Wearable Microfluidic Sensor for the Simultaneous and Continuous Monitoring of Local Sweat Rates and Electrolyte Concentrations
Abstract
:1. Introduction
2. Materials and Methods
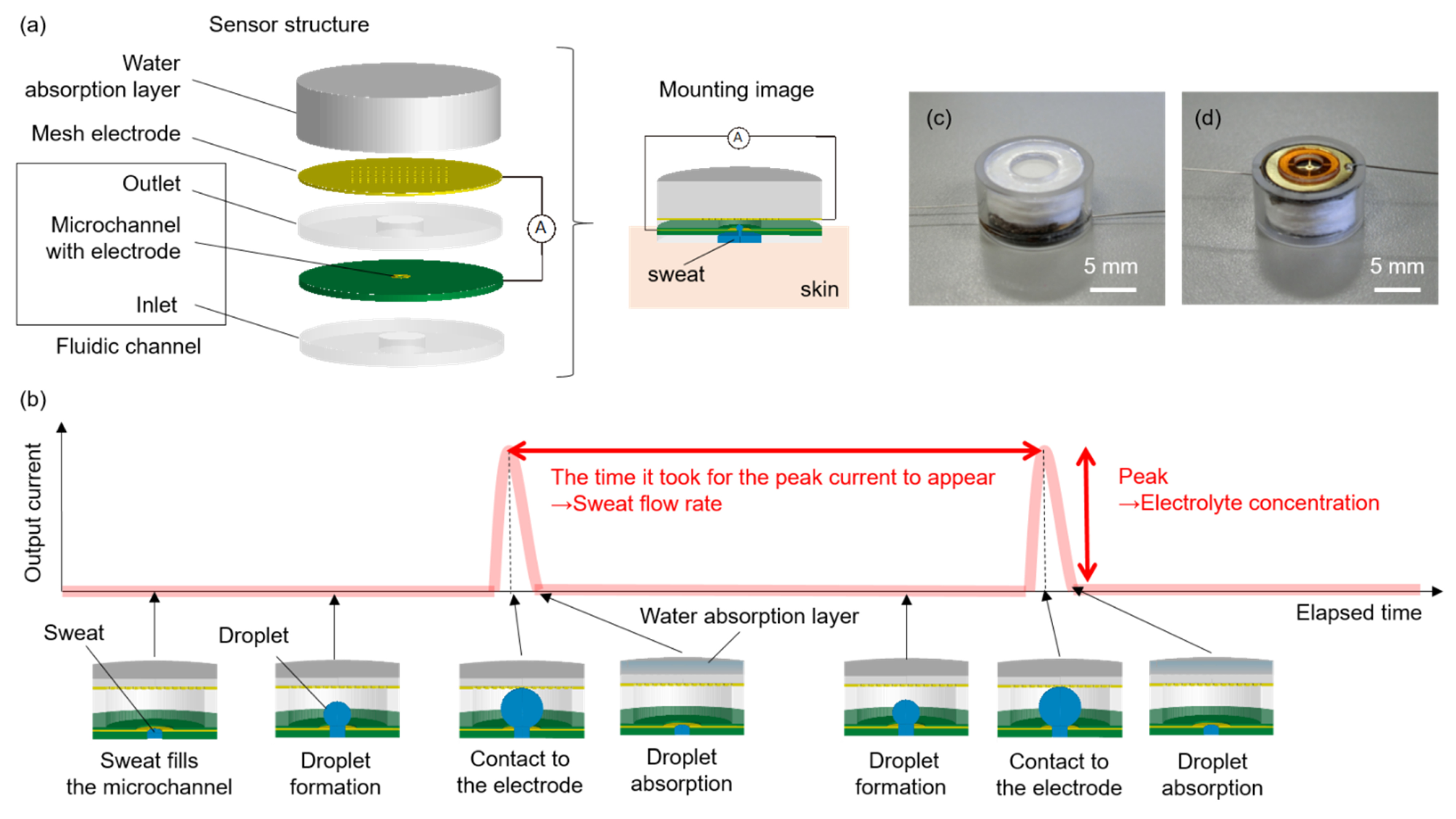
2.1. Sensing Method
2.2. Experimental Setup
2.3. Data Analysis
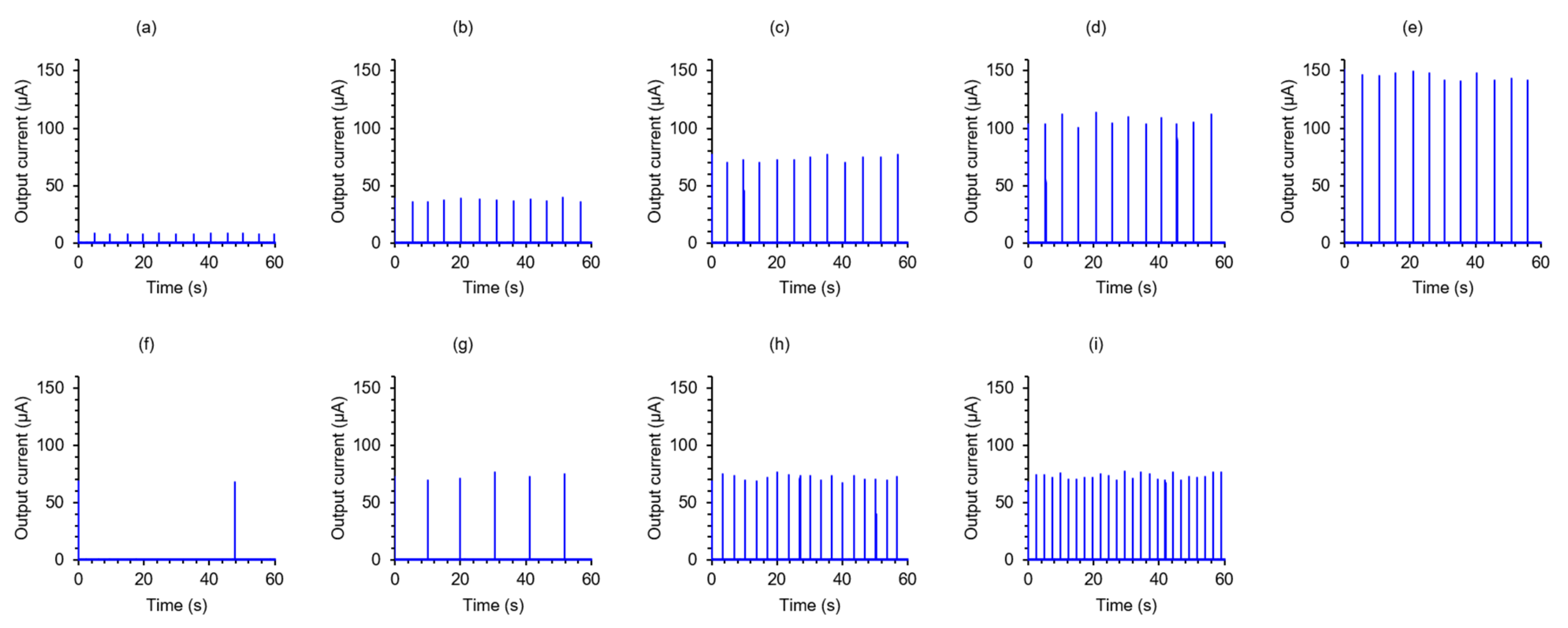
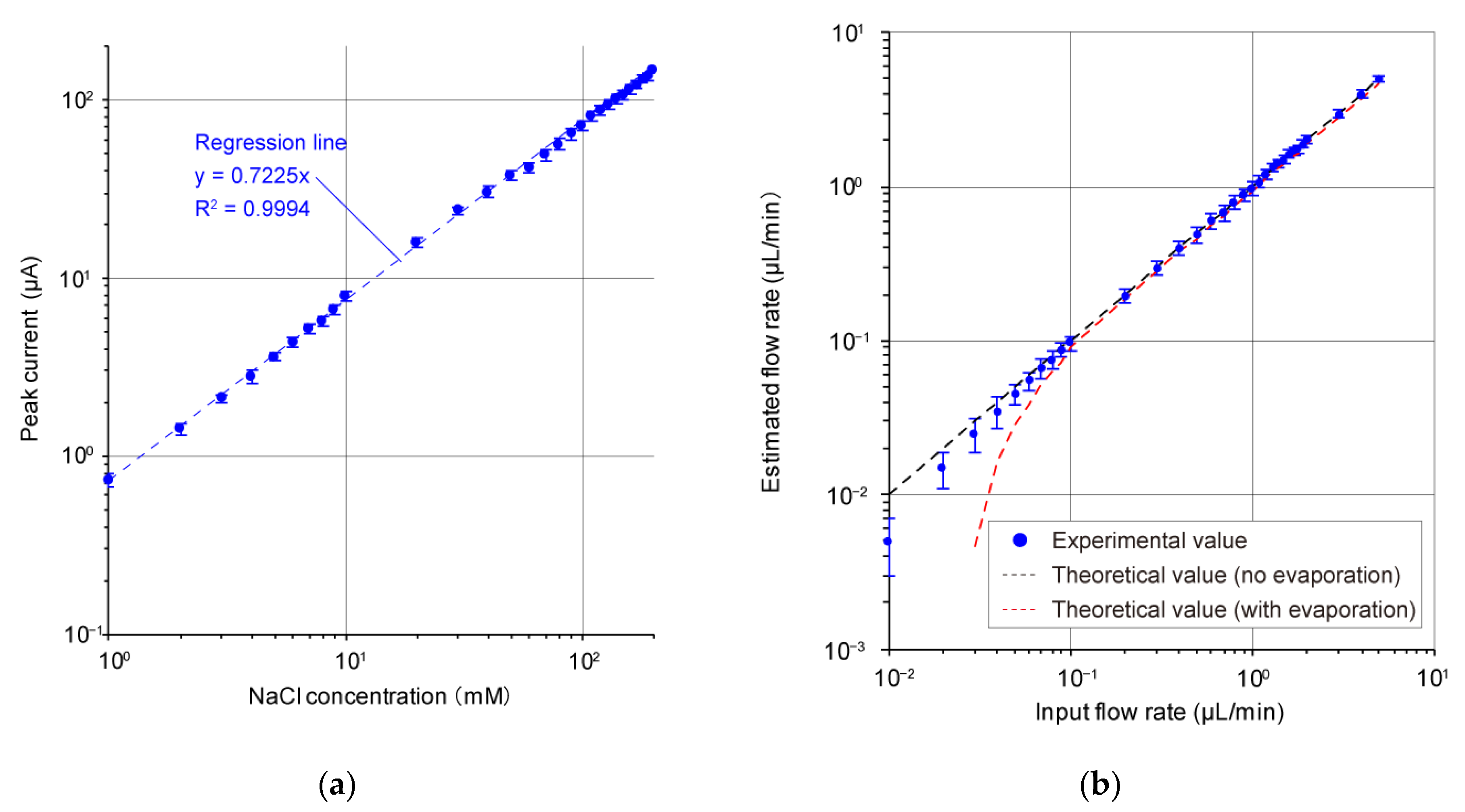
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Meehl, G.A.; Tebaldi, C. More Intense, More Frequent, and Longer Lasting Heat Waves in the 21st Century. Science 2004, 305, 994–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perkins-Kirkpatrick, S.; Gibson, P. Changes in regional heatwave characteristics as a function of increasing global temperature. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Schmeltz, M.T.; Marcotullio, P.J.; Himmelstein, S.; Woolhandler, S.; Sembajwe, G. Outcomes of hospitalizations for common illnesses associated with a comorbid heat-related illness in the United States. Clim. Chang. 2016, 138, 567–584. [Google Scholar] [CrossRef]
- Miyake, Y. Pathophysiology of heat illness: Thermoregulation, risk factors, and indicators of aggravation. Jpn. Med. Assoc. J. 2013, 56, 167–173. [Google Scholar]
- Buono, M.J.; Claros, R.; DeBoer, T.; Wong, J. Na+ secretion rate increases proportionally more than the Na+ reabsorption rate with increases in sweat rate. J. Appl. Physiol. 2008, 105, 1044–1048. [Google Scholar] [CrossRef] [Green Version]
- Baker, L.B.; Barnes, K.A.; Anderson, M.L.; Passe, D.H.; Stofan, J.R. Normative data for regional sweat sodium concentration and whole-body sweating rate in athletes. J. Sports Sci. 2016, 34, 358–368. [Google Scholar] [CrossRef]
- Amano, T.; Gerrett, N.; Inoue, Y.; Nishiyasu, T.; Havenith, G.; Kondo, N. Determination of the maximum rate of eccrine sweat glands’ ion reabsorption using the galvanic skin conductance to local sweat rate relationship. Eur. J. Appl. Physiol. 2016, 116, 281–290. [Google Scholar] [CrossRef] [Green Version]
