Mechanical Characterization and In Vitro Assay of Biocompatible Titanium Alloys
Abstract
:1. Introduction
2. Materials and Methods
2.1. Obtaining of Ti-Mo-Zr-Ta
2.2. Morphological and Structural Analysis
2.3. Tensile Testing
2.4. Fractographic Tests
2.5. In Vitro Cytocompatibility Tests
3. Results and Discussions
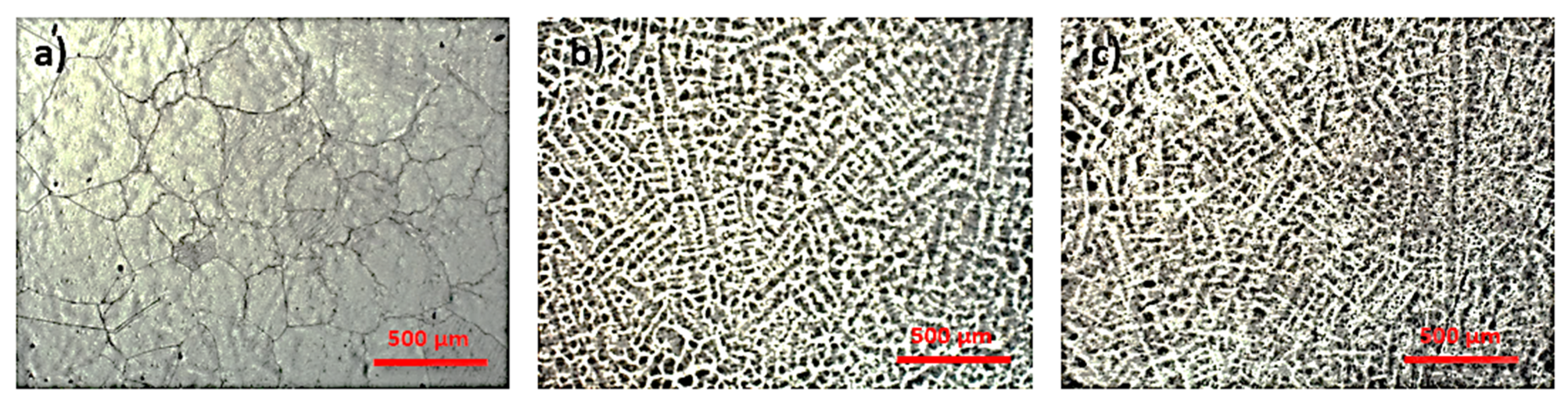
3.1. Microstructural Analysis
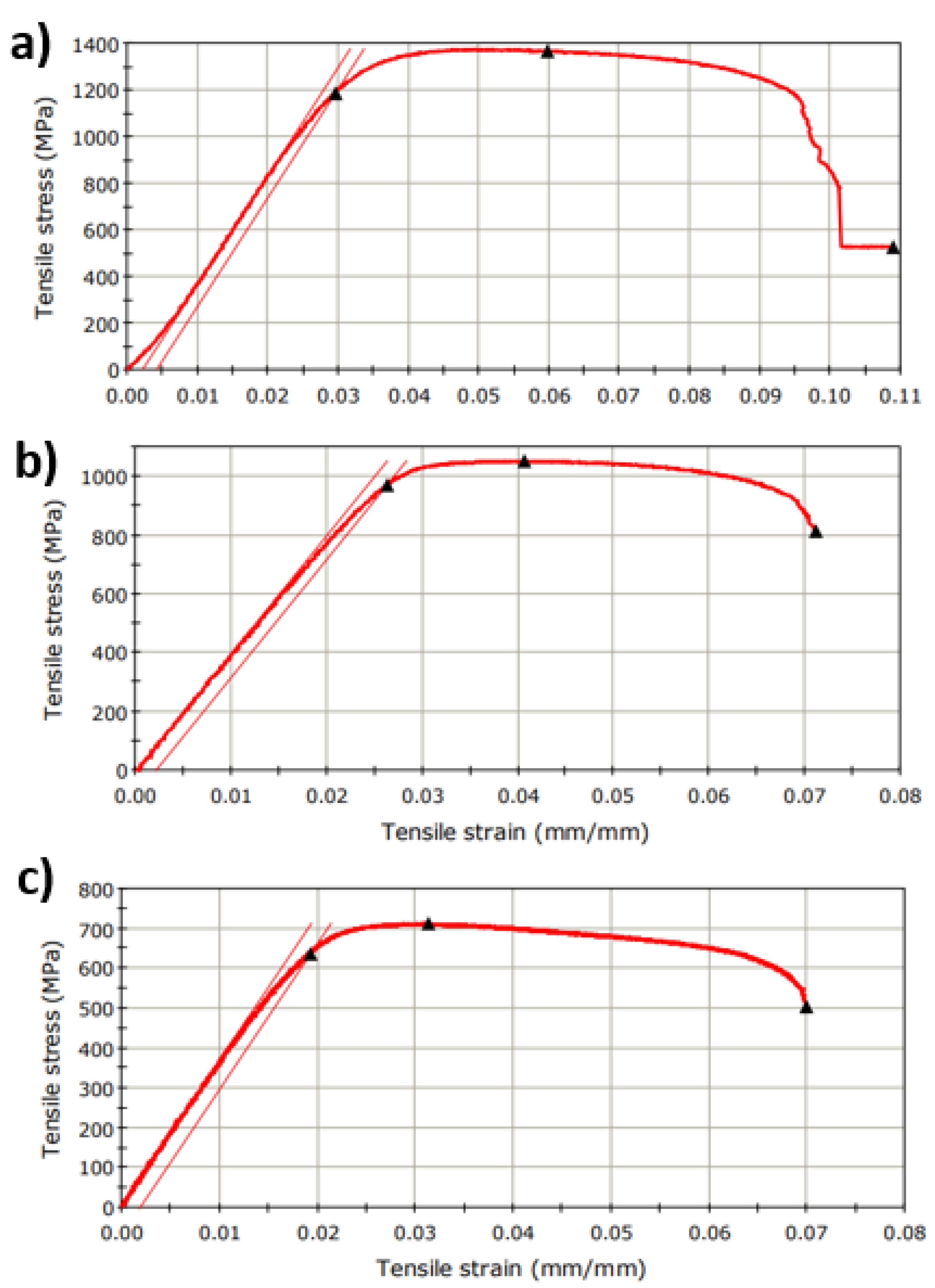
3.2. Tensile Strength Test
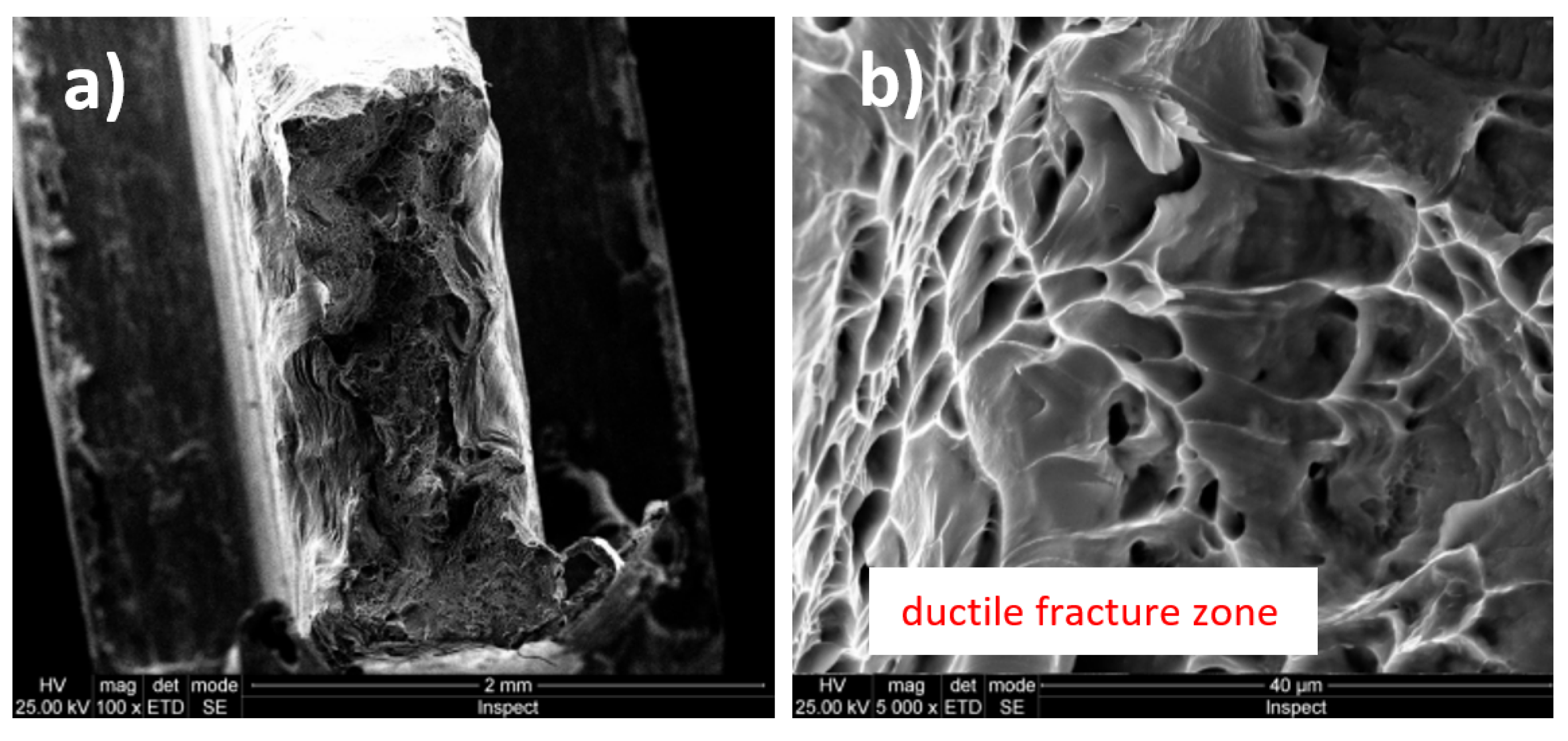
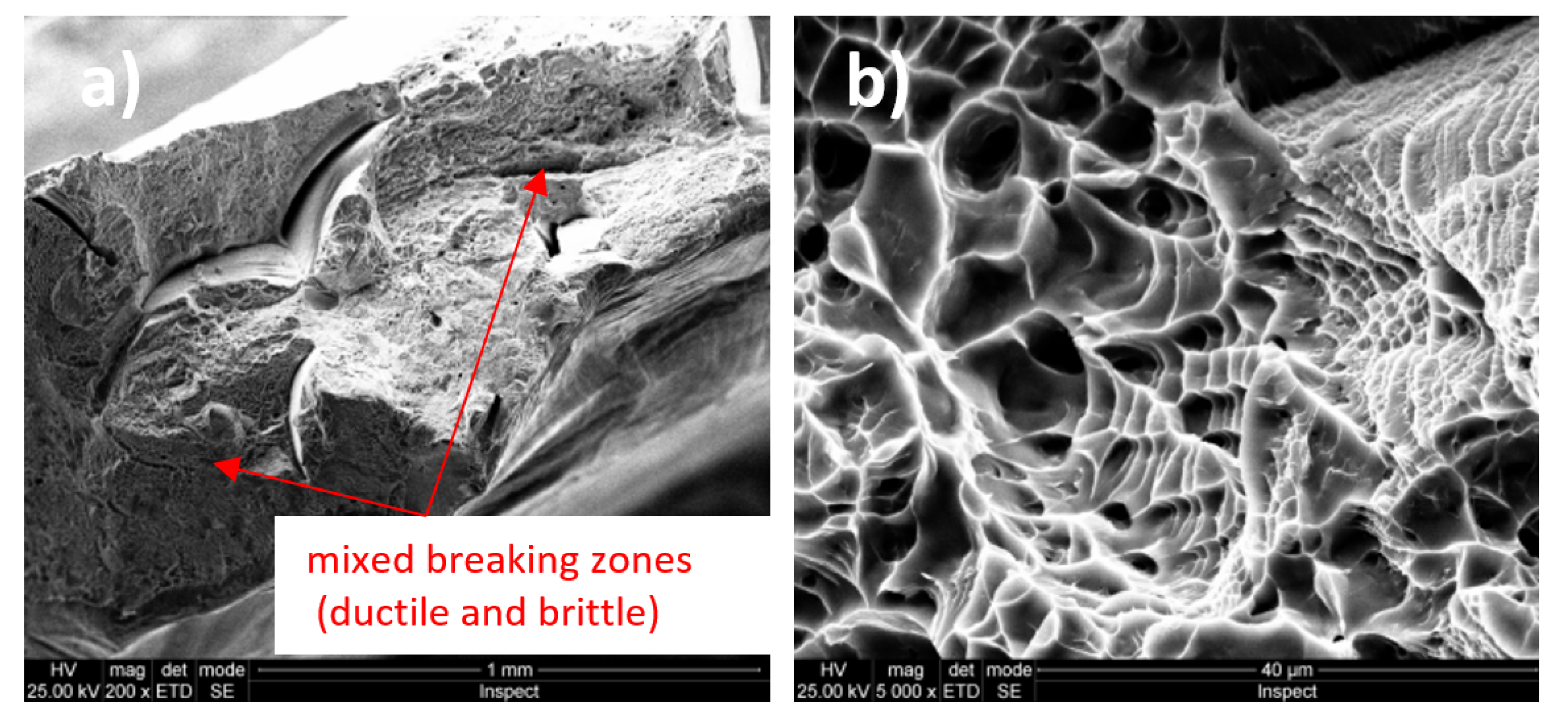
3.3. Fractographic Analysis
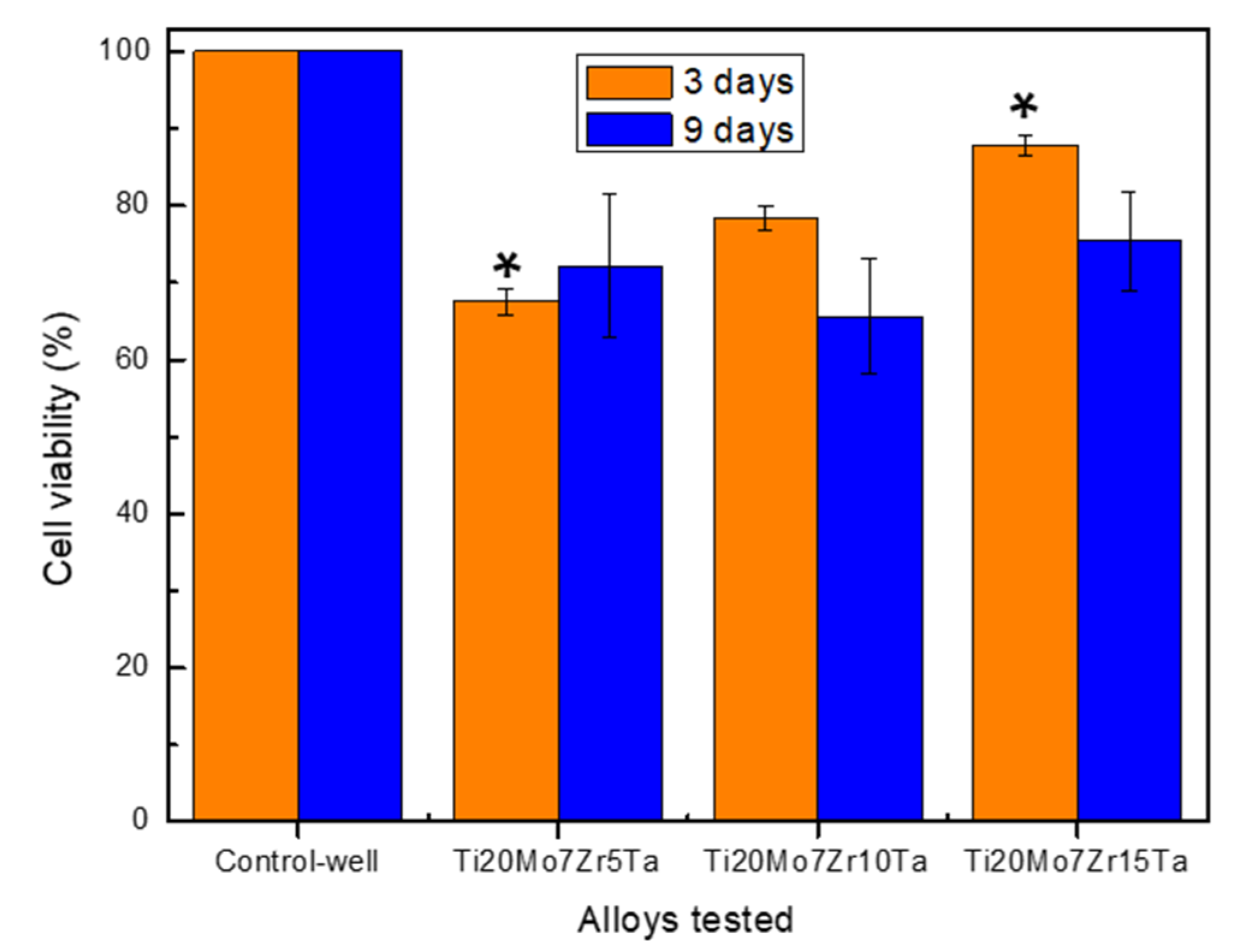
3.4. In Vitro Cytocompatibility of Ti-Mo-Zr-Ta Alloys
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Baltatu, M.S.; Vizureanu, P.; Sandu, A.V.; Munteanu, C.; Istrate, B. Microstructural Analysis and Tribological Behavior of Ti-Based Alloys with a Ceramic Layer Using the Thermal Spray Method. Coatings 2020, 10, 1216. [Google Scholar] [CrossRef]
- Verestiuc, L.; Spataru, M.C.; Baltatu, M.S.; Butnaru, M.; Solcan, C.; Sandu, A.V.; Voiculescu, I.; Geanta, V.; Vizureanu, P. New Ti-Mo-Si materials for bone prosthesis applications. J. Mech. Behav. Biomed. Mater. 2021, 113, 104198. [Google Scholar] [CrossRef]
- Spataru, M.C.; Butnaru, M.; Sandu, A.V.; Vulpe, V.; Vlad, M.D.; Baltatu, M.S.; Geanta, V.; Voiculescu, I.; Vizureanu, P.; Solcan, C. In-depth assessment of new Ti-based biocompatible materials. Mater. Chem. Phys. 2021, 258, 123959. [Google Scholar] [CrossRef]
- Zhang, T.; Chain-Tsuan Liu, C.T. Design of titanium alloys by additive manufacturing: A critical review. Adv. Powder Mater. 2021, 100014. [Google Scholar] [CrossRef]
