Biomimetic Deposition of Hydroxyapatite Layer on Titanium Alloys
Abstract
:1. Introduction
2. Materials and Methods
2.1. Obtaining of Ti-Mo-Zr-Ta Substrate Alloys
2.2. Biomimetic Method
2.3. Morphological and Structural Analysis
2.4. Micro-Indentation Test
2.5. Contact Angle Test
3. Results
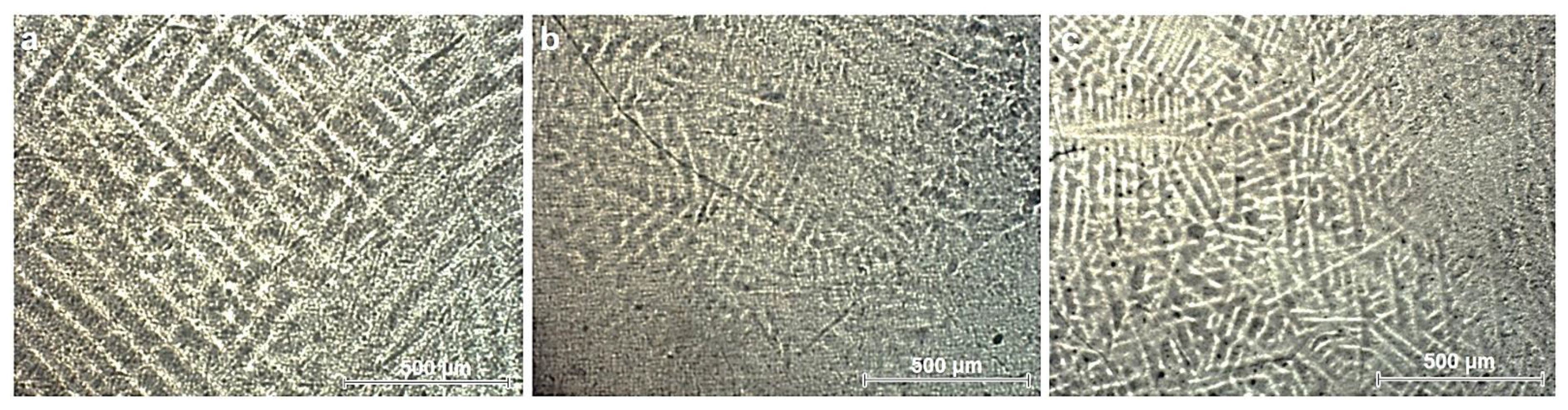
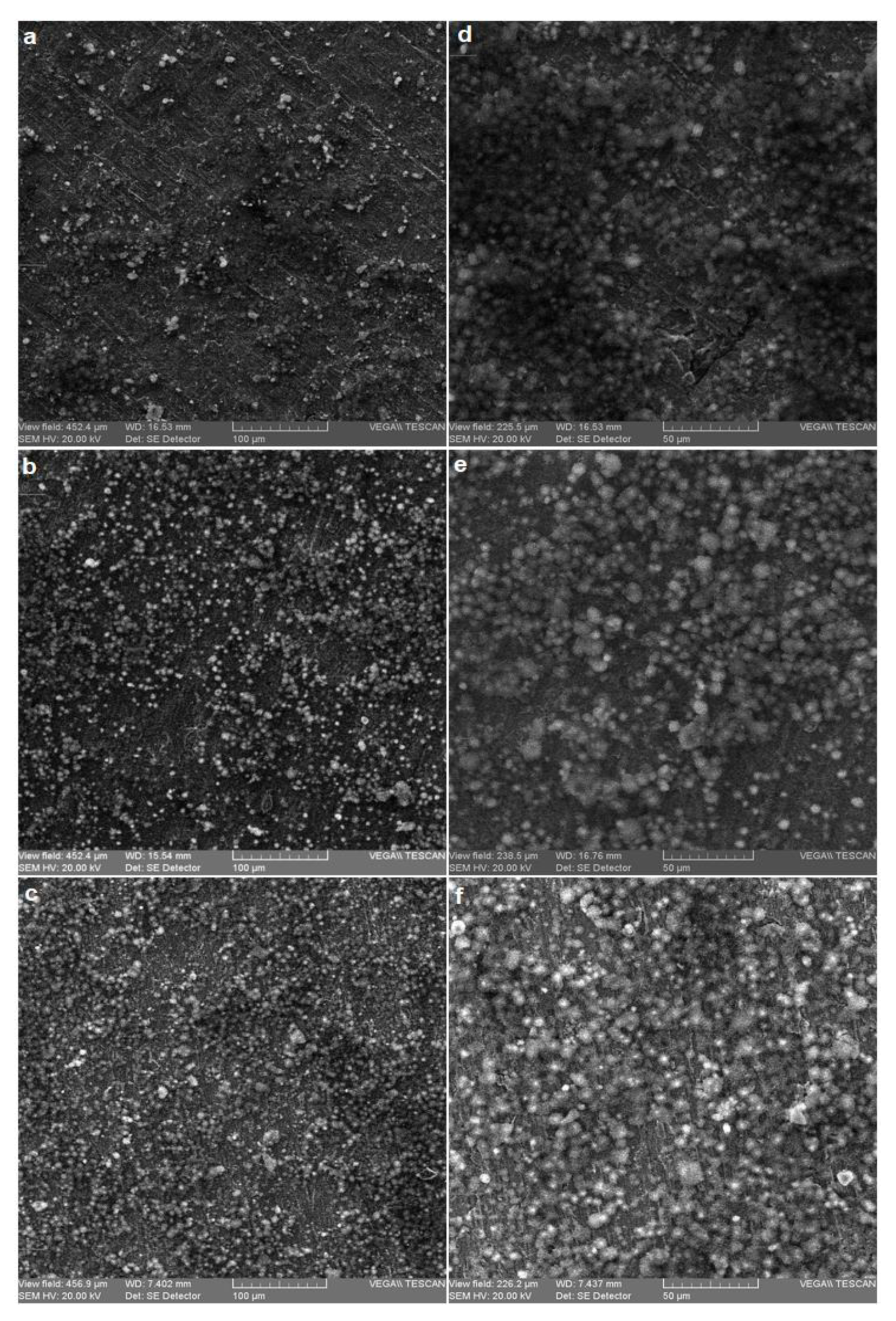
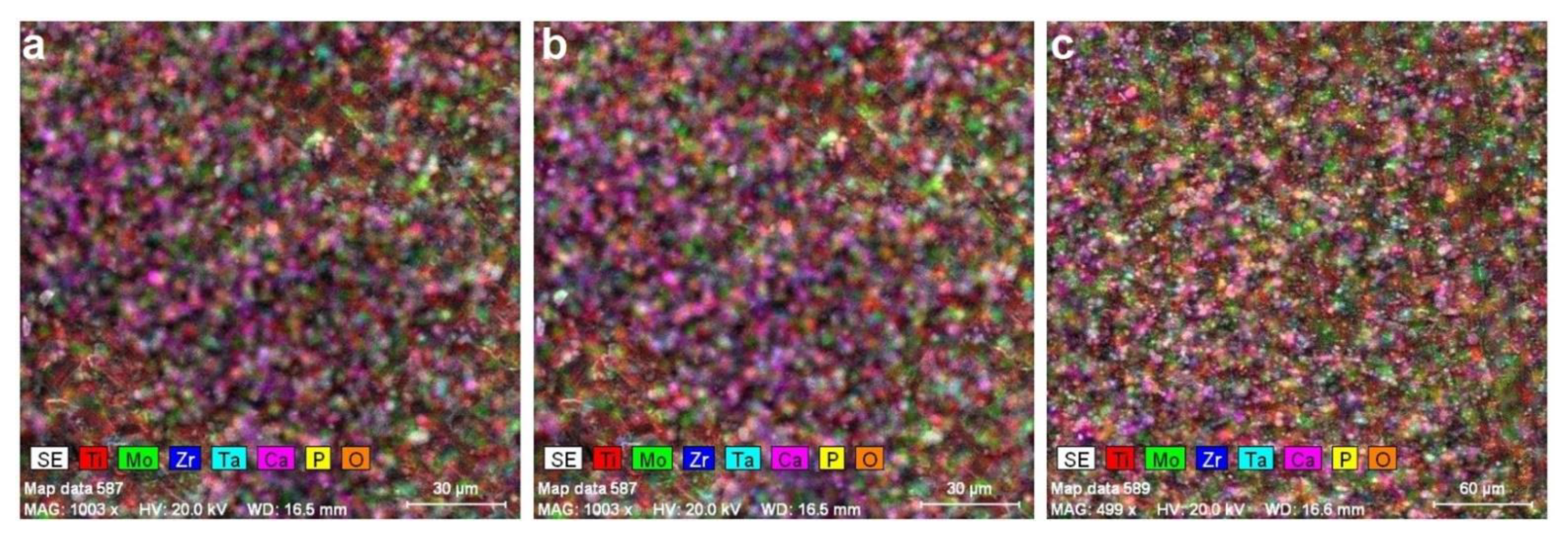
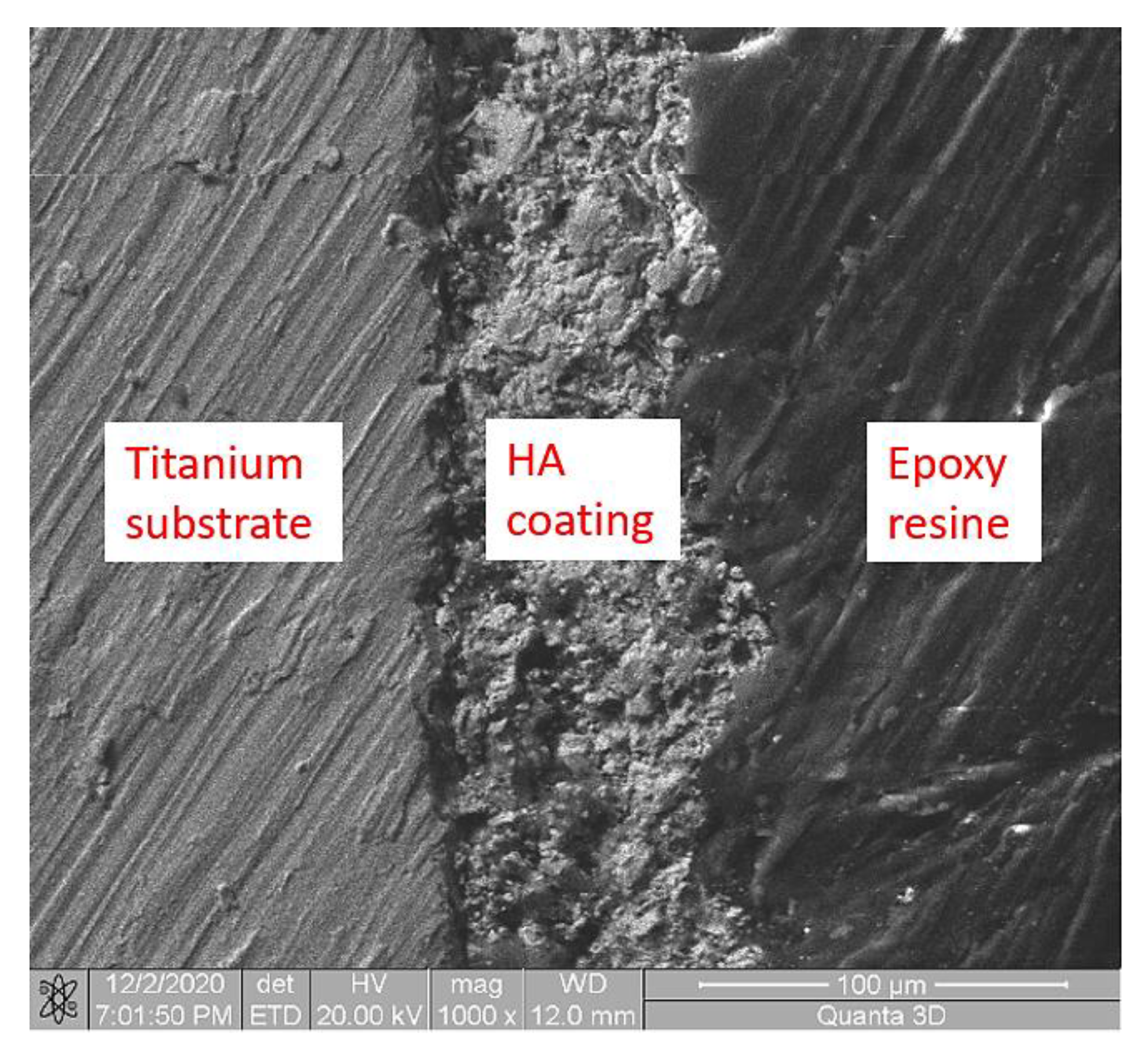
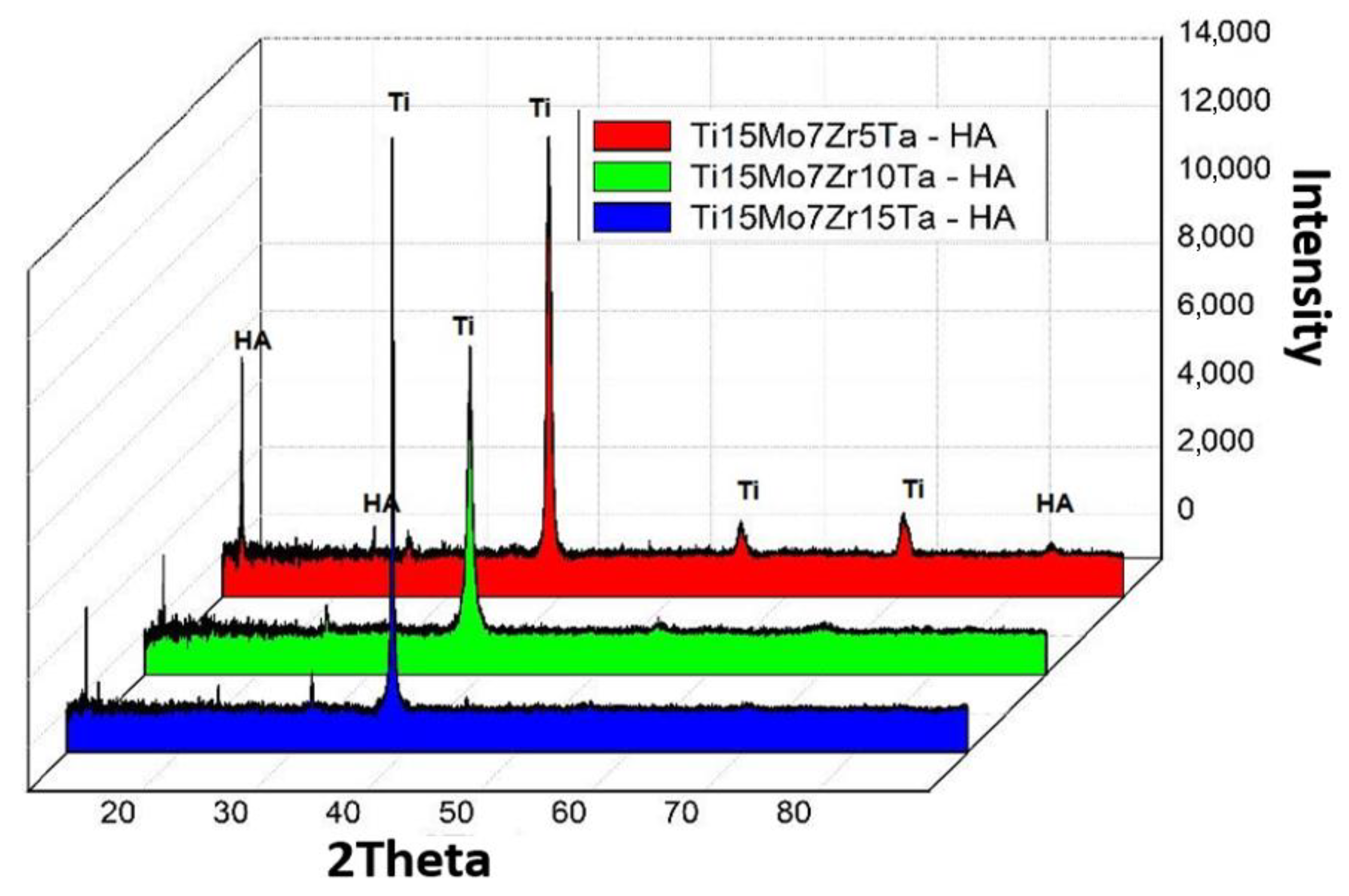
3.1. Microstructural Analysis
3.2. Micro-Indentation Analysis
3.3. Contact Angle Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bombac, D.M.; Brojan, M.; Fajfar, P.; Kosel, F.; Turk, R. Review of materials in medical applications. RMZ Mater. Geoenviron. 2007, 54, 471–499. [Google Scholar]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Song, Y.; Xu, D.S.; Yang, R.; Li, D.; Wu, W.T.; Guo, Z.X. Theoretical study of the effects of alloying elements on the strength and modulus of β-type bio-titanium alloys. Mater. Sci. Eng. A 1999, 260, 269–274. [Google Scholar] [CrossRef]
- Lupescu, S.; Istrate, B.; Munteanu, C.; Minciuna, M.G.; Focsaneanu, S.; Earar, K. Electrochemical Analysis of Some Biodegradable Mg-Ca-Mn Alloys. Rev. De Chim. 2017, 68, 1310–1315. [Google Scholar] [CrossRef]
- Minciuna, M.G.; Vizureanu, P.; Geanta, V.; Voiculescu, I.; Sandu, A.V.; Achitei, D.C.; Vitalariu, A.M. Effect of Si on the mechanical properties of biomedical CoCrMo alloys. Rev. De Chim. 2015, 66, 891–894. [Google Scholar]
- Verestiuc, L.; Spataru, M.C.; Baltatu, M.S.; Butnaru, M.; Solcan, C.; Sandu, A.V.; Voiculescu, I.; Geanta, V.; Vizureanu, P. New Ti-Mo-Si materials for bone prosthesis applications. J. Mech. Behav. Biomed. Mater. 2021, 113, 104198. [Google Scholar] [CrossRef] [PubMed]
