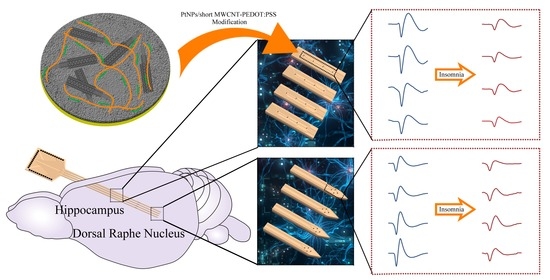
PtNPs/Short MWCNT-PEDOT: PSS-Modified Microelectrode Array to Detect Neuronal Firing Patterns in the Dorsal Raphe Nucleus and Hippocampus of Insomnia Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatus
2.2. Animal and Injection of P-Chlorophenylalanine
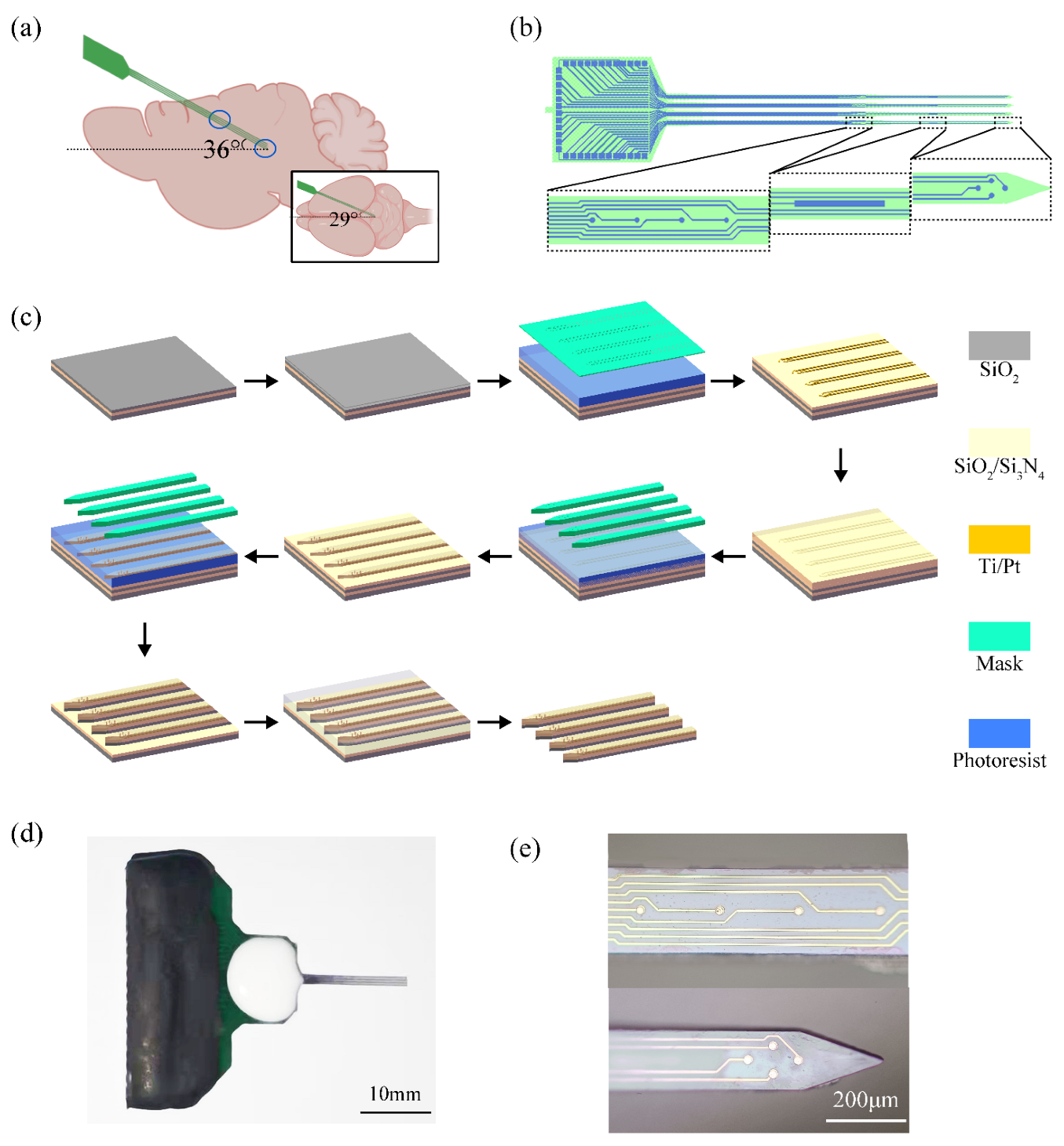
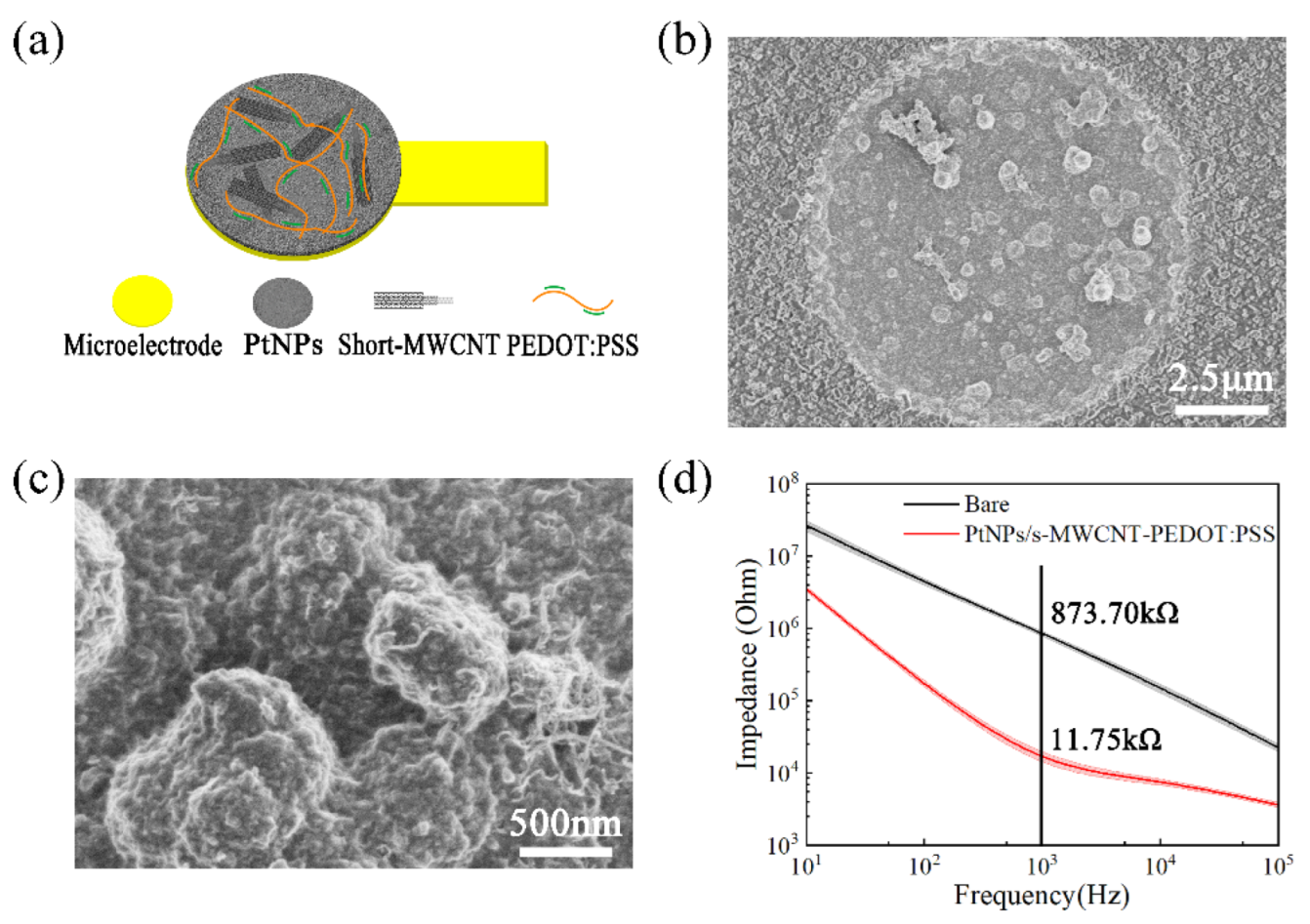
2.3. Design and Modification of Microelectrode Arrays
2.4. Surgical Procedures and Implant Pathway Check
2.5. δ Wave Slope Analysis
2.6. Data Acquisition and Statistics
3. Results
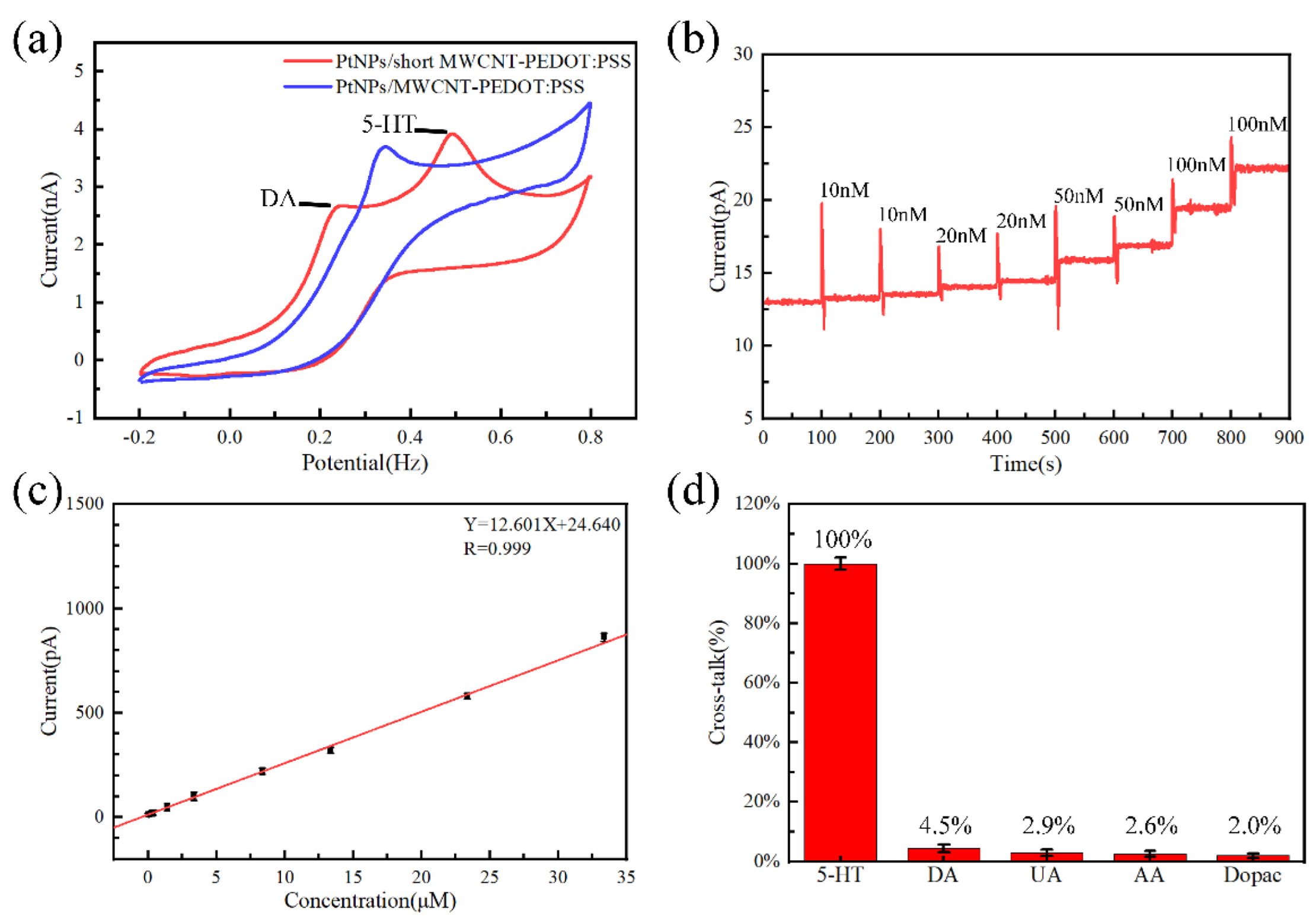
3.1. Sensor Evaluation
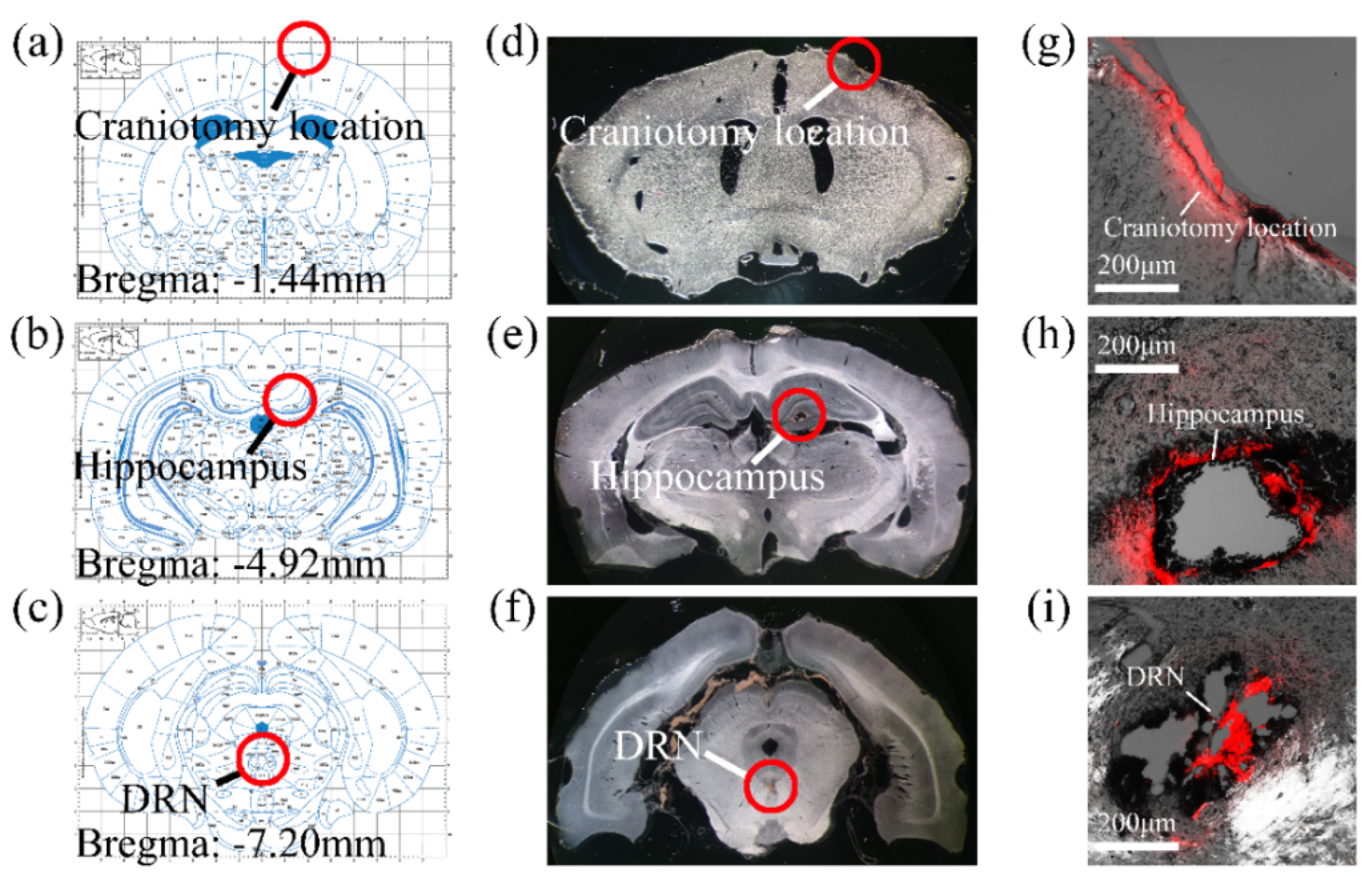
3.2. MEA Implantation Path
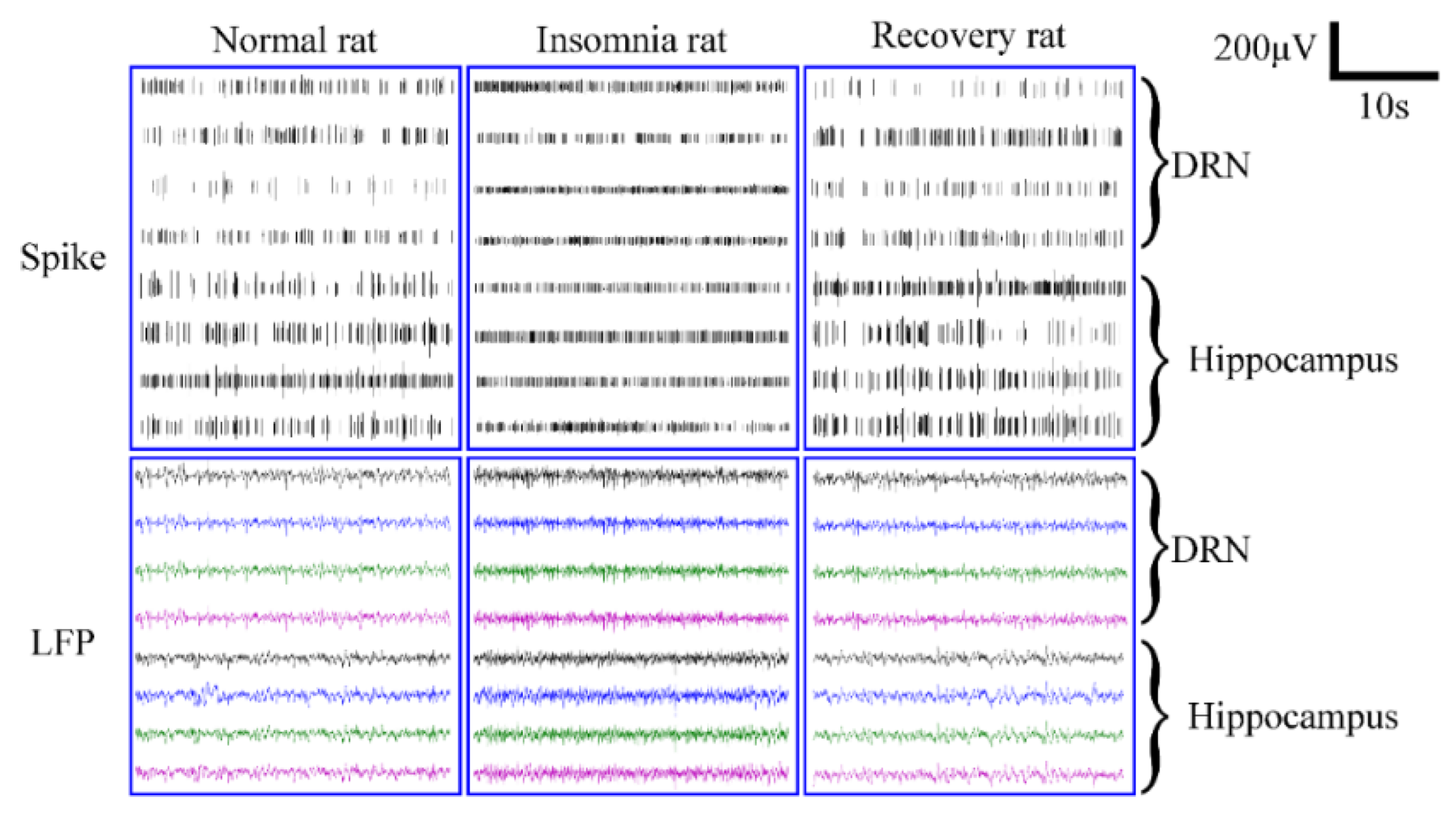
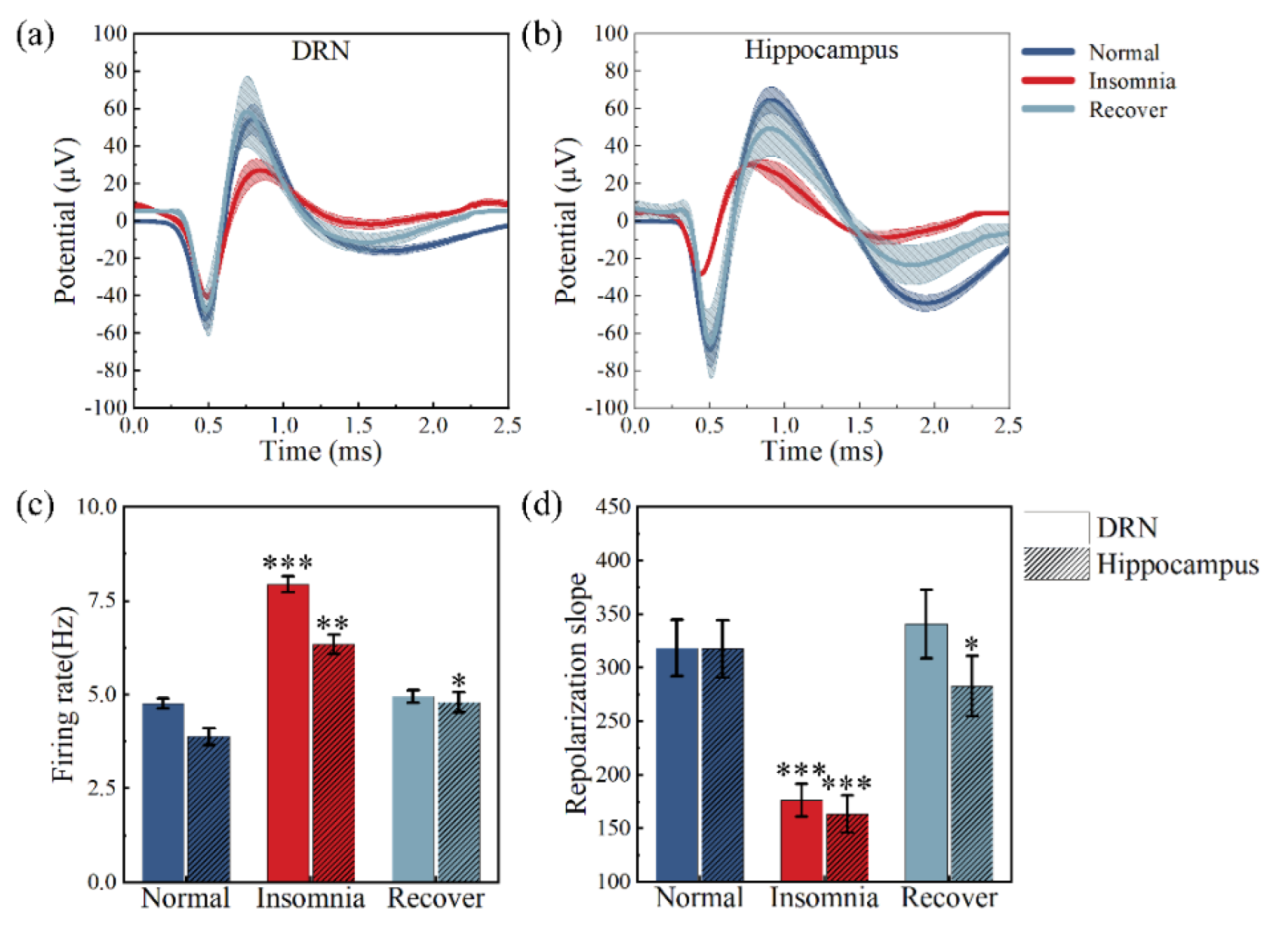
3.3. Analysis of Neural Spikes and Local Field Potentials
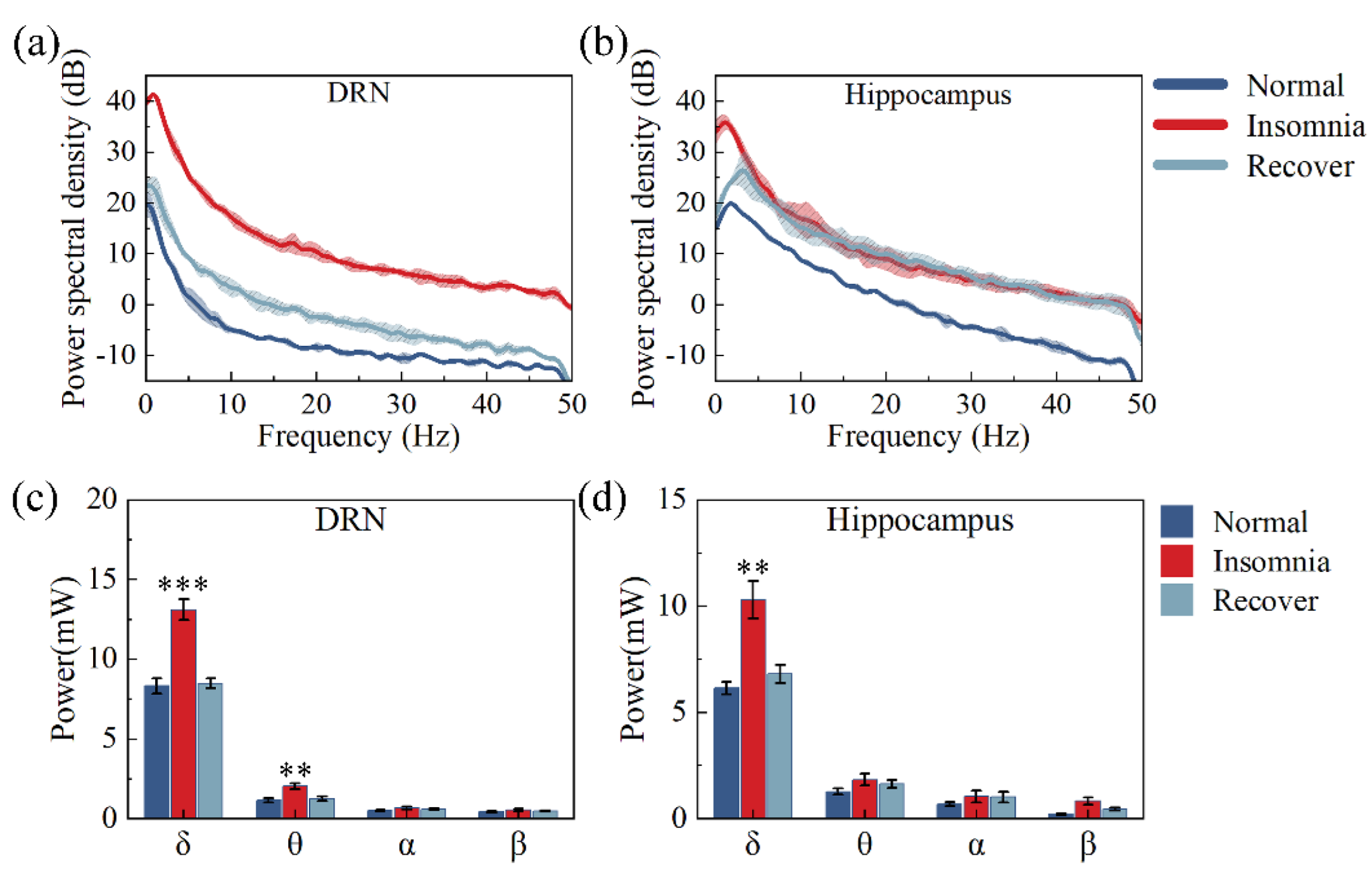
3.4. Analysis of Powers
3.5. δ Wave Slope Analysis
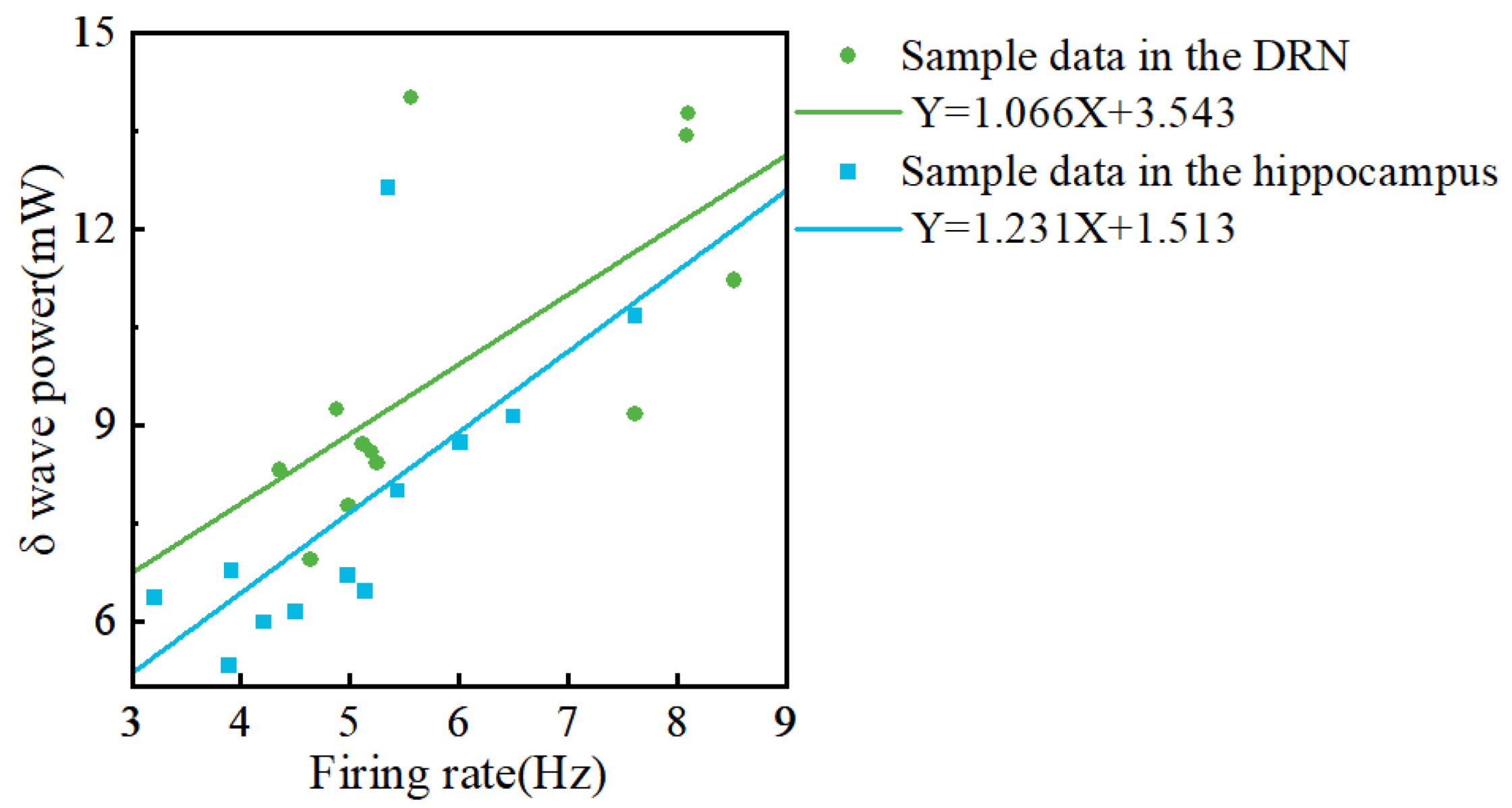
3.6. Analysis between Firing Rate and Local Field Potential
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Zambotti, M.; Trinder, J.; Silvani, A.; Colrain, I.M.; Baker, F.C. Dynamic coupling between the central and autonomic nervous systems during sleep: A review. Neurosci. Biobehav. Rev. 2018, 90, 84–103. [Google Scholar] [CrossRef]
- Knutson, K.L.; Van Cauter, E.; Rathouz, P.J.; DeLeire, T.; Lauderdale, D.S. Trends in the prevalence of short sleepers in the USA: 1975–2006. Sleep 2010, 33, 37–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlova, M.K.; Latreille, V. Sleep Disorders. Am. J. Med. 2019, 132, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Caudwell, L.; Himani, H.; Khaw, A.; Taylor, R.; White, J.; Rhodes, S.; Skinner, M. Attitudes and perceptions of health professionals towards sleep health: A systematic review. Phys. Ther. Rev. 2020, 25, 361–380. [Google Scholar] [CrossRef]