- Vizureanu, P.; Yamaguchi, S.; Le, P.; Baltatu, M. Biocompatibility Evaluation of New TiMoSi Alloys. Acta Phys. Pol. A 2020, 138, 283–286. [Google Scholar] [CrossRef]
- Ashtiani, R.E.; Alam, M.; Tavakolizadeh, S.; Abbasi, K. The Role of Biomaterials and Biocompatible Materials in Implant-Supported Dental Prosthesis. Evid.-Based Complement. Altern. Med. 2021, 2021, 3349433. [Google Scholar] [CrossRef]
- Gobbi, S.J.; Gobbi, V.J.; Rocha, Y. Requirements for Selection/Development of a Biomaterial. Biomed. J. Sci. Tech. Res. 2019, 14, 002554. [Google Scholar] [CrossRef]
- Li, Y.-H.; Wang, F.; Li, J.-J. Current developments of biomedical porous Ti–Mo alloys. Int. J. Mater. Res. 2017, 108, 619–624. [Google Scholar] [CrossRef]
- Okazaki, Y.; Rao, S.; Ito, Y.; Tateishi, T. Corrosion resistance, mechanical properties: Corrosion fatigue strength and cytocompatibility of new Ti alloys without Al and V. Biomaterials 1998, 13, 1197–1215. [Google Scholar] [CrossRef]
- Sarraf, M.; Ghomi, E.R.; Alipour, S.; Ramakrishna, S.; Sukiman, N.L. A state-of-the-art review of the fabrication and characteristics of titanium and its alloys for biomedical applications. Bio-Des. Manuf. 2021. [Google Scholar] [CrossRef]
- Bombac, D.M.; Brojan, M.; Fajfar, P.; Kosel, F.; Turk, R. Review of materials in medical applications. Mater. Geoenviron. 2007, 54, 471–499. [Google Scholar]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 844–862. [Google Scholar] [CrossRef]