- Shamsuddin, A.K.M.; Yanagimoto, S.; Kuwahara, T.; Zhang, Y.; Nomura, C.; Kondo, N. Changes in the index of sweat ion concentration with increasing sweat during passive heat stress in humans. Eur. J. Appl. Physiol. 2005, 94, 292–297. [Google Scholar] [CrossRef]
- Baker, L.B. Sweating Rate and Sweat Sodium Concentration in Athletes: A Review of Methodology and Intra/Interindividual Variability. Sports Med. 2017, 47, S111–S128. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Xu, T.; Wang, D.; Zhang, X. The role of sampling in wearable sweat sensors. Talanta 2020, 212, 120801. [Google Scholar] [CrossRef]
- Klous, L.; Folkers, M.; Daanen, H.; Gerrett, N. The effect of sweat sample storage condition on sweat content. Temperature 2021, 8, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Bariya, M.; Nyein, H.Y.Y.; Javey, A. Wearable sweat sensors. Nat. Electron. 2018, 1, 160–171. [Google Scholar]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Peck, C.A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; Lien, D.-H.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emaminejad, S.; Gao, W.; Wu, E.; Davies, Z.A.; Nyein, H.Y.Y.; Challa, S.; Ryan, S.P.; Fahad, H.M.; Chen, K.; Shahpar, Z.; et al. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc. Natl. Acad. Sci. USA 2017, 114, 4625–4630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, J.H.; Kim, S.-M.; Eom, Y.; Koo, J.M.; Cho, H.-W.; Lee, T.J.; Lee, K.G.; Park, H.J.; Kim, Y.K.; Yoo, H.-J.; et al. Extremely Fast Self-Healable Bio-Based Supramolecular Polymer for Wearable Real-Time Sweat-Monitoring Sensor. ACS Appl. Mater. Interfaces 2019, 11, 46165–46175. [Google Scholar] [CrossRef]
- He, X.; Yang, S.; Pei, Q.; Song, Y.; Liu, C.; Xu, T.; Zhang, X. Integrated Smart Janus Textile Bands for Self-Pumping Sweat Sampling and Analysis. ACS Sens. 2020, 5, 1548–1554. [Google Scholar] [CrossRef]
- Wang, S.; Wu, Y.; Gu, Y.; Li, T.; Luo, H.; Li, L.-H.; Bai, Y.; Li, L.; Liu, L.; Cao, Y.; et al. Wearable Sweatband Sensor Platform Based on Gold Nanodendrite Array as Efficient Solid Contact of Ion-selective Electrode. Anal. Chem. 2017, 89, 10224–10231. [Google Scholar] [CrossRef]
