Cobalt Phthalocyanine-Ionic Liquid Composite Modified Electrodes for the Voltammetric Detection of DNA Hybridization Related to Hepatitis B Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apparatus
2.2. Chemicals
2.3. Procedure
- Surface modification of PGEs with CoPc-ILs;
- Hybridization of HBV DNA probe with its target DNA or other oligonucleotides; NC or MM;
- Immobilization of DNA-DNA hybrids onto the surface of CoPc-IL-PGEs.
2.4. Microscopic Characterization of Electrodes
2.5. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Palchetti, I.; Mascini, M. Chapter 1. Biosensor Techniques for Environmental Monitoring. In Nucleic Acid Biosensors for Enviromental Pollution Monitoring, 1st ed.; Palchetti, I., Mascini, M., Eds.; Royal Society of Chemistry: Londra, UK, 2011; pp. 1–16. [Google Scholar]
- Zupan, S.; Flipic, B.; Babic, M. Piezoelectric Immunosensors. Acta Pharm. 1992, 42, 361–366. [Google Scholar]
- Erdem, A.; Eksin, E.; Kesici, E. Chapter 15. Biosensors for Detection of Anticancer Drug—DNA Interactions. In Biosensors and Nanotechnology: Applications in Health Care Diagnostics, 1st ed.; Altintas, Z., Ed.; Wiley Online Library: New York, NY, USA, 2017; pp. 349–365. [Google Scholar]
- Leznoff, C.C.; Lever, A.B.P. (Eds.) Phthalocyanines: Properties and Applications; Wiley-VCH: Cambridge, UK, 1993; Volume 3. [Google Scholar]
- Lukyanets, E.A.; Nemykin, V.N. The key role of peripheral substituents in the chemistry of phthalocyanines and their analogs. J. Porphyr. Phthalocyanines 2010, 14, 1–40. [Google Scholar] [CrossRef] [Green Version]
- Nemykin, V.N.; Lukyanets, E.A. Synthesis of substituted phthalocyanines. Arkivoc 2010, 1, 136. [Google Scholar] [CrossRef] [Green Version]