- Sidhu, S.S.; Singh, H.; Gepreel, M.A.H. A review on alloy design, biological response, and strengthening of beta-titanium alloys as biomaterials. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 121, 111661. [Google Scholar] [CrossRef]
- Sheremet’ev, V.A.; Akhmadkulov, O.B.; Komarov, V.S.; Korotitskii, A.V.; Lukashevich, K.E.; Galkin, S.P.; Andreev, V.A.; Prokoshkin, S.D. Thermomechanical Behavior and Structure Formation of Shape Memory Ti–Zr–Nb Alloy for Medical Applications. Met. Sci. Heat Treat. 2021, 63, 403–413. [Google Scholar] [CrossRef]
- Raganya, M.L.; Moshokoa, N.M.; Obadele, B.; Olubambi, P.A.; Machaka, R. The microstructural and mechanical characterization of the β-type Ti-11.1Mo-10.8Nb alloy for biomedical applications. IOP Conf. Ser. Mater. Sci. Eng. 2019, 655, 12025. [Google Scholar] [CrossRef]
- Song, Y.; Xu, D.; Yang, R.; Li, D.; Wu, W.; Guo, Z.X. Theoretical study of the effects of alloying elements on the strength and modulus of β-type bio-titanium alloys. Mater. Sci. Eng. A 1999, 260, 269–274. [Google Scholar] [CrossRef]
- Park, Y.J.; Song, Y.H.; An, J.H.; Song, H.J.; Anusavice, K.J. Cytocompatibility of pure metals and experimental binary titanium alloys for implant materials. J. Dent. 2013, 41, 1251–1258. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Luo, D.M. Microstructures and mechanical properties of Ti–Mo alloys cold-rolled and heat treated. Mater. Charact. 2011, 62, 931–937. [Google Scholar] [CrossRef]
- Gordin, D.M.; Gloriant, T.; Nemtoi, G.; Chelariu, R.; Aelenei, N.; Guillou, A.; Ansel, D. Synthesis, structure and electrochemical behavior of a beta Ti-12Mo-5Ta alloy as new biomaterial. Mater. Lett. 2005, 59, 2936–2941. [Google Scholar] [CrossRef]
