Thin Film Encapsulation for LCP-Based Flexible Bioelectronic Implants: Comparison of Different Coating Materials Using Test Methodologies for Life-Time Estimation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Thin Film Encapsulation (TFE) Layers
2.2. Evaluation Procedures
2.2.1. Pre-Screening of the Coatings
2.2.2. Sorption Tests and WVTR Calculation
2.2.3. Adhesion Evaluation by Adapted ASTM D1876 T-Peel Test
2.2.4. Electrochemical Impedance Spectrometry (EIS) on IDC Structures
3. Results
3.1. Pre-Screening of the Coatings by Soaking and Optical Inspection
3.2. Barrier Properties Evaluation with Sorption Test
3.3. Adhesion Evaluation with T-Peel Test
3.4. Coating Performance Evaluation with IDC Structures
4. Discussion
4.1. Methods Used for the Evaluation of the Proposed TFE Coatings
4.2. Performance of LCP and TFE Coatings
4.3. General Remarks
4.4. Choosing an Appropriate Coating for Each Application
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Swann, N.C.; De Hemptinne, C.; Thompson, M.C.; Miocinovic, S.; Miller, A.M.; Gilron, R.; Ostrem, J.L.; Chizeck, H.J.; Starr, P.A. Adaptive deep brain stimulation for Parkinson’s disease using motor cortex sensing. J. Neural Eng. 2018, 15, 046006. [Google Scholar] [CrossRef] [PubMed]
- Heck, C.N.; King-Stephens, D.; Massey, A.D.; Nair, D.R.; Jobst, B.C.; Barkley, G.L.; Salanova, V.; Cole, A.J.; Smith, M.C.; Gwinn, R.P.; et al. Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: Final results of the RNS System Pivotal trial. Epilepsia 2014, 55, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Wagner, F.; Mignardot, J.-B.; Le Goff-Mignardot, C.G.; Demesmaeker, R.; Komi, S.; Capogrosso, M.; Rowald, A.; Seáñez, I.; Caban, M.; Pirondini, E.; et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 2018, 563, 65–71. [Google Scholar] [CrossRef]
