et al. Cyclic production of biocompatible few-layer graphene ink with in-line shear-mixing for inkjet-printed electrodes and Li-ion energy storage. npj 2D Mater. Appl. 2022, 6, 3. [Google Scholar] [CrossRef]
- Zhao, S.; Xie, S.; Zhao, Z.; Zhang, J.; Li, L.; Xin, Z. Green and High-Efficiency Production of Graphene by Tannic Acid-Assisted Exfoliation of Graphite in Water. ACS Sustain. Chem. Eng. 2018, 6, 7652–7661. [Google Scholar] [CrossRef]
- Cheng, Z.L.; Kong, Y.C.; Liu, Z. Li+/Na+ Co-assisted hydrothermal exfoliation for graphite into few-layer graphene nanosheets and their excellent friction-reducing performance. ACS Sustain. Chem. Eng. 2019, 7, 19770–19778. [Google Scholar] [CrossRef]
- He, K.; Zeng, Z.; Chen, A.; Zeng, G.; Xiao, R.; Xu, P.; Huang, Z.; Shi, J.; Hu, L.; Chen, G. Advancement of Ag–Graphene Based Nanocomposites: An Overview of Synthesis and Its Applications. Small 2018, 14, 1800871. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Liu, B.; Wang, J.; Zhang, S.; Lin, Q.; Gong, P.; Ma, L.; Yang, S. A Novel Wound Dressing Based on Ag/Graphene Polymer Hydrogel: Effectively Kill Bacteria and Accelerate Wound Healing. Adv. Funct. Mater. 2014, 24, 3933–3943. [Google Scholar] [CrossRef]
- Yu, P.; Wang, X.; Zhang, K.; Zhou, D.; Wu, M.; Wu, Q.; Liu, J.; Yang, J.; Zhang, J. Aqueous cellulose solution assisted direct exfoliation of graphite to high concentration graphene dispersion. Mater. Lett. 2021, 285, 129081. [Google Scholar] [CrossRef]
- Blomquist, N.; Engström, A.-C.; Hummelgård, M.; Andres, B.; Forsberg, S.; Olin, H. Large-Scale Production of Nanographite by Tube-Shear Exfoliation in Water. PLoS ONE 2016, 11, e0154686. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.S.; Park, S.J.; Yoo, S.S.; Lee, T.-R.; Kim, T. High shear-induced exfoliation of graphite into high quality graphene by Taylor–Couette flow. RSC Adv. 2016, 6, 12003–12008. [Google Scholar] [CrossRef]
- del Río, F.; Boado, M.G.; Rama, A.; Guitián, F. A comparative study on different aqueous-phase graphite exfoliation methods for few-layer graphene production and its application in alumina matrix composites. J. Eur. Ceram. Soc. 2017, 37, 3681–3693. [Google Scholar] [CrossRef]
