Electroplated Al Press Marking for Wafer-Level Bonding
Abstract
1. Introduction
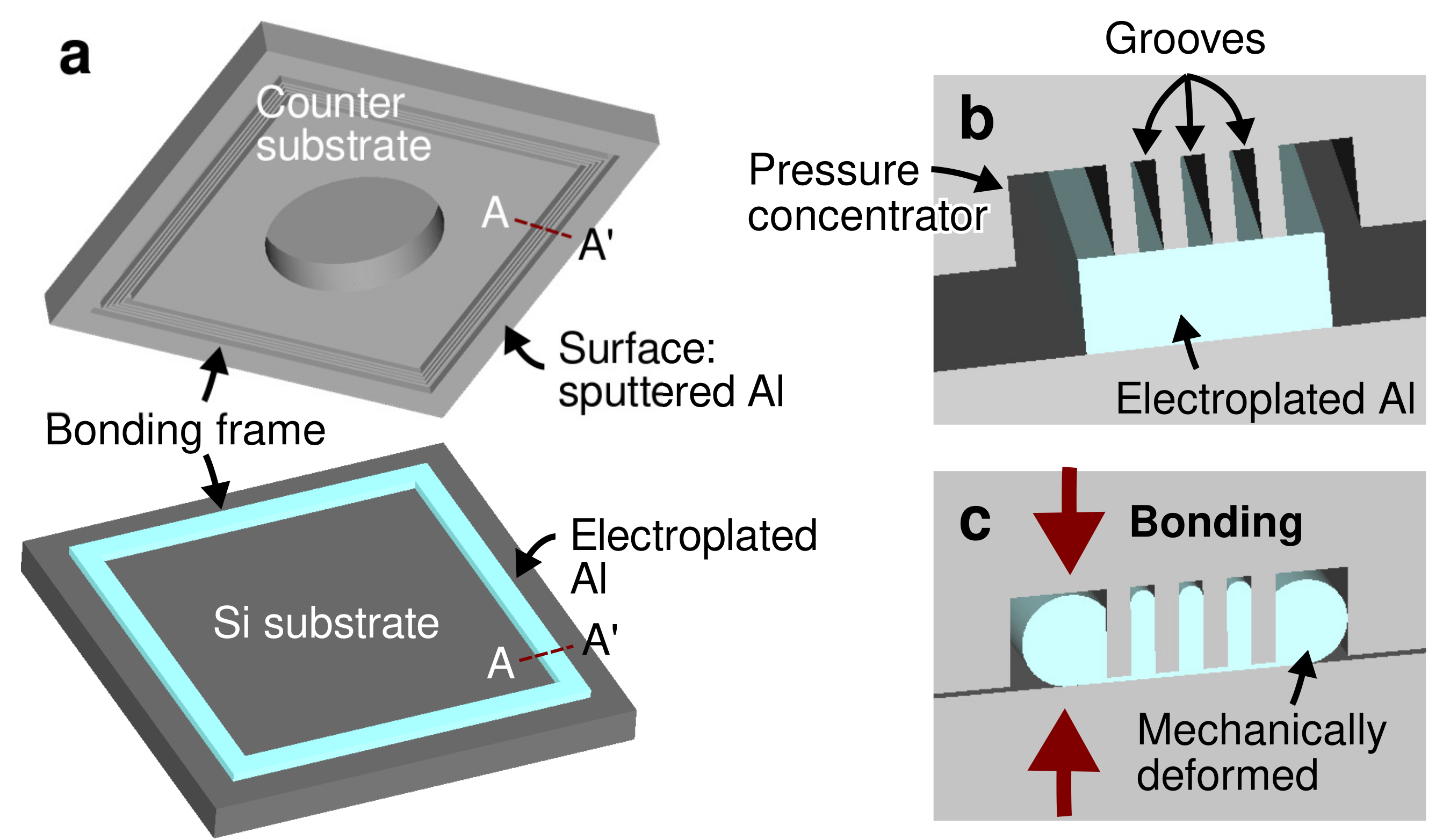
2. Materials and Methods
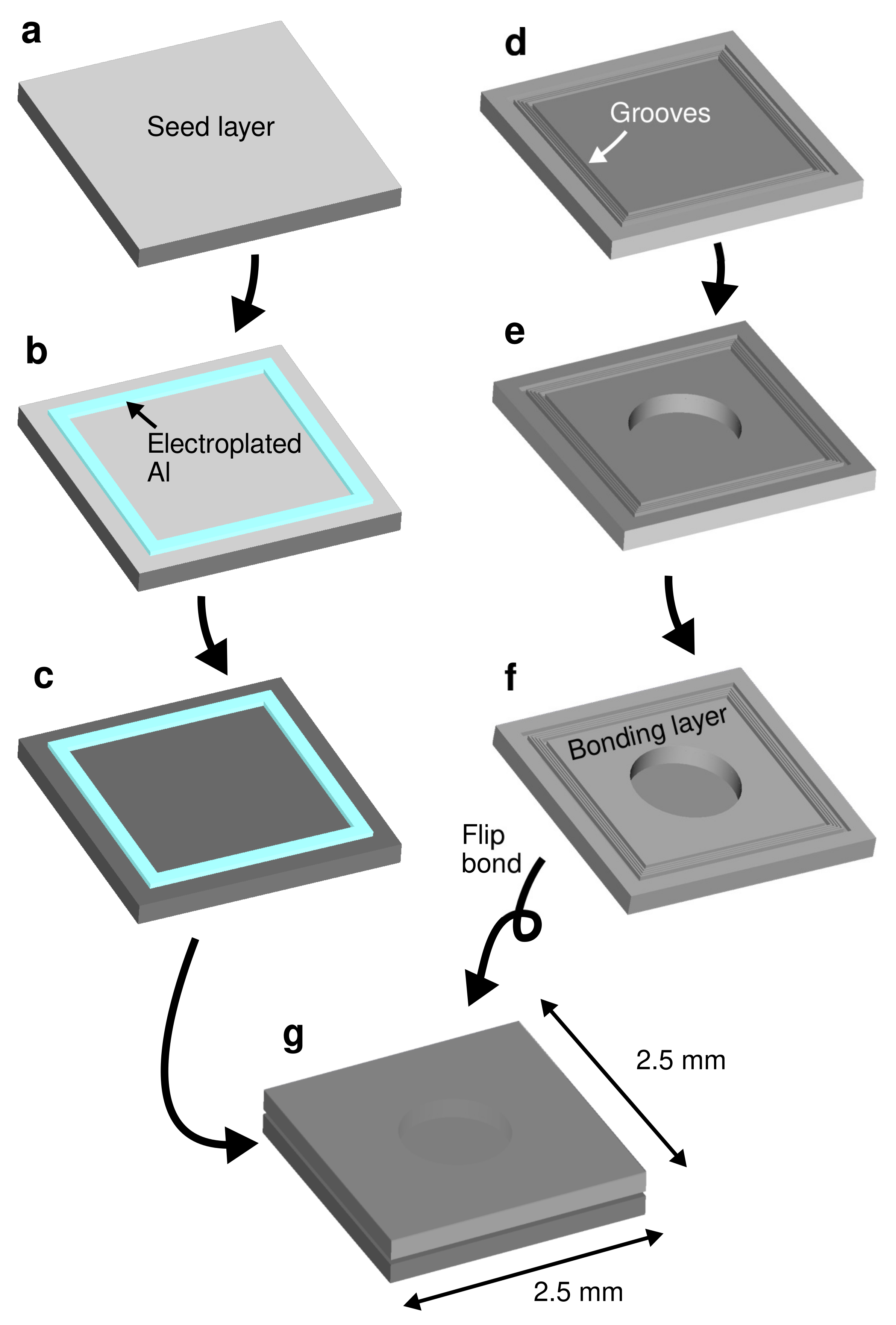
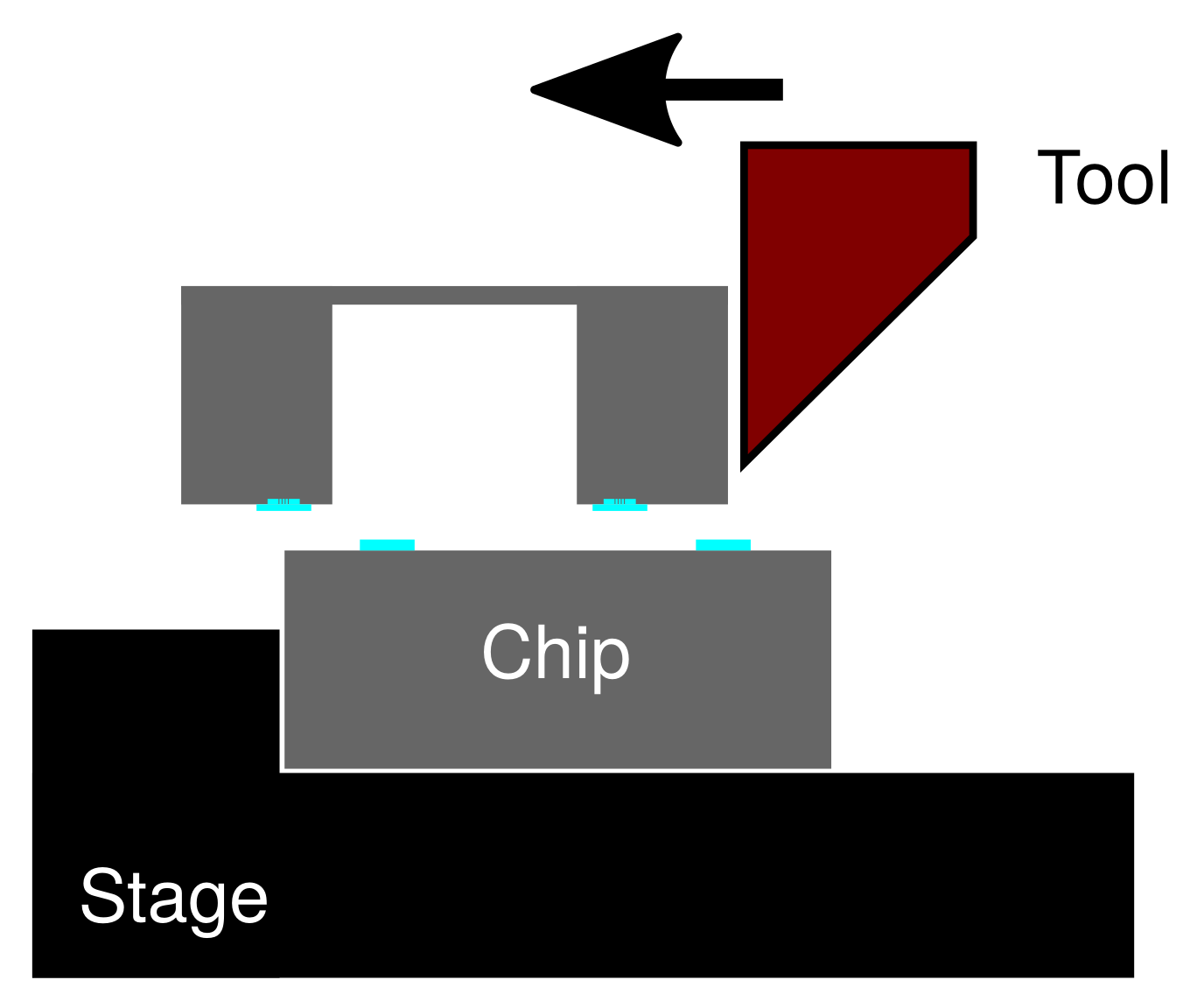
2.1. Specimen Preparation
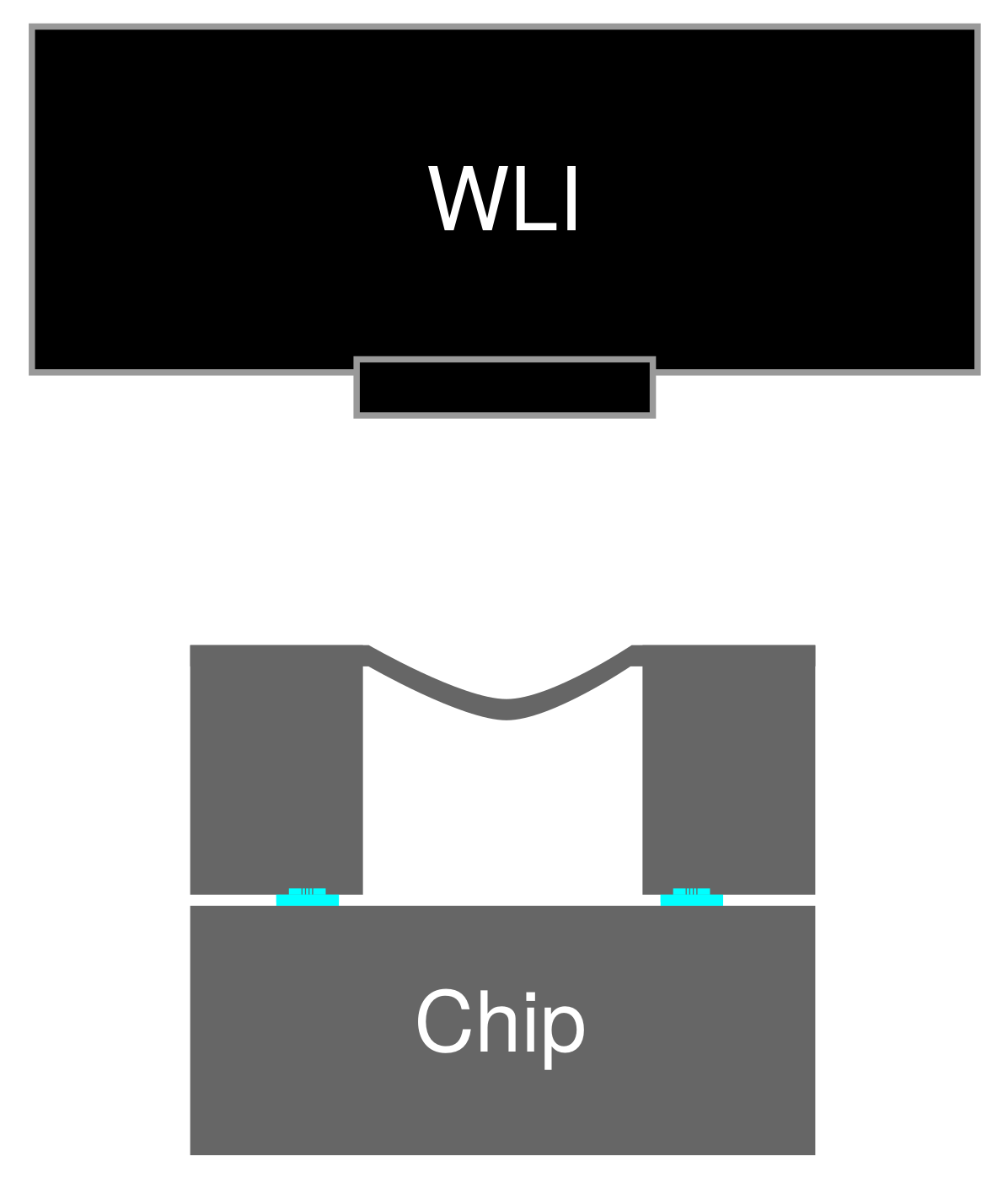
2.2. Evaluation
3. Results and Discussion
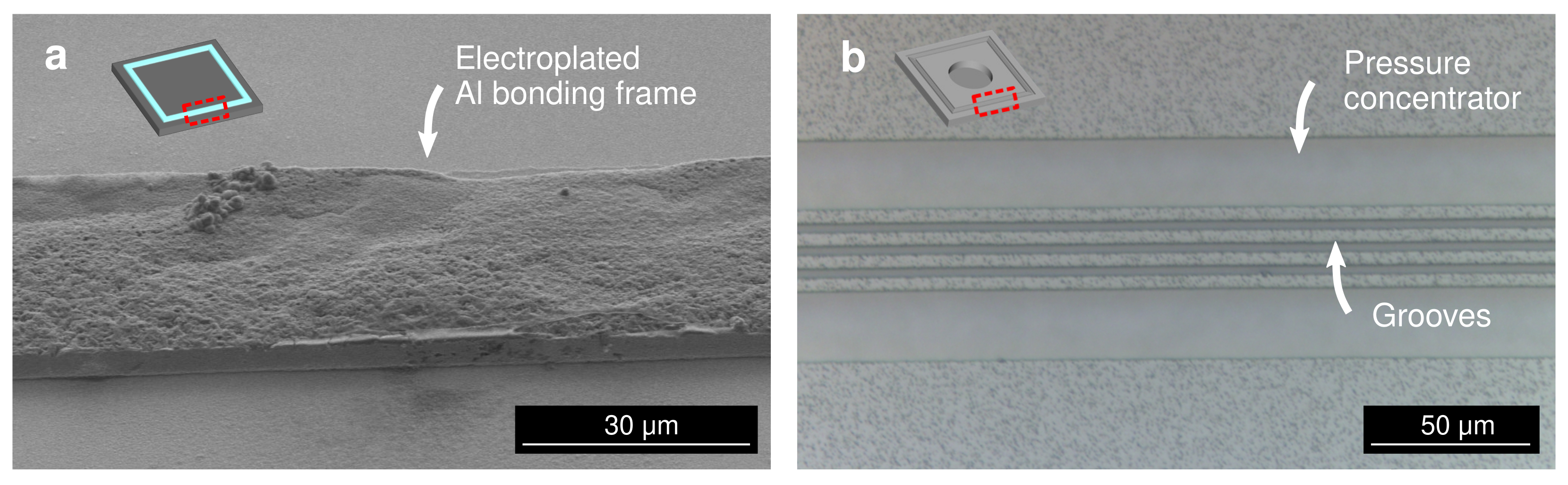
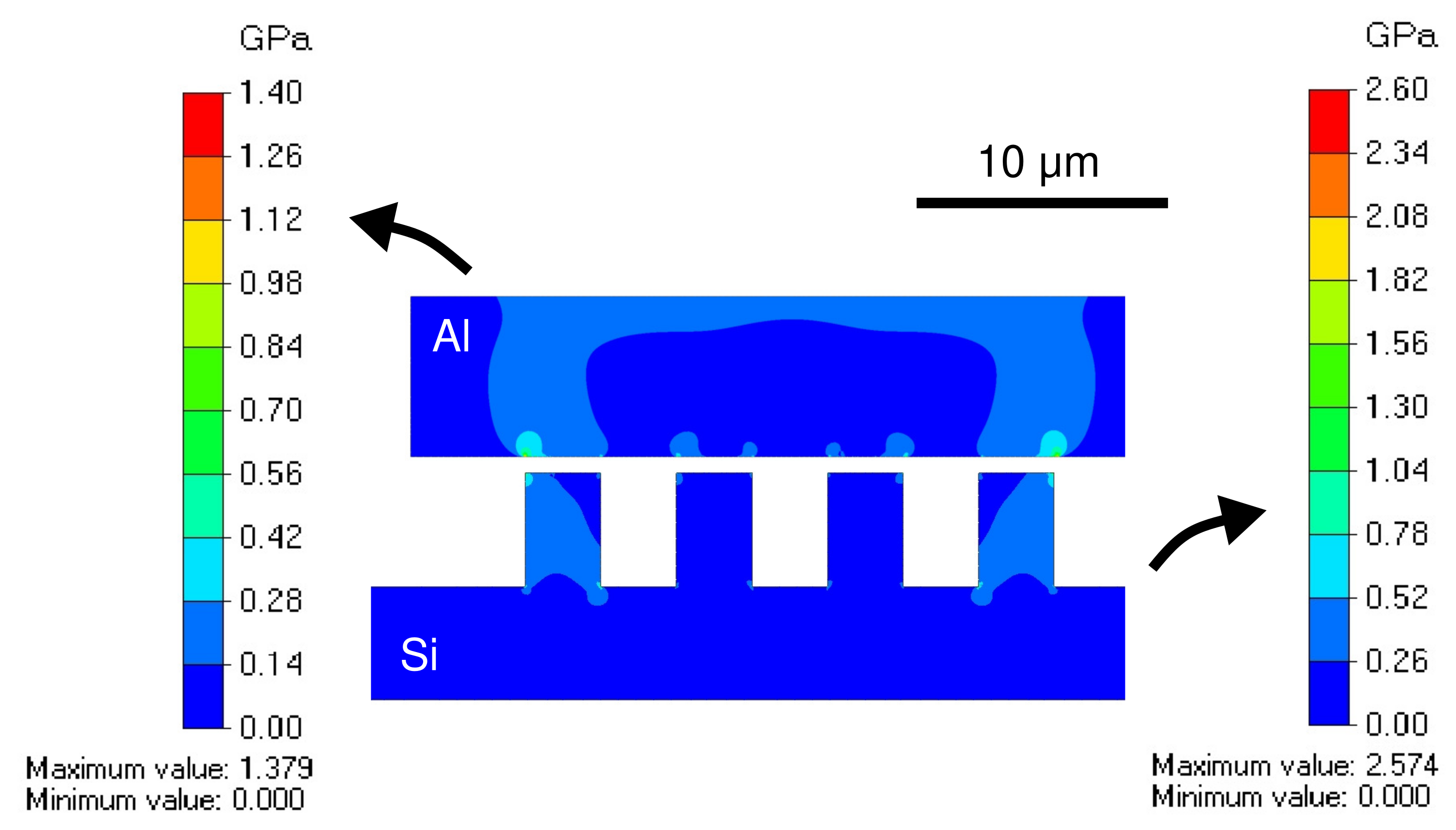
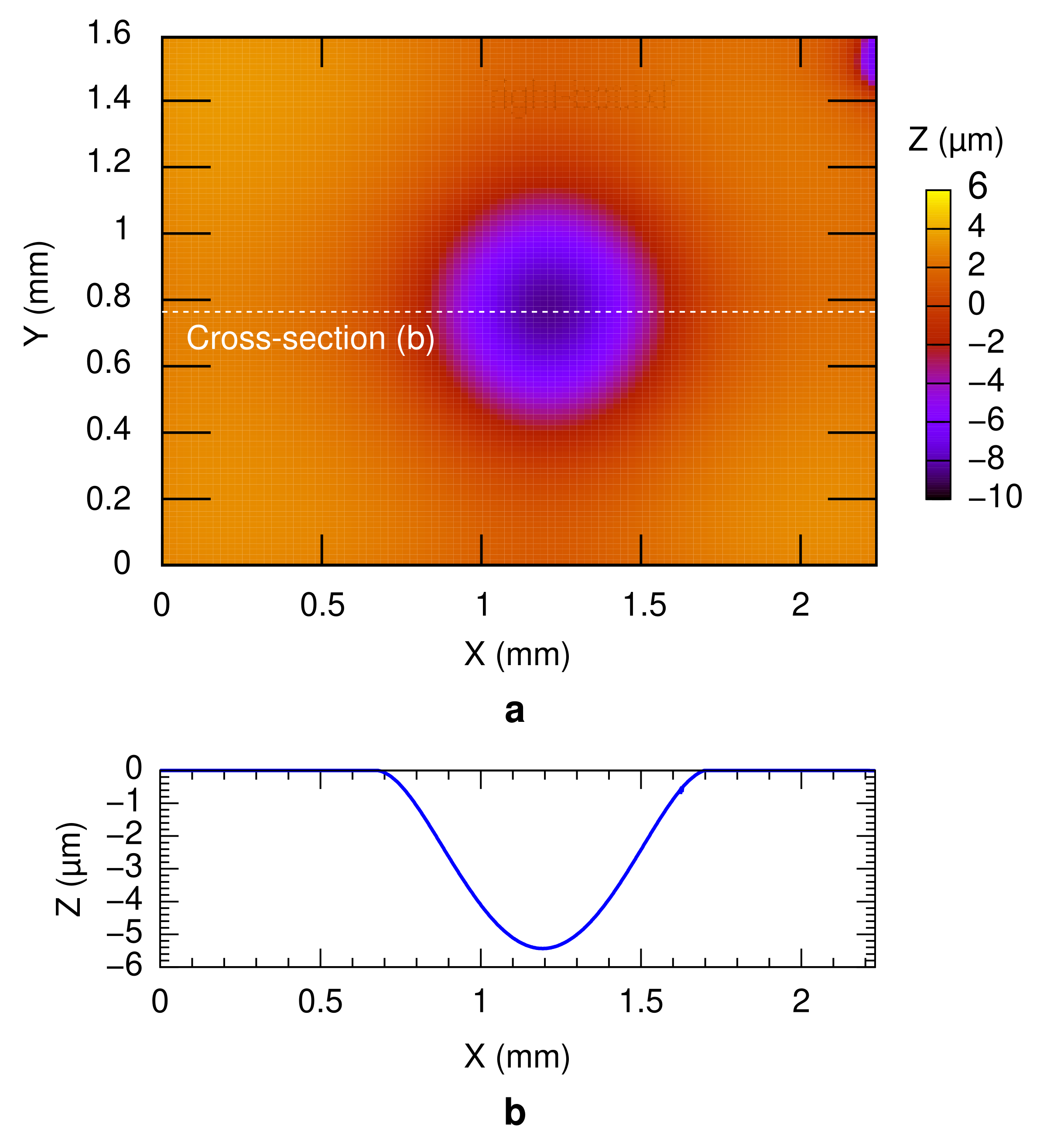
3.1. Bonding Mechanism
3.2. Hermeticity
3.3. Dicing Yield
3.4. Shear Strength
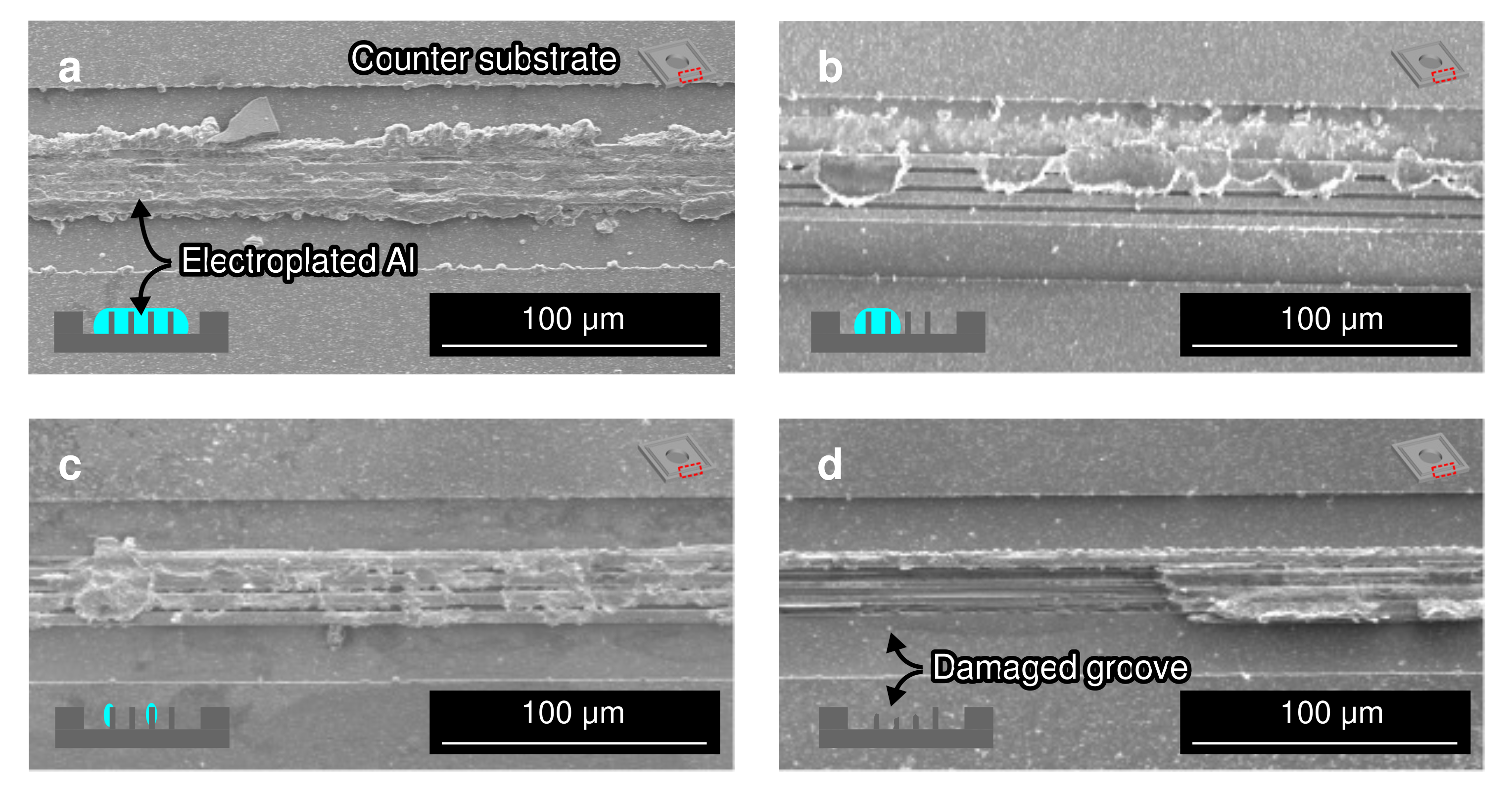
3.5. Fracture Surface
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fischer, A.C.; Forsberg, F.; Lapisa, M.; Bleiker, S.J.; Stemme, G.; Roxhed, N.; Niklaus, F. Integrating MEMS and ICs. Microsyst. Nanoeng. 2015, 1, 15005. [Google Scholar] [CrossRef]
- Reichl, H.; Grosser, V. Overview and development trends in the field of MEMS packaging. In Proceedings of the Technical Digest. MEMS 2001. 14th IEEE International Conference on Micro Electro Mechanical Systems (Cat. No. 01CH37090), Interlaken, Switzerland, 25–25 January 2001; pp. 1–5. [Google Scholar] [CrossRef]
- Tummala, R.R. SOP: What is it and why? A new microsystem-integration technology paradigm-Moore’s law for system integration of miniaturized convergent systems of the next decade. IEEE Trans. Adv. Packag. 2004, 27, 241–249. [Google Scholar] [CrossRef]
- Lapisa, M.; Stemme, G.; Niklaus, F. Wafer-level heterogeneous integration for MOEMS, MEMS, and NEMS. IEEE J. Sel. Top. Quantum Electron. 2011, 17, 629–644. [Google Scholar] [CrossRef]
- Esashi, M.; Tanaka, S. Stacked integration of MEMS on LSI. Micromachines 2016, 7, 137. [Google Scholar] [CrossRef]