- Stranges, S.; Tigbe, W.; Gómez-Olivé, F.X.; Thorogood, M.; Kandala, N.B. Sleep problems: An emerging global epidemic? Findings from the INDEPTH WHO-SAGE study among more than 40,000 older adults from 8 countries across Africa and Asia. Sleep 2012, 35, 1173–1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDonald, K.J.; Cote, K.A. Contributions of post-learning REM and NREM sleep to memory retrieval. Sleep Med. Rev. 2021, 59, 13. [Google Scholar] [CrossRef]
- Cousins, J.N.; Fernandez, G. The impact of sleep deprivation on declarative memory. In Sleep Deprivation and Cognition; VanDongen, H.P.A., Whitney, P., Hinson, J.M., Honn, K.A., Chee, M.W.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 246, pp. 27–53. [Google Scholar]
- Marshall, L.; Cross, N.; Binder, S.; Thien, T.D.V. Brain Rhythms During Sleep and Memory Consolidation: Neurobiological Insights. Physiology 2020, 35, 4–15. [Google Scholar] [CrossRef]
- Sateia, M.J. International classification of sleep disorders-third edition: Highlights and modifications. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef]
- Ditmer, M.; Gabryelska, A.; Turkiewicz, S.; Bialasiewicz, P.; Malecka-Wojciesko, E.; Sochal, M. Sleep Problems in Chronic Inflammatory Diseases: Prevalence, Treatment, and New Perspectives: A Narrative Review. J. Clin. Med. 2022, 11, 67. [Google Scholar] [CrossRef]
- Wang, M.Y.; Li, N.; Jing, S.; Wang, C.M.; Sun, J.H.; Li, H.; Liu, J.L.; Chen, J.G. Schisandrin B exerts hypnotic effects in PCPA-treated rats by increasing hypothalamic 5-HT and gamma-aminobutyric acid levels. Exp. Ther. Med. 2020, 20, 7. [Google Scholar] [CrossRef]
