4.1. General Considerations of Electrochemical Mechanisms Occurring at an Electrode Surface In Vitro and In Vivo
Cyclic voltammetry allows the investigation of electrochemical reaction mechanisms occurring at an electrode surface. Platinum is able to support a range of Faradaic and pseudo-capacitance (surface confined Faradaic) reactions as well as capacitance [
13]. In saline, this includes the reduction and oxidation of platinum oxide, adsorption and stripping of hydrogen, anion adsorption, reduction of oxygen, and reduction and oxidation of water. The mechanisms that do occur depend on the electrode surface, solution composition, and applied waveform [
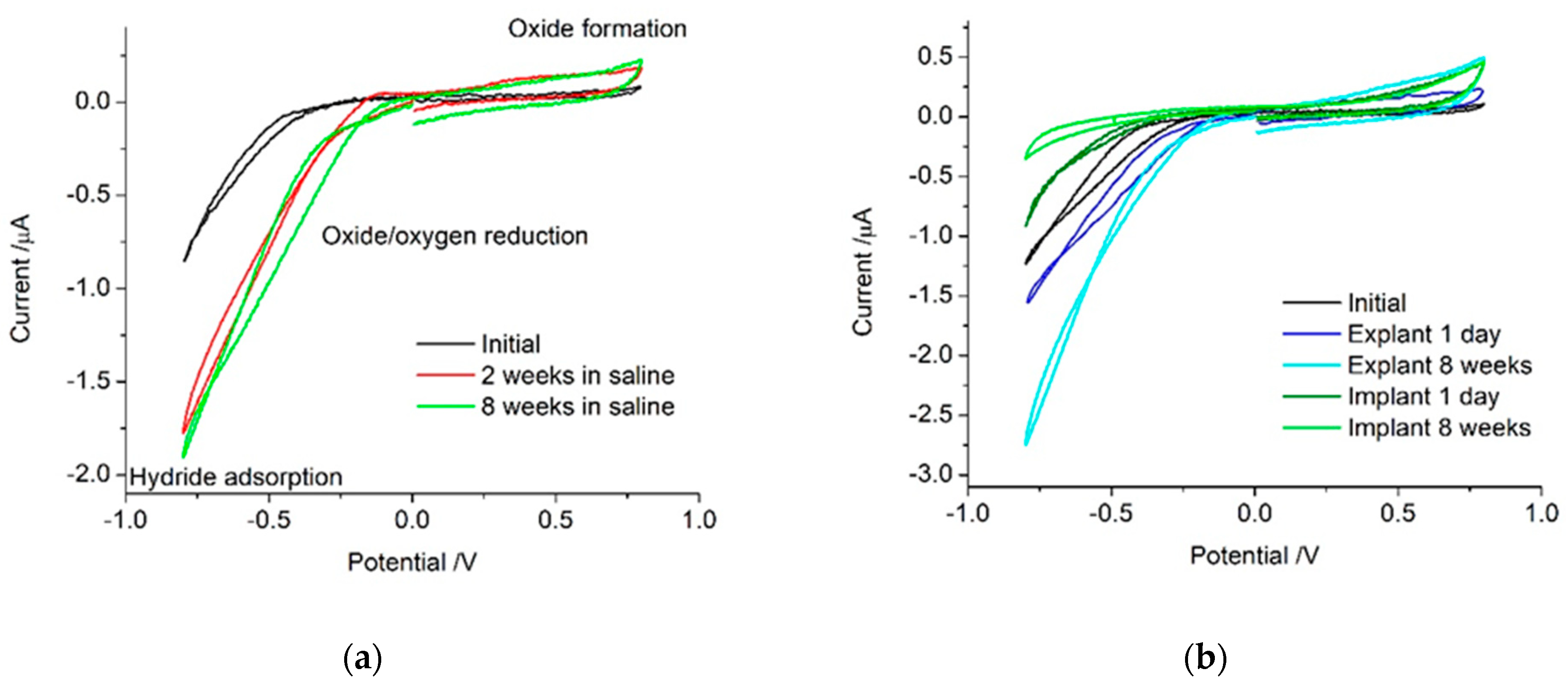
21]. The initial platinum cochlear implant electrode showed no electrochemically detectable levels of platinum oxide present (
Figure 1). On its own, voltammetry is not able to distinguish between different mechanisms occurring under the broad reduction process below −0.5 V. In non-degassed saline on a sterilised but unpolished platinum cochlear implant electrode, the reactions that occurred within the water window were most likely oxygen reduction, small amounts of platinum oxide reduction and formation, anion adsorption, capacitance, and possibly hydrogen adsorption. After multiple potential cycles, the platinum is increasingly oxidised, increasing the current associated with platinum oxide formation and removal. Electrode activation processes also reduced the over-potential required for the platinum oxide and oxygen reduction processes, shifting them to more positive potentials.