- Stefanov, C.; van Staden, J.K.F.; Stefan-van Staden, R.I. Review—Enzymatic and Non-Enzymatic (bio)sensors Based on Phthalocyanines. A Minireview. ECS J. Solid State Sci. Technol. 2020, 9, 051012. [Google Scholar] [CrossRef]
- Abbas, M.N.; Saeed, A.A.; Ali, M.B.; Errachid, A.; Zine, N.; Baraket, A.; Singh, B. Biosensor for the oxidative stress biomarker glutathione based on SAM of cobalt phthalocyanine on a thioctic acid modified gold electrode. J. Solid State Electrochem. 2019, 23, 1129–1144. [Google Scholar] [CrossRef]
- Hosseini, H.; Mahyari, M.; Bagheri, A.; Shaabani, A. A novel bioelectrochemical sensing platform based on covalently attachment of cobalt phthalocyanine to graphene oxide. Biosens. Bioelectron. 2014, 52, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Stefan-van Staden, R.I.; Ilie-Mihai, R.M.; Gugoasa, L.A.; Bilasco, A.; Visan, C.A.; Streinu-Cercel, A. Molecular recognition of IL-8, IL-10, IL-12, and IL-15 in biological fluids using phthalocyanine-based stochastic sensors. Anal. Bioanal. 2018, 410, 7723. [Google Scholar] [CrossRef] [PubMed]
- Özcan, L.; Şahin, Y.; Türk, H. Non-enzymatic glucose biosensor based on overoxidized polypyrrole nanofiber electrode modified with cobalt (II) phthalocyanine tetrasulfonate. Biosens. Bioelectron. 2008, 24, 512. [Google Scholar] [CrossRef]
- Fan, Z.; Fan, L.; Dong, C. Highly sensitive photoelectrochemical sensing of bisphenol A based on zinc phthalocyanine/TiO2 nanorod arrays. Talanta 2018, 198, 16. [Google Scholar] [CrossRef]