- Lv, Y.B.; Zhou, Q.; Yan, J.X.; Luo, L.S.; Zhang, J.L. Enzymolysis peptides from Mauremys mutica plastron improve the disorder of neurotransmitter system and facilitate sleep-promoting in the PCPA-induced insomnia mice. J. Ethnopharmacol. 2021, 274, 9. [Google Scholar] [CrossRef] [PubMed]
- Mendiguren, A.; Aostri, E.; Pineda, J. Regulation of noradrenergic and serotonergic systems by cannabinoids: Relevance to cannabinoid-induced effects. Life Sci. 2018, 192, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Monti, J.M. Serotonin control of sleep-wake behavior. Sleep Med. Rev. 2011, 15, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Diekelmann, S.; Born, J. The memory function of sleep. Nat. Rev. Neurosci. 2010, 11, 114–126. [Google Scholar] [CrossRef]
- Anacker, C.; Hen, R. Adult hippocampal neurogenesis and cognitive flexibility-linking memory and mood. Nat. Rev. Neurosci. 2017, 18, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.H.; Xu, X.; Chen, W.Q.; Nie, L.N.; Liu, Y.; Sui, N.; Liang, J. The role of hippocampus in memory reactivation: An implication for a therapeutic target against opioid use disorder. Curr. Addict. Rep. 2022, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Leerssen, J.; Wassing, R.; Ramautar, J.R.; Stoffers, D.; Lakbila-Kamal, O.; Perrier, J.; Bruijel, J.; Foster-Dingley, J.C.; Aghajani, M.; van Someren, E.J.W. Increased hippocampal-prefrontal functional connectivity in insomnia. Neurobiol. Learn. Mem. 2019, 160, 144–150. [Google Scholar] [CrossRef] [Green Version]
- Emamian, F.; Mahdipour, M.; Noori, K.; Rostampour, M.; Mousavi, S.B.; Khazaie, H.; Khodaie-Ardakani, M.; Tahmasian, M.; Zarei, M. Alterations of Subcortical Brain Structures in Paradoxical and Psychophysiological Insomnia Disorder. Front. Psychiatry 2021, 12, 10. [Google Scholar] [CrossRef]