When the electrode was implanted, the surrounding tissue contained a range of organic species that may be electrochemically active or affect the reactions detected in saline. The implanted electrode voltammetry showed a smaller reduction current magnitude below −0.5 V than seen on the explanted electrode (
Figure 1). This suggests a lower oxygen tension in tissue than in the non-degassed saline. In the body, most oxygen is bound to haemoglobin, so the oxygen tension available for reduction at the electrode surface is expected to be extremely low [
23,
28,
29]. The 1-day implanted electrode also had a larger oxidation current above 0.5 V than the explanted electrode. This may be due to the oxidation of organic molecules or anion adsorption from species not present in saline [
21,
30,
31]. After 8 weeks, the implanted electrode had an even smaller current associated with oxygen and platinum oxide reduction. However, the oxidation process appeared similar to that of the 1-day implant. This implies the electrode is not being oxidised while it is implanted and the species responsible for the oxidation current is still present. The 8-week explanted electrode still generates a large oxygen reduction current, indicating the surface is highly active and not blocked by organic species.
When judging the utility of the in vitro voltammetric analysis of electrodes as a model for in vivo performance, the solution composition is of critical importance. Similar ionic concentration and composition, low oxygen tension, and similar pH and organic composition are required [
21,
32,
33,
34,
35]. While saline or similar buffered electrolyte solutions offer reasonable, simple-to-use testing conditions, trace components in vivo may affect the voltammetric response. Furthermore, electrode polarisation can significantly modify its behaviour. Subsequently, voltammetry should be performed on implantable electrodes to gauge the range of reactions that can occur at the electrode–tissue interface. However, the simplification of this analysis to a charge storage capacity or charge density value may not provide any useful or relevant information on its in vivo performance.
4.2. Comparison of Impedance Response In Vitro and In Vivo
The impedance of bionic electrodes is typically measured with the value at 1 kHz reported. A lower electrode impedance is related to its thermal noise and increased signal-to-noise ratio for neural recording [
34]. We recently showed that impedance at low frequencies, rather than at 1 kHz, is more strongly dependant on the electrode properties and is a better predictor of thermal noise and the signal-to-noise ratio of neural recordings [
26]. These previous measurements were made on electrodes that were not electrically polarised, nor was the impedance measured in vivo. To further understand the impedance behaviour of bionic electrodes, in the current work, the impedance was measured before and after electrode polarisation and before and after implantation.
The platinum electrode behaved as a CPE in series with a resistance, in saline, when implanted in the cochlea or subcutaneously. Electrode polarisation and fibrous tissue formation had no effect on the equivalent circuit required to achieve a good fit of the impedance response. Importantly, this indicates that tissue and fibrous tissue do not introduce a new Warburg element or time constant into the impedance response.