- Roy, S.; David-Pur, M.; Hanein, Y. Carbon Nanotube-Based Ion Selective Sensors for Wearble Applications. ACS Appl. Mater. Interfaces 2017, 9, 35169–35177. [Google Scholar] [CrossRef]
- Peng, B.; Li, G.; Li, D.; Dodson, S.; Zhang, Q.; Zhang, J.; Lee, Y.H.; Demir, H.V.; Ling, X.Y.; Xiong, Q. Vertically Aligned Gold Nanorod Monolayer on Arbitrary Substrates: Self-Assembly and Femtomolar Detection of Food Contaminants. ACS Nano 2013, 7, 5993–6000. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Cheng, C.; Liu, Z.; Yuan, W.; Xu, X.; Lu, Y.; Low, S.S.; Liu, J.; Zhu, L.; Ji, D.; et al. Battery-Free and Wireless Epidarmal Electrochemical System with All-Printed Stretchable Electrode Array for Multiplexed in Situ Sweat Analysis. Adv. Mater. Technol. 2019, 4, 1800658. [Google Scholar] [CrossRef]
- Koh, A.; Kang, D.; Xue, Y.; Lee, S.; Pielak, P.M.; Kim, J.; Hwang, T.; Min, S.; Banks, A.; Bastien, P.; et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med. 2016, 8, 366ra165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandodkar, A.J.; Gutruf, P.; Choi, J.; Lee, K.; Sekine, Y.; Reeder, J.T.; Jeang, W.J.; Aranyosi, A.J.; Lee, S.P.; Model, J.B.; et al. Battery-free, skin-interfaced microfluidic/electronic system for simultaneous electrochemical, colorimetric, and volumetric analysis of sweat. Sci. Adv. 2019, 5, eaav3294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.; Bandodkar, A.J.; Reeder, J.T.; Ray, T.R.; Turnquist, A.; Kim, S.B.; Nyberg, N.; Hourlier-Fargette, A.; Model, J.B.; Aranyosi, A.J.; et al. Skin-Integrated Multifunctional Microfluidic Systems for Accurate Colorimetric Analysis of Sweat Biomarkers and Temperature. ACS Sens. 2019, 4, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Cao, Y.; Zeng, Y.; Zhou, Y.; Wen, W.; Zhang, C.; Zhao, Y.; Chen, Z. Wearable tesla valve-based sweat collection device for sweat colorimetric analysis. Talenta 2022, 240, 123208. [Google Scholar] [CrossRef]