- Spataru, M.C.; Butnaru, M.; Sandu, A.V.; Vulpe, V.; Vlad, M.D.; Baltatu, M.S.; Geanta, V.; Voiculescu, I.; Vizureanu, P.; Solcan, C. In-depth assessment of new Ti-based biocompatible materials. Mater. Chem. Phys. 2021, 258, 123959. [Google Scholar] [CrossRef]
- Baltatu, M.S.; Vizureanu, P.; Sandu, A.V.; Munteanu, C.; Istrate, B. Microstructural Analysis and Tribological Behavior of Ti-Based Alloys with a Ceramic Layer Using the Thermal Spray Method. Coatings 2020, 10, 1216. [Google Scholar] [CrossRef]
- Ciobanu, M.G.; Pop, G.; Ciobanu, O. Hydroxyapatite coatings on titanium implants. Rev. De Chim. 2007, 58, 1313–1315. [Google Scholar]
- Ciobanu, G.; Carja, G.; Ciobanu, O.; Sandu, I.; Sandu, A. SEM and EDX studies of bioactive hydroxyapatite coatings on titanium implants. Micron 2009, 40, 143–146. [Google Scholar] [CrossRef]
- Awasthi, S.; Pandey, S.K.; Arunan, E.; Srivastava, C. A review on hydroxyapatite coatings for the biomedical applications: Experimental and theoretical perspectives. J. Mater. Chem. B 2021, 9, 228–249. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Valdes, J.S.; Cortes-Hernandez, D.A.; Ortiz-Cuellar, J.C.; De la O-Baquera, E.; Escobedo-Bocardoa, J.C.; Acevedo-Davila, J.L. Laser deposition of bioactive coatings by in situ synthesis of pseudowollastonite on Ti6Al4V alloy. Opt. Laser Technol. 2021, 134, 106586. [Google Scholar] [CrossRef]
- Xue, T.; Attarilar, S.; Liu, S.F.; Liu, J.; Song, X.; Li, L.J.; Zhao, B.B.; Tang, Y.J. Surface Modification Techniques of Titanium and its Alloys to Functionally Optimize Their Biomedical Properties: Thematic Review. Front. Bioeng. Biotechnol. 2020, 8, 603072. [Google Scholar] [CrossRef]
