Electrografting a Hybrid Bilayer Membrane via Diazonium Chemistry for Electrochemical Impedance Spectroscopy of Amyloid-β Aggregation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instruments
2.3. Electrode Pretreatment
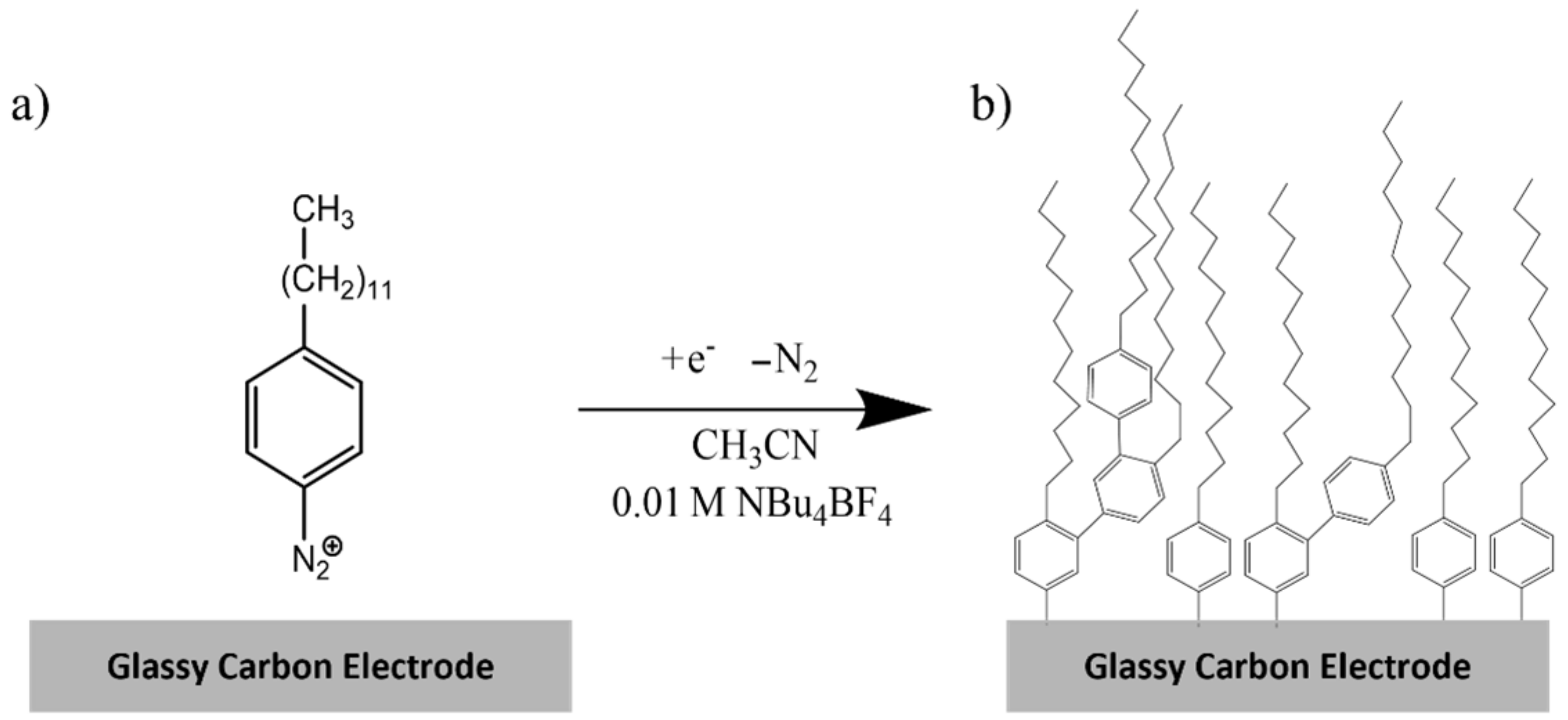
2.4. Synthesis of 4-dodecylbenzenediazonium tetrafluoroborate (DDAN)
2.5. Synthesis of 4-ethylbenzenediazonium (EDAN)
2.6. Electrode Modification
2.7. Electrochemical Measurements
2.8. Aβ1–42 Aggregation Studies
3. Results and Discussion
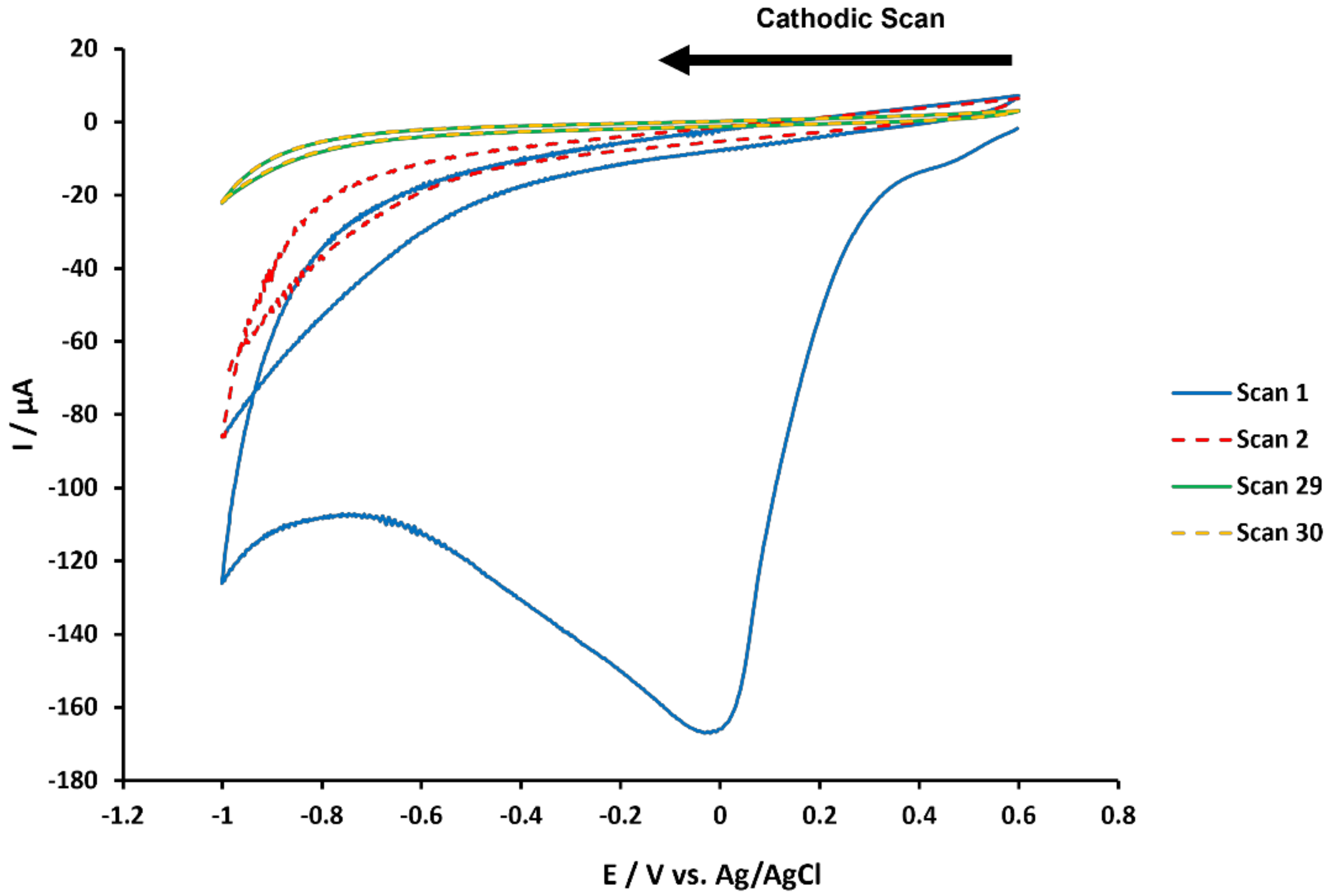
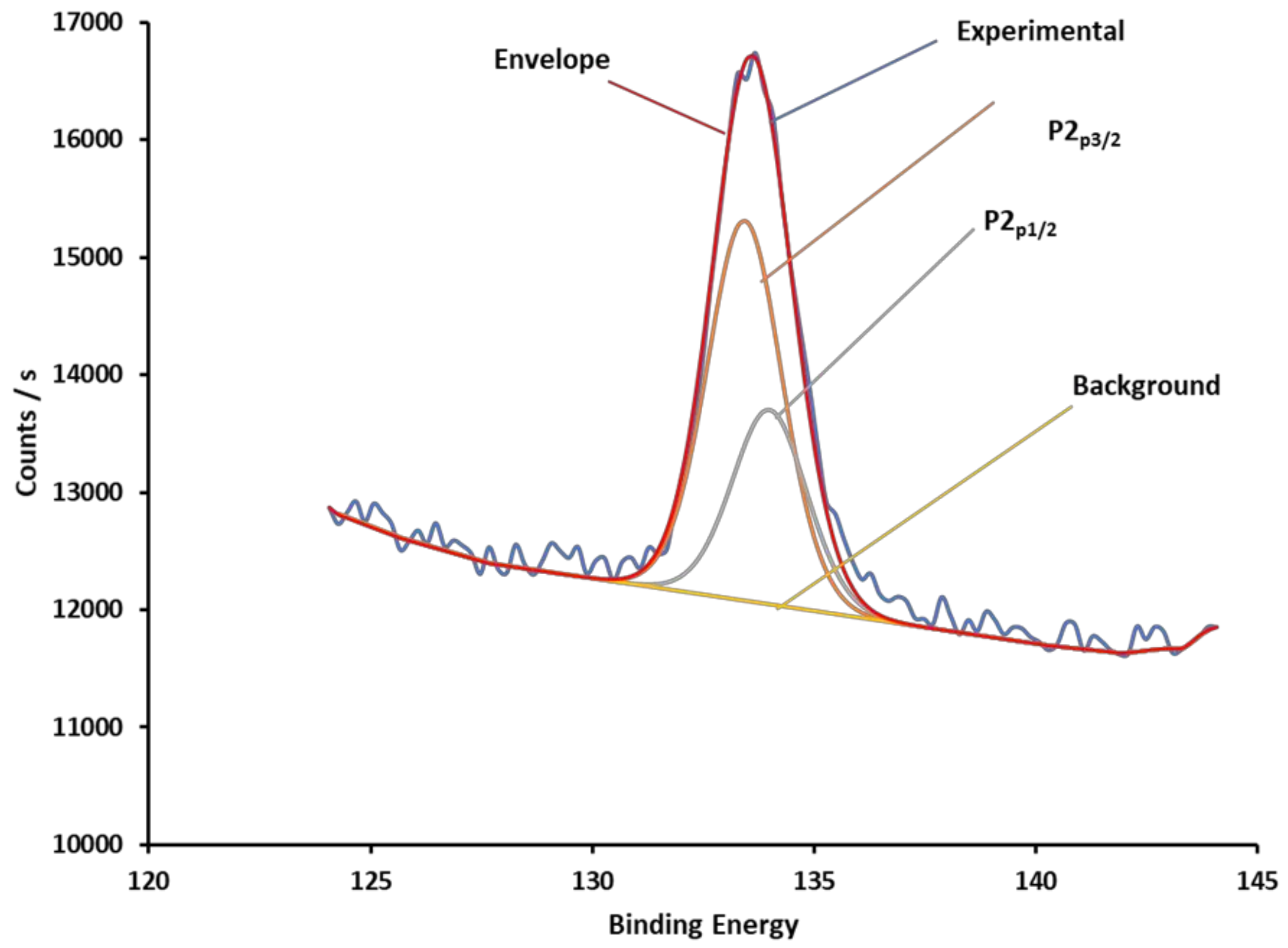
3.1. Covalent Modification of GCE Surfaces
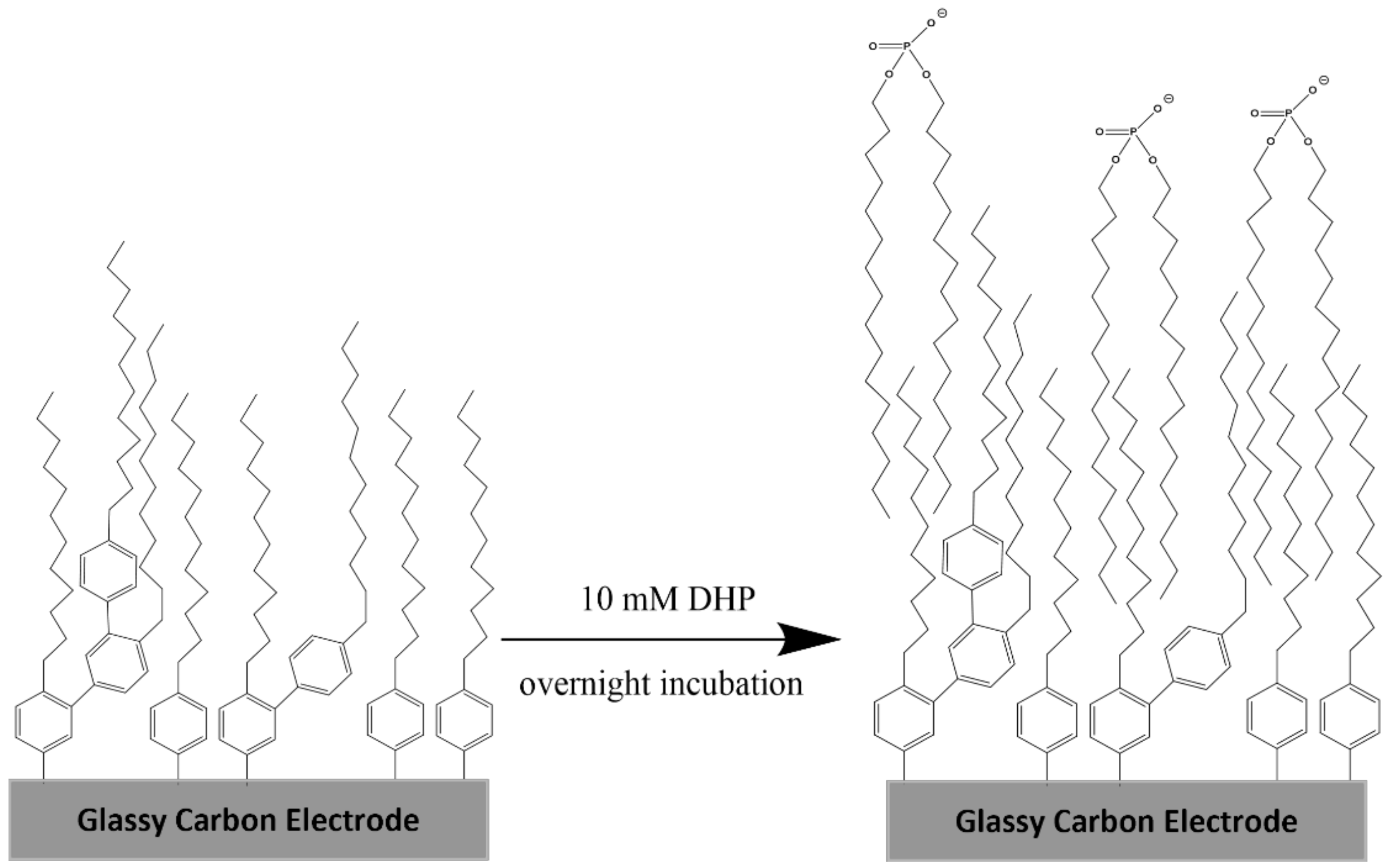
3.2. Preparation of the Hybrid Bilayer Membrane (HBLM)
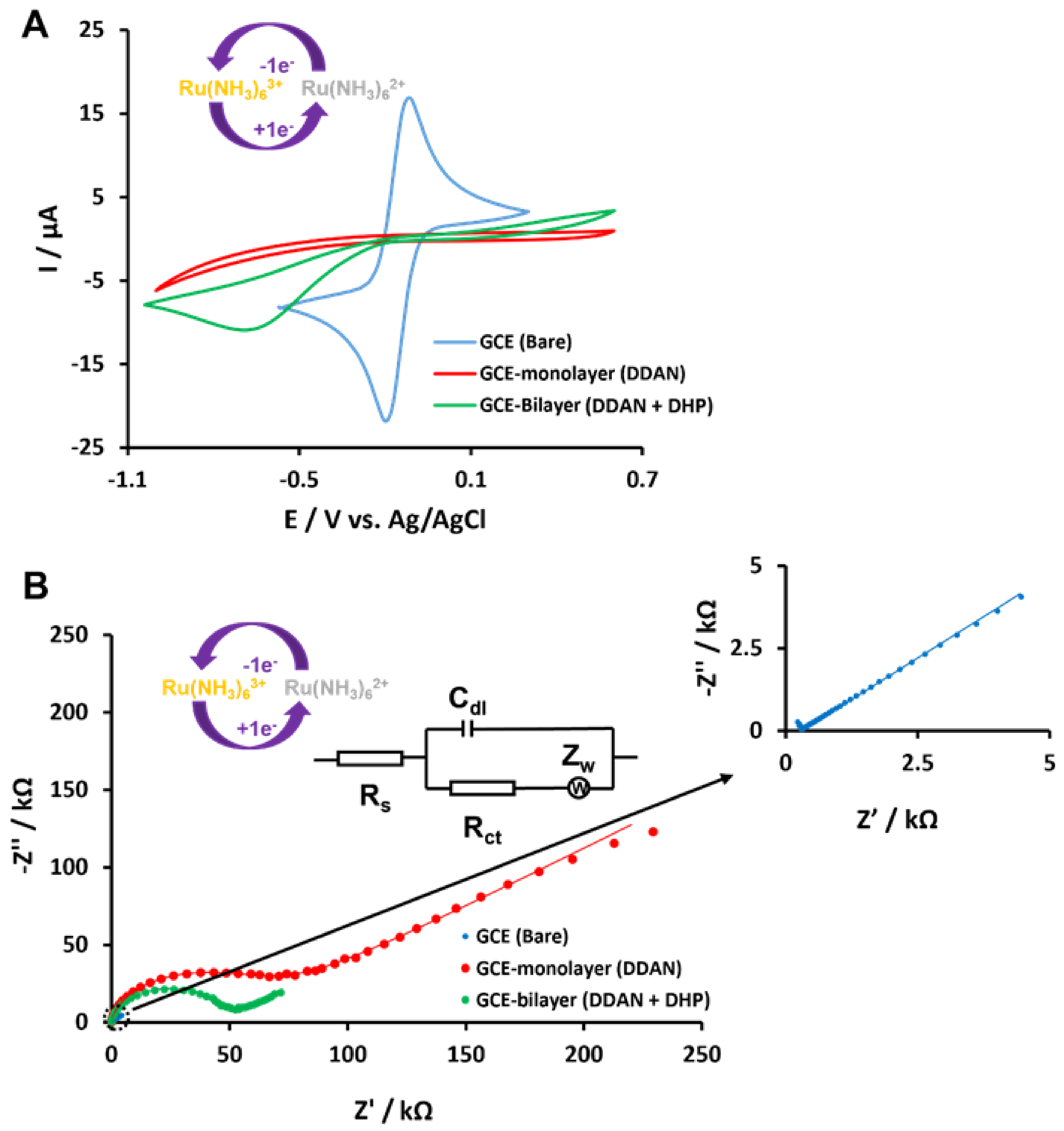
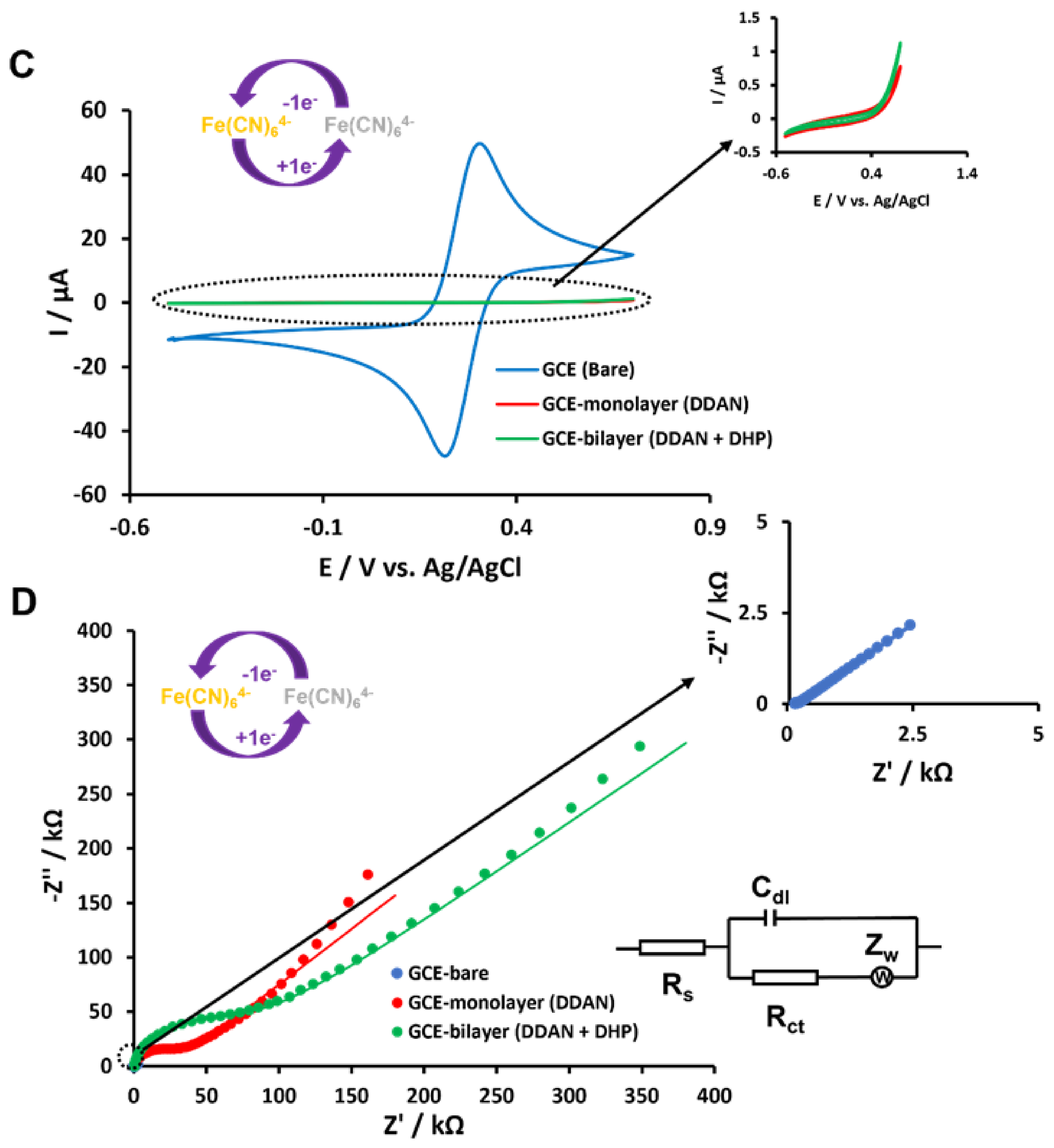
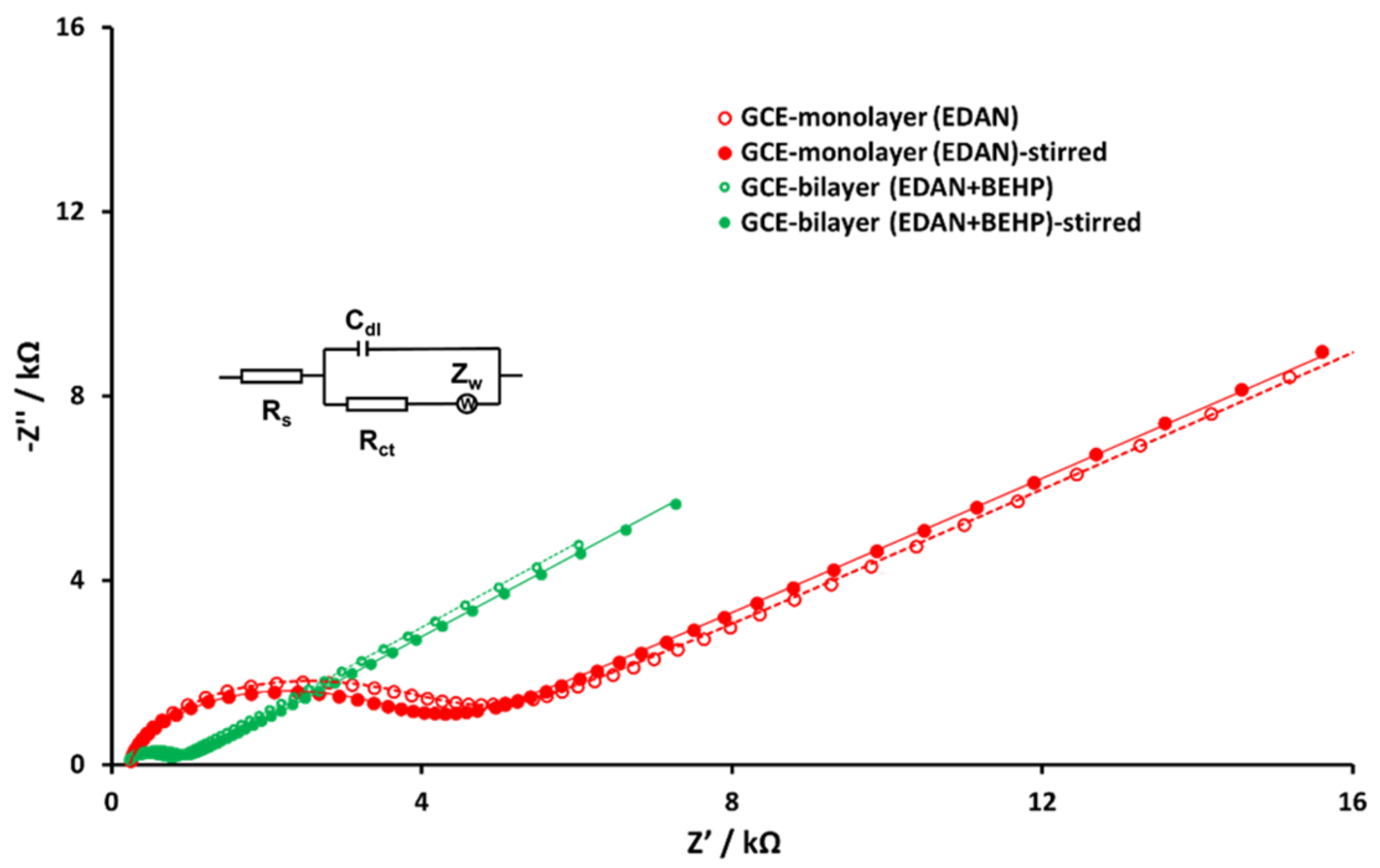
3.3. Electrochemistry of lcHBLM- and scHBLM-Modified Surfaces
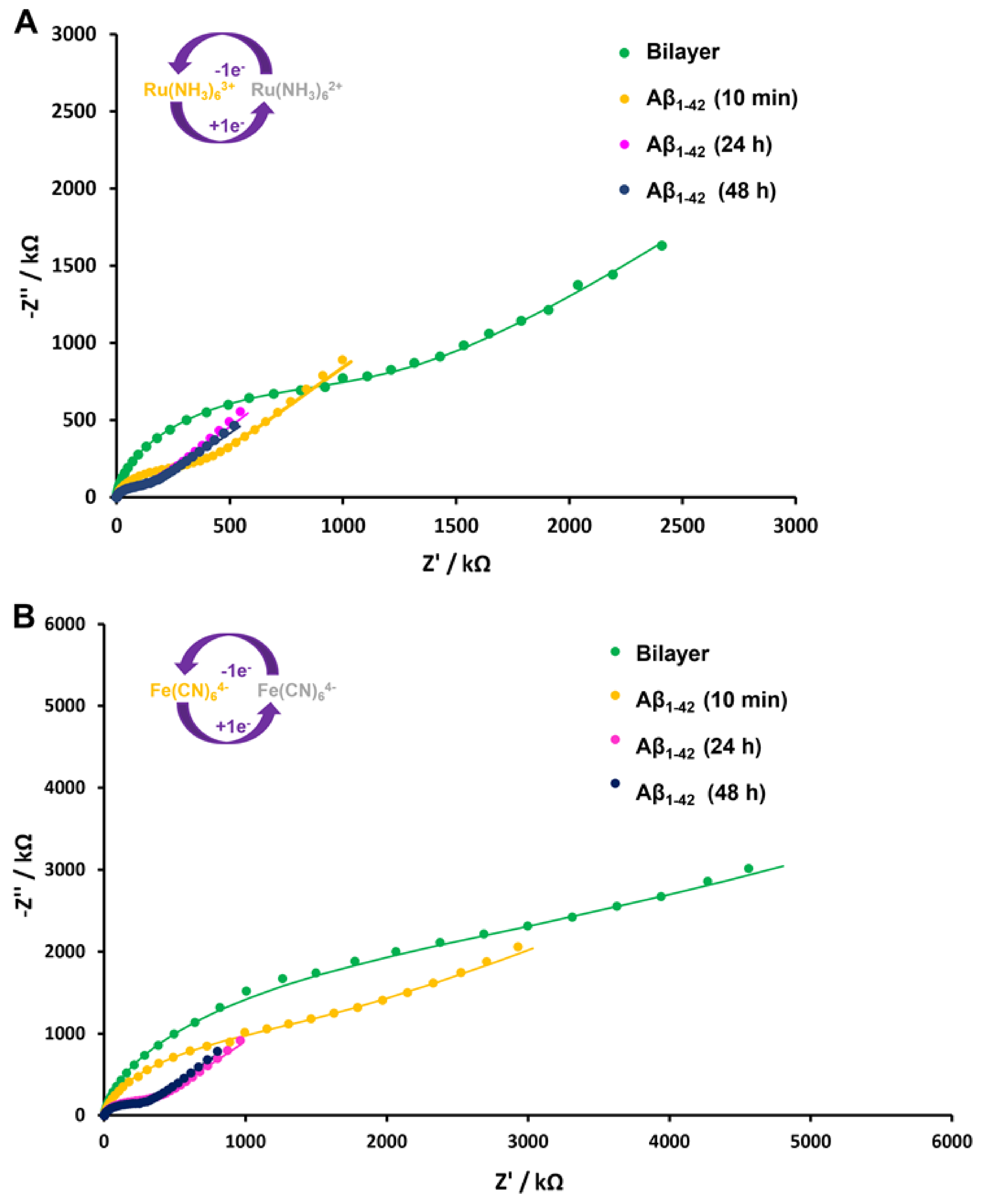
3.4. Interaction with Aβ1–42
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rebaud, S.; Maniti, O.; Girard-Egrot, A.P. Tethered bilayer lipid membranes (tBLMs): Interest and applications for biological membrane investigations. Biochimie 2014, 107, 135–142. [Google Scholar] [CrossRef]
- Shen, Y.X.; Saboe, P.O.; Sines, I.T.; Erbakan, M.; Kumar, M. Biomimetic membranes: A review. J. Memb. Sci. 2014, 454, 359–381. [Google Scholar] [CrossRef]
- Su, Z.; Leitch, J.J.; Lipkowski, J.; Maria, A.; Brett, O. Electrode-supported biomimetic membranes: An electrochemical and surface science approach for characterizing biological cell membranes. Curr. Opin. Electrochem. 2018, 12, 60–72. [Google Scholar] [CrossRef]
- Ogier, S.D.; Bushby, R.J.; Cheng, Y.; Evans, S.D.; Evans, S.W.; Jenkins, A.T.A.; Knowles, P.F.; Miles, R.E. Suspended planar phospholipid bilayers on micromachined supports. Langmuir 2000, 16, 5696–5701. [Google Scholar] [CrossRef]
- Deniaud, A.; Rossi, C.; Berquand, A.; Homand, J.; Campagna, S.; Knoll, W.; Brenner, C.; Chopineau, J. Voltage-dependent anion channel transports calcium ions through biomimetic membranes. Langmuir 2007, 23, 3898–3905. [Google Scholar] [CrossRef]
- Lautscham, L.A.; Lin, C.Y.; Auernheimer, V.; Naumann, C.A.; Goldmann, W.H.; Fabry, B. Biomembrane-mimicking lipid bilayer system as a mechanically tunable cell substrate. Biomaterials 2014, 35, 3198–3207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plant, A.L. Supported hybrid bilayer membranes as rugged cell membrane mimics. Langmuir 1999, 15, 5128–5135. [Google Scholar] [CrossRef]
- Burgess, J.D.; Rhoten, M.C.; Hawkridge, F.M. Cytochrome c oxidase immobilized in stable supported lipid bilayer membranes. Langmuir 1998, 14, 2467–2475. [Google Scholar] [CrossRef]
- Silin, V.I.; Wieder, H.; Woodward, J.T.; Valincius, G.; Offenhausser, A.; Plant, A.L. The role of surface free energy on the formation of hybrid bilayer membranes. J. Am. Chem. Soc. 2002, 124, 14676–14683. [Google Scholar] [CrossRef] [Green Version]