- Giagka, V.; Serdijn, W.A. Realizing flexible bioelectronic medicines for accessing the peripheral nerves—Technology considerations. Bioelectron. Med. 2018, 4, 8. [Google Scholar] [CrossRef]
- Greenhouse, H.; Lowry, R.; Romenesko, B. Hermeticity of Electronic Packages, 2nd ed.; William Andrew Publishing: Norwich, NY, USA, 2012; pp. 149–173. ISBN 9781437778779. [Google Scholar]
- Costello, S.; Desmulliez, M.P.Y.; McCracken, S. Review of test methods used for the measurement of hermeticity in packages containing small cavities. IEEE Trans. Compon. Packag. Manuf. Technol. 2012, 2, 430–438. [Google Scholar] [CrossRef] [Green Version]
- Klug, D.; Lacroix, D.; Savoye, C.; Goullard, L.; Grandmougin, D.; Hennequin, J.L.; Kacet, S.; Lekieffre, J. Systemic Infection Related to Endocarditis on Pacemaker Leads: Clinical presentation and management. Circulation 1997, 95, 2098–2107. [Google Scholar] [CrossRef] [PubMed]
- de Voogt, W.G. Pacemaker leads: Performance and progress. Am. J. Cardiol. 1999, 83, 187–191. [Google Scholar] [CrossRef]
- Ordonez, J.S.; Boehler, C.; Schuettler, M.; Stieglitz, T. Improved polyimide thin-film electrodes for neural implants. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; Volume 2012, pp. 5134–5137. [Google Scholar] [CrossRef]
- Chiang, C.-H.; Wang, C.; Barth, K.; Rahimpour, S.; Trumpis, M.; Duraivel, S.; Rachinskiy, I.; Dubey, A.; Wingel, K.E.; Wong, M.; et al. Flexible, high-resolution thin-film electrodes for human and animal neural research. J. Neural Eng. 2021, 18, 045009. [Google Scholar] [CrossRef]
- Choi, Y.S.; Hsueh, Y.-Y.; Koo, J.; Yang, Q.; Avila, R.; Hu, B.; Xie, Z.; Lee, G.; Ning, Z.; Liu, C.; et al. Stretchable, dynamic covalent polymers for soft, long-lived bioresorbable electronic stimulators designed to facilitate neuromuscular regeneration. Nat. Commun. 2020, 11, 5990. [Google Scholar] [CrossRef]