- Arrieta, A.; Rodriguez-Mendez, M.L.; De Saja, J.A. Langmuir—Blodgett film and carbon paste electrodes based on phthalocyanines as sensing units for taste. Sens. Actuat. B Chem. 2003, 95, 357–365. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, L.; Peng, F.; Pan, G.B. Fabrication of a cobalt phthalocyanine freestanding film on an ionic liquid surface for memory device applications. RSC Adv. 2018, 8, 5344–5349. [Google Scholar] [CrossRef] [Green Version]
- Sorokin, A.B. Phthalocyanine metal complexes in catalysis. Chem. Rev. 2013, 113, 8152–8191. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.; Devasenathipathy, R.; Chen, S.M.; Gu, J.A.; Huang, S.T. Synthesis and characterization of graphene-cobalt phthalocyanines and graphene-iron phthalocyanine composites and their enzymatic fuel cell application. Renew. Energy 2015, 74, 867–874. [Google Scholar] [CrossRef]
- Xu, H.; Xiong, H.-Y.; Zeng, Q.-X.; Jia, L.; Wang, Y.; Wang, S.-F. Direct electrochemistry and electrocatalysis of heme proteins immobilized in single-wall carbon nanotubes-surfactant films in room temperature ionic liquids. Electrochem. Commun. 2009, 11, 286–289. [Google Scholar] [CrossRef]
- Zhu, Z.; Garcia-Gancedo, L.; Flewitt, A.J.; Xie, H.; Moussy, F.; Milne, W.I. A critical review of glucose biosensors based on carbon nanomaterials: Carbon nanotubes and graphene. Sensors 2012, 12, 5996–6022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Shi, L.H.; Wang, M.J.; Li, Z.Y.; Liu, H.T.; Li, J.H. A novel room temperature ionic liquid sol-gel matrix for amperometric biosensor application. Green Chem. 2005, 7, 655–658. [Google Scholar] [CrossRef]