- Anderson, N.A.; Richter, L.J.; Stephenson, J.C.; Briggman, K.A. Characterization and control of lipid layer fluidity in hybrid bilayer membranes. J. Am. Chem. Soc. 2007, 129, 2094–2100. [Google Scholar] [CrossRef]
- Mueller, P.; Rudin, D.O.; Ti Tien, H.; Wescott, W.C. Reconstitution of cell membrane structure in vitro and its transformation into an excitable system. Nature 1962, 194, 979–980. [Google Scholar] [CrossRef] [PubMed]
- Kunitake, T.; Okahata, Y. A totally synthetic bilayer membrane. J. Am. Chem. Soc. 1977, 99, 3860–3861. [Google Scholar] [CrossRef]
- Brian, A.A.; McConnell, H.M. Allogeneic stimulation of cytotoxic T cells by supported planar membranes. Proc. Natl. Acad. Sci. USA 1984, 81, 6159–6163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, L.; Jenkins, A.T.A. The effect of the ionophore valinomycin on biomimetic solid supported lipid DPPTE/EPC membranes. Bioelectrochemistry 2007, 70, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Knoll, W.; Naumann, R.; Friedrich, M.; Robertson, J.W.F.; Lösche, M.; Heinrich, F.; McGillivray, D.J.; Schuster, B.; Gufler, P.C.; Pum, D.; et al. Solid supported lipid membranes: New concepts for the biomimetic functionalization of solid surfaces. Biointerphases 2008, 3, FA125–FA135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sackmann, E.; Tanaka, M. Supported membranes on soft polymer cushions: Fabrication, characterization and applications. Trends Biotechnol. 2000, 18, 58–64. [Google Scholar] [CrossRef]
- Smith, H.L.; Jablin, M.S.; Vidyasagar, A.; Saiz, J.; Watkins, E.; Toomey, R.; Hurd, A.J.; Majewski, J. Model lipid membranes on a tunable polymer cushion. Phys. Rev. Lett. 2009, 102, 228102. [Google Scholar] [CrossRef] [PubMed]
- Schiller, S.M.; Naumann, R.; Lovejoy, K.; Kunz, H.; Knoll, W. Archaea analogue thiolipids for tethered bilayer lipid membranes on ultrasmooth gold surfaces. Angew. Chem. Int. Ed. Engl. 2003, 42, 208–211. [Google Scholar] [CrossRef]
- Rossi, C.; Chopineau, J. Biomimetic tethered lipid membranes designed for membrane-protein interaction studies. Eur. Biophys. J. 2007, 36, 955–965. [Google Scholar] [CrossRef]
- Tse, E.C.M.; Barile, C.J.; Gewargis, J.P.; Li, Y.; Zimmerman, S.C.; Gewirth, A.A. Anion transport through lipids in a hybrid bilayer membrane. Anal. Chem. 2015, 87, 2403–2409. [Google Scholar] [CrossRef]
- Patrice, F.T.; Zhao, L.J.; Fodjo, E.K.; Li, D.W.; Qiu, K.; Long, Y.T. Highly sensitive and selective electrochemical detection of dopamine using hybrid bilayer membranes. ChemElectroChem 2019, 6, 634–637. [Google Scholar] [CrossRef]
- Favero, G.; Campanella, L.; Cavallo, S.; D’Annibale, A.; Perrella, M.; Mattei, E.; Ferri, T. Glutamate receptor incorporated in a mixed hybrid bilayer lipid membrane array, as a sensing element of a biosensor working under flowing conditions. J. Am. Chem. Soc. 2005, 127, 8103–8111. [Google Scholar] [CrossRef] [PubMed]
- Meuse, C.W.; Niaura, G.; Lewis, M.L.; Plant, A.L. Assessing the molecular structure of alkanethiol monolayers in hybrid bilayer membranes with vibrational spectroscopies. Langmuir 1998, 14, 1604–1611. [Google Scholar] [CrossRef]