- Rihani, R.; Tasnim, N.; Javed, M.; Usoro, J.O.; D’Souza, T.M.; Ware, T.H.; Pancrazio, J.J. Liquid Crystalline Polymers: Opportunities to Shape Neural Interfaces. Neuromodul. Technol. Neural Interface 2021, 1–9. [Google Scholar] [CrossRef]
- Zhang, T.; Johnson, W.; Farrell, B.; Miller, F.; Rd, B.H.; Lawrence, M.S. Advanced Packaging Using Liquid Crystalline Polymer (LCP) Substrates. In Proceedings of the 35th International Symposium on Microelectronics, Denver, CO, USA, 4–6 September 2002; pp. 1–9. [Google Scholar]
- Lee, S.W.; Min, K.S.; Jeong, J.; Kim, J.; Kim, S.J. Monolithic Encapsulation of Implantable Neuroprosthetic Devices Using Liquid Crystal Polymers. IEEE Trans. Biomed. Eng. 2011, 58, 2255–2263. [Google Scholar] [CrossRef]
- Jeong, J.; Min, K.S.; Kim, S.J. Microfabrication process for long-term reliable neural electrode arrays using liquid crystal polymer (LCP). Microelectron. Eng. 2019, 216, 111096. [Google Scholar] [CrossRef]
- Koh, K.-S.; Chin, J.; Chia, J.; Chiang, C.-L. Quantitative Studies on PDMS-PDMS Interface Bonding with Piranha Solution and its Swelling Effect. Micromachines 2012, 3, 427–441. [Google Scholar] [CrossRef]
- Yang, C.; Wang, W.; Li, Z. Optimization of corona-triggered PDMS-PDMS bonding method. In Proceedings of the 2009 4th IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Shenzhen, China, 5–8 January 2009; pp. 319–322. [Google Scholar] [CrossRef]
- Palopoli-Trojani, K.; Woods, V.; Chiang, C.-H.; Trumpis, M.; Viventi, J. In vitro assessment of long-term reliability of low-cost μECoG arrays. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; Volume 2016, pp. 4503–4506. [Google Scholar] [CrossRef]