- Rogers, T.; Kowal, J. Selection of glass, anodic bonding conditions and material compatibility for silicon-glass capacitive sensors. Sens. Actuators A Phys. 1995, 46, 113–120. [Google Scholar] [CrossRef]
- Niklaus, F.; Stemme, G.; Lu, J.Q.; Gutmann, R.J. Adhesive wafer bonding. J. Appl. Phys. 2006, 99, 2. [Google Scholar] [CrossRef]
- Farrens, S. Metal Based Wafer Level Packaging. In Proceedings of the International Wafer-Level Packaging Conference, San Jose, CA, USA, 13–16 October 2008; pp. 6–14. [Google Scholar]
- Sun, L.; Chen, M.H.; Zhang, L.; He, P.; Xie, L.S. Recent progress in SLID bonding in novel 3D-IC technologies. J. Alloys Compd. 2020, 818, 152825. [Google Scholar] [CrossRef]
- Shimatsu, T.; Mollema, R.H.; Monsma, D.; Keim, E.G.; Lodder, J.C. Metal bonding during sputter film deposition. J. Vac. Sci. Technol. A Vac. Surfaces Film. 1998, 16, 2125–2131. [Google Scholar] [CrossRef]
- Shimatsu, T.; Uomoto, M. Atomic diffusion bonding of wafers with thin nanocrystalline metal films. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2010, 28, 706–714. [Google Scholar] [CrossRef]
- Taklo, M.M.V.; Storås, P.; Schjølberg-Henriksen, K.; Hasting, H.K.; Jakobsen, H. Strong, high-yield and low-temperature thermocompression silicon wafer-level bonding with gold. J. Micromech. Microeng. 2004, 14, 884–890. [Google Scholar] [CrossRef]
- Tsau, C.H.; Spearing, S.M.; Schmidt, M.A. Characterization of wafer-level thermocompression bonds. J. Microelectromech. Syst. 2004, 13, 963–971. [Google Scholar] [CrossRef]
- Chen, K.N.; Fan, A.; Reif, R. Interfacial morphologies and possible mechanisms of copper wafer bonding. J. Mater. Sci. 2002, 37, 3441–3446. [Google Scholar] [CrossRef]