- Hou, D.; Liu, Q.; Wang, X.; Quan, Y.; Qiao, Z.; Yu, L.; Ding, S. Facile synthesis of graphene via reduction of graphene oxide by artemisinin in ethanol. J. Mater. 2018, 4, 256–265. [Google Scholar] [CrossRef]
- Deshmukh, A.R.; Kim, B.S. Bio-functionalized few-layer graphene for in situ growth of gold nanoparticles, improvement of polymer properties, and dye removal. J. Clean. Prod. 2021, 310, 127515. [Google Scholar] [CrossRef]
- Mahata, S.; Sahu, A.; Shukla, P.; Rai, A.; Singh, M.; Rai, V.K. The novel and efficient reduction of graphene oxide using Ocimum sanctum L. leaf extract as an alternative renewable bio-resource. New J. Chem. 2018, 42, 19945–19952. [Google Scholar] [CrossRef]
- Salunke, B.K.; Kim, B.S. Facile synthesis of graphene using a biological method. RSC Adv. 2016, 6, 17158–17162. [Google Scholar] [CrossRef]
- Lingaraju, K.; Naika, H.R.; Nagaraju, G.; Nagabhushana, H. Biocompatible synthesis of reduced graphene oxide from Euphorbia heterophylla (L.) and their in-vitro cytotoxicity against human cancer cell lines. Biotechnol. Rep. 2019, 24, e00376. [Google Scholar] [CrossRef] [PubMed]
- Karthik, R.; Govindasamy, M.; Chen, S.M.; Mani, V.; Lou, B.S.; Devasenathipathy, R.; Elangovan, A. Green synthesized gold nanoparticles decorated graphene oxide for sensitive determination of chloramphenicol in milk, powdered milk, honey and eye drops. J. Colloid Interface Sci. 2016, 475, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Kalali, E.N.; Guo, W.; Wang, X.; Xing, W.; Song, L.; Hu, Y. Effect of metal-based nanoparticles decorated graphene hybrids on flammability of epoxy nanocomposites. Compos. Part A Appl. Sci. Manuf. 2020, 129, 105694. [Google Scholar] [CrossRef]