- Chen, M.; Wang, X.Q.; Zhang, E.L.; Wan, Y.Z.; Hu, J. Antibacterial ability and biocompatibility of fluorinated titanium by plasma-based surface modification. Rare Met. 2021. [Google Scholar] [CrossRef]
- Wei, L.M.C.; Fu, Q.Y.; Sen, Y.; Yu, Z.T. Progress on Development and Application of Degradable Polymer Modified Coating on Magnesium Alloy Surface Feng. Rare Met. Mater. Eng. 2021, 50, 3366–3374. [Google Scholar]
- Correa, D.R.N.; Vicente, F.B.; Donato, T.A.G.; Arana-Chavez, V.E.; Buzalaf, M.A.R.; Grandini, C.R. The effect of the solute on the structure, selected mechanical properties, and biocompatibility of Ti–Zr system alloys for dental applications. Mater. Sci. Eng. C 2014, 34, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.R.S., Jr.; Nogueira, R.A.; Oliveira de Araújo, R.; Donato, T.A.G.; Chavez, V.E.A.; Claro, A.P.R.A.; Moraes, J.C.S.M.; Buzalaf, M.A.R.; Grandini, C.R. Preparation and Characterization of Ti-15Mo Alloy used as Biomaterial. Mater. Res. 2011, 14, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.L.; Lei, X.; Yu, Y.; Miao, S.; Tang, J.Y.; Fu, Y.; Ye, K.; Shen, Y.; Shi, J.Y.; Wu, H.; et al. Biological sealing and integration of a fibrinogen-modified titanium alloy with soft and hard tissues in a rat model. Biomater. Sci. 2021, 9, 5192–5208. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Zhu, B.; Yang, X.; Xie, G. Toxic elements-free low-cost Ti-Fe-Si metallic glass biomaterial developed by mechanical alloying. J. Alloys Compd. 2021, 886, 161290. [Google Scholar] [CrossRef]