- Tanaka, K.; Wang, W.S.; Baum, M.; Froemel, J.; Hirano, H.; Tanaka, S.; Wiemer, M.; Otto, T. Investigation of Surface Pre-Treatment Methods for Wafer-Level Cu-Cu Thermo-Compression Bonding. Micromachines 2016, 7, 234. [Google Scholar] [CrossRef] [PubMed]
- Malik, N.; Schjølberg-Henriksen, K.; Poppe, E.; Taklo, M.M.V.; Finstad, T.G. Al-Al thermocompression bonding for wafer-level MEMS sealing. Sens. Actuators A Phys. 2014, 211, 115–120. [Google Scholar] [CrossRef]
- Froemel, J.; Baum, M.; Wiemer, M.; Roscher, F.; Haubold, M.; Jia, C.; Gessner, T. Investigations of thermocompression bonding with thin metal layers. In Proceedings of the 2011 16th IEEE International Solid-State Sensors, Actuators and Microsystems Conference, Beijing, China, 5–9 June 2011; pp. 990–993. [Google Scholar] [CrossRef]
- Malik, N.; Schjølberg-Henriksen, K.; Poppe, E.U.; Taklo, M.M.V.; Finstad, T.G. Impact of SiO2 on Al–Al thermocompression wafer bonding. J. Micromech. Microeng. 2015, 25, 035025. [Google Scholar] [CrossRef][Green Version]
- Satoh, S.; Fukushi, H.; Esashi, M.; Tanaka, S. Role of Thin Sn Layer for Low Temperature Al-Al Thermo-compression Bonding of Wafer-Level Hermetic Sealing. Electron. Commun. Jpn. 2018, 101, 33–40. [Google Scholar] [CrossRef]
- Schulze, S.; Wietstruck, M.; Fraschke, M.; Kerepesi, P.; Kurz, H.; Rebhan, B.; Kaynak, M. Optimization of a BEOL Aluminum Deposition Process Enabling Wafer Level Al-Al Thermo-Compression Bonding. In Proceedings of the 2019 IEEE 69th Electronic Components and Technology Conference, Las Vegas, NV, USA, 28–31 May 2019; pp. 218–224. [Google Scholar] [CrossRef]