- Cristiane, K.; Vanessa, W.; Paulo, R.O.; Geovane, A.O.; Gustavo, M.; Antonio, S.M.; Marcio, F.B.; Luiz, H.M. Green method for glucose determination using microfluidic device with a non-enzymatic sensor based on nickel oxyhydroxide supported at activated biochar. Talenta 2019, 200, 518–525. [Google Scholar]
- Deonir, A.; Marcio, F.B.; Luiz, H.M. Tear glucose detection combining microfluidic thread based device, amperometric biosensor and microflow injection analysis. Biosens. Bioelectron. 2017, 98, 161–167. [Google Scholar]
- Mogi, K.; Hashimoto, Y.; Tsukahara, T.; Terano, M.; Yoshino, M.; Yamamoto, T. Nanometer-level high-accuracy molding using a photo-curable silicone elastomer by suppressing thermal shrinkage. RSC Adv. 2015, 5, 10172–10177. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Yamamoto, T. Solid state direct bonding of polymers by vacuum ultraviolet light below 160 nm. Appl. Surf. Sci. 2017, 419, 319–327. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Mogi, K.; Yamamoto, T. Vacuum ultraviolet light assisted bonding and nanoscale pattern transfer method for polydimethylsiloxane. Microelectron. Eng. 2017, 176, 116–120. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Yamamoto, T. Fabrication of an anti-reflective and super-hydrophobic structure by vacuum ultraviolet light-assisted bonding and nanoscale pattern transfer. Micromachines 2018, 9, 186. [Google Scholar] [CrossRef] [Green Version]
- Golnabi, H.; Matloob, M.R.; Bahar, M.; Sharifian, M. Investigation of Electrical Conductivity of Different Water Liquid and Electrolyte Solutions. J. Theor. Appl. Phys. 2009, 3, 24–28. [Google Scholar]
- Kamcev, J.; Sujanani, R.; Jang, E.-S.; Yan, N.; Moe, N.; Paul, D.R.; Freeman, B.D. Salt concentration dependence of ionic conductivity in ion exchange membranes. J. Membr. Sci. 2018, 547, 123–133. [Google Scholar] [CrossRef]
- Widodo, C.S.; Sela, H.; Santosa, D.R. The effect of NaCl concentration on the ionic NaCl solutions electrical impedance value using electrochemical impedance spectroscopy methods. AIP Conf. Proc. 2021, 2018, 050003. [Google Scholar]