- Yang, F.; Jiao, L.S.; Shen, Y.F.; Xu, X.Y.; Zhang, Y.J.; Niu, L. Enhanced response induced by polyelectrolyte-functionalized ionic liquid in glucose biosensor based on sol-gel organic-inorganic hybrid material. J. Electroanal. Chem. 2007, 608, 78–83. [Google Scholar] [CrossRef]
- Jin, X.X.; Yu, L.; Garcia, D.; Ren, R.X.; Zeng, X.Q. Ionic liquid high-temperature gas sensor Array. Anal. Chem. 2006, 78, 6980–6989. [Google Scholar] [CrossRef]
- Yu, L.; Diego, G.; Ren, X.R.; Zeng, X. Ionic liquid high temperature gas sensors. Chem. Commun. 2005, 2005, 2277–2279. [Google Scholar] [CrossRef]
- Jia, F.; Shan, C.; Li, F.; Niu, L. Carbon nanotube/gold nanoparticles/polyethylenimine-functionalized ionic liquid thin film composites for glucose biosensing. Biosens. Bioelectron. 2008, 24, 945–950. [Google Scholar] [CrossRef]
- Lee, W.M. Hepatitis B Virus Infection. N. Engl. J. Med. 1997, 337, 1733–1745. [Google Scholar] [CrossRef] [Green Version]
- Dusheiko, G. Oxford Textbook of Clinical Hepatology; McIntyre, N., Ed.; Oxford University Press: New York, NY, USA, 1991; p. 571. [Google Scholar]
- Purcell, R.H. The discovery of the hepatitis viruses. Gastroenterelogy 1993, 104, 955. [Google Scholar] [CrossRef]
- Mast, E.E.; Alter, M.J.; Margolis, H.S. Strategies to prevent and control hepatitis B and C virus infections: A global perspective. Vaccine 1999, 17, 1730–1733. [Google Scholar] [CrossRef]
- World Health Organization. Hepatitis. In Proceedings of the Sixty-Seventh World Health Assembly, Geneva, Switzerland, 19–24 May 2014. [Google Scholar]
- EASL Jury. EASL International Consensus Conference on Hepatitis B. 13–14 September, 2002: Geneva, Switzerland. Consensus statement (short version). J. Hepatol. 2003, 38, 533–540. [Google Scholar]