- Cheemalamarri, H.K.; Bonam, S.; Vanjari, S.R.K.; Singh, S.G. Effect of ultrathin palladium layer in achieving a low temperature and pressure wafer level aluminum to aluminum bonding. Surf. Topogr. Metrol. Prop. 2020, 8, 045008. [Google Scholar] [CrossRef]
- Takagi, H.; Maeda, R.; Chung, T.R.; Hosoda, N.; Suga, T. Effect of surface roughness on room-temperature wafer bonding by Ar beam surface activation. Jpn. J. Appl. Phys. 1998, 37, 4197–4203. [Google Scholar] [CrossRef]
- Shigetou, A.; Itoh, T.; Suga, T. Direct bonding of CMP-Cu films by surface activated bonding (SAB) method. J. Mater. Sci. 2005, 40, 3149–3154. [Google Scholar] [CrossRef]
- Kurashima, Y.; Maeda, A.; Takagi, H. Room-temperature wafer scale bonding using smoothed Au seal ring surfaces for hermetic sealing. Jpn. J. Appl. Phys. 2016, 55, 016701. [Google Scholar] [CrossRef]
- Al Farisi, M.S.; Hirano, H.; Frömel, J.; Tanaka, S. Wafer-level hermetic thermo-compression bonding using electroplated gold sealing frame planarized by fly-cutting. J. Micromech. Microeng. 2017, 27, 015029. [Google Scholar] [CrossRef]
- Al Farisi, M.S.; Hirano, H.; Tanaka, S. Low-temperature hermetic thermo-compression bonding using electroplated copper sealing frame planarized by fly-cutting for wafer-level MEMS packaging. Sens. Actuators A Phys. 2018, 279, 671–679. [Google Scholar] [CrossRef]
- Decharat, A.; Yu, J.; Boers, M.; Stemme, G.; Niklaus, F. Room-temperature sealing of microcavities by cold metal welding. J. Microelectromech. Syst. 2009, 18, 1318–1325. [Google Scholar] [CrossRef]
- Wang, X.; Bleiker, S.J.; Antelius, M.; Stemme, G.; Niklaus, F. Wafer-Level Vacuum Packaging Enabled by Plastic Deformation and Low-Temperature Welding of Copper Sealing Rings with a Small Footprint. J. Microelectromech. Syst. 2017, 26, 357–365. [Google Scholar] [CrossRef]