- Horiba. LAQUAtwin Salt-22. Available online: https://www.horiba.com/usa/water-quality/detail/action/show/Product/laquatwin-salt-22-797/ (accessed on 12 January 2022).
- Sonner, Z.; Wilder, E.; Heikenfeld, J.; Kasting, G.; Beyette, F.; Swaile, D.; Sherman, F.; Joyce, J.; Hagen, J.; Kelly-Loughnane, N.; et al. The microfluidics of the eccrine sweat gland, including biomarker partitioning, transport, and biosensing implications. AIP Biomicrofluidics 2015, 9, 031301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skinos Product. Wearable Sweat Sensor SKW-1000. Available online: http://www.skinos.co.jp/product/product11/ (accessed on 13 January 2022).
- Inoue, Y.; Shibasaki, M. Regional differences in age-related decrements of the cutaneous vascular and sweating responses to passive heating. Eur. J. Appl. Physiol. 1996, 74, 78–84. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashimoto, Y.; Ishihara, T.; Kuwabara, K.; Amano, T.; Togo, H. Wearable Microfluidic Sensor for the Simultaneous and Continuous Monitoring of Local Sweat Rates and Electrolyte Concentrations. Micromachines 2022, 13, 575. https://doi.org/10.3390/mi13040575
Hashimoto Y, Ishihara T, Kuwabara K, Amano T, Togo H. Wearable Microfluidic Sensor for the Simultaneous and Continuous Monitoring of Local Sweat Rates and Electrolyte Concentrations. Micromachines. 2022; 13(4):575. https://doi.org/10.3390/mi13040575
Chicago/Turabian StyleHashimoto, Yuki, Takako Ishihara, Kei Kuwabara, Tatsuro Amano, and Hiroyoshi Togo. 2022. "Wearable Microfluidic Sensor for the Simultaneous and Continuous Monitoring of Local Sweat Rates and Electrolyte Concentrations" Micromachines 13, no. 4: 575. https://doi.org/10.3390/mi13040575
APA StyleHashimoto, Y., Ishihara, T., Kuwabara, K., Amano, T., & Togo, H. (2022). Wearable Microfluidic Sensor for the Simultaneous and Continuous Monitoring of Local Sweat Rates and Electrolyte Concentrations. Micromachines, 13(4), 575. https://doi.org/10.3390/mi13040575