- Gabig-Ciminska, M. Developing nucleic acid-based electrical detection systems. Microb. Cell Fact. 2006, 5, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Erdem, A.; Kerman, K.; Meric, B.; Akarca, U.S.; Ozsoz, M. DNA electrochemical biosensor for the detection of short DNA sequences related to the Hepatitus B virus. Electroanalysis 1999, 11, 586–588. [Google Scholar] [CrossRef]
- Erdem, A.; Ozkan Arıksoysal, D.; Karadeniz, H.; Kara, P.; Sengonul, A.; Sayıner, A.A.; Ozsoz, M. Electrochemical genomagnetic assay for the detection of Hepatitis B virus DNA in polymerase chain amplicons by using disposable sensor technolgy. Electrochem. Commun. 2005, 7, 815–820. [Google Scholar] [CrossRef]
- Eksin, E.; Erdem, A. Chitosan-carbon nanofiber modified single-use graphite electrodes developed for electrochemical detection of DNA hybridization related to Hepatitis B virus. Electroanalysis 2016, 28, 2514–2521. [Google Scholar] [CrossRef]
- Chen, C.C.; Lai, Z.L.; Wang, G.J.; Wu, C.Y. Polymerase chain reaction-free detection of hepatitis B virus DNA using a nanostructured impedance biosensor. Biosens. Bioelectron. 2016, 77, 603–608. [Google Scholar] [CrossRef]
- Mashhadizadeh, M.H.; Talemi, R.P. Synergistic effect of magnetite and gold nanoparticles onto the response of a label-free impedimetric hepatitis B virus DNA biosensor. Mater. Sci. Eng. C 2016, 59, 773–781. [Google Scholar] [CrossRef]
- Kay, A.; Zoulim, F. Hepatitis B virus genetic variability and evolution. Virus Res. 2007, 127, 164–176. [Google Scholar] [CrossRef]
- Cummings, T.E.; Elving, P.J. Determination of the electrochemically effective electrode area. Anal. Chem. 1978, 50, 480–488. [Google Scholar] [CrossRef]