- Ortigoza-Diaz, J.; Scholten, K.; Larson, C.; Cobo, A.; Hudson, T.; Yoo, J.; Baldwin, A.; Hirschberg, A.W.; Meng, E. Techniques and Considerations in the Microfabrication of Parylene C Microelectromechanical Systems. Micromachines 2018, 9, 422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, R.J. LCP injection molded packages—Keys to JEDEC 1 performance. In Proceedings of the 54th Electronic Components and Technology Conference, Las Vegas, NV, USA, 4 June 2004; Volume 2, pp. 1807–1811. [Google Scholar]
- Au, S.L.C.; Chen, F.-Y.B.; Budgett, D.M.; Malpas, S.C.; Guild, S.-J.; McCormick, D. Injection Molded Liquid Crystal Polymer Package for Chronic Active Implantable Devices with Application to an Optogenetic Stimulator. IEEE Trans. Biomed. Eng. 2020, 67, 1357–1365. [Google Scholar] [CrossRef]
- Faheem, F.; Gupta, K.; Lee, Y.-C. Flip-chip assembly and liquid crystal polymer encapsulation for variable MEMS capacitors. IEEE Trans. Microw. Theory Tech. 2003, 51, 2562–2567. [Google Scholar] [CrossRef]
- Thompson, D.; Tentzeris, M.; Papapolymerou, J. Packaging of MMICs in multilayer LCP substrates. IEEE Microw. Wirel. Compon. Lett. 2006, 16, 410–412. [Google Scholar] [CrossRef] [Green Version]
- Jugessur, A.S.; Textor, A.; Grierson, C. Nanometer Scale Coating Using Atomic Layer Deposition Technique to Enhance Performance of Bio-Medical Devices. In Proceedings of the 2018 IEEE 12th International Conference on Nano/Molecular Medicine and Engineering (NANOMED), Waikiki Beach, HI, USA, 2–5 December 2018; pp. 146–149. [Google Scholar] [CrossRef]
- Morales, J.M.H.; Souriau, J.-C.; Simon, G. Corrosion Protection of Silicon Micro Systems with Ultra-Thin Barrier Films for Miniaturized Medical Devices. ECS Trans. 2015, 69, 9–26. [Google Scholar] [CrossRef]
- Skoog, S.A.; Elam, J.W.; Narayan, R.J. Atomic layer deposition: Medical and biological applications. Int. Mater. Rev. 2013, 58, 113–129. [Google Scholar] [CrossRef]
- George, S.M. Atomic Layer Deposition: An Overview. Chem. Rev. 2009, 110, 111–131. [Google Scholar] [CrossRef]