- Kushwaha, A.; Goswami, L.; Kim, B.S. Nanomaterial-based therapy for wound healing. Nanomaterials 2022, 12, 618. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Sharma, P.; Yadav, S.; Kumar, J.; Vij, A.; Rawat, P.; Majumder, S. A novel synthesis of the graphene oxide-silver (GO-Ag) nanocomposite for unique physiochemical applications. ACS Omega 2020, 5, 5041–5047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, C.M.; Singh, S.; Goswami, L. (Eds.) Waste-to-Energy Approaches towards Zero Waste: Interdisciplinary Methods of Controlling Waste; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Cobos, M.; De-La-Pinta, I.; Quindós, G.; Fernández, M.J.; Fernández, M.D. Graphene oxide–silver nanoparticle nanohybrids: Synthesis, characterization, and antimicrobial properties. Nanomaterials 2020, 10, 376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.P.; Zhang, L.C.; Li, J.P.; Lu, Y.; Li, H.H.; Ma, Y.N.; Yu, S.H. Facile synthesis of silver@ graphene oxide nanocomposites and their enhanced antibacterial properties. J. Mater. Chem. 2011, 21, 4593–4597. [Google Scholar] [CrossRef]
- de Medeiros, A.M.; Khan, L.U.; da Silva, G.H.; Ospina, C.A.; Alves, O.L.; de Castro, V.L.; Martinez, D.S.T. Graphene oxide-silver nanoparticle hybrid material: An integrated nanosafety study in zebrafish embryos. Ecotoxicol. Environ. Saf. 2021, 209, 111776. [Google Scholar] [CrossRef]
- Paton, K.R.; Varrla, E.; Backes, C.; Smith, R.J.; Khan, U.; O’Neill, A.; Boland, C.; Lotya, M.; Istrate, O.M.; King, P.; et al. Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids. Nat. Mater. 2014, 13, 624–630. [Google Scholar] [CrossRef]
- Kushwaha, A.; Rani, R.; Kumar, S.; Thomas, T.; David, A.A.; Ahmed, M. A new insight to adsorption and accumulation of high lead concentration by exopolymer and whole cells of lead-resistant bacterium Acinetobacter junii L. Pb1 isolated from coal mine dump. Environ. Sci. Pollut. Res. 2017, 24, 10652–10661. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.; Kushwaha, A.; Rani, R. Reduction of Hexavalent Chromium [Cr (VI)] by heavy metal tolerant Bacterium Alkalihalobacillus clausii CRA1 and its toxicity assessment through flow cytometry. Curr. Microbiol. 2022, 79, 33. [Google Scholar] [CrossRef]