- Arora, K.; Prabhakar, N.; Chand, S.; Malhotra, B.D. Ultrasensitive DNA hybridization biosensor based on polyaniline. Biosens. Bioelectron. 2007, 23, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Yang, F.; Ma, Y.; Yang, X. Electrochemical impedance detection of DNA hybridization based on dendrimer modified electrode. Biosens. Bioelectron. 2007, 22, 1716–1722. [Google Scholar] [CrossRef] [PubMed]
- Grützke, S.; Abdali, S.; Schuhmann, W.; Gebala, M. Detection of DNA hybridization using electrochemical impedance spectroscopy and surface enhanced Raman scattering. Electrochem. Commun. 2012, 19, 59–62. [Google Scholar] [CrossRef]
- Erdem, A.; Karadeniz, H.; Canavar, P.E.; Congur, G. Single-use sensor platforms based on carbon nanotubes for electrochemical detection of DNA hybridization related to Microcystis spp. Electroanalysis 2012, 24, 502–511. [Google Scholar] [CrossRef]
- Janek, R.P.; Fawcett, W.R. Impedance spectroscopy of self-assembled monolayers on au (111): Sodium ferrocyanide charge transfer at modified electrodes. Langmuir 1998, 14, 3011–3018. [Google Scholar] [CrossRef]
- Erdem, A.; Muti, M.; Mese, F.; Eksin, E. Chitosan-ionic liquid modified single-use sensor for electrochemical monitoring of sequence-selective DNA hybridization. Colloids Surf. B Biointerfaces 2014, 114, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Yaralı, E.; Kanat, E.; Erac, Y.; Erdem, A. Ionic liquid modified single-use electrode developed for voltammetric detection of miRNA-34a and its application to real samples. Electroanalysis 2020, 32, 384–393. [Google Scholar] [CrossRef]