- Majkrzak, C.F.; Berk, N.F.; Krueger, S.; Dura, J.A.; Tarek, M.; Tobias, D.; Silin, V.; Meuse, C.W.; Woodward, J.; Plant, A.L. First-principles determination of hybrid bilayer membrane structure by phase-sensitive neutron reflectometry. Biophys. J. 2000, 79, 3330–3340. [Google Scholar] [CrossRef] [Green Version]
- Mozsolits, H.; Wirth, H.J.; Werkmeister, J.; Aguilar, M.I. Analysis of antimicrobial peptide interactions with hybrid bilayer membrane systems using surface plasmon resonance. Biochim. Biophys. Acta Biomembr. 2001, 1512, 64–76. [Google Scholar] [CrossRef] [Green Version]
- Suraniti, E.; Tumolo, T.; Baptista, M.S.; Livache, T.; Calemczuk, R. Construction of Hybrid Bilayer Membrane (HBM) biochips and characterization of the cooperative binding between cytochrome-c and HBM. Langmuir 2007, 23, 6835–6842. [Google Scholar] [CrossRef]
- Meuse, C.W.; Krueger, S.; Majkrzak, C.F.; Dura, J.A.; Fu, J.; Connor, J.T.; Plant, A.L. Hybrid bilayer membranes in air and water: Infrared spectroscopy and neutron reflectivity studies. Biophys. J. 1998, 74, 1388–1398. [Google Scholar] [CrossRef] [Green Version]
- Krueger, S.; Meuse, C.W.; Majkrzak, C.F.; Dura, J.A.; Berk, N.F.; Tarek, M.; Plant, A.L. Investigation of hybrid bilayer membranes with neutron reflectometry: Probing the interactions of melittin. Langmuir 2001, 17, 511–521. [Google Scholar] [CrossRef]
- Doyle, A.W.; Fick, J.; Himmelhaus, M.; Eck, W.; Graziani, I.; Prudovsky, I.; Grunze, M.; Maciag, T.; Neivandt, D.J. Protein deformation of lipid hybrid bilayer membranes studied by sum frequency generation vibrational spectroscopy. Langmuir 2004, 20, 8961–8965. [Google Scholar] [CrossRef] [Green Version]
- Kundu, J.; Levin, C.S.; Halas, N.J. Real-time monitoring of lipid transfer between vesicles and hybrid bilayers on Au nanoshells using surface enhanced Raman scattering (SERS). Nanoscale 2009, 1, 114–117. [Google Scholar] [CrossRef]
- Millo, D.; Bonifacio, A.; Moncelli, M.R.; Sergo, V.; Gooijer, C.; van der Zwan, G. Characterization of hybrid bilayer membranes on silver electrodes as biocompatible SERS substrates to study membrane-protein interactions. Colloids Surf. B Biointerfaces 2010, 81, 212–216. [Google Scholar] [CrossRef]
- Ma, W.; Ying, Y.; Qin, L.; Gu, Z.; Zhou, H.; Li, D.; Sutherland, T.C.; Chen, H.; Long, Y. Investigating electron-transfer processes using a biomimetic hybrid bilayer membrane system. Nat. Protoc. 2013, 8, 439–450. [Google Scholar] [CrossRef]
- Gao, H.; Luo, G.A.; Feng, J.; Ottova, A.L.; Tien, H.T. Electrochemical properties of hybrid bilayer membranes and interaction with melittin. Acta Chim. Sin. 2001, 59, 220–223. [Google Scholar]
- Li, A.; Ma, Y.; Yang, F.; Yang, X. Interaction between α-actinin and negatively charged lipids membrane investigated by surface plasmon resonance and electrochemical methods. Appl. Surf. Sci. 2007, 253, 6103–6108. [Google Scholar] [CrossRef]
- Yue, M.; Zhu, X.; Zheng, Y.; Hu, T.; Yang, L.; Wu, X. Amphotericin B ion channel mimetic sensor: A new type of potassium-selective sensor based on electrode-supported hybrid bilayer membranes. Electrochim. Acta 2012, 73, 78–85. [Google Scholar] [CrossRef]