- Simoes, S. Recent Progress in the Joining of Titanium Alloys to Ceramics. Metals 2018, 8, 876. [Google Scholar] [CrossRef] [Green Version]
- Arcos, D.; Vallet-Regi, M. Substituted hydroxyapatite coatings of bone implants. J. Mater. Chem. B 2020, 8, 1781–1800. [Google Scholar] [CrossRef]
- He, D.H.; Zhang, X.X.; Liu, P.; Liu, X.K.; Chen, X.H.; Ma, F.C.; Li, W.; Zhang, K.; Zhou, H.L. Effect of hydrothermal treatment temperature on the hydroxyapatite coatings deposited by electrochemical method. Surf. Coat. Technol. 2021, 405, 126656. [Google Scholar] [CrossRef]
- Zafar, M.S.; Fareed, M.A.; Riaz, S.; Latif, M.; Habib, S.R.; Khurshid, Z. Customized Therapeutic Surface Coatings for Dental Implants. Coatings 2020, 10, 568. [Google Scholar] [CrossRef]
- Kim, S.P.; Cho, H.R.; Choe, H.C. Bioactive element coatings on nano-mesh formed Ti-6Al-4V alloy surface using plasma electrolytic oxidation. Surf. Coat. Technol. 2021, 406, 126649. [Google Scholar] [CrossRef]
- Wu, C.; Tang, Y.F.; Mao, B.B.; Zhao, K.; Cao, S.Y.; Wu, Z.X. Rapid apatite induction of polarized hydrophilic HA/PVDF bio-piezoelectric coating on titanium surface. Surf. Coat. Technol. 2021, 405, 126510. [Google Scholar] [CrossRef]
- Lyu, L.W.; Yang, S.C.; Jing, Y.; Zhang, C.Q.; Wang, J.K. Examining trabecular morphology and chemical composition of peri-scaffold osseointegrated bone. J. Orthop. Surg. Res. 2020, 15, 406. [Google Scholar] [CrossRef]