Impedance was performed at 0 V, where voltammetry indicated minimal Faradaic charge transfer was present. As a result, no Warburg element was required in the equivalent circuit. However, if the impedance was performed below −0.5 V or above 0.5 V, a Faradaic current due to oxygen reduction or platinum oxidation would have been present. While these applied potentials during EIS are unlikely to be used, the presence of Faradaic reactions at the measurement potential may occur in different conditions or on other electrode materials. The impedance response would then have been dependant on the Faradaic reaction, including the concentration of the redox species. For instance, impedance performed at −0.5 V would have shown a significant effect at low frequencies due to oxygen. This would result in variations between the non-degassed in vitro response and the low oxygen tension in vivo response. Similarly, potentials above 0.5 V may be affected by a Faradaic current associated with anions and organic species. The magnitude of the Faradaic current at these potentials also depends on the electrode surface, with electrode activation increasing the current magnitude and subsequently affecting the impedance [
21].
Great care must then be taken when performing and analysing impedance in vitro as a model of in vivo behaviour. Once again, the solution composition for an in vitro test must be similar to the in vivo environment. The choice of measurement potential and correct fitting of an equivalent circuit is crucial in correctly identifying the mechanisms occurring at the electrode–tissue interface.
Impedance performed in vitro displayed a reproducible solution resistance across all electrodes and measurement times (
Table S2). Implanted electrodes had a larger resistance, with the cochlear electrodes double that of the subcutaneous electrodes. This is consistent with the current from the cochlear electrodes having to pass through more resistive bone. There was some variable increase in cochlear electrode resistance over time, which was most likely due to bone formation in the scala tympani. The consistency in resistance over time of the subcutaneous electrodes and some cochlear electrodes indicates that fibrous tissue does not have a significant difference in resistivity compared to normal tissue. The similarity of the initial and explanted electrode resistivity also indicates that any protein fouling has a minimal impact on impedance. The effects of protein fouling on electrodes in vitro were studied previously [