- Ren, S.; Wang, Y.; Yue, F.; Cheng, X.; Dang, R.; Qiao, Q.; Sun, X.; Li, X.; Jiang, Q.; Yao, J.; et al. The paraventricular thalamus is a critical thalamic area for wakefulness. Science 2018, 362, 429–434. [Google Scholar] [CrossRef] [Green Version]
- Saper, C.B.; Fuller, P.M.; Pedersen, N.P.; Lu, J.; Scammell, T.E. Sleep state switching. Neuron 2010, 68, 1023–1042. [Google Scholar] [CrossRef] [Green Version]
- Han, M.; Manoonkitiwongsa, P.S.; Wang, C.X.; McCreery, D.B. In vivo validation of custom-designed silicon-based microelectrode arrays for long-term neural recording and stimulation. IEEE Trans. Biomed. Eng. 2012, 59, 346–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jun, J.J.; Steinmetz, N.A.; Siegle, J.H.; Denman, D.J.; Bauza, M.; Barbarits, B.; Lee, A.K.; Anastassiou, C.A.; Andrei, A.; Aydın, Ç.; et al. Fully integrated silicon probes for high-density recording of neural activity. Nature 2017, 551, 232–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoshnevisan, K.; Maleki, H.; Honarvarfard, E.; Baharifar, H.; Gholami, M.; Faridbod, F.; Larijani, B.; Faridi Majidi, R.; Khorramizadeh, M.R. Nanomaterial based electrochemical sensing of the biomarker serotonin: A comprehensive review. Mikrochim. Acta 2019, 186, 49. [Google Scholar] [CrossRef] [PubMed]
- Lecomte, A.; Descamps, E.; Bergaud, C. A review on mechanical considerations for chronically-implanted neural probes. J. Neural Eng. 2018, 15, 031001. [Google Scholar] [CrossRef]
- Murray, N.M.; Buchanan, G.F.; Richerson, G.B. Insomnia Caused by Serotonin Depletion is Due to Hypothermia. Sleep 2015, 38, 1985–1993. [Google Scholar] [CrossRef]