- Zein, R.; Alghoraibi, I.; Soukkarieh, C.; Ismail, M.T.; Alahmad, A. Influence of Polyvinylpyrrolidone Concentration on Properties and Anti-Bacterial Activity of Green Synthesized Silver Nanoparticles. Micromachines 2022, 13, 777. [Google Scholar] [CrossRef] [PubMed]
- Lund, S.; Kauppila, J.; Sirkiä, S.; Palosaari, J.; Eklund, O.; Latonen, R.-M.; Smått, J.-H.; Peltonen, J.; Lindfors, T. Fast high-shear exfoliation of natural flake graphite with temperature control and high yield. Carbon 2021, 174, 123–131. [Google Scholar] [CrossRef]
- Ismail, Z.; Kassim NF, A.; Abdullah, A.H.; Abidin AS, Z.; Ismail, F.S.; Yusoh, K. Black tea assisted exfoliation using a kitchen mixer allowing one-step production of graphene. Mater. Res. Express 2017, 4, 075607. [Google Scholar] [CrossRef]
- O’Neill, A.; Khan, U.; Nirmalraj, P.N.; Boland, J.; Coleman, J.N. Graphene Dispersion and Exfoliation in Low Boiling Point Solvents. J. Phys. Chem. C 2011, 115, 5422–5428. [Google Scholar] [CrossRef]
- Khan, U.; O’Neill, A.; Porwal, H.; May, P.; Nawaz, K.; Coleman, J.N. Size selection of dispersed, exfoliated graphene flakes by controlled centrifugation. Carbon 2012, 50, 470–475. [Google Scholar] [CrossRef]
- Lotya, M.; Hernandez, Y.; King, P.J.; Smith, R.J.; Nicolosi, V.; Karlsson, L.S.; Blighe, F.M.; De, S.; Wang, Z.; McGovern, I.T.; et al. Liquid Phase Production of Graphene by Exfoliation of Graphite in Surfactant/Water Solutions. J. Am. Chem. Soc. 2009, 131, 3611–3620. [Google Scholar] [CrossRef] [Green Version]
- Kaushal, A.; Dhawan, S.K.; Singh, V. Determination of crystallite size, number of graphene layers and defect density of graphene oxide (GO) and reduced graphene oxide (RGO). AIP Conf. Proc. 2019, 2115, 030106. [Google Scholar] [CrossRef]
- Goswami, L.; Pakshirajan, K.; Pugazhenthi, G. Biological treatment of biomass gasification wastewater using hydrocarbonoclastic bacterium Rhodococcus opacus in an up-flow packed bed bioreactor with a novel waste-derived nano-biochar based bio-support material. J. Clean. Prod. 2020, 256, 120253. [Google Scholar] [CrossRef]