35]. When an electrode was placed into a protein containing solution with a similar composition to perilymph, it partially blocked the electrode, resulting in impedance at 12 Hz increasing by ~20–30 kΩ and admittance decreasing slightly. Therefore, protein fouling is expected to be occurring on the implanted electrodes, but it has a significantly smaller effect than electrode activation and measuring within tissue (
Tables S1 and S2).
Admittance increased significantly after electrode polarisation, but no trends were visible between in vitro, either cochlea or subcutaneous implants, or over time. The admittance value is a function of surface area, roughness, and chemical functionality. Similar increases in admittance have been seen on the oxidation of platinum and iridium Utah and Michigan style bionic electrodes [
36,
37,
38]. Electrode polarisation drove changes to the electrode surface, but changes to the surrounding solution or tissue composition had a minimal impact on the admittance.
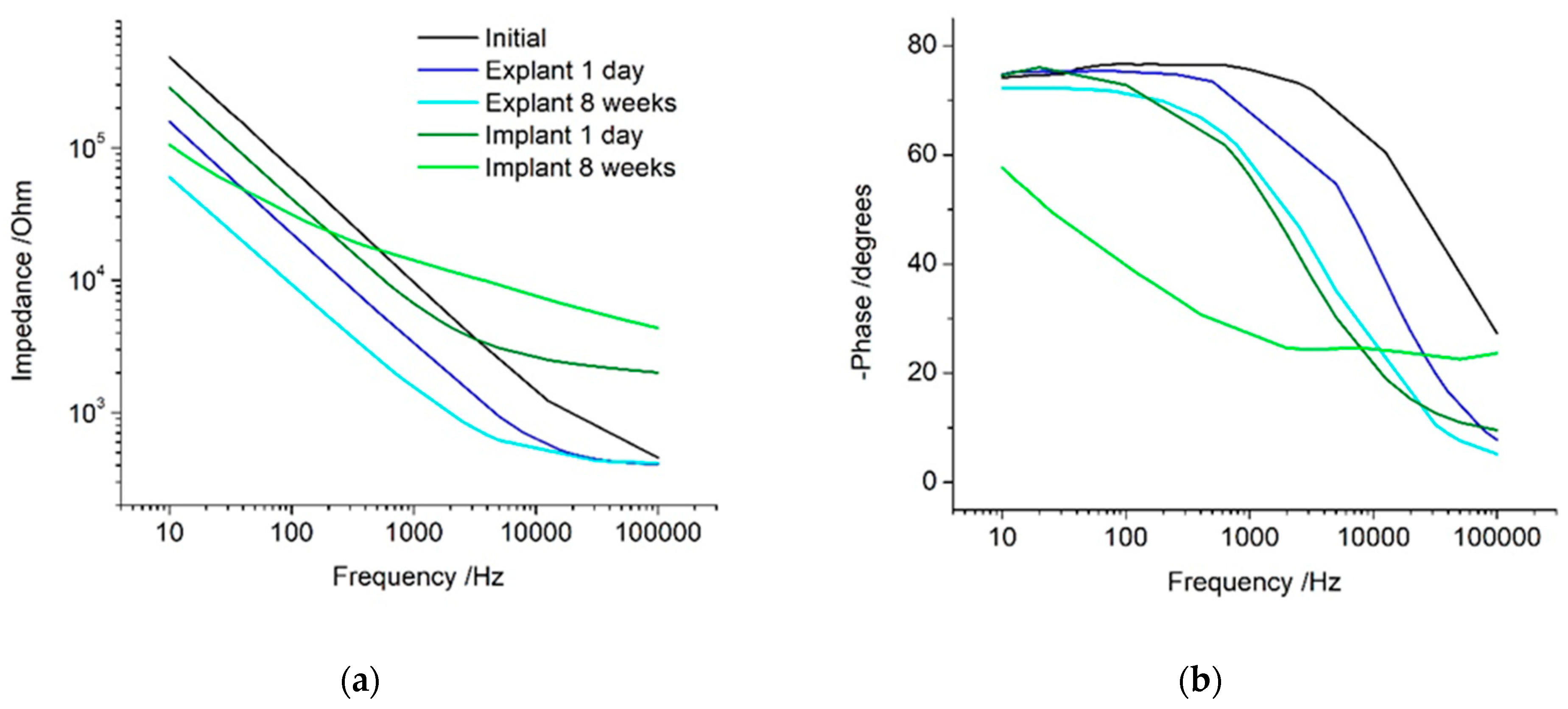
The impedance response is dominated by the electrode behaviour at low frequencies and by the solution properties at high frequencies. The transition between these two frequency regions is termed the Maxwell–Wagner frequency [
26]. This frequency depends on testing conditions and is difficult to measure and predict. When impedance analysis is limited to a measurement at 1 kHz, it is seen to decrease under all conditions, with only small differences between the implanted and control electrodes. This gives the impression that the electrode is improving in behaviour when implanted and over time, and that the in vitro and in vivo responses are similar. This measurement did not resolve any changes due to electrode activation or increases in tissue resistivity. If a Faradaic current was present, then the 1 kHz measurement would also be affected by the electron transfer reaction. The measurement frequency appears to be near the Maxwell–Wagner frequency, and therefore is susceptible to changes in most experimental conditions, which cannot be resolved. Subsequently, articles that only report the impedance at this frequency do not provide sufficient information to assess the electrode or tissue behaviour and any analysis of the results must be viewed with caution.
The impedance at low frequencies was seen to decrease with electrode activation and admittance. This frequency was previously shown to strongly correlate with electrode area, thermal noise, and signal-to-noise ratio [
26,
35]. Reporting of in vitro impedance at low frequencies would therefore be a far better predictor of in vivo performance. The impedance at high frequencies is strongly correlated with the solution resistivity. It would therefore be a much better measure of tissue resistivity over time than impedance measurements at 1 kHz.