- Hosseini, A.; Barile, C.J.; Devadoss, A.; Eberspacher, T.A.; Decreau, R.A.; Collman, J.P. Hybrid bilayer membrane: A platform to study the role of proton flux on the efficiency of oxygen reduction by a molecular electrocatalyst. J. Am. Chem. Soc. 2011, 133, 11100–11102. [Google Scholar] [CrossRef]
- Li, Y.; Tse, E.C.M.; Barile, C.J.; Gewirth, A.A.; Zimmerman, S.C. Photoresponsive Molecular switch for regulating transmembrane proton-transfer kinetics. J. Am. Chem. Soc. 2015, 137, 14059–14062. [Google Scholar] [CrossRef]
- Wilkop, T.; Xu, D.; Cheng, Q. Characterization of pore formation by streptolysin O on supported lipid membranes by impedance spectroscopy and surface plasmon resonance spectroscopy. Langmuir 2007, 23, 1403–1409. [Google Scholar] [CrossRef]
- Peng, Z.; Tang, J.; Han, X.; Wang, E.; Dong, S. Formation of a supported hybrid bilayer membrane on gold: A sterically enhanced hydrophobic effect. Langmuir 2002, 18, 4834–4839. [Google Scholar] [CrossRef]
- Liang, G.; Man, Y.; Jin, X.; Pan, L.; Liu, X. Aptamer-based biosensor for label-free detection of ethanolamine by electrochemical impedance spectroscopy. Anal. Chim. Acta 2016, 936, 222–228. [Google Scholar] [CrossRef]
- Zhi, Z.; Hasan, I.Y.; Mechler, A. Formation of alkanethiol supported hybrid membranes revisited. Biotechnol. J. 2018, 13. [Google Scholar] [CrossRef]
- Han, X.; Wang, L.; Qi, B.; Yang, X.; Wang, E. A Strategy for constructing a hybrid bilayer membrane based on a carbon substrate. Anal. Chem. 2003, 75, 6566–6570. [Google Scholar] [CrossRef]
- Anjum, S.; Qi, W.; Gao, W.; Zhao, J.; Hanif, S.; Aziz-Ur-Rehman; Xu, G. Fabrication of biomembrane-like films on carbon electrodes using alkanethiol and diazonium salt and their application for direct electrochemistry of myoglobin. Biosens. Bioelectron. 2015, 65, 159–165. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Wang, L.; Fang, Y. Ion transport through a porphyrin-terminated hybrid bilayer membrane. Electrochim. Acta 2011, 56, 1076–1081. [Google Scholar] [CrossRef]
- Lebègue, E.; Louro, R.O.; Barrière, F. Electrochemical detection of pH-responsive grafted catechol and immobilized cytochrome c onto lipid deposit-modified glassy carbon surface. ACS Omega 2018, 3, 9035–9042. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S. Glassy carbon: A promising material for micro- and nanomanufacturing. Materials 2018, 11, 1857. [Google Scholar] [CrossRef] [Green Version]
- Gam-derouich, S.; Mahouche-chergui, S.; Turmine, M.; Piquemal, J.; Hassen-Chehimi, D.B.; Omastová, M.; Chehimi, M.M. Surface Science A versatile route for surface modi fi cation of carbon, metals and semi-conductors by diazonium salt-initiated photopolymerization. Surf. Sci. 2011, 605, 1889–1899. [Google Scholar] [CrossRef]
- Saby, C.; Ortiz, B.; Champagne, G.Y.; Bélanger, D. Electrochemical modification of glassy carbon electrode using aromatic diazonium salts. 1. Blocking effect of 4-nitrophenyl and 4-carboxyphenyl groups. Langmuir 1997, 13, 6805–6813. [Google Scholar] [CrossRef]