- Borah, S.N.; Goswami, L.; Sen, S.; Sachan, D.; Sarma, H.; Montes, M.; Narayan, M. Selenite bioreduction and biosynthesis of selenium nanoparticles by Bacillus paramycoides SP3 isolated from coal mine overburden leachate. Environ. Pollut. 2021, 285, 117519. [Google Scholar] [CrossRef]
- Kumar, P.; Lahiri, I.; Mitra, A. Nickel mediated few-layer graphene growth on glass substrates by pulsed laser deposition. Results Phys. 2019, 14, 102350. [Google Scholar] [CrossRef]
- Thirumalraj, B.; Rajkumar, C.; Chen, S.M.; Palanisamy, S. One-pot green synthesis of graphene nanosheets encapsulated gold nanoparticles for sensitive and selective detection of dopamine. Sci. Rep. 2017, 7, 41213. [Google Scholar] [CrossRef] [Green Version]
- Mardlin, K.; Osazuwa, O.; Kontopoulou, M. Solvent-Free Thermomechanical Exfoliation of Graphite into Graphene Nanoplatelet Flakes: Implications for Conductive Composites. ACS Appl. Nano Mater. 2022, 5, 4938–4947. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, G.; Zhang, M.; Guo, S.; Liu, Z.H. Preparation and capacitance performance of Ag–graphene based nanocomposite. J. Power Sources 2012, 201, 376–381. [Google Scholar] [CrossRef]
- Li, S.K.; Yan, Y.X.; Wang, J.L.; Yu, S.H. Bio-inspired in situ growth of monolayer silver nanoparticles on graphene oxide paper as multifunctional substrate. Nanoscale 2013, 5, 12616–12623. [Google Scholar] [CrossRef] [PubMed]
- Nabih, S.; Hassn, S.S. Chitosan-capped Ag–Au/rGO nanohybrids as promising enzymatic amperometric glucose biosensor. J. Mater. Sci. Mater. Electron. 2020, 31, 13352–13361. [Google Scholar] [CrossRef]
- Hamad, A.; Li, L.; Liu, Z.; Zhong, X.L.; Liu, H.; Wang, T. Generation of silver titania nanoparticles from an Ag–Ti alloy via picosecond laser ablation and their antibacterial activities. RSC Adv. 2015, 5, 72981–72994. [Google Scholar] [CrossRef]
- Liang, W.; Church, T.L.; Harris, A.T. Biogenic synthesis of photocatalytically active Ag/TiO2 and Au/TiO2 composites. Green Chem. 2012, 14, 968–975. [Google Scholar] [CrossRef]
- Pham, T.D.; Lee, B.K. Feasibility of silver doped TiO2/glass fiber photocatalyst under visible irradiation as an indoor air germicide. Int. J. Environ. Res. Public Health 2014, 11, 3271–3288. [Google Scholar] [CrossRef] [Green Version]