- Shi, R.; Han, Y.; Yang, Y.; Qiao, H.Y.; He, J.; Lian, W.W.; Xia, C.Y.; Li, T.L.; Zhang, W.K.; Xu, J.K. Loganin Exerts Sedative and Hypnotic Effects via Modulation of the Serotonergic System and GABAergic Neurons. Front. Pharmacol. 2019, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.J.; Zhang, N.; Qu, Y.X.; Cao, Y.J.; Li, J.H.; Yang, Y.W.; Yang, T.G.; Sun, Y.K. Shuangxia decoction alleviates p-chlorophenylalanine induced insomnia through the modification of serotonergic and immune system. Metab. Brain Dis. 2020, 35, 315–325. [Google Scholar] [CrossRef]
- Si, Y.P.; Wang, L.L.; Lan, J.X.; Li, H.W.; Guo, T.; Chen, X.H.; Dong, C.H.; Ouyang, Z.; Chen, S.Q. Lilium davidii extract alleviates p-chlorophenylalanine-induced insomnia in rats through modification of the hypothalamic-related neurotransmitters, melatonin and homeostasis of the hypothalamic-pituitary-adrenal axis. Pharm. Biol. 2020, 58, 915–924. [Google Scholar] [CrossRef]
- Browne, C.J.; Abela, A.R.; Chu, D.; Li, Z.X.; Ji, X.D.; Lambe, E.K.; Fletcher, P.J. Dorsal raphe serotonin neurons inhibit operant responding for reward via inputs to the ventral tegmental area but not the nucleus accumbens: Evidence from studies combining optogenetic stimulation and serotonin reuptake inhibition. Neuropsychopharmacology 2019, 44, 793–804. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, B.C.; Qu, M.Z.; Zhou, G.M.; Zhang, B.L.; Yu, Z.L. A study on relations between structure and electrochemical capacitance of multi-walled carbon nanotubes. Acta Chim. Sin. 2002, 60, 1539–1542. [Google Scholar]
- Modak, S.; Roy, S.S.; Bose, R.; Chatterjee, S. Focal Epileptic Area Recognition Employing Cross EEG Rhythm Spectrum Images and Convolutional Neural Network. IEEE Sens. J. 2021, 21, 23335–23343. [Google Scholar] [CrossRef]