- Nazir, M.; Ting, O.P.; Yee, T.S.; Pushparajan, S.; Swaminathan, D.; Kutty, M.G. Biomimetic Coating of Modified Titanium Surfaces with Hydroxyapatite Using Simulated Body Fluid. Adv. Mater. Sci. Eng. 2015, 2015, 407379. [Google Scholar] [CrossRef] [Green Version]
- Saini, M.; Singh, Y.; Arora, P.; Arora, V.; Jain, K. Implant biomaterials: A comprehensive review. World J. Clin. Cases 2015, 3, 52–57. [Google Scholar] [CrossRef]
- Barrere, F.; Layrolle, P.; Van Blitterswijk, C.A.; De Groot, K. Biomimetic coatings on titanium: A crystal growth study of octacalcium phosphate. J. Mater. Sci. Mater. Med. 2001, 12, 529–534. [Google Scholar] [CrossRef]
- Bansal, P.; Singh, G.; Sidhu, H.S. Improvement of surface properties and corrosion resistance of Ti13Nb13Zr titanium alloy by plasma-sprayed HA/ZnO coatings for biomedical applications. Mater. Chem. Phys. 2021, 257, 123738. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Yu, L.H.; Fu, T.; Wang, J.; Shen, F.Q.; Cui, K.K. Microstructure evolution and growth mechanism of Si-MoSi2 composite coatings on TZM (Mo-0.5Ti-0.1Zr-0.02 C) alloy. J. Alloys Compd. 2021, 894, 162403. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, T.; Cui, K.; Shen, F.; Wang, J.; Yu, L.; Mao, H. Evolution of surface morphology, roughness and texture of tungsten disilicide coatings on tungsten substrate. Vacuum 2021, 191, 110297. [Google Scholar] [CrossRef]
- Song, S.H.; Min, B.K.; Hong, M.H.; Kwon, T.Y. Application of a Novel CVD TiN Coating on a Biomedical Co-Cr Alloy: An Evaluation of Coating Layer and Substrate Characteristics. Materials 2020, 13, 1145. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Wang, S.; Du, Y.; Qiu, L.; Chen, Z.; Chen, X.; Liu, Z.; Zhang, C. Deposition of CVD-TiCN and TiAlN coatings guided with thermodynamic calculations. Int. J. Mater. Res. 2018, 109, 277–283. [Google Scholar] [CrossRef]