- Chabot, V.; Kim, B.; Sloper, B.; Tzoganakis, C.; Yu, A. High yield production and purification of few layer graphene by Gum Arabic assisted physical sonication. Sci. Rep. 2013, 3, 1378. [Google Scholar] [CrossRef] [Green Version]


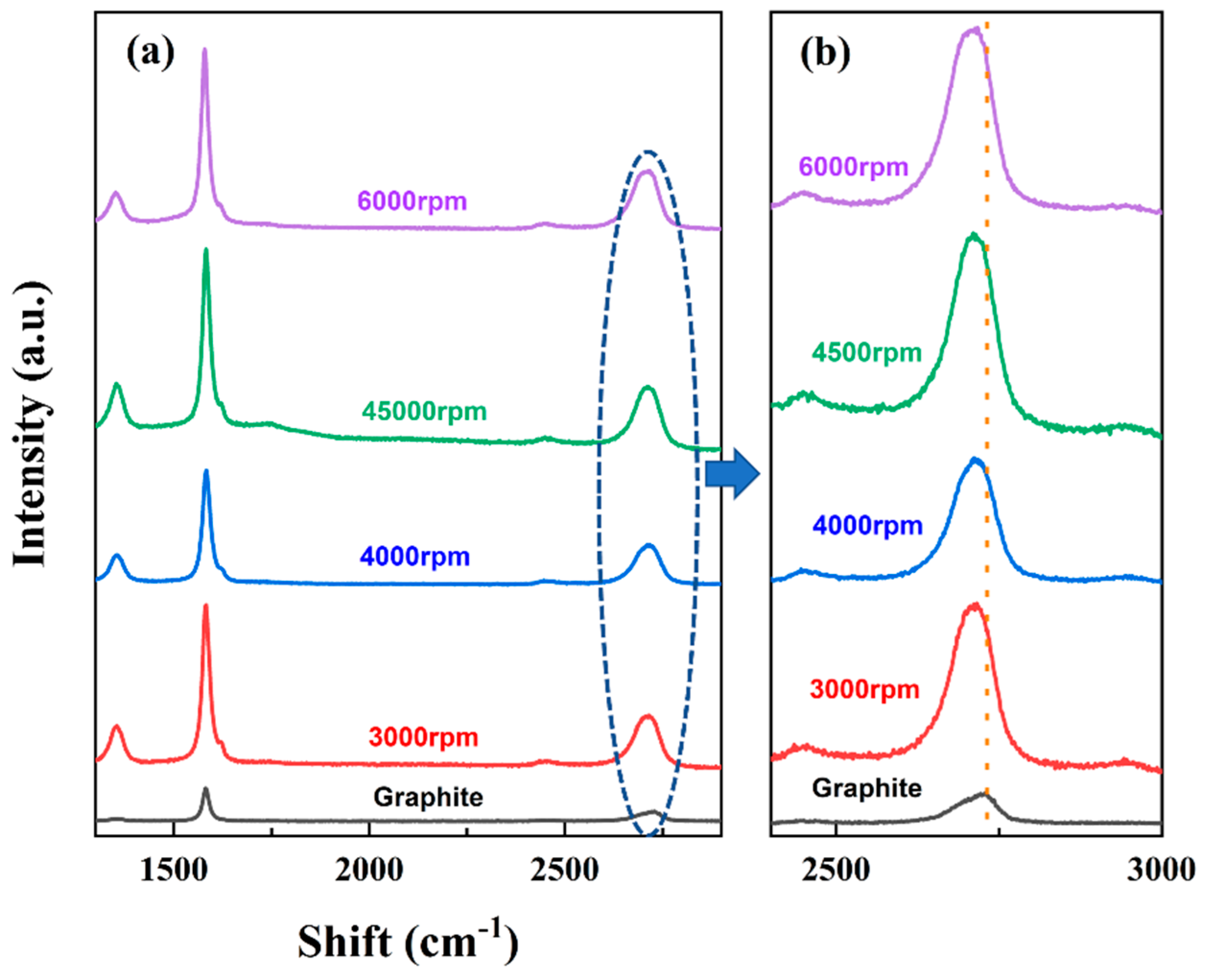

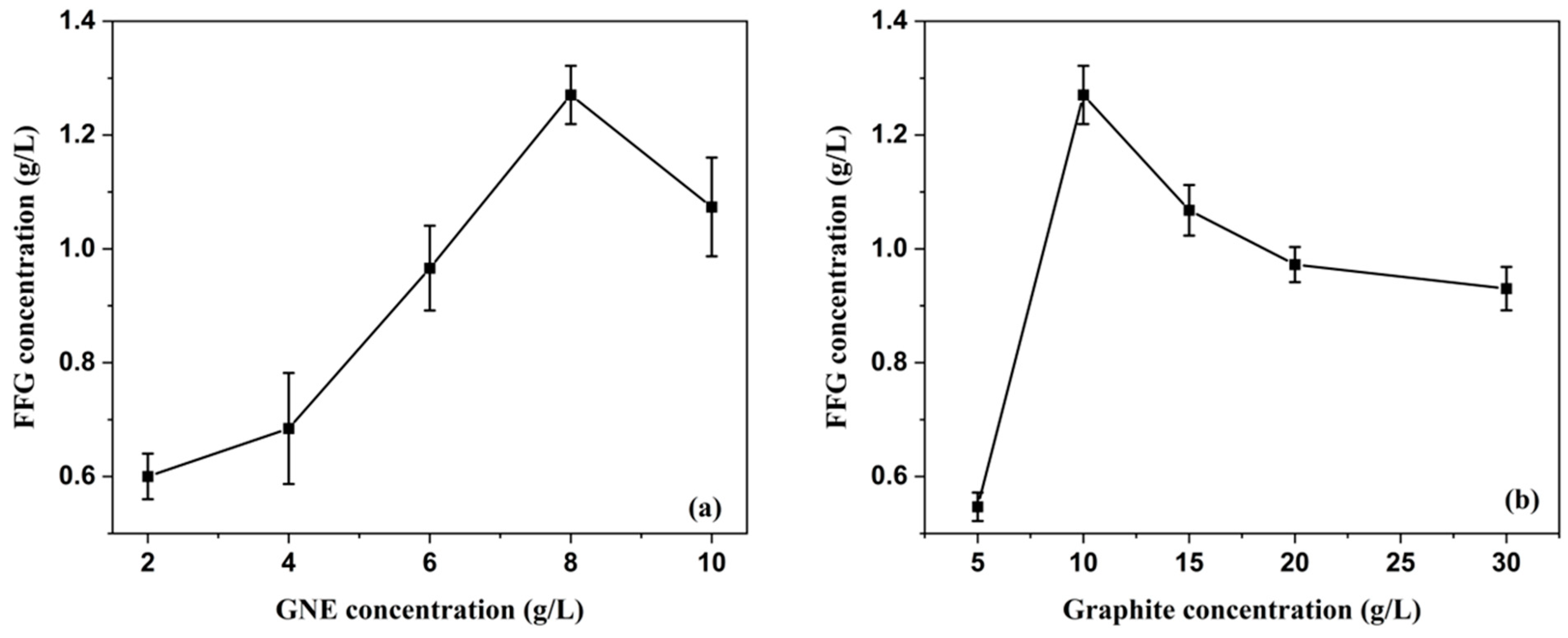
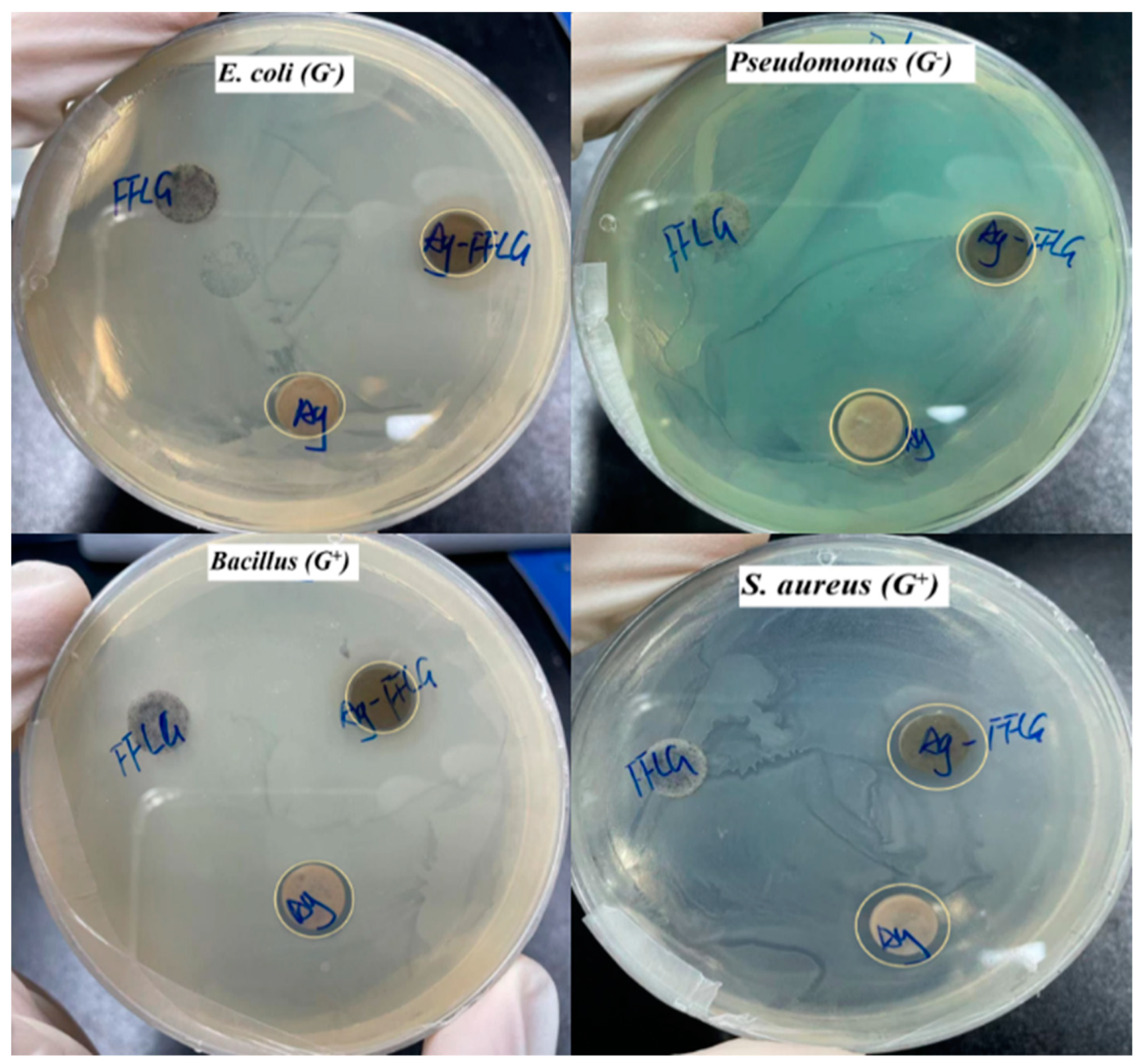
| RPM | M from Equation (1) | NG from Equation (2) | L (μm) from Equation (3) |
|---|---|---|---|
| 3000 | 0.783 | 8.610 | 1.276 |
| 4000 | 0.753 | 7.727 | 0.936 |
| 4500 | 0.751 | 7.691 | 0.76 |
| 6000 | 0.638 | 5.248 | 0.559 |
| Exfoliation Time (h) | M from Equation (1) | NG from Equation (2) | L (μm) from Equation (3) |
|---|---|---|---|
| 2 | 0.817 | 9.705 | 1.763 |
| 3 | 0.777 | 8.394 | 1.598 |
| 4 | 0.749 | 7.638 | 1.319 |
| 5 | 0.735 | 7.261 | 1.276 |
| 6 | 0.659 | 5.623 | 1.227 |
| 7 | 0.645 | 5.358 | 1.207 |
| 8 | 0.638 | 5.248 | 1.187 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bu, Y.; Kushwaha, A.; Goswami, L.; Kim, B.-S. Green Production of Functionalized Few-Layer Graphene–Silver Nanocomposites Using Gallnut Extract for Antibacterial Application. Micromachines 2022, 13, 1232. https://doi.org/10.3390/mi13081232
Bu Y, Kushwaha A, Goswami L, Kim B-S. Green Production of Functionalized Few-Layer Graphene–Silver Nanocomposites Using Gallnut Extract for Antibacterial Application. Micromachines. 2022; 13(8):1232. https://doi.org/10.3390/mi13081232
Chicago/Turabian StyleBu, Yingjie, Anamika Kushwaha, Lalit Goswami, and Beom-Soo Kim. 2022. "Green Production of Functionalized Few-Layer Graphene–Silver Nanocomposites Using Gallnut Extract for Antibacterial Application" Micromachines 13, no. 8: 1232. https://doi.org/10.3390/mi13081232
APA StyleBu, Y., Kushwaha, A., Goswami, L., & Kim, B.-S. (2022). Green Production of Functionalized Few-Layer Graphene–Silver Nanocomposites Using Gallnut Extract for Antibacterial Application. Micromachines, 13(8), 1232. https://doi.org/10.3390/mi13081232