4.3. General Experimental Considerations for the Electrochemical Analysis of Bionic Electrodes
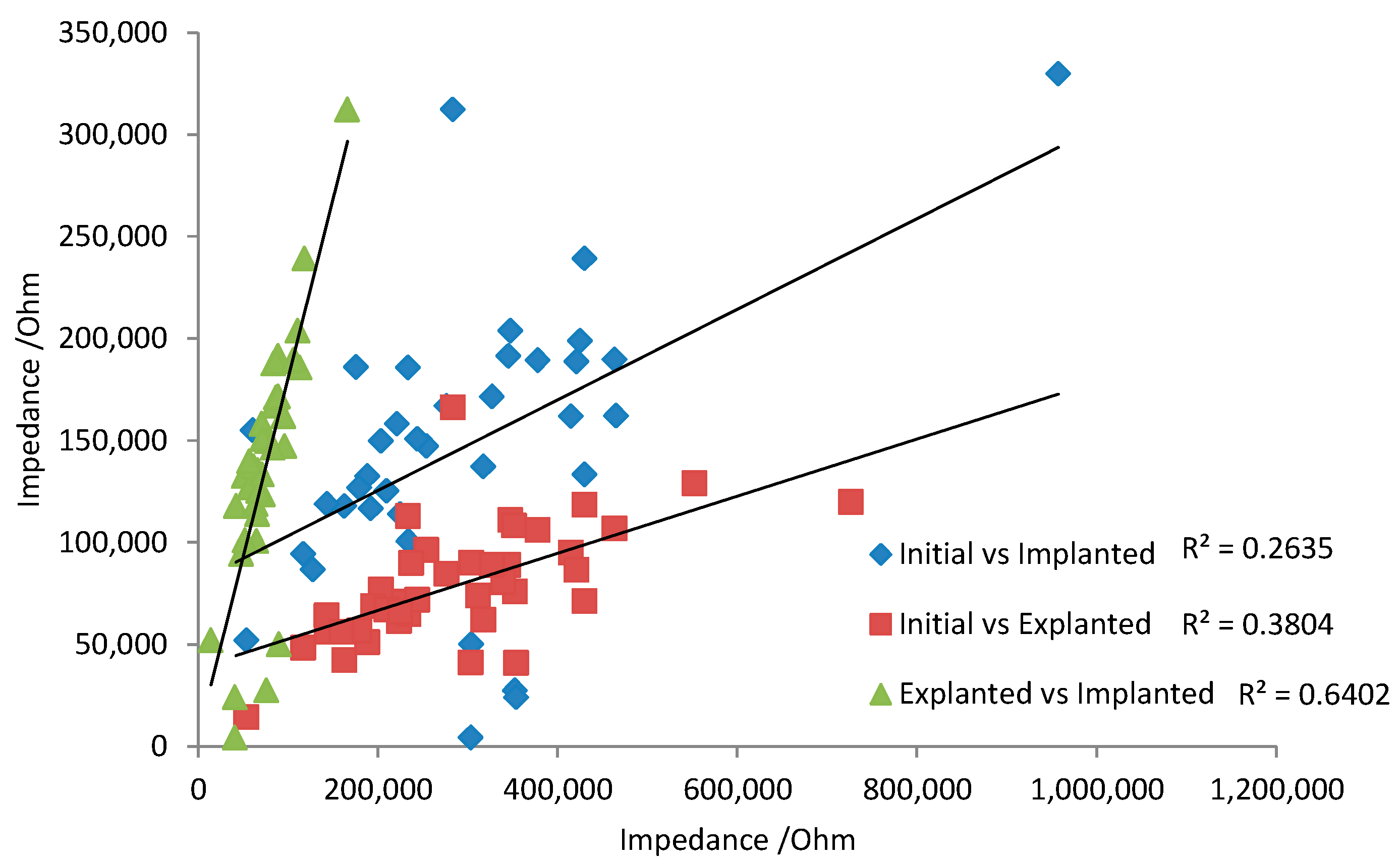
There are some general aspects that must be taken into account when translating the in vitro and in vivo electrochemical response. Over 8 weeks of implantation, there appeared to be little change in voltammetric response. This implies the tissue is not noticeably oxidising or damaging the electrode. For neural recording electrodes, the electrochemical response of platinum may therefore be relatively consistent, so that initial electrochemical measurements can be correlated with later in vivo performance. However, the electrodes were not electrically stimulated while implanted. Electrode polarisation during neural stimulation may induce electrode oxidation and surface rearrangement. Subsequently, the electrochemical behaviour of stimulated electrodes may change over time. Changes in electrode behaviour would reduce any correlation from initial electrochemical analysis with later in vivo performance. For instance, the impedance at 15 Hz was compared between initial, 1-day in vitro, and 1-day explanted responses (
Figure 6). There was a very poor prediction of in vivo response with the initial measurement due to the activation of the electrode from one voltammetric cycle. A stronger correlation of 1-day in vivo and 1-day explanted impedance was achieved, as electrode polarisation from subsequent voltammetric cycles had less impact on the impedance response.