- Goral, M.; Moskal, G.; Swadźba, L.; Hetmańczyk, M. The Influence of Silicon Amount on Structure of Si Modified Aluminide Coating Deposited on Ti46Al7Nb Alloy by Slurry Method. Key Eng. Mater. 2011, 465, 251–254. [Google Scholar] [CrossRef]
- Gautam, V.; Patnaik, A.; Bhat, I.K. Microstructure and Wear Behavior of Single layer (CrN) and Multilayered (SiN/CrN) Coatings on Particulate Filled Aluminum Alloy Composites. Silicon 2016, 8, 417–435. [Google Scholar] [CrossRef]
- Kusmierczyk, F.; Zimowski, S.; Lukaszczyk, A.; Kopia, A.; Cieniek, L.; Moskalewicz, T. Development of Microstructure and Properties of Multicomponent MoS2/HA/PEEK Coatings on a Titanium Alloy Via Electrophoretic Deposition and Heat Treatment. Metall. Mater. Trans. A 2021, 52, 3880–3895. [Google Scholar] [CrossRef]
- Nezhad, E.Z.; Qu, X.; Musharavati, F.; Jaber, F.; Appleford, M.R.; Bae, S.; Uzun, K.; Struthers, M.; Chowdhury, M.E.H.; Khandakar, A. Effects of titanium and carbon nanotubes on nano/micromechanical properties of HA/TNT/CNT nanocomposites. Appl. Surf. Sci. 2021, 538, 148123. [Google Scholar] [CrossRef]
- Kirmanidou, Y.; Sidira, M.; Drosou, M.E.; Bennani, V.; Bakopoulou, A.; Tsouknidas, A.; Michailidis, N.; Michalakis, K. New Ti-Alloys and Surface Modifications to Improve the Mechanical Properties and the Biological Response to Orthopedic and Dental Implants: A Review. BioMed Res. Int. 2016, 2016, 2908570. [Google Scholar] [CrossRef] [Green Version]
- Hung, K.Y.; Lo, S.C.; Shih, C.S.; Yang, Y.C.; Feng, H.P.; Lin, Y.C. Titanium surface modified by hydroxyapatite coating for dental implants. Surf. Coat. Technol. 2013, 231, 337–345. [Google Scholar] [CrossRef]