- Tito Patricio, M.A.; Lustosa, C.J.R.; Chaves, J.A.M.; Marques, P.W.B.; Silva, P.S.; Almeida, A.; Vilar, R.; Florêncio, O. Relationship between microstructure, phase transformation, and mechanical behavior in Ti–40Ta alloys for biomedical applications. J. Mater. Res. Technol. 2021, 14, 210–219. [Google Scholar] [CrossRef]
- Lee, S.S.; Huber, S.; Ferguson, S.J. Comprehensive in vitro comparison of cellular and osteogenic response to alternative biomaterials for spinal implants. Mater. Sci. Eng. C 2021, 127, 112251. [Google Scholar] [CrossRef]
- Correa, D.R.N.; Vicente, F.B.; Oliveira, A.R.; Lourenc, M.L.; Kuroda, P.A.B.; Buzala, M.A.R.; Grandini, C.R. Effect of the substitutional elements on the microstructure of the Ti-15Mo-Zr and Ti-15Zr-Mo systems alloys. J. Mater. Res. Technol. 2015, 4, 180–185. [Google Scholar] [CrossRef] [Green Version]
- Elias, C.N.; Lima, J.H.C.; Valiev, R.; Meyers, M.A. Biomedical applications of titanium and its alloys. JOM 2008, 60, 46–49. [Google Scholar] [CrossRef]
- Xu, D.; Lu, Z.; Wang, T.; Wang, S.; Jiang, Y.; Xu, Z.; Bi, Z.; Geng, S. Novel Ti-based alloys prepared with different heat treatment strategies as antibacterial biomedical implants. Mater. Des. 2021, 205, 109756. [Google Scholar] [CrossRef]
- Quadros, F.D.F.; Kuroda, P.A.B.; Sousa, K.D.S.J.; Donato, T.A.G.; Grandini, C.R. Preparation, structural and microstructural characterization of Ti-25Ta-10Zr alloy for biomedical applications. J. Mater. Res. Technol. 2019, 8, 4108–4114. [Google Scholar] [CrossRef]