- Endres, F. Ionic liquids: Solvents for the electrodeposition of metals and semiconductors. ChemPhysChem 2002, 3, 144–154. [Google Scholar] [CrossRef]
- Jiang, T.; Chollier Brym, M.J.; Dubé, G.; Lasia, A.; Brisard, G.M. Electrodeposition of aluminium from ionic liquids: Part II—Studies on the electrodeposition of aluminum from aluminum chloride (AICl3)—Trimethylphenylammonium chloride (TMPAC) ionic liquids. Surf. Coat. Technol. 2006, 201, 10–18. [Google Scholar] [CrossRef]
- Berretti, E.; Giaccherini, A.; Martinuzzi, S.M.; Innocenti, M.; Schubert, T.J.S.; Stiemke, F.M.; Caporali, S. Aluminium electrodeposition from ionic liquid: Effect of deposition temperature and sonication. Materials 2016, 9, 719. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.X.; El Abedin, S.Z.; Endres, F. Electroplating of mild steel by aluminium in a first generation ionic liquid: A green alternative to commercial Al-plating in organic solvents. Surf. Coat. Technol. 2006, 201, 1352–1356. [Google Scholar] [CrossRef]
- Bardi, U.; Caporali, S.; Craig, M.; Giorgetti, A.; Perissi, I.; Nicholls, J.R. Electrodeposition of aluminium film on P90 Li-Al alloy as protective coating against corrosion. Surf. Coat. Technol. 2009, 203, 1373–1378. [Google Scholar] [CrossRef]
- Lin, M.C.; Gong, M.; Lu, B.; Wu, Y.; Wang, D.Y.; Guan, M.; Angell, M.; Chen, C.; Yang, J.; Hwang, B.J.; et al. An ultrafast rechargeable aluminium-ion battery. Nature 2015, 520, 324–328. [Google Scholar] [CrossRef]
- Wang, S.; Yu, Z.; Tu, J.; Wang, J.; Tian, D.; Liu, Y.; Jiao, S. A Novel Aluminum-Ion Battery: Al/AlCl3-[EMIm]Cl/Ni3S2@Graphene. Adv. Energy Mater. 2016, 6, 1600137. [Google Scholar] [CrossRef]