- Chi, Y.X.; An, S.P.; Xu, Y.P.; Liu, M.D.; Zhang, J. In vitro biocompatibility of a sandblasted, acid-etched HA composite coating on ultrafine-grained titanium. RSC Adv. 2021, 11, 6124–6130. [Google Scholar] [CrossRef]
- Ciobanu, G.; Harja, M. Investigation on hydroxyapatite coatings formation on titanium surface. IOP Conf. Ser. Mater. Sci. Eng. 2018, 444, 032007. [Google Scholar] [CrossRef]
- Mansoor, P.; Dasharath, S.M. Synthesis and characterization of wollastonite (CaSio(3))/titanium oxide (TiO2) and hydroxyapatite (HA) ceramic composites for bio-medical applications fabricated by spark plasma sintering technology. Mater. Today-Proc. 2021, 45, 332–337. [Google Scholar] [CrossRef]
- Liu, J.Q.; Liu, J.; Attarilar, S.; Wang, C.; Tamaddon, M.; Yang, C.L.; Xie, K.G.; Yao, J.G.; Wang, L.Q.; Liu, C.Z.; et al. Nano-Modified Titanium Implant Materials: A Way Toward Improved Antibacterial Properties. Front. Bioeng. Biotechnol. 2020, 8, 576969. [Google Scholar] [CrossRef]
- Mahri, M.; Shen, N.; Berrizbeitia, F.; Rodan, R.; Daer, A.; Faigan, M.; Taqi, D.; Wu, K.Y.; Ahmadi, M.; Ducret, M.; et al. Osseointegration Pharmacology: A Systematic Mapping Using Artificial Intelligence. Acta Biomater. 2021, 119, 284–302. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Liu, P.; Liu, X.; Ma, F.; Chen, X.; Li, W.; Du, J.; Wang, P.; Zhao, J. Characterization of hydroxyapatite coatings deposited by hydrothermal electrochemical method on NaOH immersed Ti6Al4V. J. Alloys Compd. 2016, 672, 336–343. [Google Scholar] [CrossRef]