- Song, Y.H.; Kim, M.K.; Park, E.J.; Song, H.J.; Anusavice, K.J.; Park, Y.J. Cytotoxicity of alloying elements and experimental titanium alloys by WST-1 and agar overlay tests. Dent. Mater. 2014, 30, 977–983. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Choe, H.C.; Brantley, W.A. Nanostructured thin film formation on femtosecond laser-textured Ti-35Nb-xZr alloy for biomedical applications. Thin Solid Films 2011, 519, 4668–4675. [Google Scholar] [CrossRef]
- Zhan, Y.; Li, C.; Jiang, W. β-type Ti-10Mo-1.25Si-xZr biomaterials for applications in hard tissue replacements. Mater. Sci. Eng. C 2012, 32, 1664–1668. [Google Scholar] [CrossRef]
- Zhang, L.B.; Wang, K.Z.; Xu, L.J.; Xiao, S.L.; Chen, Y.Y. Effect of Nb addition on microstructure, mechanical properties and castability of type TiMo alloys. Trans Nonferrous Met. Soc. China 2015, 25, 2214–2220. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- ISO 6892-1:2010: Metallic Materials. Tensile Test. Part 1: Ambient Temperature Test Method. Available online: https://www.iso.org/obp/ui/#iso:std:iso:6892:-1:ed-3:v1:en (accessed on 21 January 2022).
- Vlad, M.D.; Valle, L.J.; Poeată, I.; Barracó, M.; López, J.; Torres, R.; Fernández, E. Injectable iron-modified apatitic bone cement intended for kyphoplasty: Cytocompatibility study. J. Mater. Sci. Mater. Med. 2008, 19, 3575–3583. [Google Scholar] [CrossRef]
- Vlad, M.D.; Valle, L.J.; Poeată, I.; López, J.; Torres, R.; Barracó, M.; Fernández, E. Biphasic calcium sulfate dihydrate/iron-modified alpha-tricalcium phosphate bone cement for spinal applications: In vitro study. Biomed. Mater. 2010, 5, 025006. [Google Scholar] [CrossRef]