- Li, M.; Jin, Z.-X.; Zhang, W.; Bai, Y.-H.; Cao, Y.; Li, W.-M.; Wu, D.; Li, A.-D. Comparison of chemical stability and corrosion resistance of group IV metal oxide films formed by thermal and plasma-enhanced atomic layer deposition. Sci. Rep. 2019, 9, 10438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Cauwe, M.; Yang, Y.; Schaubroeck, D.; Mader, L.; De Beeck, M.O. Ultra-Long-Term Reliable Encapsulation Using an Atomic Layer Deposited HfO2/Al2O3/HfO2 Triple-Interlayer for Biomedical Implants. Coatings 2019, 9, 579. [Google Scholar] [CrossRef] [Green Version]
- Nanbakhsh, K.; Ritasalo, R.; Serdijn, W.A.; Giagka, V. Long-term Encapsulation of Platinum Metallization Using a HfO2 ALD—PDMS Bilayer for Non-hermetic Active Implants. In Proceedings of the 2020 IEEE 70th Electronic Components and Technology Conference (ECTC), Orlando, FL, USA, 3–30 June 2020; pp. 467–472. [Google Scholar]
- Kim, K.; Van Gompel, M.; Wu, K.; Schiavone, G.; Carron, J.; Bourgeois, F.; Lacour, S.P.; Leterrier, Y. Extended Barrier Lifetime of Partially Cracked Organic/Inorganic Multilayers for Compliant Implantable Electronics. Small 2021, 17, 2103039. [Google Scholar] [CrossRef] [PubMed]
- Graff, G.L.; Williford, R.E.; Burrows, P.E. Mechanisms of vapor permeation through multilayer barrier films: Lag time versus equilibrium permeation. J. Appl. Phys. 2004, 96, 1840–1849. [Google Scholar] [CrossRef]
- Vanhoestenberghe, A.; Donaldson, N. Corrosion of silicon integrated circuits and lifetime predictions in implantable electronic devices. J. Neural Eng. 2013, 10, 031002. [Google Scholar] [CrossRef] [PubMed]
- Nanbakhsh, K.; Kluba, M.; Pahl, B.; Bourgeois, F.; Dekker, R.; Serdijn, W.; Giagka, V. Effect of Signals on the Encapsulation Performance of Parylene Coated Platinum Tracks for Active Medical Implants. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; Volume 2019, pp. 3840–3844. [Google Scholar]