- Ortiz, B.; Saby, C.; Champagne, G.Y.; Bélanger, D. Electrochemical modification of a carbon electrode using aromatic diazonium salts. 2. Electrochemistry of 4-nitrophenyl modified glassy carbon electrodes in aqueous media. J. Electroanal. Chem. 1998, 455, 75–81. [Google Scholar] [CrossRef]
- Shul, G.; Ruiz, C.A.C.; Rochefort, D.; Brooksby, P.A.; Bélanger, D. Electrochemical functionalization of glassy carbon electrode by reduction of diazonium cations in protic ionic liquid. Electrochim. Acta 2013, 106, 378–385. [Google Scholar] [CrossRef]
- Breton, T.; Bélanger, D. Modification of carbon electrode with aryl groups having an aliphatic amine by electrochemical reduction of in situ generated diazonium cations. Langmuir 2008, 24, 8711–8718. [Google Scholar] [CrossRef] [Green Version]
- Schirowski, M.; Abellán, G.; Nuin, E.; Pampel, J.; Dolle, C.; Wedler, V.; Fellinger, T.P.; Spiecker, E.; Hauke, F.; Hirsch, A. Fundamental insights into the reductive covalent cross-linking of single-walled carbon nanotubes. J. Am. Chem. Soc. 2018, 140, 3352–3360. [Google Scholar] [CrossRef]
- Peiris, C.R.; Vogel, Y.B.; Le Brun, A.P.; Aragonès, A.C.; Coote, M.L.; Díez-Pérez, I.; Ciampi, S.; Darwish, N. Metal-single-molecule-semiconductor junctions formed by a radical reaction bridging gold and silicon electrodes. J. Am. Chem. Soc. 2019, 141, 14788–14797. [Google Scholar] [CrossRef]
- Lin, S.; Lin, C.; Jhang, J.; Hung, W. Electrodeposition of long-chain alkylaryl layers on au surfaces. J. Phys. Chem. C 2012, 116, 17048–17054. [Google Scholar] [CrossRef]
- Gautier, C.; López, I.; Breton, T. A post-functionalization toolbox for diazonium (electro)-grafted surfaces: Review of the coupling methods. Mater. Adv. 2021, 2, 2773–2810. [Google Scholar] [CrossRef]
- Actis, P.; Caulliez, G.; Shul, G.; Opallo, M.; Mermoux, M.; Marcus, B.; Boukherroub, R.; Szunerits, S. Functionalization of glassy carbon with diazonium salts in ionic liquids. Langmuir 2008, 24, 6327–6333. [Google Scholar] [CrossRef]
- Liu, G.; Liu, J.; Bo, T.; Eggers, P.K.; Gooding, J.J. The modification of glassy carbon and gold electrodes with aryl diazonium salt: The impact of the electrode materials on the rate of heterogeneous electron transfer. Chem. Phys. 2005, 319, 136–146. [Google Scholar] [CrossRef]
- Hetemi, D.; Noël, V.; Pinson, J. Grafting of diazonium salts on surfaces: Application to biosensors. Biosensors 2020, 10, 4. [Google Scholar] [CrossRef] [Green Version]
- Bourdillon, C.; Delamar, M.; Demaille, C.; Hitmi, R.; Moiroux, J.; Pinson, J. Immobilization of glucose oxidase on a carbon surface derivatized by electrochemical reduction of diazonium salts. J. Electroanal. Chem. 1992, 336, 113–123. [Google Scholar] [CrossRef]
- Jiang, C.; Alam, M.T.; Silva, S.M.; Taufik, S.; Fan, S.; Gooding, J.J. Unique sensing interface that allows the development of an electrochemical immunosensor for the detection of tumor necrosis factor α in whole blood. ACS Sensors 2016, 1, 1432–1438. [Google Scholar] [CrossRef]