- Vyazovskiy, V.V.; Riedner, B.A.; Cirelli, C.; Tononi, G. Sleep homeostasis and cortical synchronization: II. A local field potential study of sleep slow waves in the rat. Sleep 2007, 30, 1631–1642. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, J.; Gent, T.C.; Hoekstra, M.M.B.; Emmenegger, Y.; Mongrain, V.; Landolt, H.P.; Adamantidis, A.R.; Franken, P. Rapid fast-delta decay following prolonged wakefulness marks a phase of wake-inertia in NREM sleep. Nat. Commun. 2020, 11, 3130. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Gaire, J.; McDowell, S.P.; Otto, K.J. The effect of site placement within silicon microelectrodes on the long-term electrophysiological recordings. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; Volume 2014, pp. 465–468. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Xu, S.; Wang, H.; Liu, J.; Dai, Y.; Xie, J.; Song, Y.; Wang, Y.; Wang, Y.; Qu, L.; et al. Total sleep deprivation-induced electrophysiological activities changes in rat hippocampal CA1 detected by microelectrode arrays. Sens. Actuators A Phys. 2021, 331, 112983. [Google Scholar] [CrossRef]
- Penington, N.J.; Fox, A.P. Effects of LSD on Ca++ currents in central 5-HT-containing neurons-5-HT1A receptors may play a role in hallucinogenesis. J. Pharmacol. Exp. Ther. 1994, 269, 1160–1165. [Google Scholar] [PubMed]
- Bean, B.P. The action potential in mammalian central neurons. Nat. Rev. Neurosci. 2007, 8, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Jacklet, J.W.; Grizzaffi, J.; Tieman, D.G. Serotonin and cAMP induce excitatory modulation of a serotonergic neuron. J. Neurobiol. 2006, 66, 499–510. [Google Scholar] [CrossRef]
- Parker, D. Serotonergic modulation of locust motor-neurons. J. Neurophysiol. 1995, 73, 923–932. [Google Scholar] [CrossRef]