- Al Farisi, M.S.; Hertel, S.; Wiemer, M.; Otto, T. Aluminum Patterned Electroplating from AlCl3–[EMIm]Cl Ionic Liquid towards Microsystems Application. Micromachines 2018, 9, 589. [Google Scholar] [CrossRef] [PubMed]
- Al Farisi, M.S.; Tsukamoto, T.; Tanaka, S. Tailoring material properties of electrochemically deposited Al film from chloroaluminate ionic liquid for microsystem technology using pulsed deposition. Sens. Actuators A Phys. 2020, 316, 112384. [Google Scholar] [CrossRef]
- Al Farisi, M.S.; Tsukamoto, T.; Tanaka, S. Mechanical hardening of electrochemically deposited aluminum from chloroaluminate ionic liquid. Scr. Mater. 2022, 213, 114599. [Google Scholar] [CrossRef]
- Al Farisi, M.S.; Tsukamoto, T.; Tanaka, S. Fully CMOS-Compatible Wafer Bonding Based on Press Marking Using Thick Electroplated Aluminum. In Proceedings of the 2021 21st IEEE International Conference on Solid-State Sensors, Actuators and Microsystems (Transducers), Online, 20–24 June 2021; pp. 1138–1141. [Google Scholar] [CrossRef]
- Timoshenko, S.; Woinowsky-Krieger, S. Theory of Plates and Shells; McGraw-Hill: New York, NY, USA, 1987. [Google Scholar]
- Tanaka, S.; Fukushi, H. Comprehensive study on wafer-level vacuum packaging using anodically-bondable LTCC wafer and thin film getter. In Proceedings of the 2015 Transducers-2015 IEEE 18th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS), Anchorage, AK, USA, 21–25 June 2015; pp. 468–471. [Google Scholar] [CrossRef]
- Al Farisi, M.S.; Hirano, H.; Tanaka, S. Zero-Balance Method for Evaluation of Sealed Cavity Pressure Down to Single Digit Pa Using Thin Silicon Diaphragm. J. Microelectromech. Syst. 2020, 29, 418–426. [Google Scholar] [CrossRef]