- Tschernitschek, H.; Borchers, L.; Geurtsen, W. Nonalloyed titanium as a bioinert metal—A review. Quintessence Int. 2005, 36, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Baltatu, M.S.; Vizureanu, P.; Goanţă, V.; Tugui, C.A.; Voiculescu, I. Mechanical tests for Ti-based alloys as new medical materials. IOP Conf. Ser. Mater. Sci. Eng. 2019, 572, 012029. [Google Scholar] [CrossRef] [Green Version]
- Ohtsu, N.; Hiromoto, S.; Yamane, M.; Satoh, K.; Tomozawa, M. Chemical and crystallographic characterizations of hydroxyapatite- and octacalcium phosphate-coatings on magnesium synthesized by chemical solution deposition using XPS and XRD. Surf. Coat. Technol. 2013, 218, 114–118. [Google Scholar] [CrossRef]
- Niinomi, M. Titanium Alloys. In Encyclopedia of Biomedical Engineering; Elsevier: Cambridge, MA, USA, 2019; pp. 213–224. ISBN 9780128051443. [Google Scholar]


| Alloy | Element (g) | Batch Weight (g) | Ingot Weight (g) | Efficiency (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Ti | Mo | Zr | Ta | |||||
| S1 | Ti15Mo7Zr5Ta | 51.20 | 10.52 | 5.00 | 3.62 | 70.34 | 70.33 | 99.99 |
| S2 | Ti15Mo7Zr10Ta | 63.02 | 15.08 | 7.04 | 15.00 | 70.55 | 70.45 | 99.86 |
| S3 | Ti15Mo7Zr15Ta | 44.11 | 10.70 | 5.07 | 10.61 | 70.49 | 70.20 | 99.59 |
| Sample | Ti (wt.%) | Mo (wt.%) | Zr (wt.%) | Ta (wt.%) | |
|---|---|---|---|---|---|
| S1 | Average | 73.94 | 14.57 | 6.75 | 4.74 |
| Stdev | ±0.20 | ±0.30 | ±0.10 | ±0.10 | |
| S2 | Average | 68.75 | 15.10 | 6.80 | 9.35 |
| Stdev | ±0.20 | ±0.10 | ±0.10 | ±0.30 | |
| S3 | Average | 63.71 | 14.85 | 6.99 | 14.45 |
| Stdev | ±0.50 | ±0.30 | ±0.2 | ±0.20 | |
| Ion | Na+ | Ca2+ | Mg2+ | K+ | Cl− | (HPO4)2− | (SO4)2− | (HCO3)− |
|---|---|---|---|---|---|---|---|---|