- Miller, J.N.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry; Pearson Education: Essex, UK, 2005; pp. 121–123. [Google Scholar]
- Zhang, W.; Yang, T.; Jiang, C.; Jiao, K. DNA hybridization and phosphinothricin acetyltransferase gene sequence detection based on zirconia/nanogold film modified electrode. Appl. Surf. Sci. 2008, 254, 4750–4756. [Google Scholar] [CrossRef]
- Ye, Y.K.; Zhao, J.H.; Yan, F.; Zhu, Y.L.; Ju, H.X. Electrochemical behavior and detection of hepatitis B virus DNA PCR production at gold electrode. Biosens. Bioelectron. 2003, 18, 1501–1508. [Google Scholar] [CrossRef]
- Hassen, W.M.; Chaix, C.; Abdelghani, A.; Bessueille, F.; Leonard, D.; Jaffrezic-Renault, N. An impedimetric DNA sensor based on functionalized magnetic nanoparticles for HIV and HBV detection. Sens. Actuators B 2008, 134, 755–760. [Google Scholar] [CrossRef]
- Liu, W.T.; Guo, H.; Wu, J.H. Effects of target length on the hybridization efficiency and specificity of rRNA-based oligonucleotide microarrays. Appl. Environ. Microbiol. 2007, 73, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Erdem, A.; Eksin, E. Electrochemical detection of solution phase hybridization related to single nucleotide mutation by carbon nanofibers enriched electrodes. Materials 2019, 12, 3377. [Google Scholar] [CrossRef] [Green Version]
- Erdem, A.; Congur, G. Hydroxyapatite nanoparticles modified graphite electrodes for electrochemical DNA detection. Electroanalysis 2018, 30, 67–74. [Google Scholar] [CrossRef]
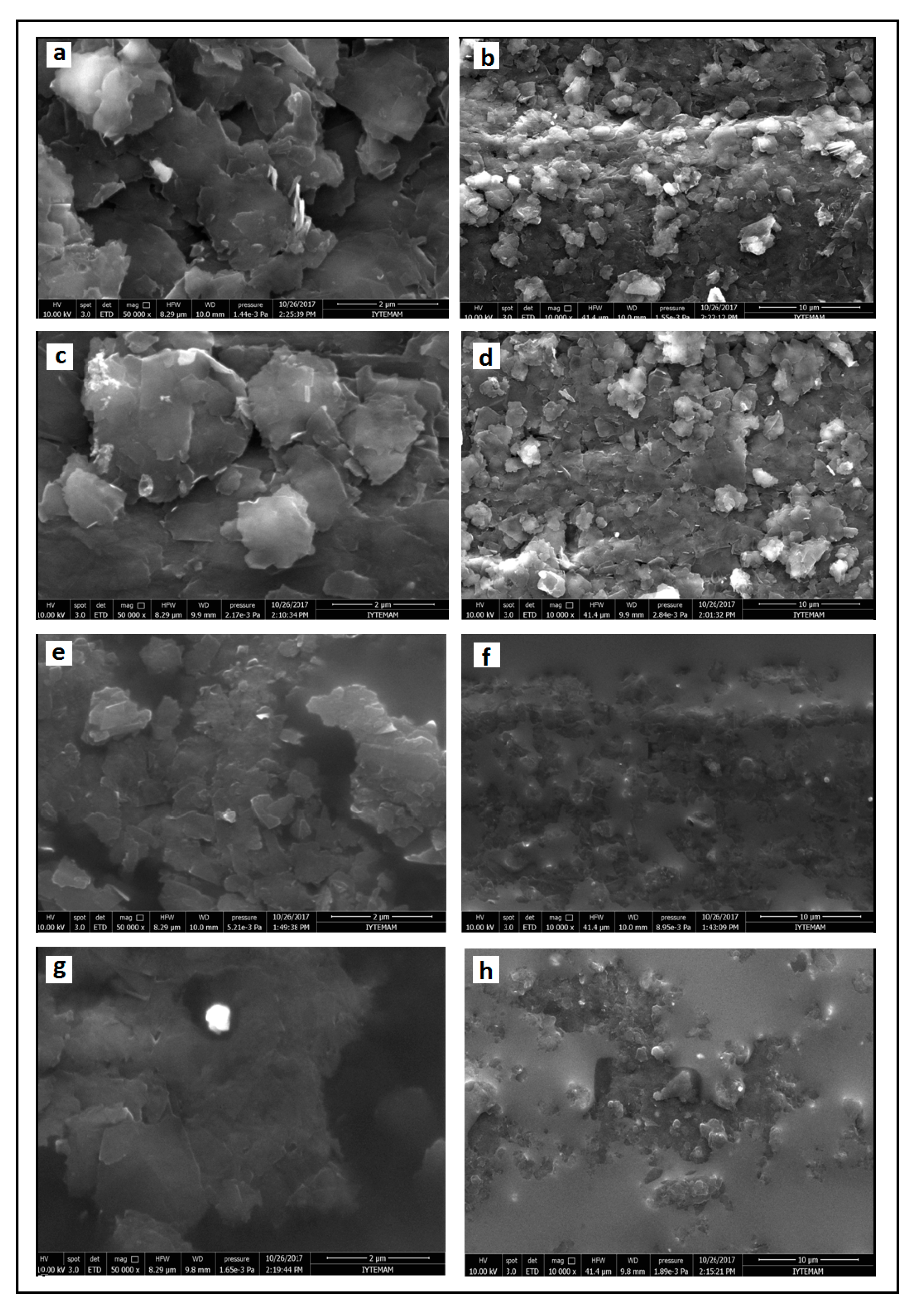
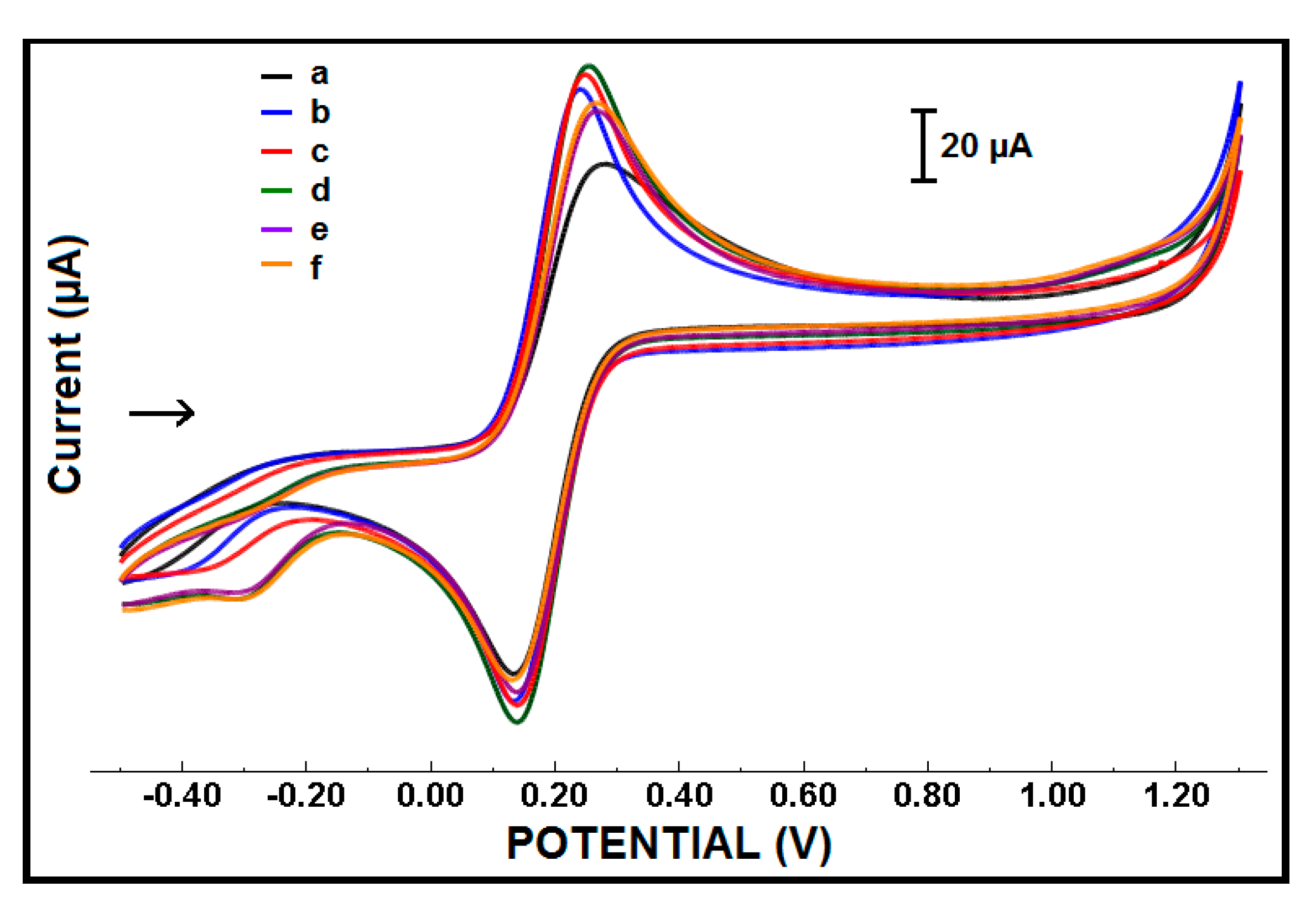
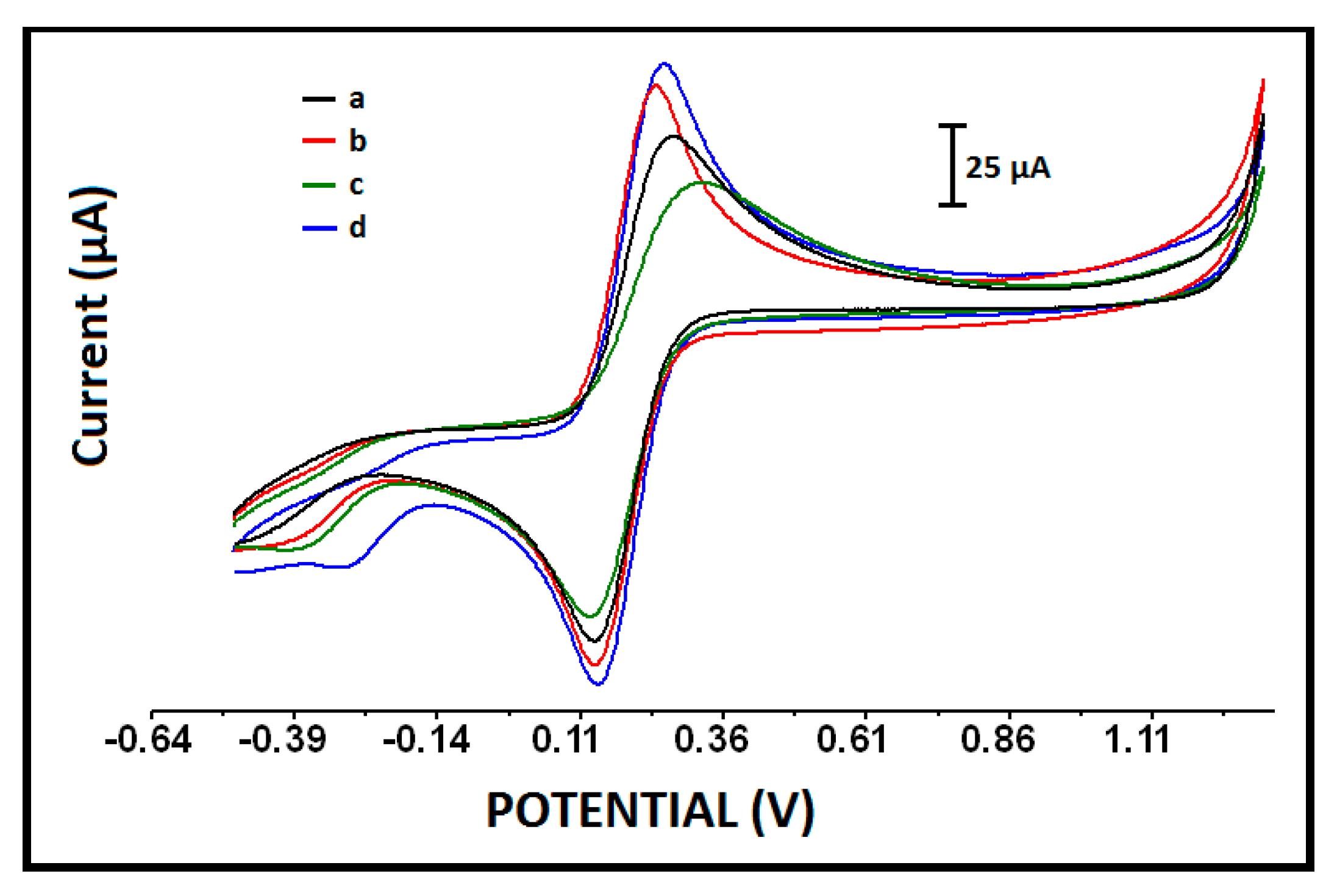
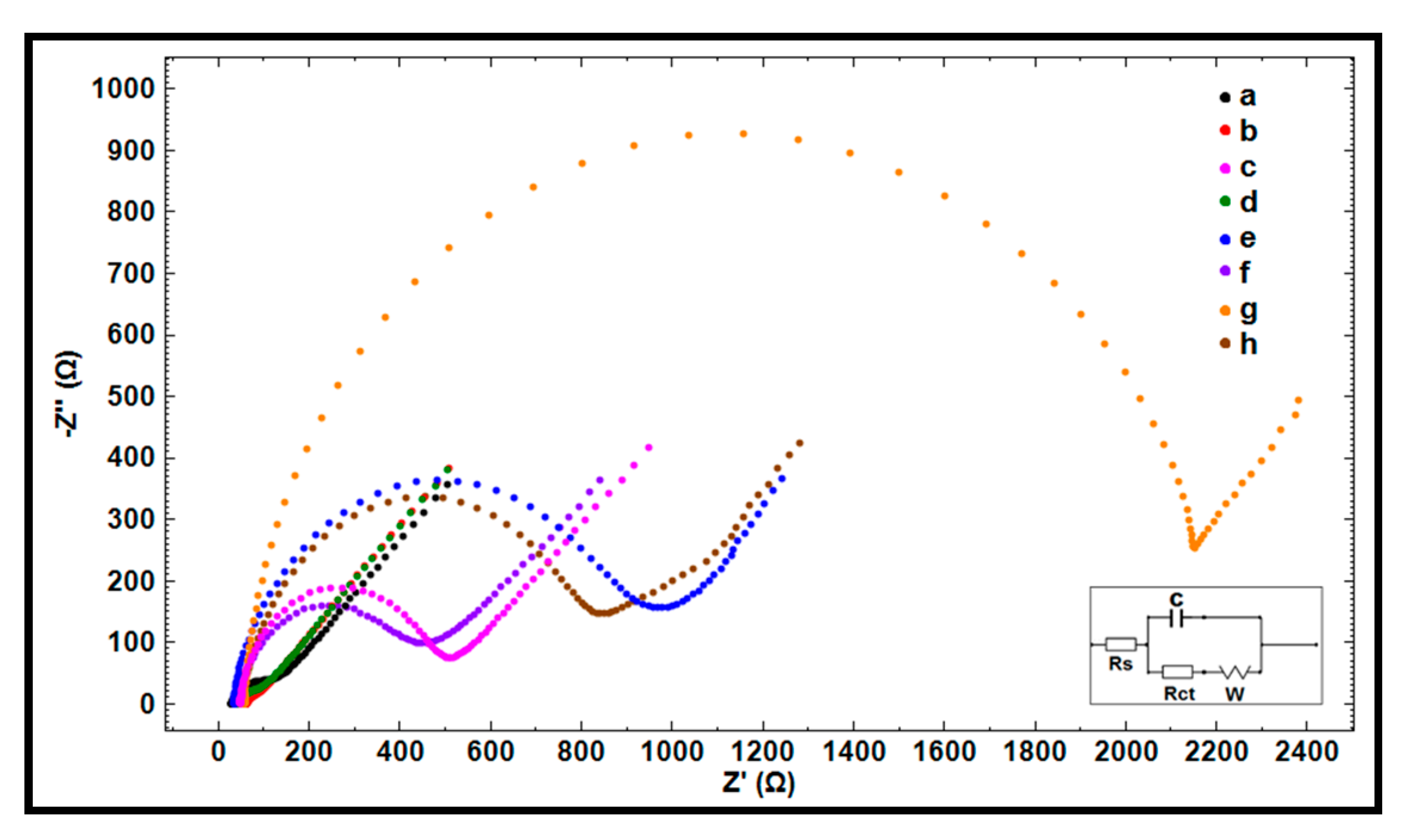