- Donaldson, N.; Lamont, C.; Idil, A.S.; Mentink, M.; Perkins, T. Apparatus to investigate the insulation impedance and accelerated life-testing of neural interfaces. J. Neural Eng. 2018, 15, 066034. [Google Scholar] [CrossRef]
- Jeong, J.; Bae, S.H.; Seo, J.-M.; Chung, H.; Kim, S.J. Long-term evaluation of a liquid crystal polymer (LCP)-based retinal prosthesis. J. Neural Eng. 2016, 13, 025004. [Google Scholar] [CrossRef]
- Lamont, C.A. Non-Hermetic Protection of Thin-Film Metallisation Layers Intended for Implanted Electronic Medical Devices. Ph.D. Thesis, University College London, London, UK, 2020. [Google Scholar]
- Lamont, C.; Grego, T.; Nanbakhsh, K.; Idil, A.S.; Giagka, V.; Vanhoestenberghe, A.; Cogan, S.; Donaldson, N. Silicone encapsulation of thin-film SiOx, SiOxNy and SiC for modern electronic medical implants: A comparative long-term ageing study. J. Neural Eng. 2021, 18, 055003. [Google Scholar] [CrossRef]
- Hukins, D.; Mahomed, A.; Kukureka, S. Accelerated aging for testing polymeric biomaterials and medical devices. Med. Eng. Phys. 2008, 30, 1270–1274. [Google Scholar] [CrossRef]
- Potter-Baker, K.A.; Capadona, J.R. Reducing the “Stress”: Antioxidative Therapeutic and Material Approaches May Prevent Intracortical Microelectrode Failure. ACS Macro Lett. 2015, 4, 275–279. [Google Scholar] [CrossRef]
- Schaubroeck, D.; Verplancke, R.; Cauwe, M.; Cuypers, D.; Baumans, K. Polyimide-ald-polyimide layers as hermetic encapsulant for implants. In Proceedings of the XXXI International Conference on Surface Modification Technologies (SMT31), Mons, Belgium, 5–7 July 2017. [Google Scholar]