The electrochemistry performed in saline was with a defined and stable reference electrode. This allows the potentials of any processes to be measured accurately. The solution composition can also be carefully modified to assign voltammetric responses to specific reaction mechanisms. In contrast, the in vivo measurements were made versus a quasi-reference electrode. The potential of a quasi-reference electrode is unknown and can change over time with changes in tissue composition and electrode surface, even within a single experiment. For instance, oxygen concentration, pH, platinum oxide formation, anion concentration, and organic species adsorption can alter the reference electrode potential. The potential of a platinum electrode coated with varies proteins changed by nearly 100 mV [
37]. It is possible to calibrate a quasi-reference electrode by measuring the potential of a known redox process. Typical redox species used for calibrating a quasi-reference electrode include ferrocene, ferricyanide and ruthenium hexaamine. When the concentration of these species is known, the oxidation and reduction peaks of their voltammetric response can be directly translated to the potential on a known reference electrode. However, the addition of these species into an animal may induce an undesirable biological response. Furthermore, the concentration of the chemical would not be accurately known. A well-defined surface confined redox process can also be used to calibrate the electrode potentials. The platinum oxide reduction process and hydrogen adsorption are surface confined; however, in opposition to previous reports [
39], these processes are poorly defined and depend on solution composition (pH and anion concentration) that prevent their use as a calibration process. As a result, the voltammetric response obtained in vivo may not align with the measurements in saline. This will affect the potential range used for defining the safe water electrolysis window, assigning potentials to specific redox reactions, and obtaining equivalent charge and CSC values from in vitro and in vivo experiments. Great care must then be taken in assigning specific potentials and reaction mechanisms for redox processes from the in vivo electrochemistry.
EIS was performed at 0 V vs. the reference electrodes, with the in vivo quasi-reference electrode being different to the well-defined Ag/AgCl reference electrode. The actual working electrode potential was therefore different for the in vitro and in vivo experiments. No Faradaic process was present at this potential, so the equivalent circuit was valid for in vitro and in vivo measurements. Changes to electrode potential do not affect the solution resistance, but will change the admittance value by ~10–20% [
35]; however, this is significantly smaller than the order of magnitude change seen from electrode activation (
Table S2).
All electrochemical measurements performed in this manuscript were undertaken in a 3-electrode system. A 3-electrode system ensures the majority of the current flows from the working electrode to a counter electrode while the working electrode potential is controlled by the reference electrode. In a commonly used 2-electrode system, the current flows from the working electrode through the reference electrode [
39]. Passing the current through the reference electrode will drive changes in its composition and subsequently its potential. This will further complicate any voltammetric response obtained and affect any analysis of measured potentials and reaction mechanisms.
These issues associated with the stability of the reference electrode potential are only of concern when measuring the dc potentials of an electrochemical system as described in this manuscript. When measuring the electrophysiological behaviour of tissue, the response is normally high-pass filtered, removing the dc component of the data. As a result, changes in reference electrode potential due to solution composition and electrode polarisation do not affect local field potential and spike data assessed from high frequency electrophysiological recordings.