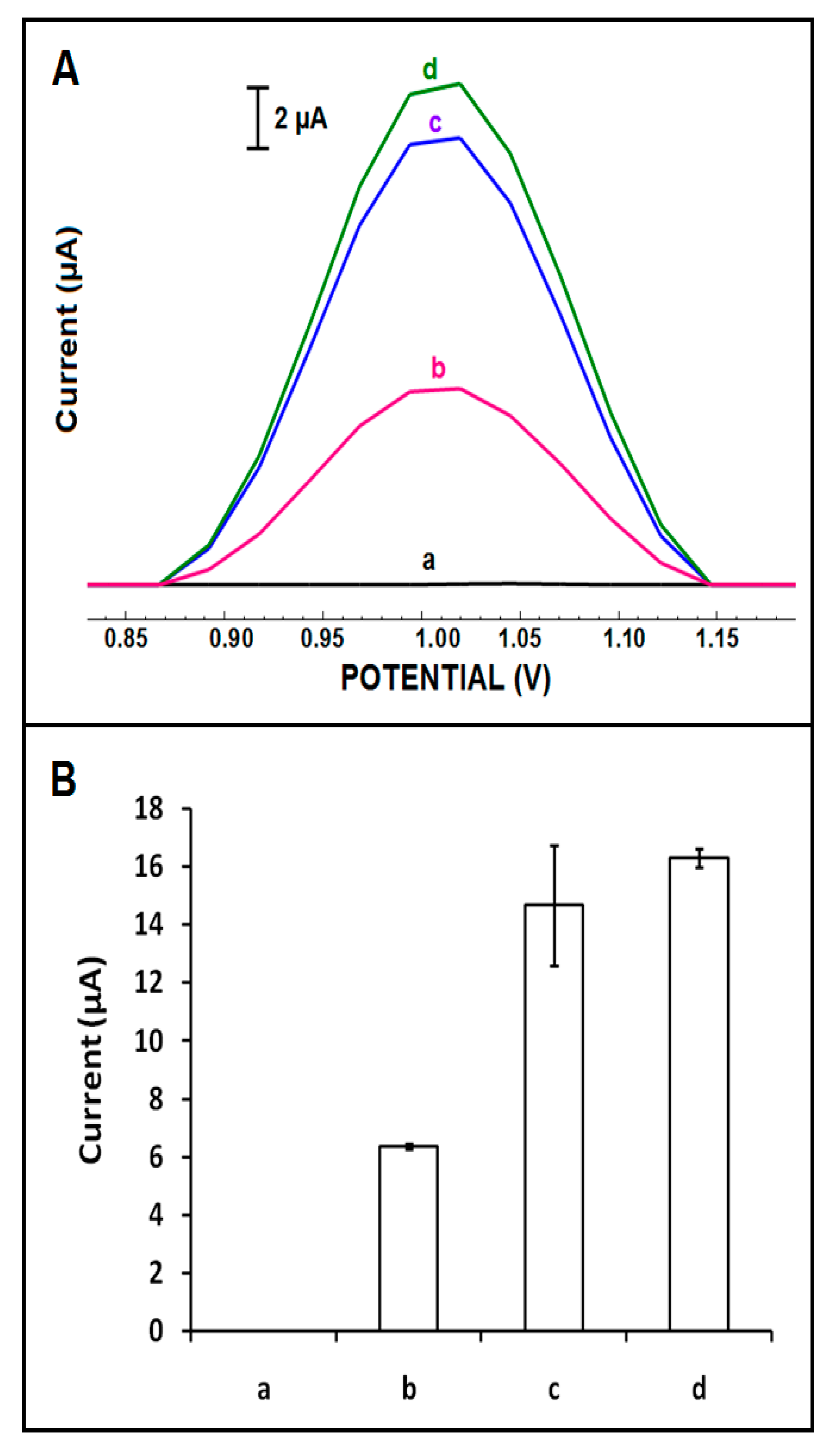
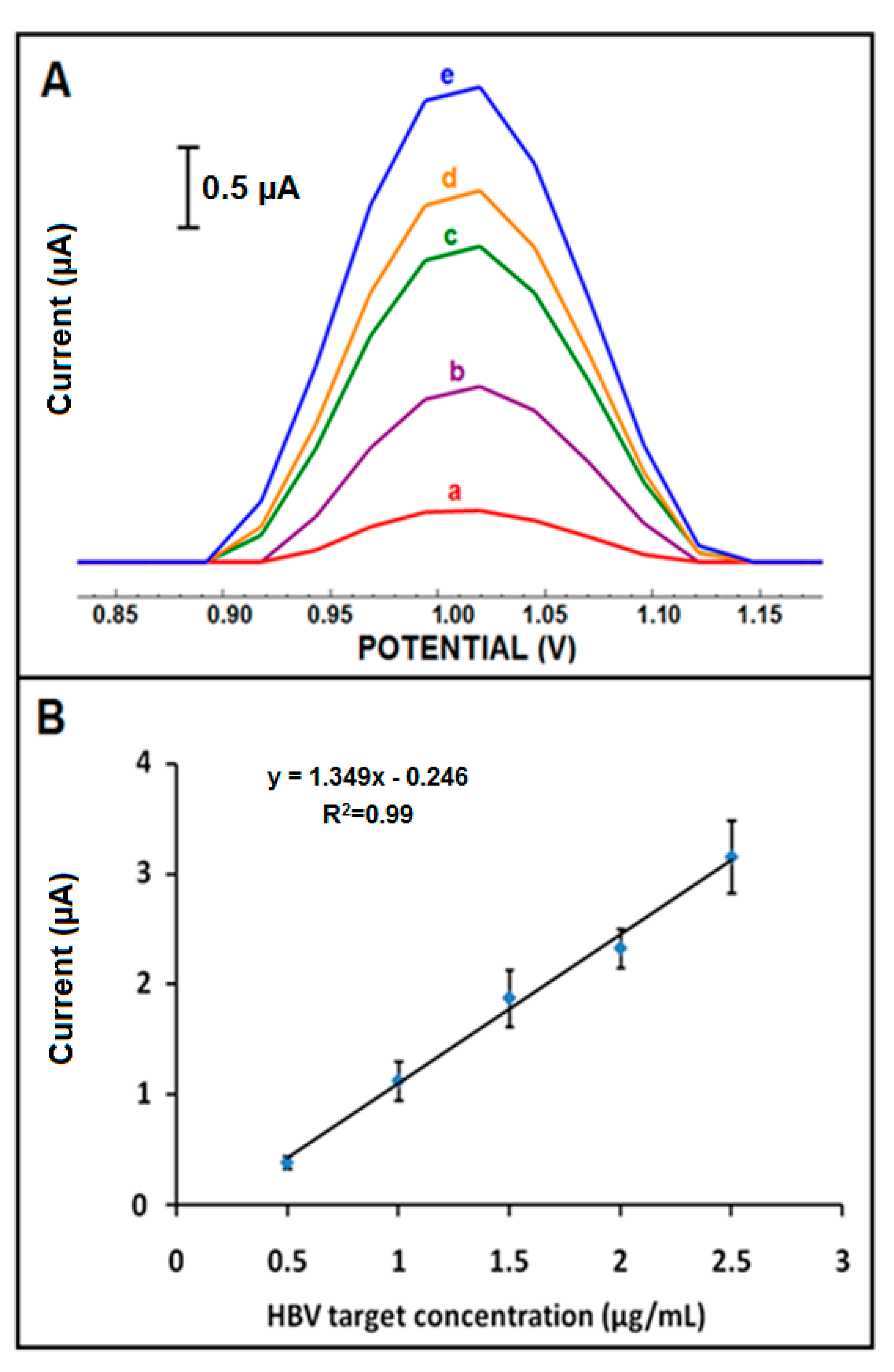
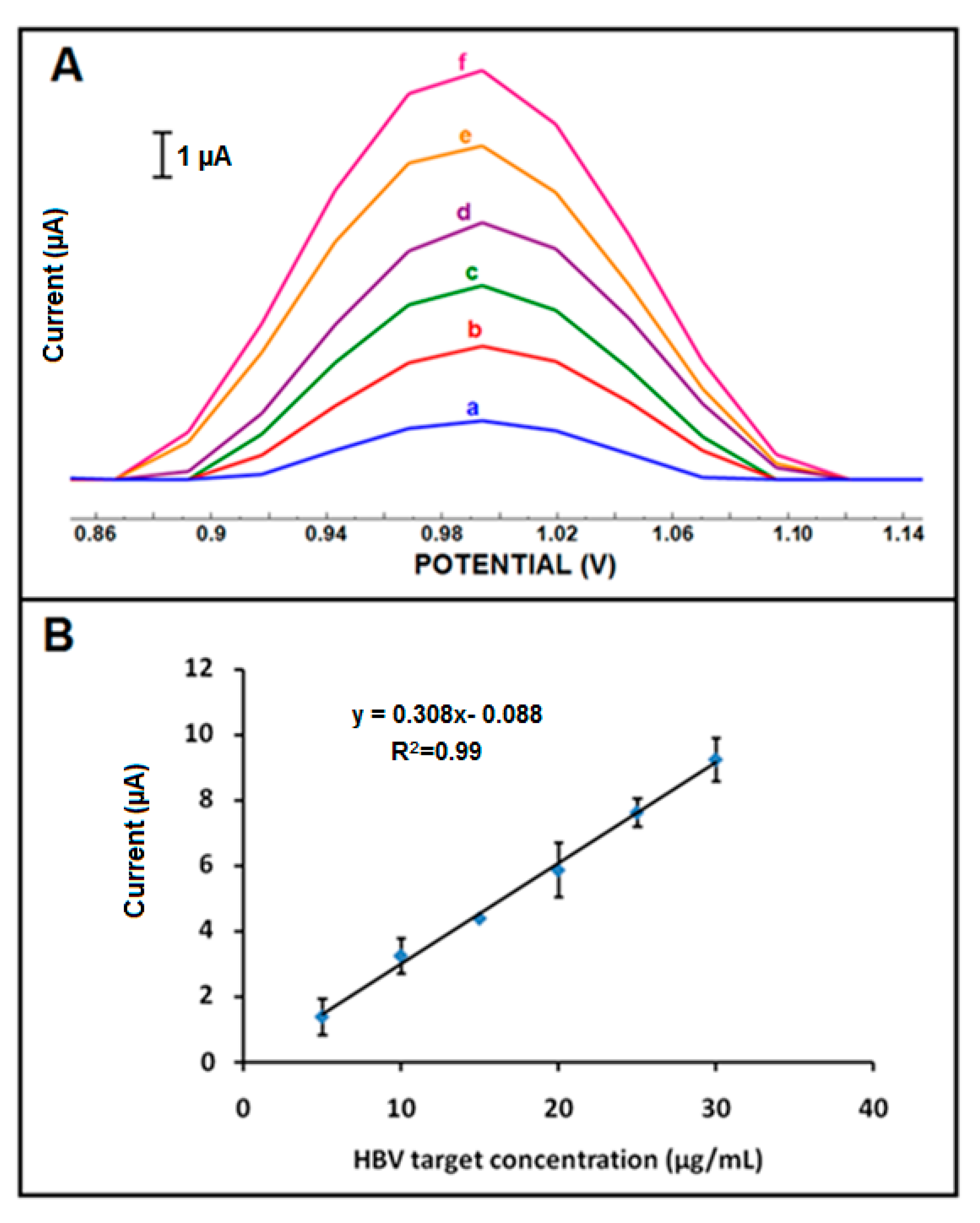
| Electrode | Ia (µA) | Ic (µA) | Qa (mC) | Qc (mC) |
|---|---|---|---|---|
| PGE | 75.58 ± 15.44 | 82.45 ± 9.17 | 1.29 | 0.96 |
| IL-PGE | 96.39 ± 14.27 | 90.91 ± 7.41 | 1.33 | 1.02 |
| 25 µg/mL CoPc-IL-PGE | 100.96 ± 10.56 | 88.43 ± 7.06 | 1.38 | 1.11 |
| 50 µg/mL CoPc-IL-PGE | 104.60 ± 14.22 | 90.55 ± 11.17 | 1.50 | 1.24 |
| 100 µg/mL CoPc-IL-PGE | 96.00 ± 15.70 | 82.59 ± 11.82 | 1.44 | 1.19 |
| 200 µg/mL CoPc-IL-PGE | 96.72 ± 11.65 | 81.50 ± 10.18 | 1.50 | 1.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaralı, E.; Erdem, A. Cobalt Phthalocyanine-Ionic Liquid Composite Modified Electrodes for the Voltammetric Detection of DNA Hybridization Related to Hepatitis B Virus. Micromachines 2021, 12, 753. https://doi.org/10.3390/mi12070753
Yaralı E, Erdem A. Cobalt Phthalocyanine-Ionic Liquid Composite Modified Electrodes for the Voltammetric Detection of DNA Hybridization Related to Hepatitis B Virus. Micromachines. 2021; 12(7):753. https://doi.org/10.3390/mi12070753
Chicago/Turabian StyleYaralı, Ece, and Arzum Erdem. 2021. "Cobalt Phthalocyanine-Ionic Liquid Composite Modified Electrodes for the Voltammetric Detection of DNA Hybridization Related to Hepatitis B Virus" Micromachines 12, no. 7: 753. https://doi.org/10.3390/mi12070753
APA StyleYaralı, E., & Erdem, A. (2021). Cobalt Phthalocyanine-Ionic Liquid Composite Modified Electrodes for the Voltammetric Detection of DNA Hybridization Related to Hepatitis B Virus. Micromachines, 12(7), 753. https://doi.org/10.3390/mi12070753