- King, B.M. Analysis of Variance, in International Encyclopedia of Education, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Konovalenko, I.; Maruschak, P.; Prentkovskis, O. Automated Method for Fractographic Analysis of Shape and Size of Dimples on Fracture Surface of High-Strength Titanium Alloys. Metals 2018, 8, 161. [Google Scholar] [CrossRef] [Green Version]
- Mulkins, M.A.; Manolagas, S.C.; Deftos, L.J.; Sussman, H.H. 1,25- dihydroxyvitamin D3 increases bone alkaline phosphatase isoenzyme levels in human osteogenic sarcoma cells. J. Biol. Chem. 1983, 258, 621–6225. [Google Scholar] [CrossRef]
- Franceschi, R.T.; Linson, C.J.; Peter, T.C.; Romano, P.R. Regulation of cellular adhesion and fibronectin synthesis by 1α, 25-dihydroxyvitamin D3. J. Biol. Chem. 1987, 262, 4165–4171. [Google Scholar] [CrossRef]
- Milkovic, L.; Hoppe, A.; Detsch, R.; Boccaccini, A.R.; Zarkovic, N. Effects of cu-doped 45S5 bioactive glass on the lipid peroxidation associated growth of human osteoblast-like cells in vitro. J. Biomed. Mater. Res. A 2014, 102, 3556–3561. [Google Scholar] [CrossRef]
- Raucci, M.G.; Adesanya, K.; Di Silvio, L.; Catauro, M.; Ambrosio, L. The biocompatibility of silver-containing Na2O.CaO.2SiO2 glass prepared by sol-gel method: In vitro studies. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 92, 102–110. [Google Scholar] [CrossRef]
- ISO 10993-5:2009—Biological Evaluation of Medical Devices–Part 5: Tests for In Vitro Cytotoxicity. Available online: https://nhiso.com/wp-content/uploads/2018/05/ISO-10993-5-2009.pdf.s (accessed on 21 January 2022).