- Niu, Z.; Zhang, Z.; Zhao, W.; Yang, J. Interactions between amyloid β peptide and lipid membranes. Biochim. Biophys. Acta Biomembr. 2018, 1860, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Khondker, A.; Alsop, R.J.; Rheinstädter, M.C. Membrane-accelerated Amyloid-β aggregation and formation of cross-β sheets. Membranes 2017, 7, 49. [Google Scholar] [CrossRef] [Green Version]
- Bharadwaj, P.; Solomon, T.; Malajczuk, C.J.; Mancera, R.L.; Howard, M.; Arrigan, D.W.M.; Newsholme, P.; Martins, R.N. Role of the cell membrane interface in modulating production and uptake of Alzheimer’s beta amyloid protein. Biochim. Biophys. Acta Biomembr. 2018, 1860, 1639–1651. [Google Scholar] [CrossRef]
- Drolle, E.; Hane, F.; Lee, B.; Leonenko, Z. Atomic force microscopy to study molecular mechanisms of amyloid fibril formation and toxicity in Alzheimer’s disease. Drug Metab. Rev. 2014, 46, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, D.J.; Wesén, E.; Björkeroth, J.; Rocha, S.; Esbjörner, E.K. Lipid membranes catalyse the fibril formation of the amyloid-β (1–42) peptide through lipid-fibril interactions that reinforce secondary pathways. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1921–1929. [Google Scholar] [CrossRef]
- Chang, C.C.; Edwald, E.; Veatch, S.; Steel, D.G.; Gafni, A. Interactions of amyloid-β peptides on lipid bilayer studied by single molecule imaging and tracking. Biochim. Biophys. Acta Biomembr. 2018, 1860, 1616–1624. [Google Scholar] [CrossRef]
- Kandel, N.; Matos, J.O.; Tatulian, S.A. Structure of amyloid β 25–35 in lipid environment and cholesterol-dependent membrane pore formation. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Capone, R.; Quiroz, F.G.; Prangkio, P.; Saluja, I.; Sauer, A.M.; Bautista, M.R.; Turner, R.S.; Yang, J.; Mayer, M. Amyloid-β-induced ion flux in artificial lipid bilayers and neuronal cells: Resolving a controversy. Neurotox. Res. 2009, 16, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Lal, R.; Lin, H.; Quist, A.P. Amyloid beta ion channel: 3D structure and relevance to amyloid channel paradigm. Biochim. Biophys. Acta Biomembr. 2007, 1768, 1966–1975. [Google Scholar] [CrossRef] [Green Version]
- Bode, D.C.; Freeley, M.; Nield, J.; Palma, M.; Viles, J.H. Amyloid-β oligomers have a profound detergent-like effect on lipid membrane bilayers, imaged by atomic force and electron microscopy. J. Biol. Chem. 2019, 294, 7566–7572. [Google Scholar] [CrossRef]
- Schenk, D.; Barbour, R.; Dunn, W.; Gordon, G.; Grajeda, H.; Guldo, T.; Hu, K.; Huang, J.; Johnson-Wood, K.; Khan, K.; et al. Immunization with amyloid-β attenuates Alzheimer disease-like pathology in the PDAPP mouse. Nature 1999, 400, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.P.; Barlow, A.K.; Chromy, B.A.; Edwards, C.; Freed, R.; Liosatos, M.; Morgan, T.E.; Rozovsky, I.; Trommer, B.; Viola, K.L.; et al. Diffusible, nonfibrillar ligands derived from Aβ1-42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. USA 1998, 95, 6448–6453. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, A.; Kaneko, N.; Villemagne, V.L.; Kato, T.; Doecke, J.; Doré, V.; Fowler, C.; Li, Q.X.; Martins, R.; Rowe, C.; et al. High-performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature 2018, 554, 249–254. [Google Scholar] [CrossRef]