- Babaroud, N.B.; Dekker, R.; Serdijn, W.; Giagka, V. PDMS-Parylene Adhesion Improvement via Ceramic Interlayers to Strengthen the Encapsulation of Active Neural Implants. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; Volume 2020, pp. 3399–3402. [Google Scholar] [CrossRef]
- Babaroud, N.B.; Dekker, R.; Holk, O.; Tiringer, U.; Taheri, P.; Horvath, D.; Nanasi, T.; Ulbert, I.; Serdijn, W.; Giagka, V. Investigation of the long-term adhesion and barrier properties of a PDMS-Parylene stack with PECVD ceramic interlayers for the conformal encapsulation of neural implants. In Proceedings of the 2021 23rd European Microelectronics and Packaging Conference & Exhibition (EMPC), Gothenburg, Sweden, 13–16 September 2021; pp. 1–7. [Google Scholar]
- Lee, C.J.; Oh, S.J.; Song, J.K.; Kim, S.J. Neural signal recording using microelectrode arrays fabricated on liquid crystal polymer material. Mater. Sci. Eng. C 2004, 24, 265–268. [Google Scholar] [CrossRef]
- Min, K.S.; Lee, C.J.; Jun, S.B.; Kim, J.; Lee, S.E.; Shin, J.; Chang, J.W.; Kim, S.J. A Liquid Crystal Polymer-Based Neuromodulation System: An Application on Animal Model of Neuropathic Pain. Neuromodul. Technol. Neural Interface 2014, 17, 160–169. [Google Scholar] [CrossRef] [PubMed]
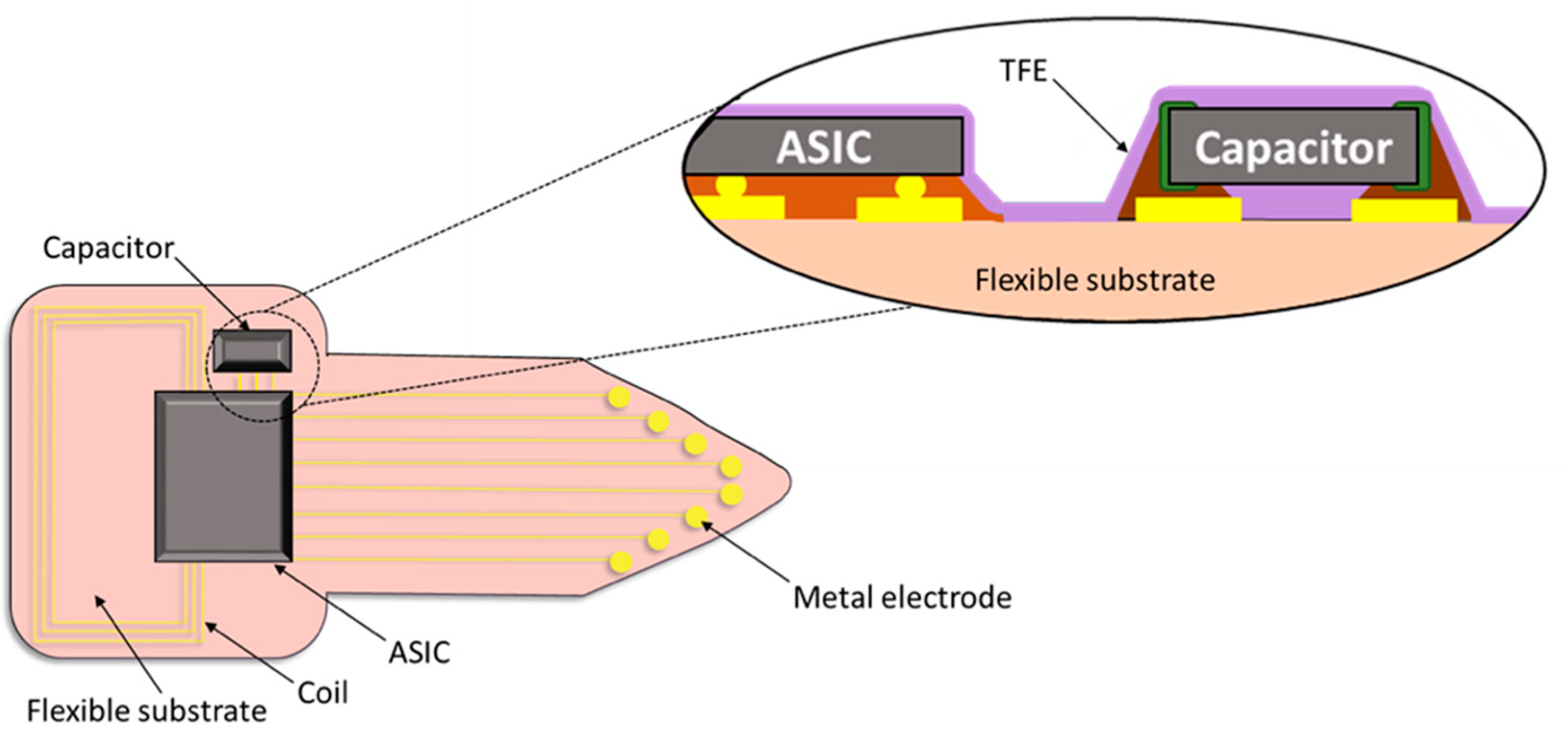

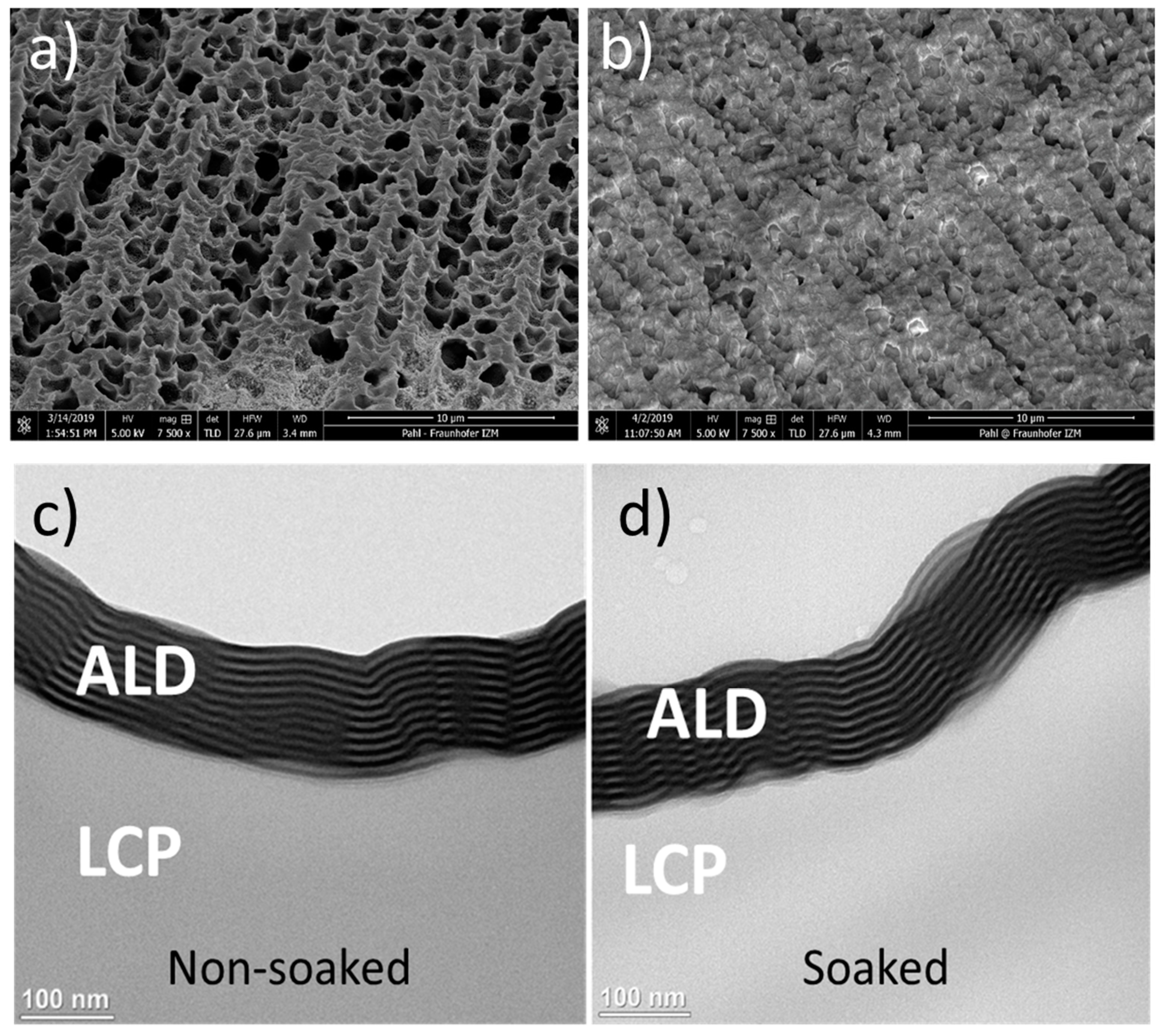
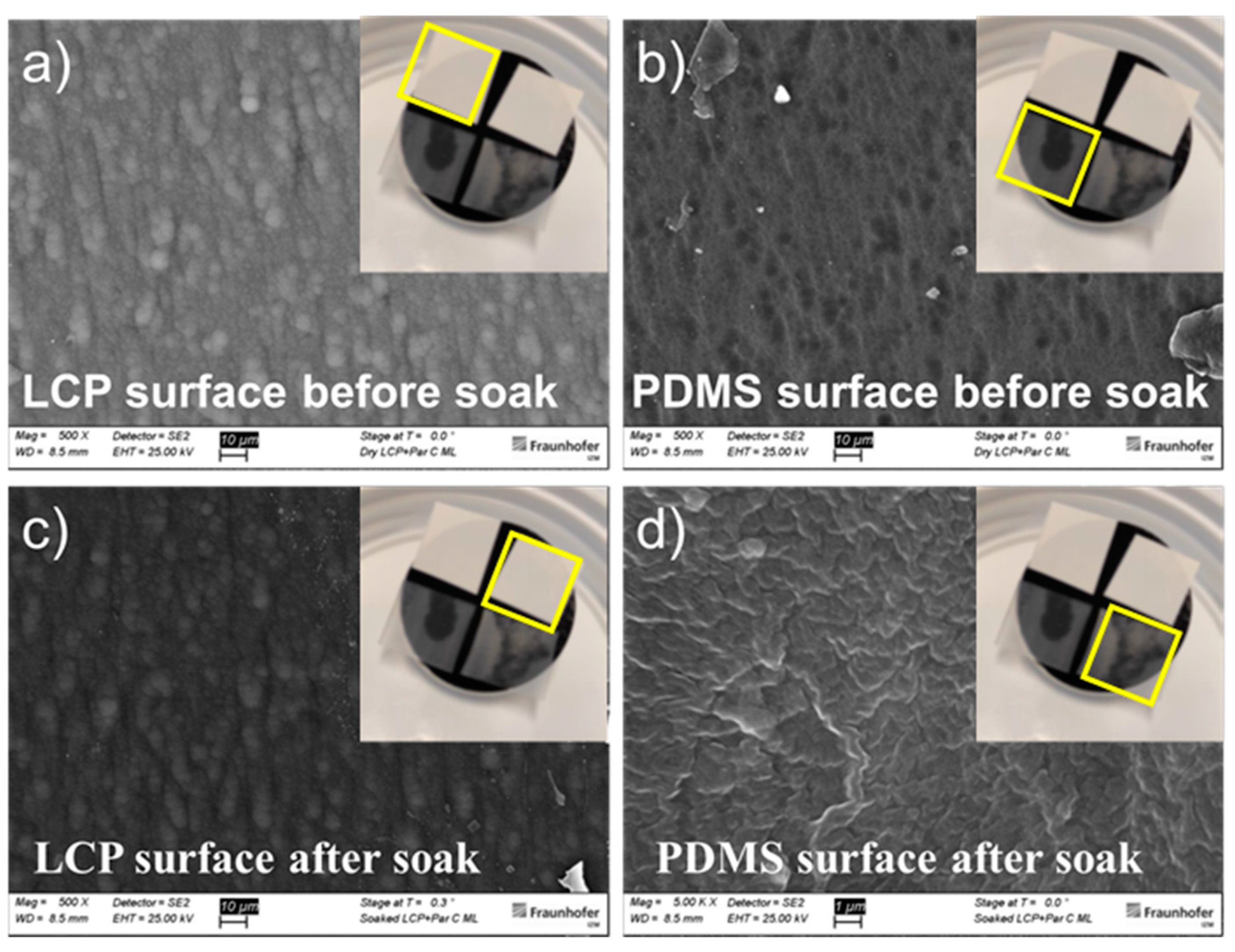
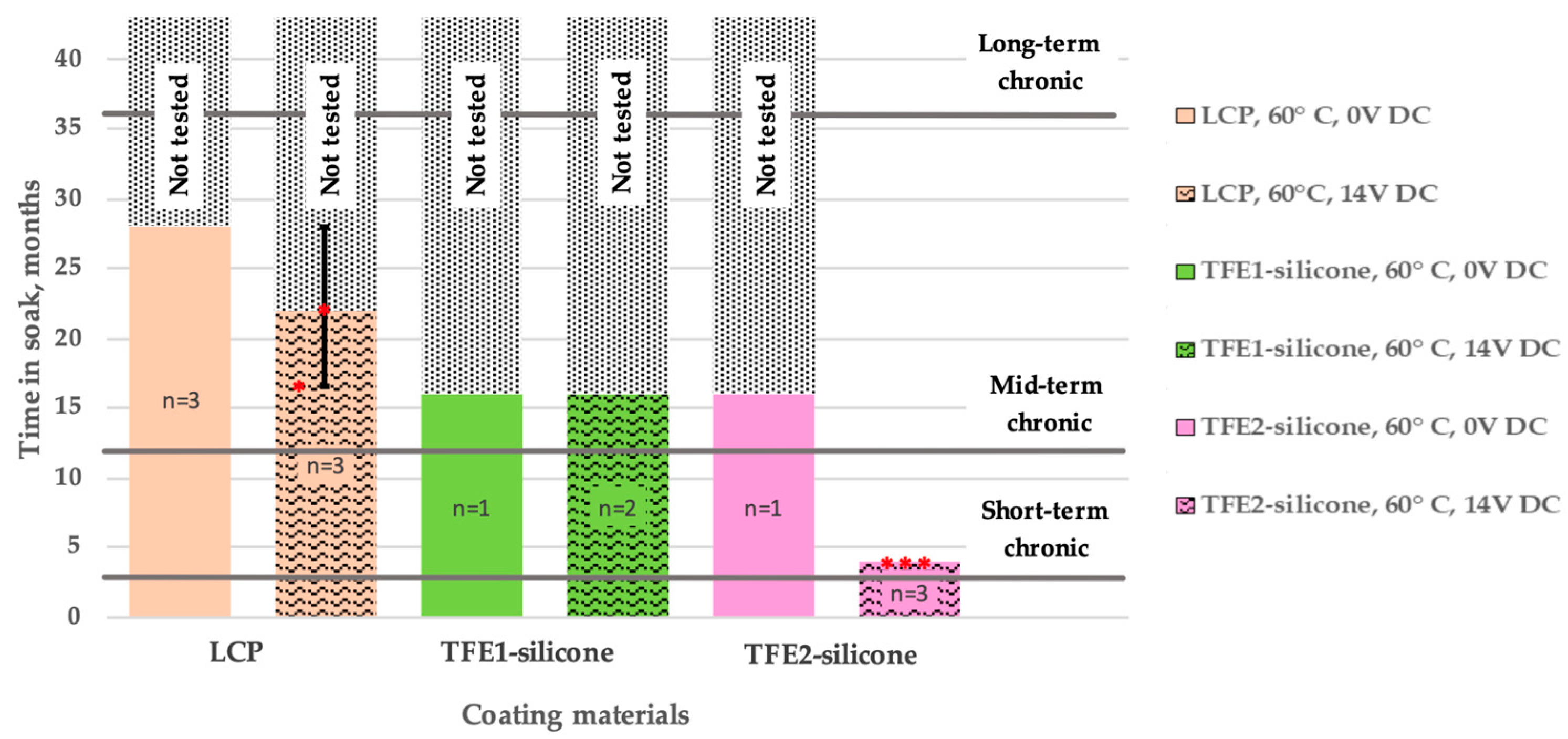
| Materials Stack | Thickness | WVTR | WVTR |
|---|---|---|---|
| (After Bending 1) | |||
| LCP | 100 µm | 202.05 mg/m2 day | 202.05 mg/m2 day |
| LCP–TFE1 | 100 + 0.1 | 2.87 mg/m2 day | 68.61 mg/m2 day |
| LCP–TFE2 | 100 + 7 | 2.23 mg/m2 day | 20.66 mg/m2 day |
| Materials Stack | Peel Force 1 (Before Soak) | Peel Force 2 (After Soak 1) |
|---|---|---|
| LCP-LCP | failed to peel | failed to peel |
| LCP-TFE1-silicone | 8 N (btw. TFE1 and LCP) | 8 N (btw. TFE1 and LCP) |
| LCP-TFE2-silicone | 0.1 N (btw. TFE2 and silicone) | 0.1 N (btw. TFE2 and silicone) |