| Characteristics | Ti20Mo7Zr5Ta | Ti20Mo7Zr10Ta | Ti20Mo7Zr15Ta |
|---|---|---|---|
| Extension at Tensile Strength (mm) | 0.91 | 0.60 | 0.51 |
| Load at Tensile Strength (N) | 4616.39 | 3617.76 | 2511.38 |
| Tensile Strain at Tensile Strength (mm/mm) | 0.06 | 0.04 | 0.03 |
| Tensile Stress at Tensile Strength (MPa) | 1365.27 | 1051.74 | 712.29 |
| Energy at Break (Standard) (J) | 5.86 | 2.97 | 2.35 |
| Load at Break (Standard) (N) | 1788.90 | 2802.12 | 1779.06 |
| Extension at Break (Standard) (mm) | 1.65 | 1.04 | 1.15 |
| Tensile Strain at Break (Standard) (mm/mm) | 0.11 | 0.07 | 0.07 |
| Tensile Stress at Break (Standard) (MPa) | 529.06 | 814.62 | 504.58 |
| Modulus (Automatic Young’s) (MPa) | 46,508.25 | 40,123.67 | 36,390.64 |
| Load at Yield (Offset 0.2%) (N) | 4011.49 | 3336.26 | 2242.77 |
| Tensile Strain at Yield (Offset 0.2%) (mm/mm) | 0.03 | 0.0003 | 0.02 |
| Tensile Stress at Yield (Offset 0.2%) (MPa) | 1186.37 | 969.90 | 636.10 |
| Area (mm^2) | 3.38 | 3.44 | 3.53 |
| Geometry | Rectangular | ||
| Length (mm) | 15.20 | 14.68 | 16.40 |
| Thickness (mm) | 1.17 | 1.17 | 1.22 |
| Width (mm) | 2.89 | 2.94 | 2.89 |
| Elongation (%) | 10.85 | 7.08 | 7.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baltatu, I.; Sandu, A.V.; Vlad, M.D.; Spataru, M.C.; Vizureanu, P.; Baltatu, M.S. Mechanical Characterization and In Vitro Assay of Biocompatible Titanium Alloys. Micromachines 2022, 13, 430. https://doi.org/10.3390/mi13030430
Baltatu I, Sandu AV, Vlad MD, Spataru MC, Vizureanu P, Baltatu MS. Mechanical Characterization and In Vitro Assay of Biocompatible Titanium Alloys. Micromachines. 2022; 13(3):430. https://doi.org/10.3390/mi13030430
Chicago/Turabian StyleBaltatu, Iustinian, Andrei Victor Sandu, Maria Daniela Vlad, Mihaela Claudia Spataru, Petrica Vizureanu, and Madalina Simona Baltatu. 2022. "Mechanical Characterization and In Vitro Assay of Biocompatible Titanium Alloys" Micromachines 13, no. 3: 430. https://doi.org/10.3390/mi13030430
APA StyleBaltatu, I., Sandu, A. V., Vlad, M. D., Spataru, M. C., Vizureanu, P., & Baltatu, M. S. (2022). Mechanical Characterization and In Vitro Assay of Biocompatible Titanium Alloys. Micromachines, 13(3), 430. https://doi.org/10.3390/mi13030430