Electrochemical measurements made in saline use a high conductivity electrolyte, limiting any iRu effects. When electrochemistry was performed in vivo, the tissue resistance was significantly higher than in saline. Fitting the EIS produced a solution resistance of several kΩ. The effects of iRu become visible in cyclic voltammetry at a level of ~1 mV or more. The voltammetry in this work produced currents up to ~1 μA, so an R of 5 kΩ would result in iRu = 5 mV. For the implanted electrode voltammetry, regions where the current is large may have a small shift in measured potentials as a result of iRu. The impact of iRu will be greater when the resistance or current is larger. This may occur when using faster voltammetric scan rates, larger electrodes, or the tissue is more resistive (due to larger electrode distances or higher bone content between the electrodes). When iRu is present, electrochemical analysis is further complicated, as variations in resistance will also impact its response (i.e., variations in electrode size, distances, and tissue resistivity will affect electrochemical behaviour).
In the current work, the electrode distances were different between animals and over time. As a result, the tissue resistivity will be slightly affected by experimental error. The electrode area also varied along the implant and between each handmade device. This increased the variation across the data set.
The impacts of varying reference electrode potential and iRu are not normally assessed or even detected during in vitro measurements. The presence of these effects in vivo can significantly impact the relevance of the in vitro measurements. Appropriate care must be taken to prevent these effects or to measure their impact.
4.4. Implications for Controlled Current Stimulation of Tissue
Electrochemical analysis of bionic electrodes is typically confined to voltammetry and EIS; however, they are normally used clinically with short (μs) current controlled (chronopotentiometric) pulses. In general, the charge storage capacity is related to the change in electrode potential during a current pulse [
24]. The maximum current can then be limited so the electrode potential does not obtain values which enable water electrolysis. However, it is difficult to assign specific reaction mechanisms to a chronopotentiometric curve. The voltammetry performed in this work indicates that oxygen reduction, platinum oxide reduction and formation, anion adsorption, capacitance, hydrogen adsorption, and water electrolysis can occur during current pulsing. These reactions can have slow kinetics and be irreversible [
38]. As a result, a biphasic waveform may be charge balanced, but the reactions on the oxidation and reduction pulses may be different. This can lead to electrode activation over multiple current pulses and a change in the chemical composition of the electrode–tissue interface.
Electrode activation reduced the over-potential for oxygen reduction and platinum oxide reduction and formation. Electrode activation may occur during current pulsing in vivo, so changes in electrode potential would decrease over time. The low oxygen tension in vivo would significantly reduce the charge available from this reaction, so the reduction current would need to be supplied by a different mechanism, and would most likely lead to a more negative reduction potential. The high in vivo resistance will lead to iRu effects, with larger currents having a greater impact. Thus, while the electrode potential may overshoot the water electrolysis potential, the true electrode potential may still be safe.
In some commercial devices, electrode performance is assessed by an impedance test [
39,
40]. This test measures the electrode potential at some point during a current pulse and applies Ohm’s law to calculate a resistance value. An early publication using this technique measured the potential at the end of a current pulse and argued that in vivo changes in response were initially due to fluid build-up, followed by increasing amounts of fibrous tissue growth [
40]. Changes in the impedance test have been detected with cell growth over the electrode in vitro and after fibrous tissue formation in vivo, but not with protein fouling in vitro [
41,
42]. However, these previous reports all perform this impedance test in a different manner, preventing any comparison of results. Measuring a point on a smooth chronopotentiometric curve is prone to significant error, limiting the accuracy of this technique. More fundamentally, Ohm’s law is only applicable to electrical circuits composed of uniform conductors, not electrochemical systems. The results obtained from an impedance test are not equivalent to electrochemical impedance spectroscopy. Values obtained from the impedance test are not real and the sole attribution of changes in response to biological processes is too simplistic. Changes in tissue or solution resistance, bone formation, distance between electrodes, electrode size or roughness, electrode activation, and solution composition could affect the electrode potential during a current pulse and hence the values measured from an impedance test.
4.5. Recommended In Vitro Testing Method for Bionic Electrodes
While there has been a tendency to reduce electrochemical information about bionic electrodes to impedance at 1 kHz and a cathodic CSC, this and previous work by our group has shown this type of analysis is too simplistic. Electrochemical reactions are not reducible to Ohm’s law, and require more detailed analyses to understand charge transfer at the electrode–tissue interface.