- Limbocker, R.; Chia, S.; Ruggeri, F.S.; Perni, M.; Cascella, R.; Heller, G.T.; Meisl, G.; Mannini, B.; Habchi, J.; Michaels, T.C.T.; et al. Trodusquemine enhances Aβ 42 aggregation but suppresses its toxicity by displacing oligomers from cell membranes. Nat. Commun. 2019, 10, 225. [Google Scholar] [CrossRef] [Green Version]
- Bode, D.C.; Baker, M.D.; Viles, J.H. Ion channel formation by amyloid-β42 oligomers but not amyloid-β40 in cellular membranes. J. Biol. Chem. 2017, 292, 144–1413. [Google Scholar] [CrossRef] [Green Version]
- Korshavn, K.J.; Satriano, C.; Lin, Y.; Zhang, R.; Dulchavsky, M.; Bhunia, A.; Ivanova, M.I.; Lee, Y.H.; La Rosa, C.; Lim, M.H.; et al. Reduced lipid bilayer thickness regulates the aggregation and cytotoxicity of amyloid-β. J. Biol. Chem. 2017, 292, 4638–4650. [Google Scholar] [CrossRef] [Green Version]
- Pobandt, T.; Knecht, V. Free energy of lipid bilayer defects affected by Alzheimer’s disease-associated amyloid-β42 monomers. J. Phys. Chem. B 2014, 118, 3507–3516. [Google Scholar] [CrossRef]
- Ambroggio, E.E.; Kim, D.H.; Separovic, F.; Barrow, C.J.; Barnham, K.J.; Bagatolli, L.A.; Fidelio, G.D. Surface behavior and lipid interaction of Alzheimer β-amyloid peptide 1–42: A membrane-disrupting peptide. Biophys. J. 2005, 88, 2706–2713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demuro, A.; Smith, M.; Parker, I. Single-channel Ca2+ imaging implicates Aβ 1–42 amyloid pores in Alzheimer’s disease pathology. J. Cell Biol. 2011, 195, 515–524. [Google Scholar] [CrossRef] [Green Version]
- Miyashita, N.; Straub, J.E.; Thirumalai, D. Structures of β-amyloid peptide 1–40, 1–42, and 1–55-the 672–726 fragment of APP-in a membrane environment with implications for interactions with γ-secretase. J. Am. Chem. Soc. 2009, 131, 17843–17852. [Google Scholar] [CrossRef] [Green Version]
- Mirkhalaf, F.; Paprotny, J.; Schiffrin, D.J. Synthesis of metal nanoparticles stabilized by metal-carbon bonds. J. Am. Chem. Soc. 2006, 128, 7400–7401. [Google Scholar] [CrossRef]
- Stine, W.B.; Jungbauer, L.; Yu, C.; LaDu, M.J. Preparing Synthetic Aβ in Different Aggregation States. In Alzheimer’s Disease and Frontotemporal Dementia: Methods and Protocols; Roberson, E.D., Ed.; Humana Press: Totowa, NJ, USA, 2010; ISBN 978-1-60761-744-0. [Google Scholar]
- Menanteau, T.; Levillain, E.; Breton, T. Electrografting via diazonium chemistry: From multilayer to monolayer using radical scavenger. Chem. Mater. 2013, 25, 2905–2909. [Google Scholar] [CrossRef] [Green Version]
- Menanteau, T.; Dias, M.; Levillain, E.; Downard, A.J.; Breton, T. Electrografting via diazonium chemistry: The key role of the aryl substituent in the layer growth mechanism. J. Phys. Chem. C 2016, 120, 4423–4429. [Google Scholar] [CrossRef]