| SBF | 142.0 | 2.5 | 1.5 | 5.0 | 147.8 | 1.0 | 0.5 | 4.2 |
| Blood plasma | 142.0 | 2.5 | 1.5 | 5.0 | 103.0 | 1.0 | 0.5 | 27.0 |
| Sample | Layer Thickness (µm) |
|---|---|
| S1-HA | 35 ± 3 |
| S2-HA | 29 ± 4 |
| S3-HA | 31 ± 2 |
| Sample | Loading Deformation (N) | Release Deformation (μm) | Young Modulus (GPa) | Stiffness (N/μm) | Specimen Poisson Ration |
|---|---|---|---|---|---|
| S1-HA | 13.35 ± 0.4 | 12.75 ± 0.3 | 55.35 ± 0.3 | 6.35 ± 0.1 | 0.27 |
| S2-HA | 13.25 ± 0.5 | 12.10 ± 0.1 | 56.25 ± 0.2 | 6.25 ± 0.2 | 0.27 |
| S3-HA | 13.75 ± 0.4 | 12.35 ± 0.2 | 56.45 ± 0.3 | 5.75 ± 0.2 | 0.27 |
| Alloy | S1-HA | S2-HA | S3-HA |
|---|---|---|---|
| Liquid used | Water | ||
| Contact angle (degrees) | 73.61 | 45.64 | 57.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baltatu, M.S.; Sandu, A.V.; Nabialek, M.; Vizureanu, P.; Ciobanu, G. Biomimetic Deposition of Hydroxyapatite Layer on Titanium Alloys. Micromachines 2021, 12, 1447. https://doi.org/10.3390/mi12121447
Baltatu MS, Sandu AV, Nabialek M, Vizureanu P, Ciobanu G. Biomimetic Deposition of Hydroxyapatite Layer on Titanium Alloys. Micromachines. 2021; 12(12):1447. https://doi.org/10.3390/mi12121447
Chicago/Turabian StyleBaltatu, Madalina Simona, Andrei Victor Sandu, Marcin Nabialek, Petrica Vizureanu, and Gabriela Ciobanu. 2021. "Biomimetic Deposition of Hydroxyapatite Layer on Titanium Alloys" Micromachines 12, no. 12: 1447. https://doi.org/10.3390/mi12121447
APA StyleBaltatu, M. S., Sandu, A. V., Nabialek, M., Vizureanu, P., & Ciobanu, G. (2021). Biomimetic Deposition of Hydroxyapatite Layer on Titanium Alloys. Micromachines, 12(12), 1447. https://doi.org/10.3390/mi12121447