- Guillaumin, M.C.C.; McKillop, L.E.; Cui, N.; Fisher, S.P.; Foster, R.G.; de Vos, M.; Peirson, S.N.; Achermann, P.; Vyazovskiy, V.V. Cortical region-specific sleep homeostasis in mice: Effects of time of day and waking experience. Sleep 2018, 41, zsy079. [Google Scholar] [CrossRef] [Green Version]
- Vassalli, A.; Franken, P. Hypocretin (orexin) is critical in sustaining theta/gamma-rich waking behaviors that drive sleep need. Proc. Natl. Acad. Sci. USA 2017, 114, E5464–E5473. [Google Scholar] [CrossRef] [Green Version]
- Jones, S.G.; Vyazovskiy, V.V.; Cirelli, C.; Tononi, G.; Benca, R.M. Homeostatic regulation of sleep in the white-crowned sparrow (Zonotrichia leucophrys gambelii). BMC Neurosci. 2008, 9, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Gonzalez, D.; Lesku, J.A.; Rattenborg, N.C. Increased EEG spectral power density during sleep following short-term sleep deprivation in pigeons (Columba livia): Evidence for avian sleep homeostasis. J. Sleep Res. 2008, 17, 140–153. [Google Scholar] [CrossRef]
- Watson, B.O.; Ding, M.X.; Buzsaki, G. Temporal coupling of field potentials and action potentials in the neocortex. Eur. J. Neurosci. 2018, 48, 2482–2497. [Google Scholar] [CrossRef]
- Mander, B.A.; Winer, J.R.; Walker, M.P. Sleep and Human Aging. Neuron 2017, 94, 19–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medic, G.; Wille, M.; Hemels, M.E.H. Short- and long-term health consequences of sleep disruption. Nat. Sci. Sleep 2017, 9, 151–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, Y.D.; Kang, J.M.; Cho, S.J.; Lee, J.S.; Hwang, H.Y.; Kang, S.G. fMRI brain activation in patients with insomnia disorder during a working memory task. Sleep Breath. 2018, 22, 487–493. [Google Scholar] [CrossRef]