- Legtenberg, R.; Tilmans, H.A.C. Electrostatically driven vacuum-encapsulated polysilicon resonators Part I. Design and fabrication. Sens. Actuators A Phys. 1994, 45, 57–66. [Google Scholar] [CrossRef]
- Frömel, J.; Billep, D.; Geßner, T.; Wiemer, M. Application of micromechanical resonant structures for measuring the sealing of bonded sensor systems. Microsyst. Technol. 2006, 12, 481–483. [Google Scholar] [CrossRef]
- Stark, B.; Mei, Y.; Zhang, C.; Najafi, K. A Doubly Anchored Surface Micromachined Pirani Gauge for Vacuum Package Characterization. In Proceedings of the Sixteenth Annual International Conference on Micro Electro Mechanical Systems, Kyoto, Japan, 23–23 January 2003; pp. 506–509. [Google Scholar] [CrossRef]
- Mailly, F.; Dumas, N.; Pous, N.; Latorre, L.; Garel, O.; Martincic, E.; Verjus, F.; Pellet, C.; Dufour-Gergam, E.; Nouet, P. Pirani pressure sensor for smart wafer-level packaging. Sens. Actuators A Phys. 2009, 156, 201–207. [Google Scholar] [CrossRef]
- Santagata, F.; Creemer, J.F.; Iervolino, E.; Sarro, P.M. Tube-shaped Pirani gauge for in situ hermeticity monitoring of SiN thin-film encapsulation. J. Micromech. Microeng. 2012, 22, 105025. [Google Scholar] [CrossRef]
- Asano, S.; Muroyama, M.; Nakayama, T.; Hata, Y.; Nonomura, Y.; Tanaka, S. 3-Axis Fully-Integrated Capacitive Tactile Sensor with Flip-Bonded CMOS on LTCC Interposer. Sensors 2017, 17, 2451. [Google Scholar] [CrossRef] [PubMed]
- Bahari, J.; Jones, J.D.; Leung, A.M. Sensitivity improvement of micromachined convective accelerometers. J. Microelectromech. Syst. 2012, 21, 646–655. [Google Scholar] [CrossRef]