- Greenwood, J.; Phan, T.H.; Fujita, Y.; Li, Z.; Ivasenko, O.; Vanderlinden, W.; Van Gorp, H.; Frederickx, W.; Lu, G.; Tahara, K.; et al. Covalent modification of graphene and graphite using diazonium chemistry: Tunable grafting and nanomanipulation. ACS Nano 2015, 9, 5520–5535. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.A.I.; Bhatia, R.; Lal, R. Amyloid-beta protein forms ion channels: Implications for Alzheimer’s disease pathophysiology. FASEB J. 2001, 15, 2433–2444. [Google Scholar] [CrossRef] [Green Version]
- Tempra, C.; Scollo, F.; Pannuzzo, M.; Lolicato, F.; La Rosa, C. A unifying framework for amyloid-mediated membrane damage: The lipid-chaperone hypothesis. Biochim. Biophys. Acta Proteins Proteomics 2022, 1870, 140767. [Google Scholar] [CrossRef]
- Ragaliauskas, T.; Mickevicius, M.; Budvytyte, R.; Niaura, G.; Carbonnier, B.; Valincius, G. Adsorption of β-amyloid oligomers on octadecanethiol monolayers. J. Colloid Interface Sci. 2014, 425, 159–167. [Google Scholar] [CrossRef]
- Budvytyte, R.; Ambrulevičius, F.; Jankaityte, E.; Valincius, G. Electrochemical assessment of dielectric damage to phospholipid bilayers by amyloid β-oligomers. Bioelectrochemistry 2022, 145, 108091. [Google Scholar] [CrossRef]
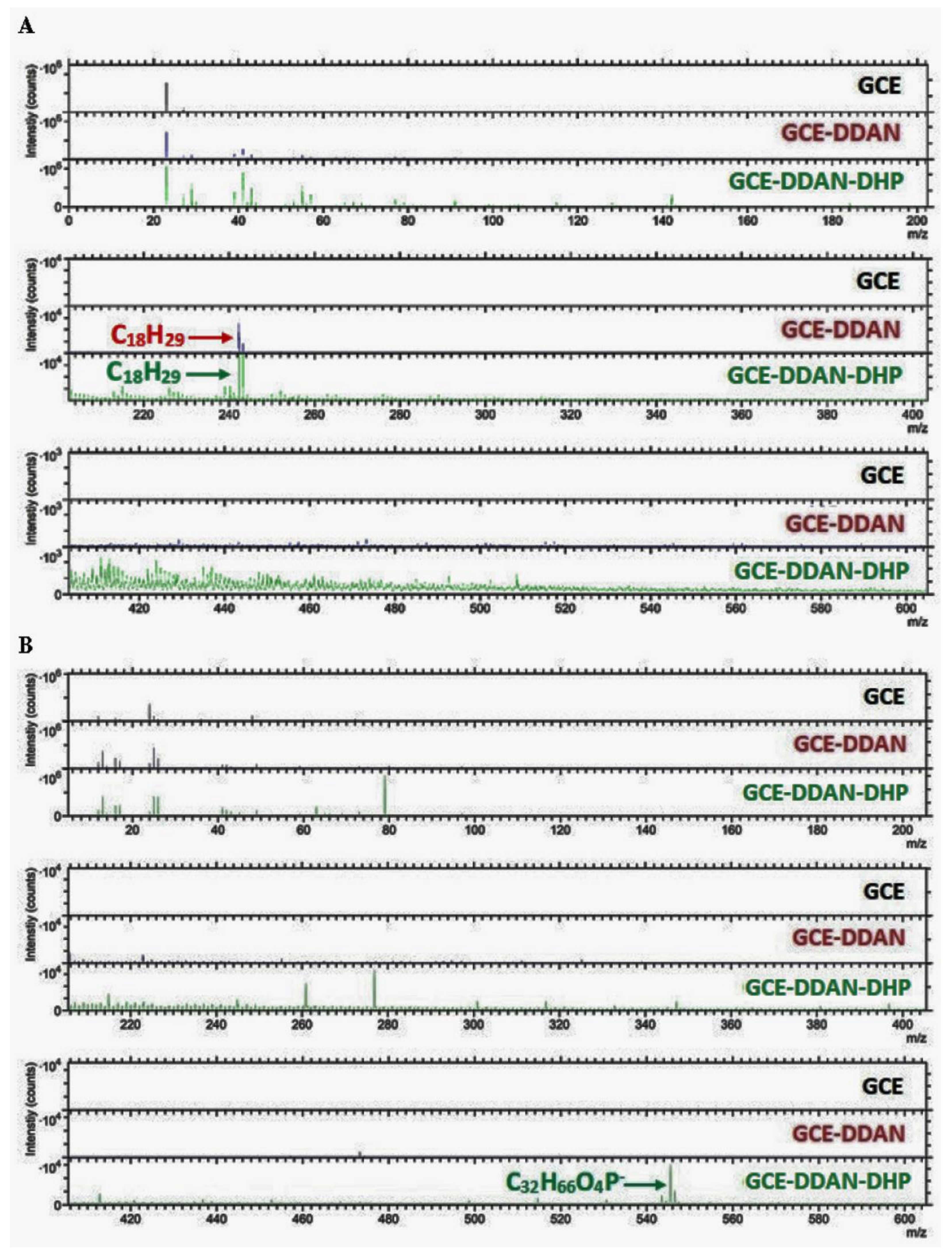
| Electrode | Contact Angle Measured (°) | Electrode | Contact Angle Measured (°) | Electrode | Contact Angle Measured (°) |
|---|---|---|---|---|---|
| Bare GCE 1 | 75.864 | GCE-DDAN 1 | 89.482 | GCE-DDAN-DHP 1 | 80.702 |
| Bare GCE 2 | 66.880 | GCE-DDAN 2 | 85.151 | GCE-DDAN-DHP 2 | 80.441 |
| Bare GCE 3 | 71.702 | GCE-DDAN 3 | 83.490 | GCE-DDAN-DHP 3 | 81.125 |
| Bare GCE 4 | 75.156 | GCE-DDAN 4 | 84.299 | GCE-DDAN-DHP 4 | 75.250 |
| Bare GCE 5 | 73.963 | GCE-DDAN 5 | 86.724 | GCE-DDAN-DHP 5 | 78.196 |
| Bare GCE 6 | 76.869 | GCE-DDAN 6 | 86.220 | GCE-DDAN-DHP 6 | 79.021 |
| Average Contact Angle (°), n = 6 | 73.405 | 85.894 | 79.122 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fini, H.; Hassan, Q.; Noroozifar, M.; Kerman, K. Electrografting a Hybrid Bilayer Membrane via Diazonium Chemistry for Electrochemical Impedance Spectroscopy of Amyloid-β Aggregation. Micromachines 2022, 13, 574. https://doi.org/10.3390/mi13040574
Fini H, Hassan Q, Noroozifar M, Kerman K. Electrografting a Hybrid Bilayer Membrane via Diazonium Chemistry for Electrochemical Impedance Spectroscopy of Amyloid-β Aggregation. Micromachines. 2022; 13(4):574. https://doi.org/10.3390/mi13040574
Chicago/Turabian StyleFini, Hamid, Qusai Hassan, Meissam Noroozifar, and Kagan Kerman. 2022. "Electrografting a Hybrid Bilayer Membrane via Diazonium Chemistry for Electrochemical Impedance Spectroscopy of Amyloid-β Aggregation" Micromachines 13, no. 4: 574. https://doi.org/10.3390/mi13040574
APA StyleFini, H., Hassan, Q., Noroozifar, M., & Kerman, K. (2022). Electrografting a Hybrid Bilayer Membrane via Diazonium Chemistry for Electrochemical Impedance Spectroscopy of Amyloid-β Aggregation. Micromachines, 13(4), 574. https://doi.org/10.3390/mi13040574