- Dai, X.J.; Jiang, J.; Zhang, Z.Q.; Nie, X.; Liu, B.X.; Pei, L.; Gong, H.H.; Hu, J.P.; Lu, G.M.; Zhan, Y. Plasticity and Susceptibility of Brain Morphometry Alterations to Insufficient Sleep. Front. Psychiatry 2018, 9, 16. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.R.; Chun, M.H.; Han, E.Y. Effects of Hypnotics on Sleep Patterns and Functional Recovery of Patients with Subacute Stroke. Am. J. Phys. Med. Rehabil. 2010, 89, 315–322. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, M.; Dai, Y.; Song, Y.; Wang, Y.; Lu, B.; Li, Y.; Cai, X. PtNPs/Short MWCNT-PEDOT: PSS-Modified Microelectrode Array to Detect Neuronal Firing Patterns in the Dorsal Raphe Nucleus and Hippocampus of Insomnia Rats. Micromachines 2022, 13, 488. https://doi.org/10.3390/mi13030488
Wang Y, Wang M, Dai Y, Song Y, Wang Y, Lu B, Li Y, Cai X. PtNPs/Short MWCNT-PEDOT: PSS-Modified Microelectrode Array to Detect Neuronal Firing Patterns in the Dorsal Raphe Nucleus and Hippocampus of Insomnia Rats. Micromachines. 2022; 13(3):488. https://doi.org/10.3390/mi13030488
Chicago/Turabian StyleWang, Yun, Mixia Wang, Yuchuan Dai, Yilin Song, Yiding Wang, Botao Lu, Yinghui Li, and Xinxia Cai. 2022. "PtNPs/Short MWCNT-PEDOT: PSS-Modified Microelectrode Array to Detect Neuronal Firing Patterns in the Dorsal Raphe Nucleus and Hippocampus of Insomnia Rats" Micromachines 13, no. 3: 488. https://doi.org/10.3390/mi13030488
APA StyleWang, Y., Wang, M., Dai, Y., Song, Y., Wang, Y., Lu, B., Li, Y., & Cai, X. (2022). PtNPs/Short MWCNT-PEDOT: PSS-Modified Microelectrode Array to Detect Neuronal Firing Patterns in the Dorsal Raphe Nucleus and Hippocampus of Insomnia Rats. Micromachines, 13(3), 488. https://doi.org/10.3390/mi13030488