| Before Soak | After Soak | |||
|---|---|---|---|---|
| Element | On LCP | On PDMS | On LCP | On PDMS |
| C | 80.41% | 36.8% | 80.13% | 36.91% |
| O | 0% | 30.72% | 0% | 31.15% |
| Si | 0.07% | 32.48% | 0.26% | 31.94% |
| Cl | 19.12% | 0% | 18.85% | 0% |
| Al | 0.23% | 0% | 0.34% | 0% |
| Ti | 0.17% | 0% | 0.11% | 0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pak, A.; Nanbakhsh, K.; Hölck, O.; Ritasalo, R.; Sousa, M.; Van Gompel, M.; Pahl, B.; Wilson, J.; Kallmayer, C.; Giagka, V. Thin Film Encapsulation for LCP-Based Flexible Bioelectronic Implants: Comparison of Different Coating Materials Using Test Methodologies for Life-Time Estimation. Micromachines 2022, 13, 544. https://doi.org/10.3390/mi13040544
Pak A, Nanbakhsh K, Hölck O, Ritasalo R, Sousa M, Van Gompel M, Pahl B, Wilson J, Kallmayer C, Giagka V. Thin Film Encapsulation for LCP-Based Flexible Bioelectronic Implants: Comparison of Different Coating Materials Using Test Methodologies for Life-Time Estimation. Micromachines. 2022; 13(4):544. https://doi.org/10.3390/mi13040544
Chicago/Turabian StylePak, Anna, Kambiz Nanbakhsh, Ole Hölck, Riina Ritasalo, Maria Sousa, Matthias Van Gompel, Barbara Pahl, Joshua Wilson, Christine Kallmayer, and Vasiliki Giagka. 2022. "Thin Film Encapsulation for LCP-Based Flexible Bioelectronic Implants: Comparison of Different Coating Materials Using Test Methodologies for Life-Time Estimation" Micromachines 13, no. 4: 544. https://doi.org/10.3390/mi13040544
APA StylePak, A., Nanbakhsh, K., Hölck, O., Ritasalo, R., Sousa, M., Van Gompel, M., Pahl, B., Wilson, J., Kallmayer, C., & Giagka, V. (2022). Thin Film Encapsulation for LCP-Based Flexible Bioelectronic Implants: Comparison of Different Coating Materials Using Test Methodologies for Life-Time Estimation. Micromachines, 13(4), 544. https://doi.org/10.3390/mi13040544