- Khan, M.J.; Tsukamoto, T.; Al Farisi, M.S.; Tanaka, S. Fabrication method of micromachined quartz glass resonator using sacrificial supporting structures. Sens. Actuators A Phys. 2020, 305, 111922. [Google Scholar] [CrossRef]



| Material | Deposition | Features |
|---|---|---|
| Au | Sputtering/evaporation |
|
| Electroplating |
| |
| Cu | Sputtering/evaporation |
|
| Electroplating |
| |
| Al | Sputtering/evaporation |
|
| Technology | Temp. (C) | Strength (MPa) | Note | Ref. |
|---|---|---|---|---|
| Ultra thin film | RT | NA | Consecutive deposition-bonding | [11] |
| Sputtered film | 450 | 339 | Sputtered thin film | [17] |
| 300–450 | 11–80 | [18] | ||
| Surface passivated | 360–390 | 32–209 | Sn passivation | [19] |
| Impurity included | 300–500 | NA | Cu impurity | [20] |
| Impurity + passivation | 250 | 30 | Cu impurity, Pd passivation | [21] |
| This study | 250–450 | 8–100 | Pure Al press marking |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Farisi, M.S.; Tsukamoto, T.; Tanaka, S. Electroplated Al Press Marking for Wafer-Level Bonding. Micromachines 2022, 13, 1221. https://doi.org/10.3390/mi13081221
Al Farisi MS, Tsukamoto T, Tanaka S. Electroplated Al Press Marking for Wafer-Level Bonding. Micromachines. 2022; 13(8):1221. https://doi.org/10.3390/mi13081221
Chicago/Turabian StyleAl Farisi, Muhammad Salman, Takashiro Tsukamoto, and Shuji Tanaka. 2022. "Electroplated Al Press Marking for Wafer-Level Bonding" Micromachines 13, no. 8: 1221. https://doi.org/10.3390/mi13081221
APA StyleAl Farisi, M. S., Tsukamoto, T., & Tanaka, S. (2022). Electroplated Al Press Marking for Wafer-Level Bonding. Micromachines, 13(8), 1221. https://doi.org/10.3390/mi13081221