There is now an effort to prescribe a single testing solution which is valid for all implantable electrodes [
43]. This would enable a simple comparison of all stimulation waveforms, new electrode materials, and geometries. However bionic electrodes are used in vastly different regions of the body (e.g., within the brain, in the cochlea, on the retina, within blood vessels, and within muscle). These electrodes may be in contact with different types and concentrations of salt, protein, and other organic species and different cell types. Moreover, a model solution of the brain may have poor relevance to the cochlea.
An electrode material can have specific and non-specific interactions; for instance, proteins will adsorb on most electrodes, partially blocking them [
35], while specific anions and amino acids will attach to a platinum electrode but may not interact with other electrode materials [
31]. Omitting amino acids which adsorb to platinum from a model solution limits its relevance. Simple solutions, such as PBS [
43], are very poor in vivo models for platinum, as the high phosphate concentration adsorbs on the surface, altering the charge transfer mechanisms [
21]. Commonly used sulfuric acid solutions used to clean and assess platinum electrodes [
44] are also not relevant, as highly polished electrodes are not used clinically; the charge available from hydride and anion adsorption and the protein stability and adsorption in highly acidic solutions are not relevant to the in vivo environment [
21]. Moreover, a model solution tailored specifically to platinum may have poor relevance for understanding other materials.
Furthermore, the electrode–tissue interface is dynamic, with changes in composition over time and from electrical stimulation, and protein adsorption is time dependent [
3]. Recommending a testing solution composition for reproducible comparisons must also include the method for its use.
We recommend a series of electrochemical testing solutions and experiments for assessing bionic electrodes. These experiments are aimed at understanding the range of behaviours of an electrode, but are limited in their predictive response as the impact of implantation, iRu, variable reference electrode potential, and local variations in chemical and tissue composition are not captured in an in vitro test.
The electrode should be tested in a simple saline solution (e.g., 0.9%/0.15 M NaCl) which has been degassed with nitrogen or argon for at least 10 min. A second test should then be performed in a degassed artificial interstitial fluid, perilymph, or cerebrospinal fluid to assess the impact of other ions on the electrochemical performance. Different proteins, amino acids, and other organics can then be added to these solutions at typical concentrations and with varying ratios. The electrode should be exposed to the proteins and amino acids for 10 min before testing to ensure reproducible surface adsorption occurs. Finally, electrodes should be tested in degassed foetal bovine serum after 10 min exposure to assess the impact of other chemical species on the electrochemical performance.
Electrochemistry should be performed in a 3-electrode system with a well-defined reference electrode and large counter electrode. Proteins will adsorb on the reference electrode, affecting its potential, so it must be appropriately calibrated. EIS should be performed at 0 V with ~10 mV amplitude over a wide frequency range (e.g., 0.1–100,000 Hz). The entire response should be reported and fit with a simple equivalent circuit. The impedance at low frequencies (e.g., 15 Hz) can be reported. Cyclic voltammetry is used to understand the reaction mechanisms and kinetics of an electrochemical system. The response varies with electrode material, preparation, and solution composition. This prevents the prescription of a single testing protocol. The voltammetric response should be tested over a wide potential range to determine the reactions that occur and their potentials, the changes in response with different model solutions, and after repeated voltammetric cycles. In general, the voltammetric scan rate should be low to minimize
iRu and aid in translating to any in vivo performance. Current pulsing can be performed, but the response varies significantly with applied waveform and is affected by
iRu, a variable reference electrode, and the local chemical and tissue composition that occurs after implantation [
24].
The effective electrode area can be assessed by the addition of a redox mediator such as ruthenium hexamine to the solution at a known concentration and by appropriate fitting of the voltammetric response, depending on the electrode geometry [
21,
22,
37]. The blocking of the electrode by protein adsorption can be assessed by similar methods [
35].